 Open Access
Open Access
ARTICLE
Classifying Cardiac Anomalies in Right and Left Isomerism: Concordant and Discordant Patterns
1 Mediterranean Pediatric Cardiology Center, Bambino Gesù Children’s Hospital, Via Sirina, Taormina, Italy
2 Internal Medicine Unit, Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
3 Department of Pediatric Cardiology and Cardiac Surgery, Bambino Gesù Children’s Hospital, Roma, Italy
4 Advanced Cardiovascular Imaging Unit, Department of Imaging, Bambino Gesù Children’s Hospital, Roma, Italy
5 Radiology Department, S. Vincenzo Hospital, Taormina, Italy
6 Biosciences Institute, Newcastle University, Newcastle upon Tyne, UK
* Corresponding Author: Lilia Oreto. Email:
Congenital Heart Disease 2023, 18(1), 97-111. https://doi.org/10.32604/chd.2022.023619
Received 05 June 2022; Accepted 15 August 2022; Issue published 09 January 2023
Abstract
Aims: Evidence is emerging that, in the setting of isomerism, the atrial and bronchial arrangement are not always concordant, nor are these patterns always harmonious with the arrangement of the abdominal organs. We aimed to evaluate the concordance between these features in a cohort of patients with cardiac malformations in the setting of known isomerism, seeking to determine whether it was feasible to assess complexity on this basis, in this regard taking note of the potential value of bronchial as opposed to appendage morphology. Methods and Results: We studied 78 patients known to have isomerism of the bronchuses, 43 with right and 35 with left isomerism. Appendage anatomy could be determined in 49 cases (63%), all but one of these being concordant with bronchial anatomy. When assessing abdominal features, in only 59 cases (76%) was splenic morphology in keeping with the thoracic findings. As expected, right isomerism was associated with greater complexity of cardiac malformations, with an odds ratio of 6.53, with confidence intervals from 2.2–19.3 (p < 0.001). The odds were slightly decreased with thoraco-abdominal disharmony, when lesions shown to carry higher risk were then found in the setting of left isomerism. Conclusion: Harmony is excellent between bronchial and appendage isomerism, but less so with the arrangement of the abdominal organs. Right isomerism in our cohort, was indicative of a six-fold increase in intracardiac complexity. When discordance was found between the systems, however, the cardiac anomalies were less typical of the anticipated findings for right vs. left isomerism of the appendages.Graphic Abstract
Keywords
Isomerism is an abnormal condition in which the arrangement of thoracic organs no longer show the anticipated morphological lateralization into left and right sides. Thus, in right isomerism, the lungs, bronchuses and atrial appendages are morphologically right on both sides, while all are morphologically left in left isomerism. Although the abdominal organs are themselves never isomeric in these settings, it is anticipated that structures that are typically left-sided, such as the spleen, will be absent, whereas markers of right-sidedness, such as the inferior caval vein, will be absent in left isomerism. When the atrial appendages are isomeric, then the finding is invariably associated with congenital heart disease, which is usually complex. It follows, therefore, that a thorough characterization of both cardiac and extracardiac arrangements is paramount in patients with abnormalities of bodily lateralization, the specific diagnosis being known to have a strong impact on treatment strategy and outcome [1–4]. Although it had been suggested that isomerism may be no more than a “useful mnemonic”, molecular approaches showed that knocking out the Pitx2 or Cited-2 genes produced unequivocal right isomerism, whereas knocking out the Lefty-1 gene produced left isomerism [5–7].
It has usually been presumed that the arrangements of the atrial appendages, the bronchuses, and the abdominal organs are harmonious. Increasing evidence is now emerging, however, that different patterns of lateralization may co-exist in the same individual, generating unexpected associations [8–11]. The isomeric variants, furthermore, are usually grouped together as so-called “heterotaxy”, which historically was differentiated in terms of “asplenia” and “polysplenia” [12]. Abnormalities of the spleen, however, are often discordant with the type of thoracic isomerism, while absence of the spleen is not necessarily indicative of “heterotaxy” [13]. In the light of these ongoing uncertainties, we aimed to evaluate our own cohort of patients known to have isomeric features, ascertaining the concordance between the morphology of atrial appendages and bronchuses, and then between thoracic and abdominal features. We classified complexity by creating a score from zero to three based on the associated intracardiac lesions. This then permitted us to estimate the predictive values of bronchial as opposed to appendage morphology, taking into account also the associated abdominal features.
We conducted a retrospective search for the terms “right isomerism”, “left isomerism”, “asplenia” and “polysplenia” using the databases established between 2014 and 2020 by the Departments of Pediatric Cardiology and Radiology of Bambino Gesù Children’s Hospitals in Taormina and Rome. Inclusion criteria were the presence of at least one complete thoraco-abdominal imaging study with contrast-enhanced computed tomography or magnetic resonance revealing the presence of two symmetrical bronchuses or atrial appendages. Where available, we also collected echocardiographic data. Informed consent was obtained from patients or their parents. The study was approved by the local ethics committee (Messina Ethical Committee, protocol number 50–18). We compared the data regarding the morphology of bronchuses and atrial appendages, the presence, number and location of the spleen or spleens, the arrangement of the superior and inferior caval veins, the pulmonary veins, and the intracardiac cardiac anatomy.
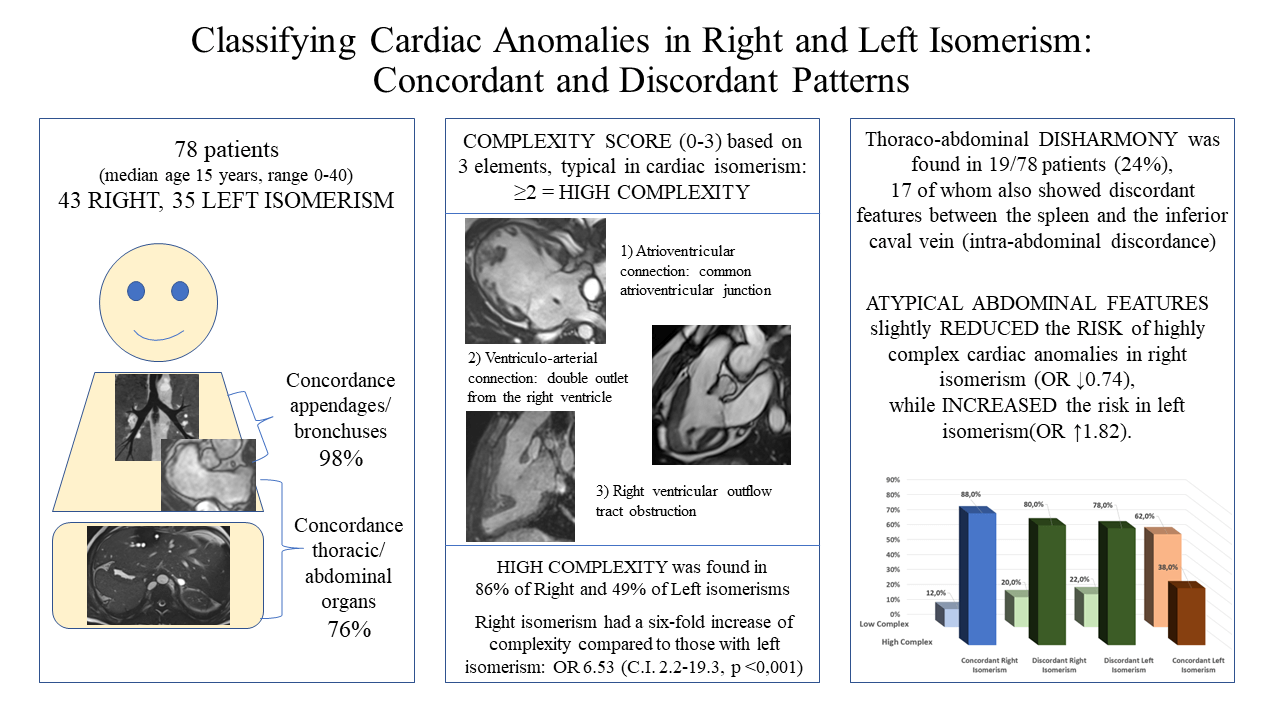
We classified appendages as showing right morphology when presenting with a broad junction with the remainder of the atrial chamber, and with the pectinate muscles within the appendage encircling the atrioventricular vestibule to reach the crux cordis [14]. Left morphology was diagnosed in presence of a narrow junction between the appendage and the remainder of the atrium, and with a smooth vestibule interposing between the extent of the pectinate muscles and the crux (Fig. 1).
Figure 1: Morphology of atrial appendages based on the extent of pectinate muscles. The upper panels show morphologically right appendages (A, ultrasound imaging; B–E magnetic resonance imaging, arrow pointing pectinate muscles extending all along the atrial wall); the lower panels show morphologically left appendages
For right bronchial morphology, we sought a shorter principal bronchus, with its first bifurcation giving rise to a superior lobar branch in eparterial fashion, along with a tri-lobed lung. Conversely, we expected the morphologically left lung to be bi-lobed, and to be supplied by a longer bronchus branching in hypoarterial fashion [15]. For the spleen, we distinguished between its absence, or the presence of multiple or polylobular spleens.
Since the normal arrangement is to find both superior and inferior caval veins (ICV) located in the right hemithorax, we noted, in all cases, presence or absence of right or left superior and inferior caval veins, presence of azygos or hemiazygos veins, and instances in which the ICV crossed from one side to the other when extending from the abdomen into the thorax. We deemed interruption of the ICV to be atypical for right isomerism, whereas normal termination of the ICV was considered atypical for left isomerism. The normal arrangement of the pulmonary veins is to enter an atrium with a morphologically left appendage. On this basis, we took the stance that totally anomalous pulmonary venous connection must have been present in individuals with isomeric right appendages. So as to avoid any potential misunderstanding between anatomy and nomenclature, we also deemed the connections to be anomalous whenever one or more of the pulmonary veins did not enter an atrial chamber, irrespective of its right- or left-sidedness, thus distinguishing partially as opposed to totally anomalous connection as we usually do in the setting of usual atrial arrangement. We also recognized the situation in which the pulmonary veins joined together to form a confluence prior to entering one or other of the atrial chambers, which in our cohort was often in midline fashion. This finding is of significance since the venoatrial junction can become stenotic.
When assessing intracardiac anatomy, we followed the sequential-segmental approach [16], accounting for the types and modes of atrioventricular and ventriculo-arterial connections, and noting the obstruction of the right or left ventricular outflow tracts if present. In this regard, in the setting of the biventricular arrangement, the atrioventricular connections must be mixed. In these instances, therefore, we also described the direction of looping of the ventricular mass. The mixed connections were distinguished from double inlet or absent atrioventricular connections. Ventriculo-arterial connections were described as being concordant, discordant, double outlet, or single outlet [16]. We did not find any examples of common arterial trunk. We described dextrocardia when the cardiac apex was pointing to the right, and levocardia when pointing leftward. The aortic arch was classified as being left- or right-sided according to its relationship to the trachea, with note taken of any anomalies of origin of the brachiocephalic arteries.
Since several variables were categorical or dichotomous, we chose a standard non-parametric approach, presenting continuous variables as median and range, while expressing numerically the categorical ones. Frequencies and percentages were estimated. The concordance between appendages and bronchuses was initially assessed, and then statistically verified by Chi-square testing, as was concordance between the spleen and ICV (intra-abdominal concordance). Concordance between the thoracic and abdominal features was then further tested using the Chi-square approach. Cohen’s Kappa test was also implemented to verify the agreement between appendages and bronchuses. Since the features of the cardiac anomalies were categorical, we created a complexity score based on 3 key findings most typically associated with isomerism of the appendages, namely the commonality of the atrioventricular junction, the ventriculo-arterial connection of double outlet right ventricle, and the presence of obstruction of the right ventricular outflow tract. We chose these three features since they are present in the great majority of cases with isomerism (common atrioventricular junction in 58%, double outlet right ventricle in 54%, right outflow obstruction in 78%), and therefore we considered them as the most typical expression of complex congenital heart disease in our population. We considered patients with two or more of these features to have high complexity, while a score of one or zero was deemed to represent low complexity. Testing the complexity scores then permitted consistent estimations of the odds ratio.
We excluded 2 patients from our initial trawl, one with absence of the spleen, and the other with multiple spleens, because the thoracic organs were lateralized. We also excluded 3 patients in whom bronchial morphology could not be determined with certainty. This left 78 individuals, 40 being male, known to have bronchial isomerism. Their median age was 15 years, with a range from birth to 40 years. Right bronchial morphology was found in 43, and left morphology in 35 patients. Due to the limitations of the images available, appendage morphology could be determined in only 49 patients, with right isomerism found in 26 cases (Fig. 2).
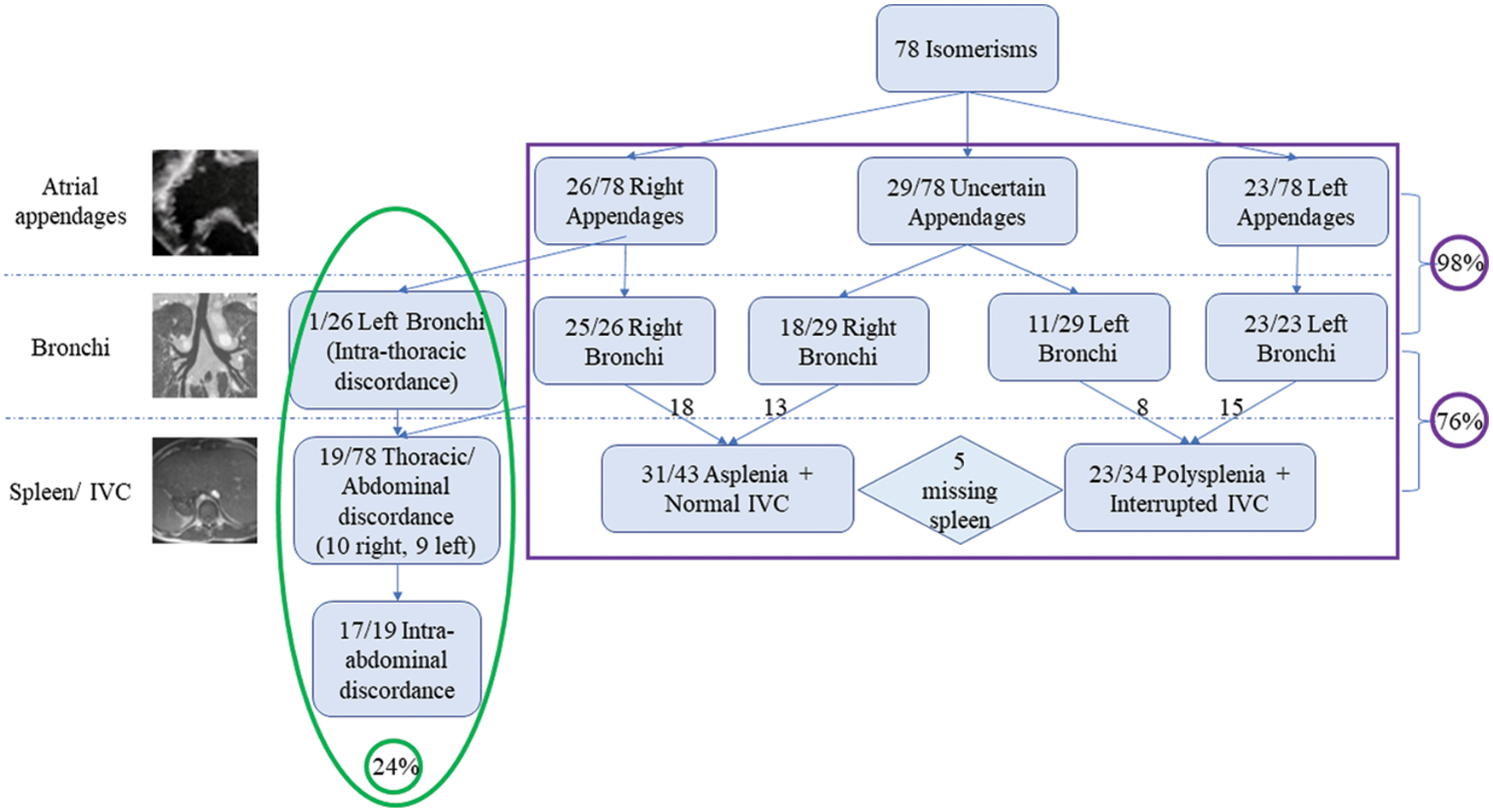
Figure 2: Classification of our population based on the features of atrial appendages, bronchial pattern, and our chosen abdominal characteristics. The purple box includes cases showing concordance within the systems, while green box includes discordant cases
In all these patients bar one, there was harmony between the arrangements of appendages and bronchuses, giving 98% concordance. The discordant individual had isomeric right atrial appendages with left bronchial isomerism. In the 29 patients in whom we could not characterize the appendages, 18 had right, and 11 left bronchial isomerism.
When comparing the abdominal features with bronchial isomerism, only 59 (76%) cases showed the anticipated arrangements of the spleen and the ICV. Of these, 33 had right and 26 left bronchial isomerism. In 5 patients we were unable, with certainty, to determine splenic morphology, but ICV anatomy was still consistent with the thoracic features. In these 19 individuals with thoraco-abdominal disharmony, 10 showed right and 9 left bronchial isomerism. In 17 of these individuals, furthermore, there was additional discordance between the morphologies of the spleen and the IVC (Table 1).
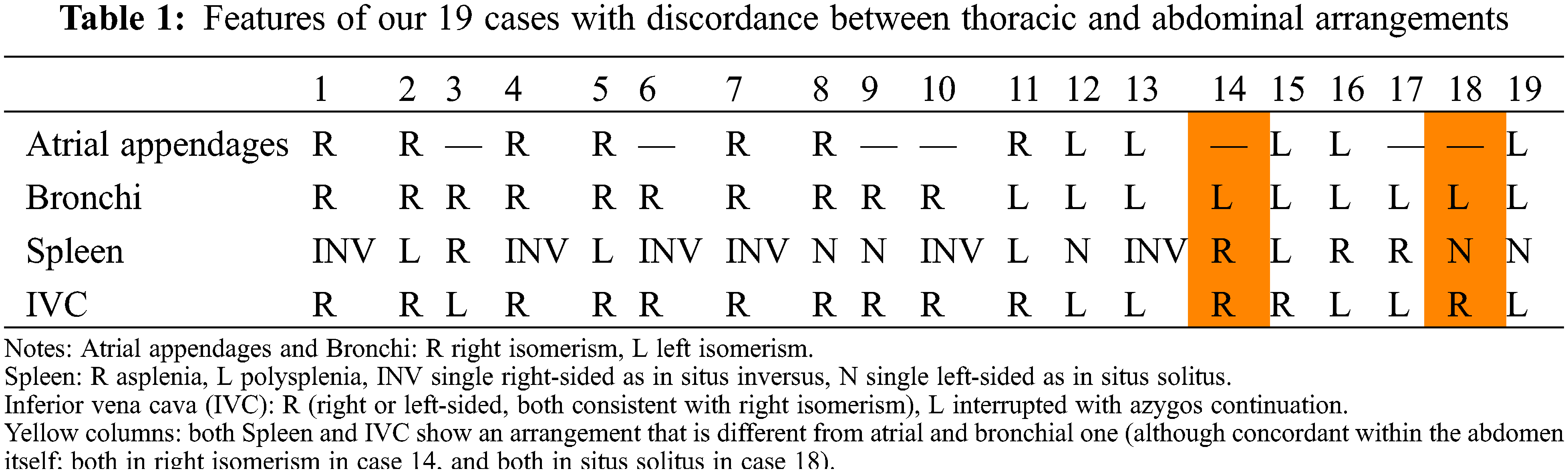
In 14 individuals, the discordance was splenic, with the arrangement of the ICV being unexpected in 3 cases. Both the spleen and the ICV showed discordant features in two patients with left bronchial isomerism. When taking account of the features in the 49 individuals known to have isomeric appendages, we found a very high agreement with bronchial morphology (kappa 0.959, p < 0.0001). When assessing the same relationship for the abdominal features, the concordance was complete. The overall patterns of splenic arrangement, caval and pulmonary venous anatomy, segmental-sequential cardiac anatomy and associated cardiac defects are shown in Table 2 and illustrated in Figs. 3–6.
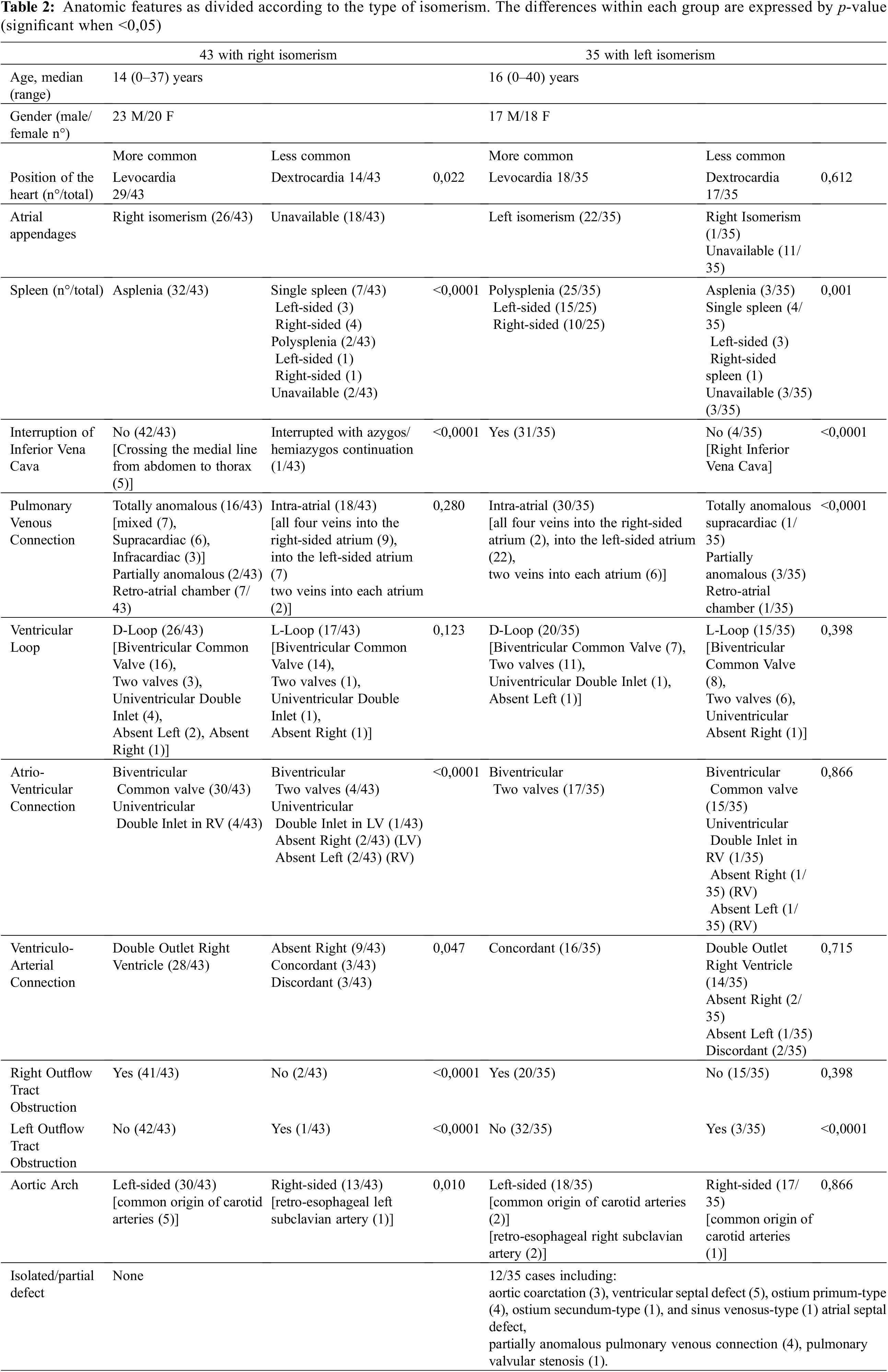
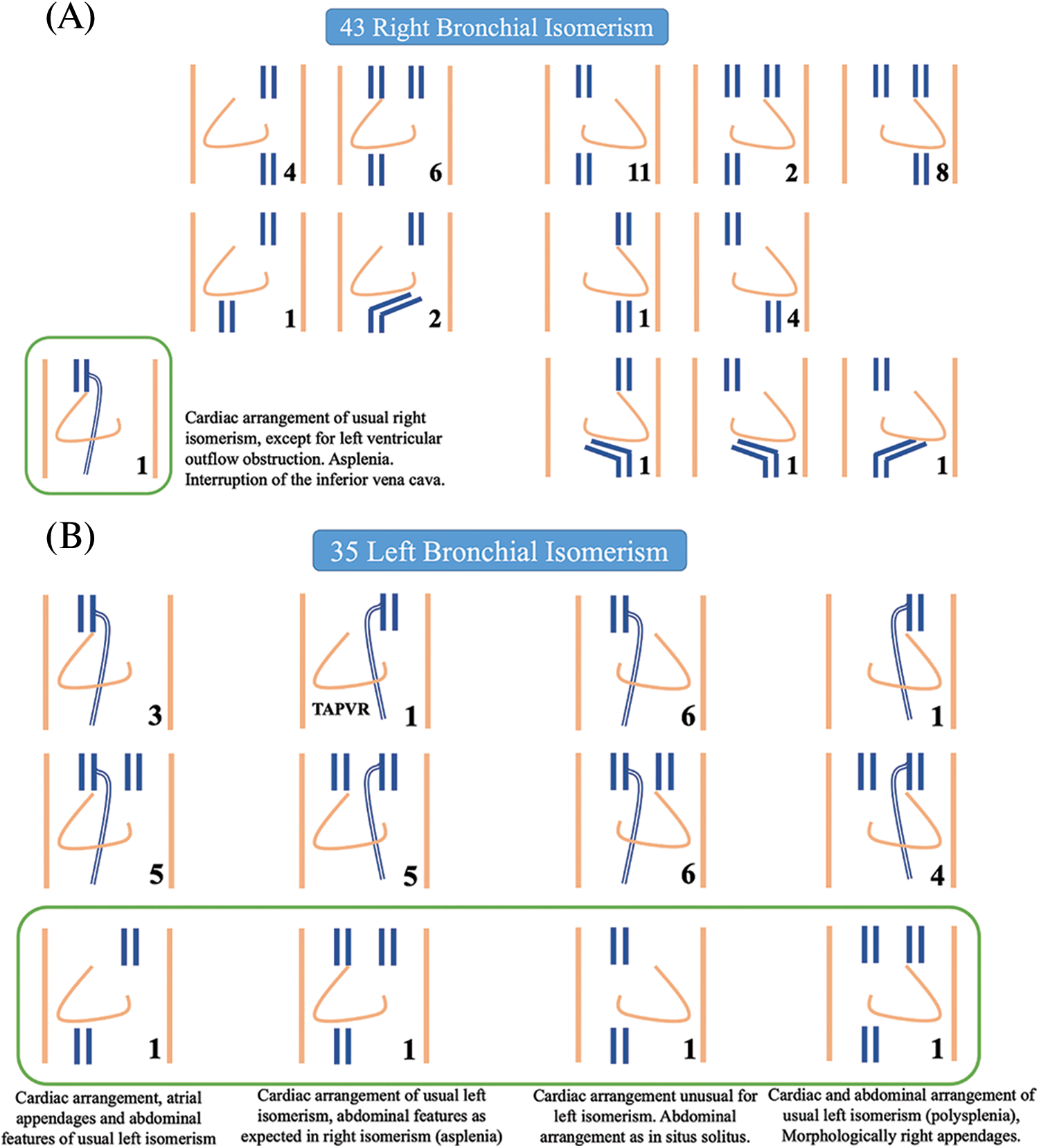
Figure 3: Cartoon showing all the different patterns of presence and location of superior and inferior caval veins observed in our patients with right (Panel A) and left isomerism (Panel B). Levocardia and dextrocardia are reported on the right and left side of each figure, respectively. The numbers indicate how many patients exhibited that particular arrangement. The green box contains cases of «atypical» arrangement of the caval veins with respect to bronchial isomerism. TAPVR, totally anomalous pulmonary venous return
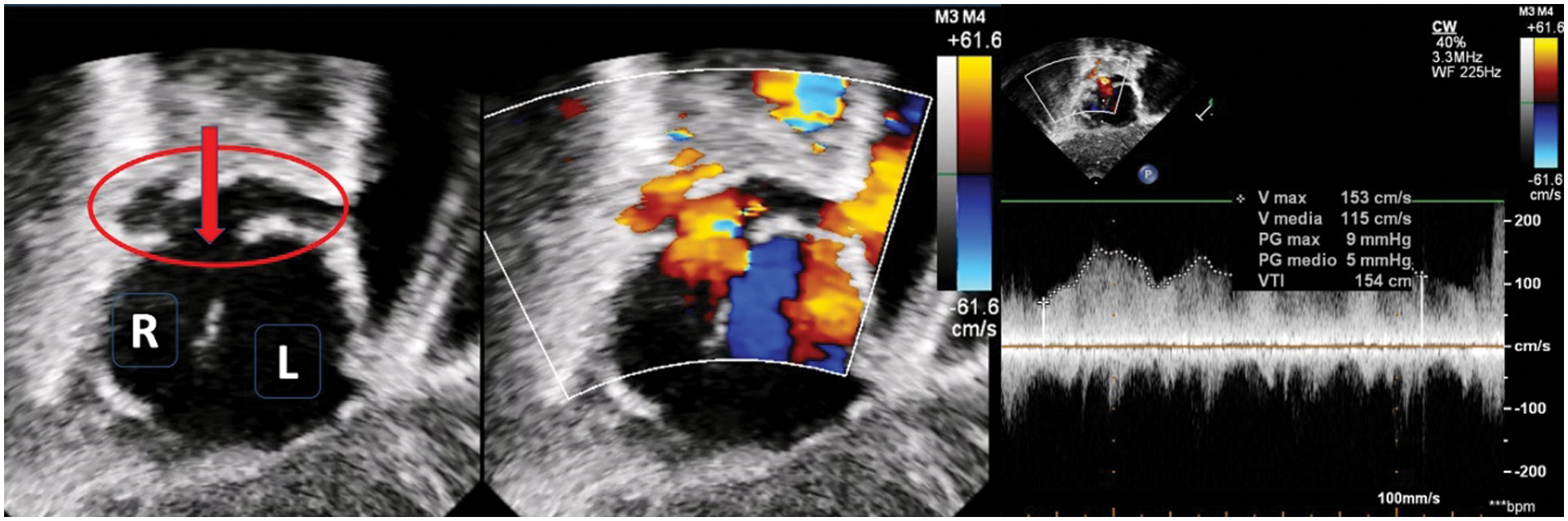
Figure 4: Ultrasound imaging (subcostal view) in a patient with right isomerism and dextrocardia. The left panel shows right-positioned and left-positioned atrial chambers. The red circle shows a retro-atrial confluence of the pulmonary veins which drains into the atrium through an open connection (red arrow). Color-Doppler in the middle and pulsed-wave Doppler in the right panel account for a slightly increased flow velocity through the connection (mean Doppler gradient 5 mmHg)
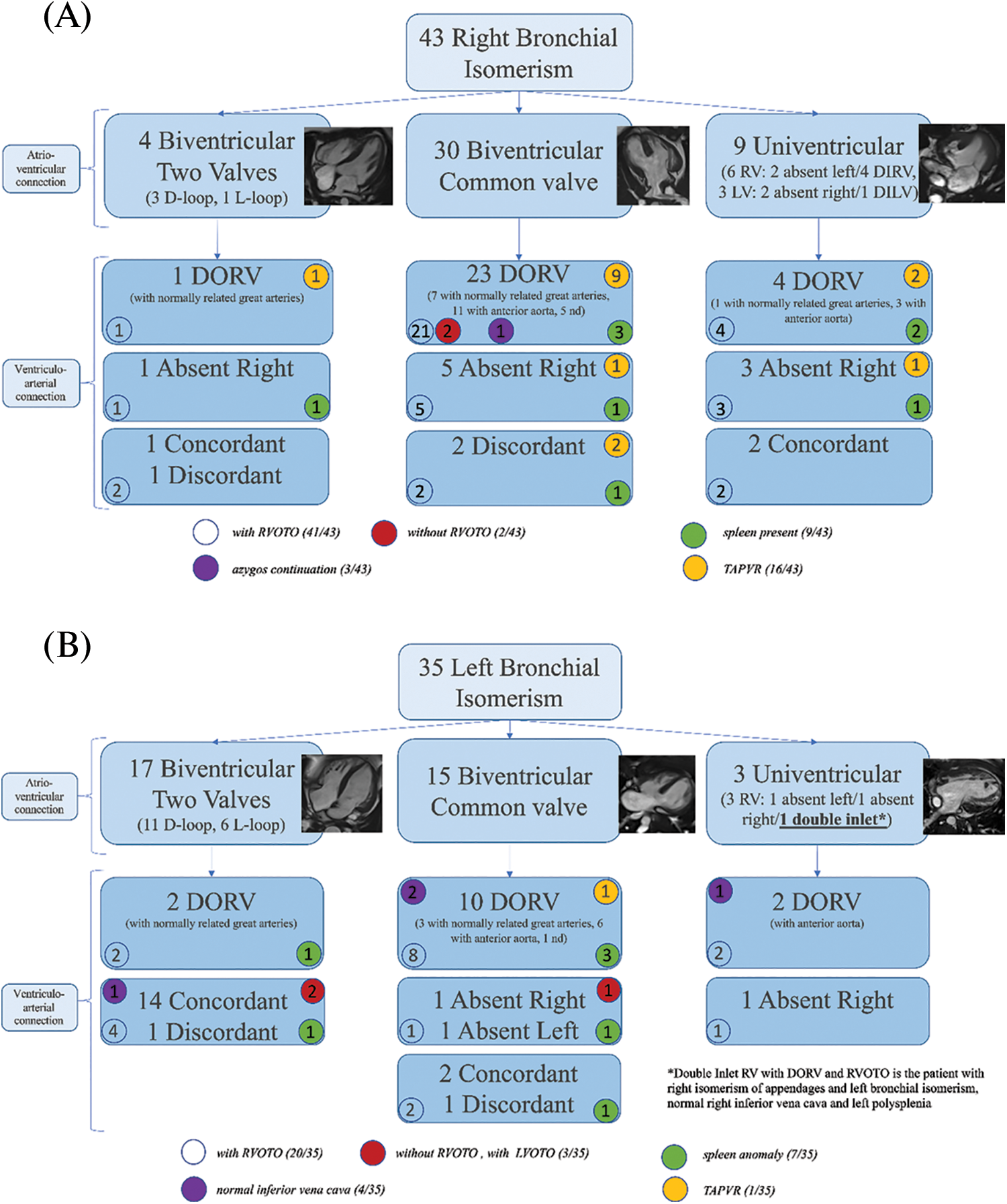
Figure 5: Classification of patients based on the sequential-segmental approach, for right (Panel A) and left isomerism (Panel B). Biventricular connections, by definition, are mixed. The univentricular connections are related not to the atrium, but to the dominant ventricle. “Absent left”, therefore, is indicative of a dominant morphologically right ventricle, and vice versa. Numbers within the circles represent the number of patients expressing that feature. AV, atrioventricular; RV, right ventricle; LV, left ventricle; DIRV double-inlet right ventricle; DILV, double-inlet left ventricle; DORV, double-outlet right ventricle; RVOTO, right ventricular outflow tract obstruction; TAPVR, totally anomalous pulmonary venous drainage
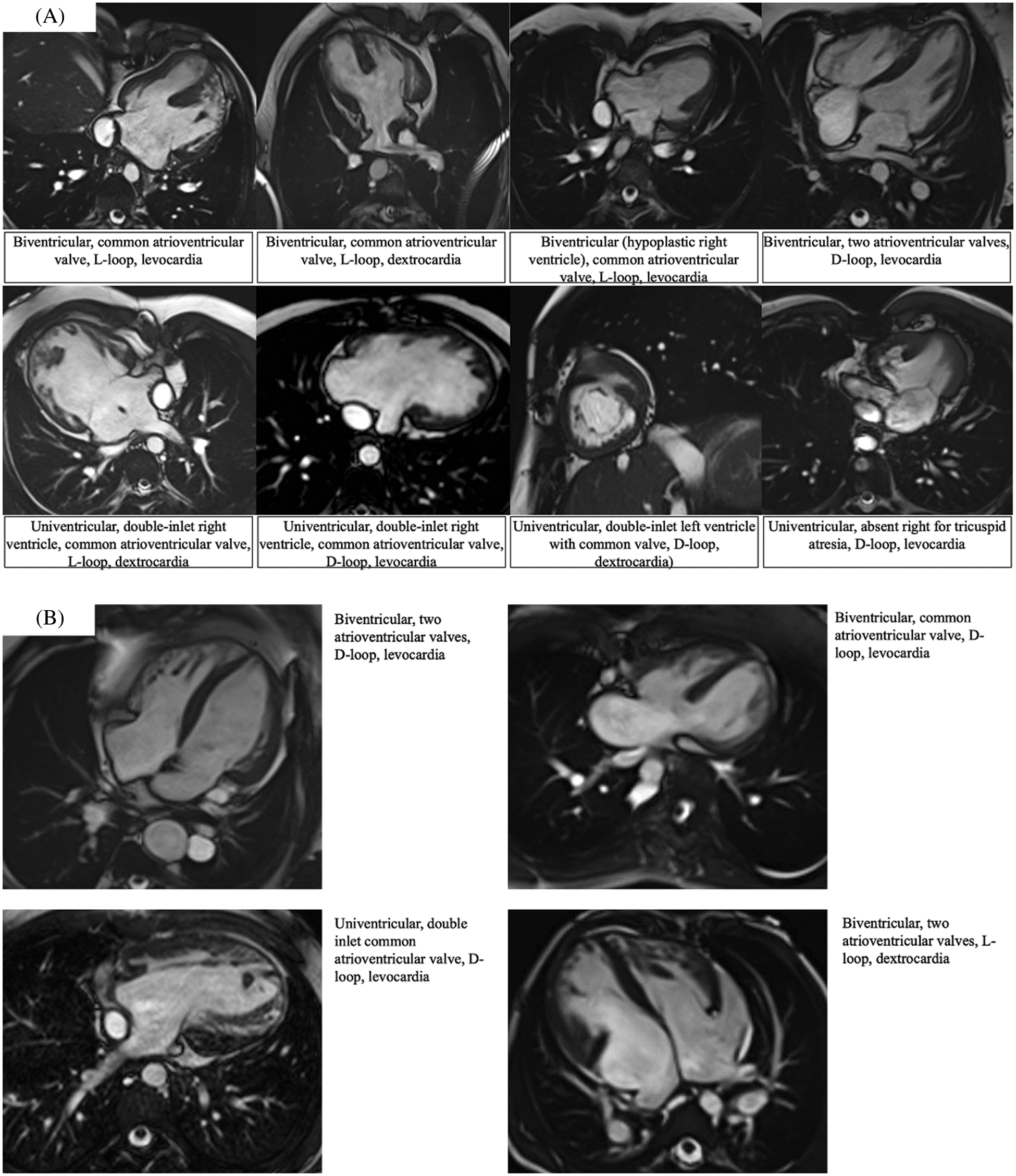
Figure 6: Magnetic resonance imaging showing the different atrioventricular connections as observed in right {Panel A} vs. left {Panel B} isomerism. Each panel is supported by a short description
Our complexity score revealed that 86% of those with right isomerism had highly complex lesions. In contrast, only 49% of those with left isomerism had highly complex lesions (Fig. 7).
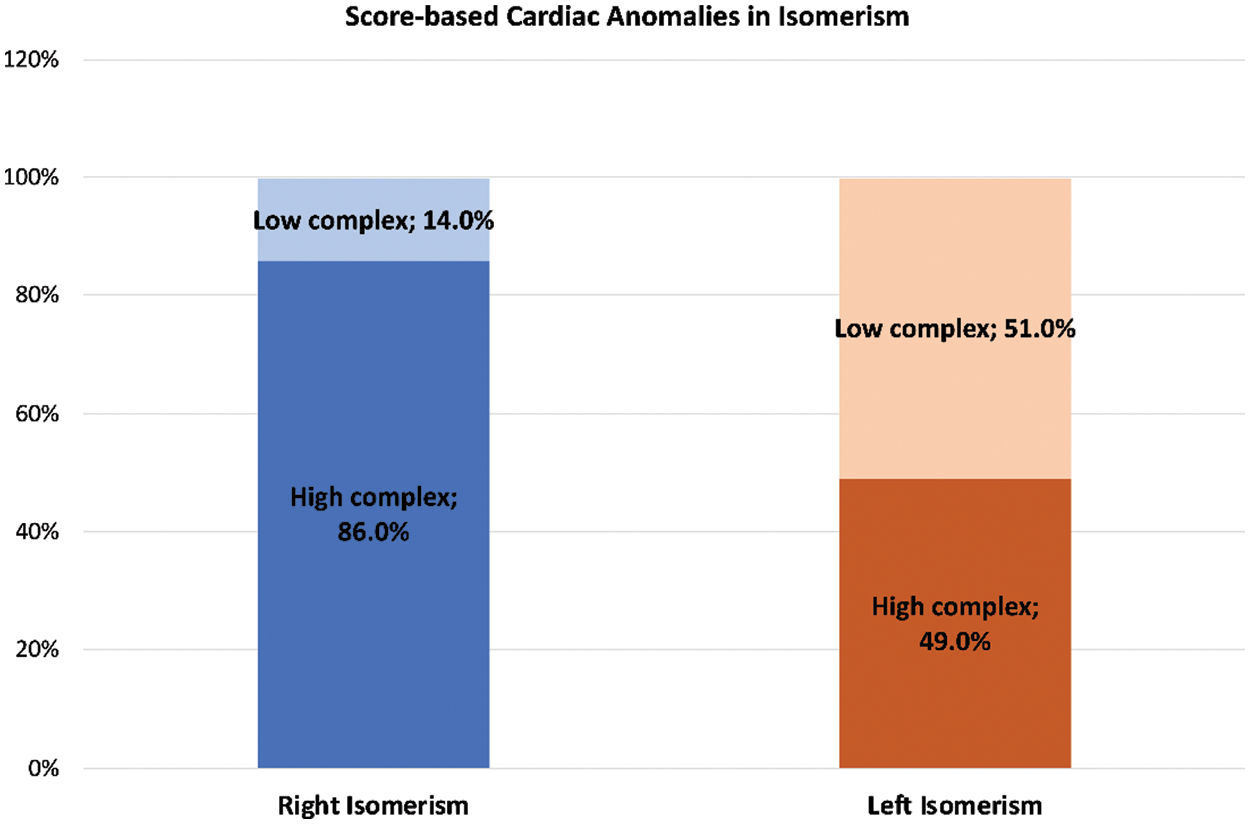
Figure 7: Bar graphs illustrating the frequencies of highly complex (dark colour) and less complex (light colour) cardiac anomalies as observed in the settings of right (blue) and left isomerism (orange)
The significantly higher complexity in right as opposed to left isomerism produced an odds ratio of 6.53 (CI 2.2–19.3; p < 0.001). This relationship was then further influenced when there was thoraco-abdominal discordance (Fig. 8).
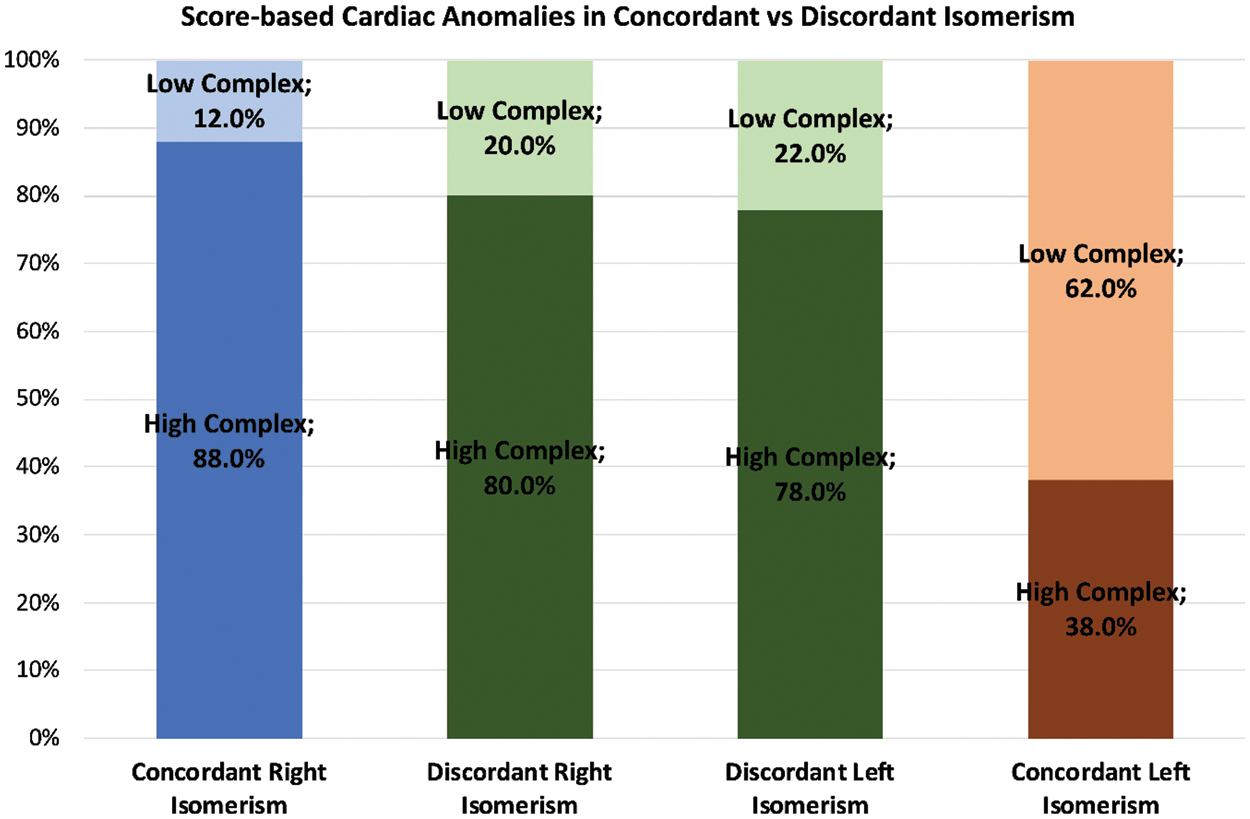
Figure 8: The bar graphs, using the same system as described for Fig. 7, show how the complexities are modified when there is concordance, shown in blue/orange, as opposed to discordance, shown in green, between the thoracic and abdominal organs
Disharmony in the setting of right isomerism then decreased the odds ratio by 0.741 (CI 0.12–4.6), albeit without achieving statistical significance. On the other hand, thoracic-abdominal discordance increased the risk of cardiac anomalies producing high complexity when found in the setting of left isomerism. The odds ratio was then 1.82, (CI 0.44–7.47), although again this change did not achieve statistical significance. Taken overall, therefore, right as compared to left isomerism was associated with a six-fold increased risk of high complexity. Presence of atypical abdominal features minimally reduced this risk for those with right isomerism, but increased the risk 1.8-fold in the setting of left isomerism.
Our study confirms that isomeric features, when present, are found only for the thoracic organs. In this regard, all our patients could be distinguished as having either right or left bronchial morphology. Bronchial morphology, moreover, then proved highly predictive for the morphology of the atrial appendages, with only one of our patients showing discordance in this respect. Applying the score designed to grade complexity, we then found that those with right isomerism had a six-fold increase of complexity compared to those with left isomerism, albeit that almost half of these latter individuals also had highly complex intracardiac lesions. In keeping with previous accounts, the commonest combination of lesions in our patients with right isomerism was an atrioventricular septal defect with common atrioventricular junction, double outlet right ventricle, and obstruction of the right ventricular outflow tract. This combination produces the highest complexity score of 3. In those with functionally univentricular atrioventricular connections, the dominant ventricle was usually morphologically right. One of the less complex combinations in our population with right isomerism, yielding a score of only one, was tetralogy of Fallot. The complexity in the setting of right isomerism, of course, is further increased by the universal presence of anomalous pulmonary venous connection. This feature is totally anomalous, in anatomical terms, even if the pulmonary veins return to the heart. In more than half of our patients, nonetheless, the veins drained in extracardiac fashion. Any planned surgical interventions will potentially be more complex when there is extracardiac drainage. Again, in keeping with previous experiences, we found left isomerism much more frequently to be associated with “simple” defects, such as isolated ventricular septal defects or isolated aortic coarctation. Anomalous pulmonary venous drainage was found in only 5 patients, which except for one individual was always partial. Lesions deemed to confer high complexity, nonetheless, were still found in almost half of our cohort (Table 2).
A major finding was the frequent occurrence of discordance between the systems of organs. In the presence of such disharmony, furthermore, the associated cardiac anomalies are less typical of the anticipated patterns for right or left isomerism. The combinations proved to be ameliorative in terms of complexity for right isomerism, but exerted detrimental effects in the setting of left isomerism.
The thoraco-abdominal discordance, furthermore, was associated with discrepancies between the arrangements within the abdominal cavity. In only 2 of the 19 cases with such discordance were the arrangements of the spleen and ICV in harmony, in both instances being as expected for right isomerism while the atrial appendages and bronchuses showed left isomerism. Interruption of the ICV emerges as the most accurate discriminating feature, with splenic anatomy presenting a wider variability. Neither feature, however, proved uniformly predictive of the thoracic arrangement.
We are well aware that such findings of discordance in the setting of so-called “heterotaxy” between the arrangement of appendages, bronchuses, and abdominal organs have already received emphasis [8,9,17–19]. Such discordances, however, do not mean that segregation of “heterotaxy” on the basis of isomerism should be discarded. On the contrary, the anomalies of lateralization should simply be specified, paying attention to the arrangement of the appendages, the bronchial pattern, and the abdominal features [5,9]. To the best of our knowledge, however, the possibility that such discordances may influence the expression of the associated cardiac anomalies has not previously been explored. If confirmed by more extensive studies, this data will offer useful prognostic information from the time of initial evaluation, which may well be in fetal life. Previous studies have already demonstrated that postnatal survival is greater for those with left as opposed to right isomerism, at least in the first two decades of life [3,17].
The point of clinical distinction between the appendages is key, since it is this feature which dictates cardiac isomerism. In our study, we were able to characterize morphology of the appendages with certainty in only two-thirds of patients. This may be due to the fact that we adhered strictly to the notion that the shape of the appendages was insufficient to attribute right or left morphology. Instead, we tried, as far as possible, to use the feature of the extent of pectinate muscles within the appendages relative to the atrioventricular junctions, as is the case for morphologists when holding the hearts in their hands [5]. Echocardiography in neonates can offer useful signs regarding shape adequately to characterize atrial morphology (Fig. 1) [20]. Assessment of the extent of the pectinate muscles, or presence of terminal crests, however, may not be always possible during life for several reasons, since the images provided may not be sufficiently clear, patients have already undergone multiple surgeries, often inducing some kind of volume overload. When seeking to assess the pectinate muscles, our rate of discrimination was lower compared to the findings reported by Yim et al. [9]. Morphologists are usually able to define the anatomy of the appendages in all hearts with so-called heterotaxy. In the investigation of Frescura et al. [21], a perfect agreement was then found between the arrangements of appendages, the bronchuses, and the abdominal organs. Tremblay et al. [22], in contrast, who examined almost 200 hearts with unequivocal isomerism of the appendages, in keeping with our observations, found discordances between the atrial and bronchial patterns.
We are aware of the limitations of our own study, the main ones being its retrospective nature, and the relatively high incidence of patients in whom we were unable to classify the precise morphology of the appendages. Our experience has highlighted the complex tridimensional nature of the appendages and their precise identification requires a dedicated multiplanar approach that it is unlikely to be available in retrospective studies. We also recognize that the small number of patients in our cohort reduced the predictive value and the statistical significance of our complexity score. We anticipate that ongoing studies will serve to clarify our hypothesis regarding the impact of disharmony between the systems on the complexity of the associated cardiac anomalies. We also expect that prospective studies will significantly improve the rate of identification of the atrial appendages by imaging during life.
In conclusion, our study confirms that isomeric features are confined to the thoracic organs. We found bronchial morphology then to be highly predictive for the arrangement of the appendages. The concordance between the thoracic arrangement and the anticipated abdominal features, in contrast, was far from perfect. When calculating a score for intracardiac complexity, we found that right isomerism was associated with a six-fold increase compared to left isomerism. When there was discordance within the systems, the associated cardiac anomalies were less characteristic of the anticipated findings for right as opposed to left isomerism. This finding itself, of course, could well have been conditioned by our inability always to identify specifically the morphology of the atrial appendages. Additional studies will now be required to clarify this aspect.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: LO, FSI, ADP, RHA, SA; data collection: LO, PC, TPS, AC; analysis and interpretation of results: LO, GM, PR, PG, PG, LG; draft manuscript preparation: LO, AS, RHA, SA. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Geddes, G. C., Samudrala, S. S., Earing, M. G. (2020). Neonatal assessment of infants with heterotaxy. Clinics in Perinatology, 47(1), 171–182. [Google Scholar]
2. Landis, B. J., Cooper, D. S., Hinton, R. B. (2016). CHD associated with syndromic diagnoses: Peri-operative risk factors and early outcomes. Cardiology in the Young, 26(1), 30–52. DOI 10.1017/S1047951115001389. [Google Scholar] [CrossRef]
3. Loomba, R. S., Nijhawan, K., Anderson, R. (2016). Impact of era, type of isomerism, and ventricular morphology on survival in heterotaxy: Implications for therapeutic management. World Journal for Pediatric and Congenital Heart Surgery, 7(1), 54–62. [Google Scholar]
4. Qureshi, A. U., Kazmi, U., Kazmi, T., Sadiq, M. (2012). Congenital heart defects associated with atrial heterotaxy. Journal of College of Physicians and Surgeons Pakistan, 22(9), 549–552. [Google Scholar]
5. Loomba, R. S., Hlavacek, A. M., Spicer, D. E., Anderson, R. H. (2015). Isomerism or heterotaxy: Which term leads to better understanding? Cardiology in the Young, 25(6), 1037–1043. [Google Scholar]
6. Bamforth, S. D., Bragança, J., Farthing, C. R., Schneider, J. E., Broadbent, C. et al. (2004). Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nature Genetics, 36(11), 1189–1196. [Google Scholar]
7. Meno, C., Shimono, A., Saijoh, Y., Yashiro, K., Mochida, K. et al. (1998). Lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell, 94(3), 287–297. [Google Scholar]
8. Loomba R. S., Pelech A. N., Shah P. H., Anderson R. H. (2016). Determining bronchial morphology for the purposes of segregating so-called heterotaxy. Cardiology in the Young, 26(4), 725–737. DOI 10.1017/S1047951115001195. [Google Scholar] [CrossRef]
9. Yim, D., Nagata, H., Lam, C. Z., Grosse-Wortmann, L., Seed, M. et al. (2018). Disharmonious patterns of heterotaxy and isomerism: How often are the classic patterns breached? Circulation Cardiovascular Imaging, 11(2), e006917. [Google Scholar]
10. Sanders, S. P., Geva, T. (2018). Classifying heterotaxy syndrome: Time for a new approach. Circulation: Cardiovascular Imaging, 11(2), e007490. DOI 10.1161/CIRCIMAGING.118.007490. [Google Scholar] [CrossRef]
11. Loomba, R. S., Tretter, J. T., Anderson, R. H. (2018). Letter by loomba et al regarding article, “Disharmonious patterns of heterotaxy and isomerism: How often are the classic patterns breached?” Circulation Cardiovascular Imaging, 11(6), e007718. [Google Scholar]
12. Van Mierop, L., Gessner, I., Schliebler, G. (1972). Asplenia and polysplenia syndrome. Birth Defects, 1, 74–82. [Google Scholar]
13. Waldman, J. D., Rosenthal, A., Smith, A. L., Shurin, S., Nadas, A. S. (1977). Sepsis and congenital asplenia. The Journal of Pediatrics, 90(4), 555–559. DOI 10.1016/S0022-3476(77)80365-X. [Google Scholar] [CrossRef]
14. Anderson, R. H., Brown, N. A., Meno, C., Spicer, D. E. (2015). The importance of being isomeric. Clinical Anatomy, 28(4), 477–486. DOI 10.1002/ca.22517. [Google Scholar] [CrossRef]
15. Jacobs, J. P., Anderson, R. H., Weinberg, P. M., Walters, H. L., Tchervenkov, C. I. et al. (2007). The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiology in the Young, 17(Suppl 2), 1–28. [Google Scholar]
16. Anderson, R. H., Shirali, G. (2009). Sequential segmental analysis. Annals of Pediatric Cardiology, 2(1), 24–35. [Google Scholar]
17. Baban, A., Cantarutti, N., Adorisio, R., Lombardi, R., Calcagni, G. et al. (2018). Long-term survival and phenotypic spectrum in heterotaxy syndrome: A 25-year follow-up experience. International Journal of Cardiology, 268, 100–105. [Google Scholar]
18. Thiene, G., Frescura, C. (2019). Asplenia and polysplenia syndromes: Time of successful treatment and updated terminology. International Journal of Cardiology, 274, 117–119. [Google Scholar]
19. Tawfik, A. M., Batouty, N. M., Zaky, M. M., Eladalany, M. A., Elmokadem, A. H. (2013). Polysplenia syndrome: A review of the relationship with viscero-atrial situs and the spectrum of extra-cardiac anomalies. Surgical and Radiologic Anatomy, 35(8), 647–653. DOI 10.1007/s00276-013-1100-x. [Google Scholar] [CrossRef]
20. Teele, S. A., Jacobs, J. P., Border, W. L., Chanani, N. K. (2015). Heterotaxy syndrome: Proceedings from the 10th international PCICS meeting. World Journal for Pediatric and Congenital Heart Surgery, 6(4), 616–629. DOI 10.1177/2150135115604470. [Google Scholar] [CrossRef]
21. Frescura, C., Ho, S. Y., Giordano, M., Thiene, G. (2020). Isomerism of the atrial appendages: Morphology and terminology. Cardiovascular Pathology, 47, 107205. DOI 10.1016/j.carpath.2020.107205. [Google Scholar] [CrossRef]
22. Tremblay, C., Loomba, R. S., Frommelt, P. C., Perrin, D., Spicer, D. E. et al. (2017). Segregating bodily isomerism or heterotaxy: Potential echocardiographic correlations of morphological findings. Cardiology in the Young, 27(8), 1470–1480. DOI 10.1017/S104795111700049X. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools