 Open Access
Open Access
CASE REPORT
Neocuspidization of the Pulmonary Valve with Autologous Pericardium in the Adult Patient with Ventricular Septal Defect and Infective Endocarditis: A Case Report and Review of the Literature
1 Department of Adult Cardiac Surgery, Heart Institute, Kyiv, Ukraine
2 Department of Pediatric Cardiac Surgery, Heart Institute, Kyiv, Ukraine
* Corresponding Author: Igor Mokryk. Email:
Congenital Heart Disease 2022, 17(6), 641-646. https://doi.org/10.32604/chd.2022.025096
Received 22 June 2022; Accepted 09 August 2022; Issue published 11 October 2022
Abstract
Congenital heart disease (CHD) is one of the risk factors for developing infective endocarditis (IE). Right-sided IE occurs in 5%–10% of endocarditis cases, and pulmonary valve (PV) is involved in less than 2% of such patients. Literature data are few, and optimal treatment methods, indications for surgery, and types of operative techniques are still under debate. We present an adult patient with a rare combination of the ventricular septal defect (VSD) and PV IE who underwent surgical treatment. Neocuspidization with autologous pericardium was utilized for the reconstruction of his PV. We discuss details of this novel surgical technique.Graphic Abstract
Keywords
Right-sided infective endocarditis (RSIE) accounts for 5% to 10% of all IE cases [1]. It is most often associated with Intravenous drug abuse (IVDA). Patients with intracardiac devices, long-standing central venous catheters, and underlying CHD also have a higher risk for the development of RSIE [2–4]. Pulmonary valve IE (PVIE) is rare and occurs in less than 2% of endocarditis cases. Literature data regarding the management of PVIE are scarce [4]. Therefore, medical strategies, indications for surgery, and the type of operative technique are still under debate in this clinical situation.
The aortic valve neocuspidization (AVNeo) technique, described by Ozaki et al. [5] is a significant advancement in the field of aortic valve surgery. Excellent mid-term results are reported. We used the same principle for the PV neocuspidization (PVNeo) in the adult patient with VSD and PVIE.
A 46-years old male was admitted to a local hospital with complaints of low-grade fever, fatigue, and dyspnoea on physical exertion. During three weeks he received treatment, including antibiotics. There was no positive clinical effect. After a mobile mass (vegetation) on PV was visualized at transthoracic echocardiography (TTE), the patient was referred to our clinic. A man was diagnosed with perimembranous VSD in childhood. He did not receive any surgical correction, and he did not visit his cardiologist on a regular basis. The patient notes periods of low-grade fever over the past thirty months. His family physician diagnosed him with upper respiratory and pulmonary infections associated with pulmonary overflow and prescribed courses of antibiotic therapy. Also, the patient was diagnosed with glomerulonephritis two years ago. The man has no history of IVDA or recent dental work.
Physical examination revealed hepatosplenomegaly and mild pedal oedema. Auscultation of the precordium revealed a pan-systolic murmur best heard at the left lower sternal border. A pan-diastolic murmur was also heard at the left upper sternal border with the epicenter in 2nd intercostal space.
The patient’s blood work was significant for: C-reactive protein 22.7 mg/L; procalcitonin 0.495 ng/ml; GFR 44 mL/min; NT-proBNP 7334 pg/mL. His blood culture was negative.
TTE detected a perimembranous VSD 8 mm in diameter with a left-to-right shunt; Qp/Qs was 1.6/1; Pulmonary Artery (PA) pressure was 73 mm Hg. PV was severely insufficient with large fixed and mobile vegetations.
Based on Duke Criteria patient was diagnosed with IE: one major criteria (echocardiographic evidence of endocardial involvement) and three minor criteria (predisposing heart disorder (VSD), fever, and immunologic phenomena (glomerulonephritis)) [2]. The patient received medical treatment with intravenous antibiotics (vancomycin and meropenem). After one week of such therapy, the clinical condition did not improve. His Laboratory Data and Echocardiographic presentation also had no positive dynamics. The patient was qualified for surgery: Pulmonary valve repair/replacement and VSD closure.
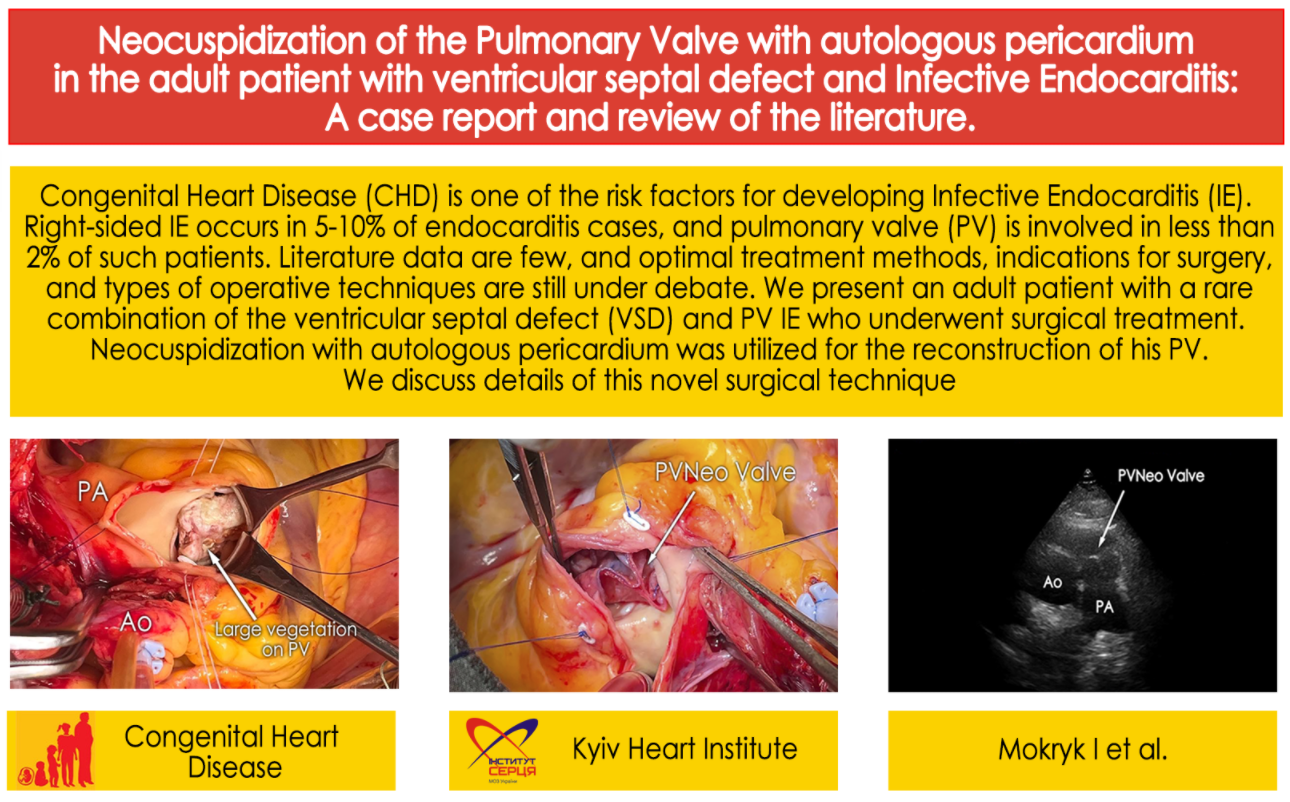
An operation was performed through a midline sternotomy. A 7 cm × 8 cm pericardial flap was harvested. It was treated with 0.625% buffered glutaraldehyde solution for 10 min, followed by triple rinsing in saline for 6 min each time. The central aortic and bicaval cannulation was used to initiate cardiopulmonary bypass. The heart was arrested with crystalloid cardioplegia. After right atriotomy, the intracardiac anatomy was inspected: no vegetations were revealed on the tricuspid valve, and a perimembranous VSD (8 mm in diameter) was visualized. A pulmonary valve was approached as a next step to remove infected material from the surgical field. We used transverse pulmonary arteriotomy 1 cm above commissures to visualize the PV: all cusps were destroyed with massive vegetations (Fig. 1).
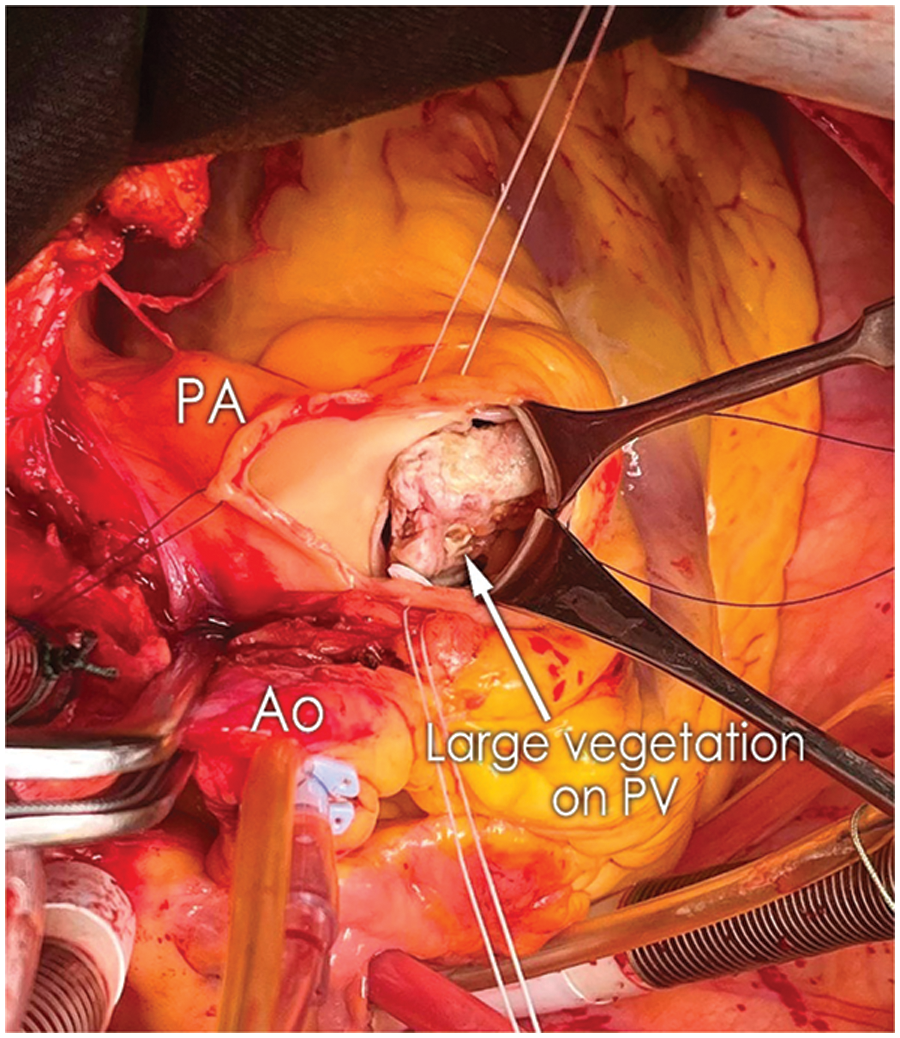
Figure 1: Intraoperative view demonstrating large vegetations involving all three cusps of the pulmonary valve (Ao, aorta; PA, pulmonary artery)
The PV was excised, and the local endocardium was topically treated with a povidone-iodine solution. Next, the VSD was closed with a patch of glutaraldehyde-treated autopericardium (GTAP) using the running suture technique. A PVNeo was performed as a final step of the procedure. Intercommissural distance was measured, and the corresponding leaflets (two 23-mm and one 25-mm) were trimmed using sizers and a template developed by Ozaki et al. [5]. The leaflets were fixed to the annulus using a 4.0 polypropylene running suture. The order of leaflet implantation left, non-facing, right. The smooth pericardial surface was oriented toward the right ventricle outflow tract (RVOT) to avoid postoperative thrombocytopenia. Suture starts at the nadir and goes toward the commissure. The size of the PVNeo leaflets is larger than in corresponding native ones. Therefore, a significant plication should be performed during implantation. Bigger bites are taken on the patch (ratio 3:1) while suturing near the nadir. More equal ones (2:1 and 1:1)-while approaching the commissures. At the top of the commissures, sutures are brought outside of the PA and fixed on pledgets. The final alignment of leaflets was achieved by applying a vertical 5.0 Polypropylene suture at the top of commissures (Fig. 2).
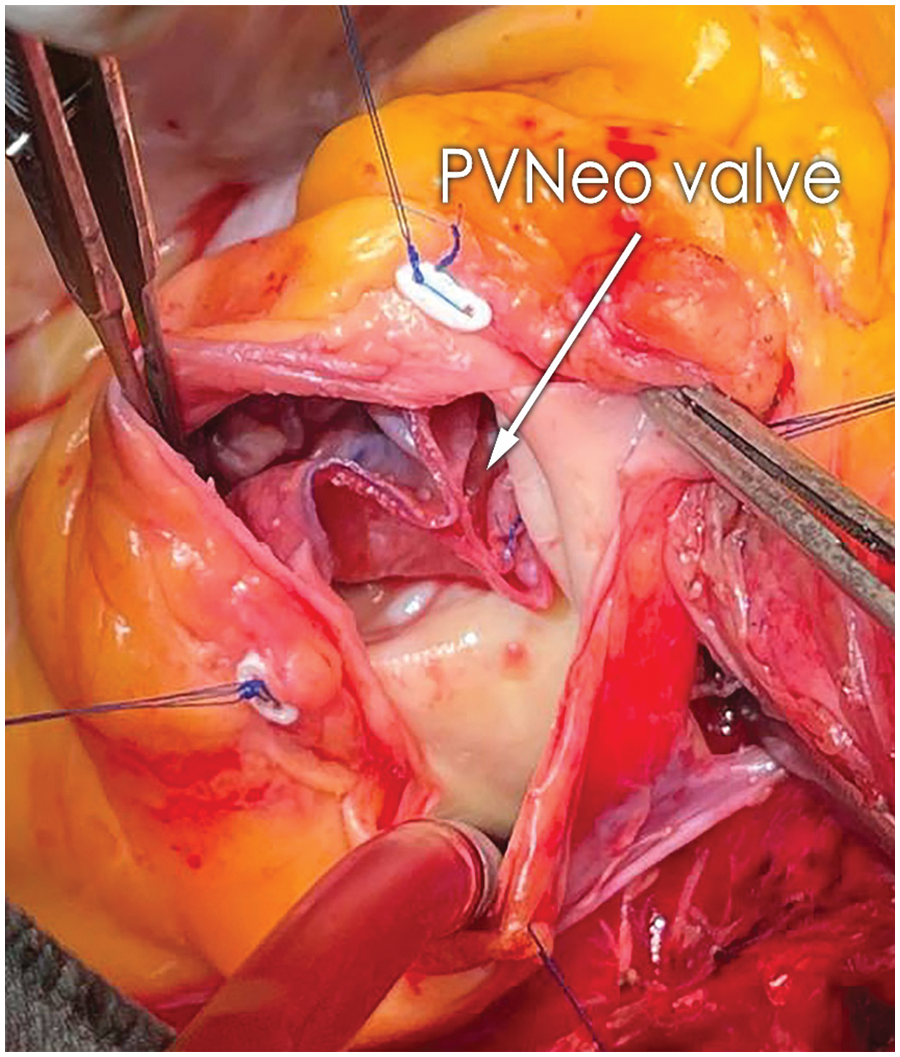
Figure 2: Final result of pulmonary valve reconstruction with PVNeo technique (PVNeo, pulmonary valve neocuspidization)
Intraoperative transesophageal echocardiography (TEE) demonstrated excellent PVNeo valve geometry and function with a peak pressure gradient of 7 mm Hg across the valve and only trace insufficiency. A detailed description and video of the PVNeo technique have been presented in our previous report [6].
The postoperative period was uneventful. Histopathology of the material from the PV demonstrated old vegetation with zones of fibrinous debris, organizing fibrosis, and chronic inflammation. It did not show any bacterial growth upon evaluation. The patient was discharged on the 9th postoperative day. At the 3-months follow-up, he is in good condition. No clinical and laboratory evidence of relapse endocarditis. On TTE: no residual VSD; PA pressure 37 mm Hg; PVNeo valve is symmetric, with a pressure gradient of 5 mm Hg and trace insufficiency (Figs. 3A and 3B). The patient was given warfarin for one month postoperatively to prevent PVNeo valve thrombosis. Then he was left on low-dose aspirin for six months.
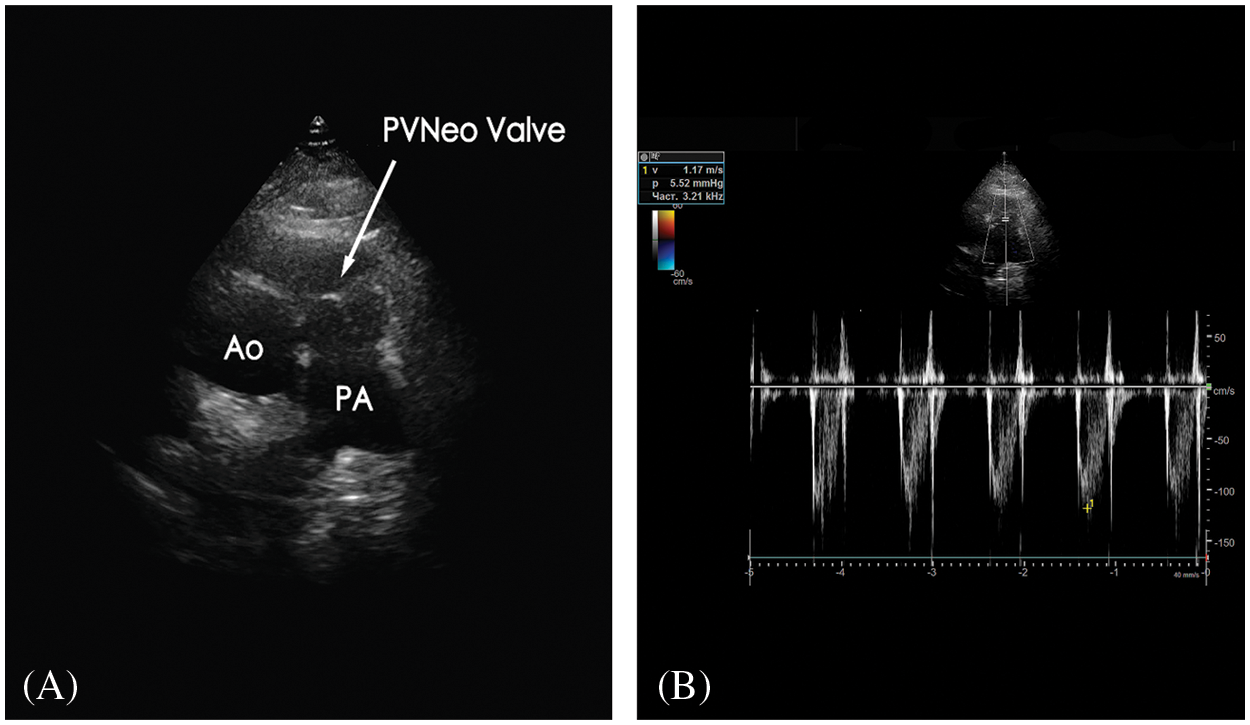
Figure 3: A) Transthoracic echocardiography (modified short-axis view) presents good geometry and function of the reconstructed pulmonary valve. B) Doppler image demonstrates excellent hemodynamic performance of the PVNeo valve at three-month follow-up Ao, aorta; PA, pulmonary artery; PVNeo, pulmonary valve neocuspidization
The lifetime incidence of IE in patients with CHD may be as high as 8.5% compared to 0.7% in controls with anatomically normal hearts [3]. Among various types of CHD, endocarditis is most commonly associated with VSD [3].
We assume that the current clinical event was an episode of repeat subacute endocarditis. Our patient started receiving antibiotic treatment two and a half years ago due to recurrent upper respiratory infections. We speculate that this was the manifestation of endocarditis. The diagnosis was missed because of the lack of echocardiography control. The absence of bacterial growth in blood culture and vegetation material is associated with the antibiotic treatment the patient received before admission to our hospital. Endocardial infection in our case was complicated by glomerulonephritis. Based on these facts, we stress the importance of regular cardiology controls with expert TTE evaluation for all patients with CHD. Infective endocarditis alertness in this subgroup should be high, especially in case of clinical or anamnestic data of recurrent fever, vascular or immunologic phenomena [2].
RSIE responds well to optimal medical therapy. Among patients with anatomically normal hearts and RSIE, only 5%–40% undergo surgery [1]. An optimal strategy for cases with underlying hemodynamically significant CHD would be an aggressive infection treatment with intravenous antibiotics. After the eradication of the bacterial agent, a surgical correction of CHD and IE may be performed on an elective basis. Expedited surgery should be reserved for cases with a poor response to optimal medical treatment, intractable hemodynamic compromise due to severe destruction of right-sided valves, large vegetations on PV, and septic embolization to the lungs [4,6]. The decision to perform surgical correction in our case was based on clinical, laboratory, and instrumental evidence of persistent infection and severe hemodynamic compromise despite optimal medical therapy.
Our patient had a rare combination of VSD and PV IE. A standard technique of VSD closure with a GTAP patch was used. The absolute majority of publications on PV replacement (PVR) are dedicated to patients who previously underwent RVOT reconstruction [7]. Literature data on the replacement of PV with originally normal anatomy are scarce. PVIE is the main indication for PVR in this subgroup of patients [4]. Methods and details of the operative technique are not standardized [4,8]. To avoid a higher risk of valvular thrombosis associated with right-sided mechanical valves, as well as complications of lifelong anticoagulation therapy, we opted for PVR with a biological valve in our patient [9].
PVNeo was favored because we believe this technique had several significant advantages over standard PVR with biological prostheses. The method was initially developed by Ozaki et al. for aortic valve replacement and has demonstrated excellent mid-term results [5]. Differences in the geometric anatomy of aortic and pulmonary roots are minimal [10]. This permits to use Ozaki concept for PV reconstruction. There are few reports on such experiences in the current literature [6,8,11]. All patients had normal anatomy of the PV annulus. The feasibility of performing PVNeo was demonstrated in every case. PVNeo does not have any rigid stent. This major advantage preserves the natural mobility of the right ventricular outflow tract and results in excellent postoperative hemodynamics [5,12]. In our case, the peak transvalvular gradient was 5 mm Hg with only trace insufficiency. The absence of any synthetic materials placed in the bloodstream potentially decreases the risk of prosthetic endocarditis on reconstructed PV [13]. A unique feature of PVNeo is the flexibility of the technique. Only destroyed cusps of PV may be replaced, while intact leaflets are preserved [6,11]. GTAP, while used for aortic valve reconstruction, has demonstrated results comparable to those of biological prostheses in the mid-term follow-up [5]. This material is readily available, and the method is economically attractive. There are cases when it is impossible to use the patient’s autologous pericardium: previous open-heart surgery or pericarditis. In such situations, other commercially available xenopericardial materials may be used for PV reconstruction [14].
We assume that advantageous characteristics of the Ozaki technique will result in better hemodynamic performance and higher resistance to infection of the PVNeo valve compared to mechanical and biological prostheses. However, we acknowledge the limitations of our study, including a single-case observation and short-term follow-up. A more significant number of patients and longer follow-up is needed to prove our theory. In patients with CHD, the level of alertness for IE should be high, especially if recurrent fever, immunologic and vascular phenomena are present.
Authorship: MI. Concept and design of the study; original draft preparation and editing; analysis and interpretation of the results. DV. Review and editing of the manuscript; analysis and interpretation of the results. VV. Draft review and editing. NI. Draft editing and data collection. TB. Methodology; draft review and editing; analysis and interpretation of the results.
Ethical Approval: The informed consent is obtained, and the patient granted permission to publish the images for this article.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Shmueli, H., Thomas, F., Flint, N., Setia, G., Janjic, A. et al. (2020). Right-sided infective endocarditis 2020: Challenges and updates in diagnosis and treatment. Journal of the American Heart Association, 9(15), e017293. DOI 10.1161/JAHA.120.017293. [Google Scholar] [CrossRef]
2. Wang, A., Gaca, J. G., Chu, V. H. (2018). Management considerations in infective endocarditis: A review. JAMA, 320(1), 72–83. DOI 10.1001/jama.2018.7596. [Google Scholar] [CrossRef]
3. Snygg-Martin, U., Giang, K. W., Dellborg, M., Robertson, J., Mandalenakis, Z. (2021). Cumulative incidence of infective endocarditis in patients with congenital heart disease: A nationwide, case-control study over nine decades. Clinical Infectious Diseases, 73(8), 1469–1475. DOI 10.1093/cid/ciab478. [Google Scholar] [CrossRef]
4. Datar, Y., Yin, K., Wang, Y., Lawrence, K. W., Awtry, E. H. et al. (2022). Surgical outcomes of pulmonary valve infective endocarditis: A US population-based analysis. International Journal of Cardiology, 361, 50–54. DOI 10.1016/j.ijcard.2022.05.033. [Google Scholar] [CrossRef]
5. Ozaki, S., Kawase, I., Yamashita, H., Uchida, S., Takatoh, M. et al. (2018). Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. The Journal of Thoracic and Cardiovascular Surgery, 155(6), 2379–2387. DOI 10.1016/j.jtcvs.2018.01.087. [Google Scholar] [CrossRef]
6. Todurov, B., Mokryk, I., Batsak, B., Ponych, N. (2022). Pulmonary valve neocuspidization and tricuspid valve replacement in intravenous drug abusers with infective endocarditis: Report of two cases. Interactive Cardiovascular and Thoracic Surgery, 35(2), ivac063. DOI 10.1093/icvts/ivac063. [Google Scholar] [CrossRef]
7. Ligon, R. A., Latson, L. A., Ruzmetov, M. M., Chan, K., Roth, T. et al. (2021). A systematic approach to pulmonary valve replacement in the current era. Congenital Heart Disease, 16(3), 285–297. DOI 10.32604/CHD.2021.014373. [Google Scholar] [CrossRef]
8. Jaswal, V., Singh Thingnam, S. K., Kumar, V., Munirathinam, G. K. (2020). Surgical valve repair of native pulmonary valve endocarditis using the ozaki technique. The Annals of Thoracic Surgery, 110(6), e509–e511. DOI 10.1016/j.athoracsur.2020.03.125. [Google Scholar] [CrossRef]
9. Pragt, H., van Melle, J. P., Javadikasgari, H., Seo, D. M., Stulak, J. M. et al. (2017). Mechanical valves in the pulmonary position: An international retrospective analysis. The Journal of Thoracic and Cardiovascular Surgery, 154(4), 1371–1378.e1. DOI 10.1016/j.jtcvs.2017.04.072. [Google Scholar] [CrossRef]
10. Berdajs, D., Zünd, G., Schurr, U., Camenisch, C., Turina, M. I. et al. (2007). Geometric models of the aortic and pulmonary roots: Suggestions for the ross procedure. European Journal of Cardio-Thoracic Surgery, 31(1), 31–35. DOI 10.1016/j.ejcts.2006.10.037. [Google Scholar] [CrossRef]
11. Kumar, R., Halder, V., Gourav, K. P., Patel, R., Munirathimnam, G. K. et al. (2020). Pulmonary valve neocuspidisation with glutaraldehyde-treated autologous pericardium–A novel technique in pulmonary valve endocarditis. Journal of Cardiac Surgery, 35(7), 1725–1728. DOI 10.1111/jocs.14659. [Google Scholar] [CrossRef]
12. Cocomello, L., Meloni, M., Rapetto, F., Baquedano, M., Ordoñez, M. V. et al. (2019). Long-term comparison between pulmonary homograft versus bioprosthesis for pulmonary valve replacement in tetralogy of fallot. Journal of the American Heart Association, 8(24), e013654. DOI 10.1161/JAHA.119.013654. [Google Scholar] [CrossRef]
13. Ali-Ghosh, H., Barlow, C. W. (2021). Commentary: Bioprosthetic pulmonary valve endocarditis: Another “arrow in our quiver”. JTCVS Techniques, 6, 73–74. DOI 10.1016/j.xjtc.2021.01.039. [Google Scholar] [CrossRef]
14. Mourad, F., Shehada, S. E., Lubarski, J., Serrano, M., Demircioglu, E. et al. (2019). Aortic valve construction using pericardial tissue: Short-term single-centre outcomes. Interactive Cardiovascular and Thoracic Surgery, 28(2), 183–190. DOI 10.1093/icvts/ivy230. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools