 Open Access
Open Access
ARTICLE
Interventricular Septal Hematoma after Congenital Cardiac Defects Repair at a Single Institution
1 Department of Echocardiography and Ultrasound, Hunan Children’s Hospital, Changsha, China
2 Heart Center, Hunan Children’s Hospital, Changsha, China
3 Pediatrics Research Institute of Hunan Province, Hunan Children’s Hospital, Changsha, China
4 Departments of Surgery and Pediatrics, Children’s Hospital of Georgia, Augusta University, Augusta, Georgia, USA
* Corresponding Author: Xicheng Deng. Email:
Congenital Heart Disease 2022, 17(6), 687-695. https://doi.org/10.32604/chd.2022.024333
Received 04 July 2022; Accepted 14 September 2022; Issue published 11 October 2022
Abstract
Background: Interventricular septal hematoma is a rare complication after congenital cardiac repair. The management varies according to the literature. We present our experience with this rare complication. Methods: Echocardiography database were reviewed with the term ‘‘hematoma’’ or “hypoechoic mass” for patients who underwent congenital heart surgery from January 2018 to December 2021 at our institution to identify potential interventricular septal hematoma cases. Relevant data of the patients identified were collected. Focus was put on the presentation, management, outcomes according to patent medical charts and serial echocardiographic report data. Results: In total, there were 5 patients included. The mean age and weight at surgery were 5.5 ± 3.6 months and 5.5 ± 1.4 kg, respectively. Four patients were diagnosed with ventricular septal defect and the other one being double outlet of the right ventricle. While all patients had intraoperative transesophageal echocardiography, 80% (4 of 5) of Interventricular septal hematoma were revealed intraoperatively. Only one patient received hematoma drainage intraoperatively while the other 3 identified in the operating room were only closely observed. One after ventricular septal defect repair presented continuous dysfunction of the left ventricle at the last follow-up, while the others were doing well. All hematomas resolved completely with a mean time to interventricular septal hematoma resolution of 35.8 ± 16.9 days. Conclusion: Infants seem to be at a higher risk for Interventricular septal hematoma following congenital heart surgery. While the majority of interventricular septal hematoma has a benign postoperative course, some may result in ventricular dysfunction. Management strategies may be chosen on a case-by-case basis.Graphic Abstract
Keywords
List of abbreviations
| VSD | ventricular septal defect |
| DORV | double outlet right ventricle |
| IVSH | interventricular septal hematoma |
| CICU | cardiac intensive care unit |
| TEE | transesophageal echocardiography |
| TTE | transthoracic echocardiography |
Various complications following repair of congenital cardiac defects have been reported, such as atrioventricular block, patch dehiscence and thrombus formation [1], among which an interventricular septal hematoma (IVSH) is an extremely rare complication. The true incidence of IVSH after ventricular septal defect (VSD) repair is unknown. It was first reported after patch closure of VSD in 2005. Since then, a few cases of IVSH have been reported [2–13]. Here we present our own series of five cases and discuss its etiology, management and prognosis.
After approval of institutional review board of Hunan Children’s Hospital, China, inpatient record database was reviewed for patients presenting with IVSH following congenital heart repair from January 2018 to December 2021. Echocardiography images were reviewed for diagnosis correctiveness and progression of the hematoma by an echocardiogram specialist. Patient records were also reviewed to collect relevant data for analysis.
2.1 Surgical Technique and Echocardiography
All patients were operated on under general anesthesia and cardiopulmonary bypass with mild to moderate hypothermia via a routine middle sternotomy. VSD repair was performed via the right atrium using an autologous pericardial patch with 6/0 polypropylene running suture. The double outlet right ventricle (DORV) patient was of subaortic VSD type and was repaired with an autologous pericardial patch. All patients underwent preoperative transthoracic echocardiography (TTE) and all but the DORV case underwent intraoperative transesophageal echocardiography (TEE) before and after cardiopulmonary bypass. The transthoracic echocardiography was performed using PHILIPS EPIQ 7C ultrasound system, with probe S8-3, 5–8 mHz (Philips Medical Systems, Andover, MA, USA). Transesophageal echocardiography was performed using PHILIPS IE33 ultrasonic diagnostic apparatus, with probe S7-3t, 3–7 mHz (Philips Medical Systems, Andover, MA, USA). The left heart function was evaluated using M-type echocardiogram with an ejection fraction reference of at least 55%.
2.2 Postoperative Echocardiography
TTE was routinely performed 5 to 7 days after surgery before discharged and 1, 3, 6 months, 1 year and then once a year after surgery for follow-up of the ventricular septal hematoma, with focus on change in the thickness of the interventricular septum, resolution of the hematoma and heart function over time.
3.1 Five Cases of Interventricular Septal Hematoma
In total, 5 children were identified, including 4 males and 1 female, aged 45 days to 11 months (mean 5.5 ± 3.6 months), and weighing 3.2 to 6.6 kg (mean 5.5 ± 1.4 kg). Four were perimembranous type VSD and the other one was VSD type DORV. Associated anomalies included patent ductus arteriosus (n = 3), atrial septal defect (n = 3), right ventricular double lumen (n = 1), right coronary cusp prolapse (n = 1). All patients were repaired as described in the Method section. Four IVSH were identified intraoperatively with TEE, and the other one was discovered postoperatively in the cardiac intensive care unit (CICU) with TTE (Table 1).

3.2 Intraoperative TEE Monitoring
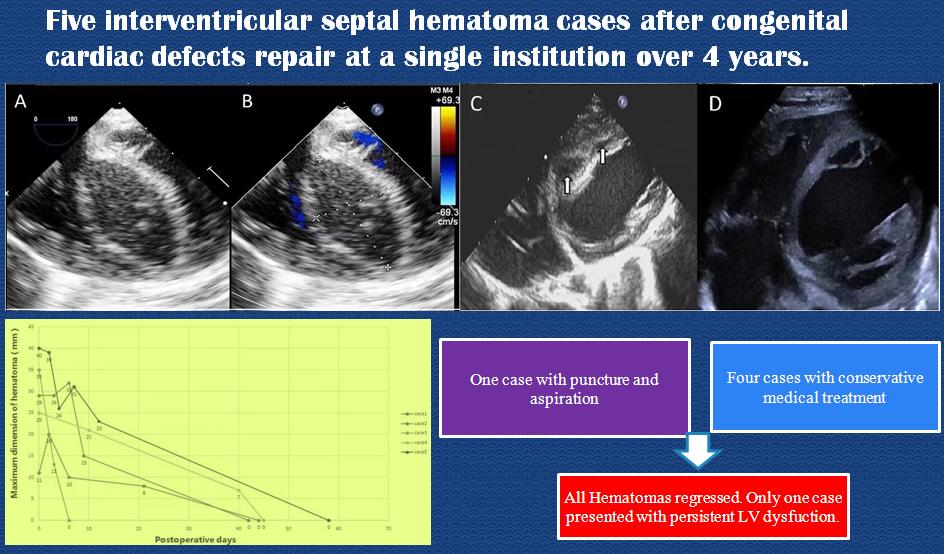
The thickness of the ventricular septum was normal before cardiopulmonary bypass in all the 5 cases. There was no residual shunt around the ventricular septal patch postoperatively. Postoperative TEE monitoring of the interventricular septum typically showed a fusiform thickening, mostly located in the middle of the ventricular septum at the lower end of the patch (range: 11 mm × 6 mm–40 mm × 29 mm in maximum as shown in echocardiographic views). Mixed echoic areas dominated by hypoechoic areas could be seen inside, with irregular borders and no visible ventricular cavity connected (Fig. 1). In one case with DORV, a similar mixed echoic area was found in the free wall of the right ventricle, with a hypoechoic area predominantly connected to the mixed echoic area of the interventricular septum. The right ventricular outflow tract was compressed and narrowed. The ejection fractions were normal in three, moderately impaired in one and severely impaired in the last one.
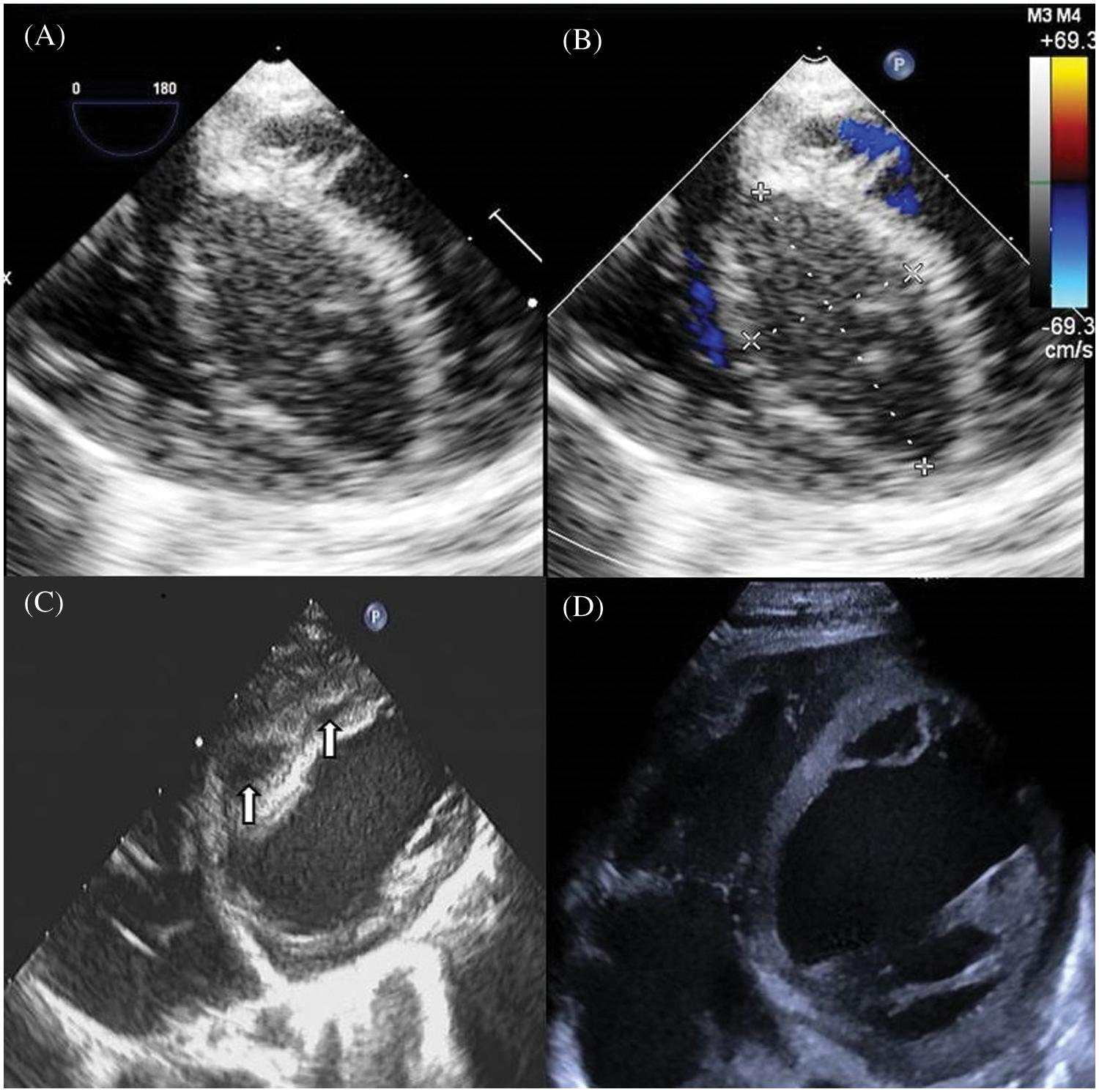
Figure 1: Echocardiographic views of an infant presenting with an interventricular septal hematoma after VSD repair. (A) TEE (four-chamber view) image demonstrates a large interventricular septal hypoechoic hematoma measuring 36 mm × 18 mm, with clear boundary. (B) Color Doppler shows no visible blood flow signal in the hematoma. (C) Ten days after surgery, TTE shows diminishing hematomas separated as two masses sized 15 mm × 9 mm, 10 mm × 4 mm, respectively, with echolucency. (D) 42 days after surgery TTE shows the thickness of the septum is normal, without echolucency
3.3 Management and Complications during Hospitalization
One of the four patients with IVSD identified intraoperatively were put on a second-round bypass and the hematoma was incised for drainage. The other 3 were left untouched as there was no significant hemodynamic compromise. Three patients with ventricular wall normokinesia and normal heart function postoperatively. The two patients with moderate or severe left ventricular dysfunction were closely observed and managed with medical treatment with prolonged length of hospitalization. All patients were discharged successfully.
All patients were followed up regularly, typically 1 month, 3 months, 6 months and 1 year and then once a year postoperatively. 4 patients were doing well with no further hospitalization. The hematomas disappeared, and the thickness and echolucent mass of the ventricular septum returned to normal at 11–56 days (39.8 ± 16.9 days) (Figs. 1 and 2). The patient with impaired cardiac function remained to have significantly enlarged left ventricle (Z score 5.24) with a low ejection fraction of 29%. He was re-hospitalized 146 days after operative for cardiac failure and pneumonia. His NT-pro B-type natriuretic peptide, dropped from 8051.0 pg/ml right after operation to 789.8 pg/ml one year later. He is now doing basically well with no shortness of breath and is gaining weight normally. Close observation is warranted.
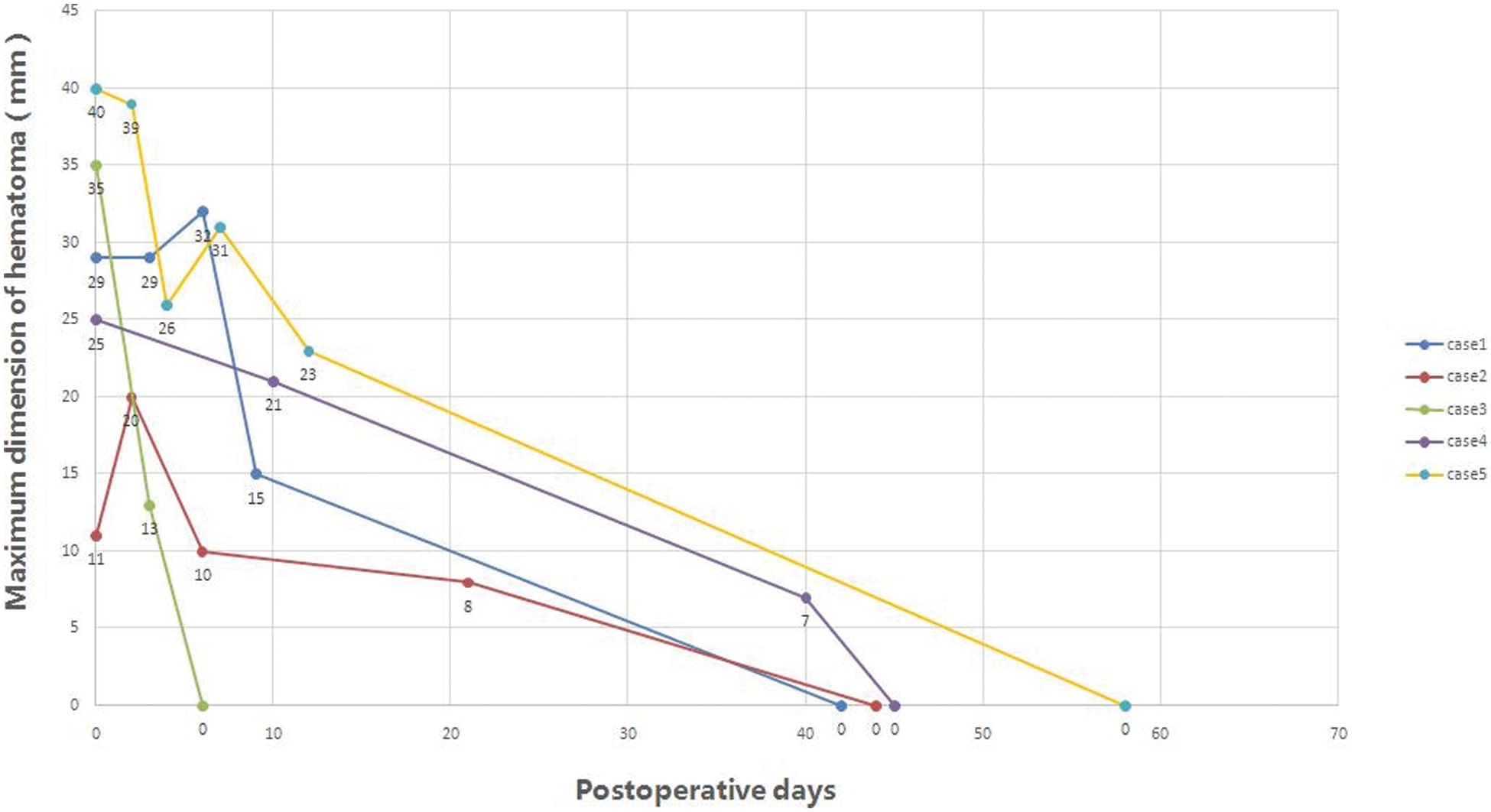
Figure 2: Hematomas diminished and disappeared over time in all 5 patients
Note: Case 5 underwent hematoma evacuation. Case 1 continued to have left ventricular dysfunction.
As a rare complication after cardiac surgery, the incidence and etiology are largely unknown. Currently, there have been no studies reporting the incidence of IVSH. During the whole 4 years of this study period, we performed 2093 congenital heart surgeries in total, of which 1337 were VSD repair as index procedure, hence the incidence was 5/1337 or 0.37% (including VSD type DORV case). We believe this is credible as we routinely perform TEE for all patients weighing >5 kg. Those whose weight <5 kg with VSD as index procedure made only a small fraction of all VSD cases of this period. Jegatheeswaran et al. [14] documented 12 patients during a 12-year period which is comparable with our data. As for the occurrence of IVSH, a popular theory attributes this to injury of the septal perforating arterial branch (SPA) [15]. James and Burch noted the SPA crosses the lower border of the anterior limb of the septomarginal trabeculation and ends at the base of the medial papillary muscle of the tricuspid valve [16]. Hosseinpour et al. [17] observed that the areas delineated by the outlet septum, the medial papillary muscle, and the VSD margin are generally devoid of the first SPA. In our cases, it could be seen a rapid postoperative increase in the diameter of the interventricular septum which suggests surgical trauma of the coronary septal branch during placement of the septal patch sutures. Anticoagulated, the bleeding probably dissects along a plane beneath the endocardium, resulting in a hematoma that bulges into the ventricular cavity [5], confirmed by postoperative echocardiogram. This rationale makes technical issues a potential contributor to IVSH occurrence. We discussed these cases and started to use finer suture and needles (7/o) and tried to avoid biting deep when sewing a patch to the VSD. But it still requires more time to say if it works.
Another potential cause is assumed to be high-pressure waterjet injury which has been reported occasionally in the literature [18,19]. The cases in the present study all had a VSD and after repair we routinely checked the competence of the tricuspid valve with saline jet using a syringe connected with a soft plastic needle. Occasionally, the needle was not right in the cavity of the right ventricle but pricked against the septal or free wall of the ventricle when injecting saline. Though not having hard evidence, we speculate this mechanism could have resulted in myocardium injury and epicardium dissection. This is plausible especially in infants whose heart tissue is very fragile.
In terms of demographics, we speculate that patients who are younger and have a lower weight may potentially be at an increased risk of IVSH due to difficult visualization as evidenced by an average age of 5.5 months and a median weight of 5.5 kg in our series, in line with previous study [12]. A large defect in a small baby may be a risk factor for occurrence of IVSH. However, this requires further studies to confirm.
According to the literature, natural history of the IVSH varies. It may be contained and resolve over time, dissect between spiral planes of the myocardium leading to septal rupture and a VSD, extend on to the left ventricle free wall with the potential for myocardial rupture, communicate between the ventricles across their inferior wall without septal rupture, or it may result in cardiogenic shock, heart block, outflow tract obstruction, cardiac tamponade, abscess transformation, and death [8,20]. Most reports are in the adult population where IVSH has been described after myocardial infarction, percutaneous intervention and surgical bypass grafting of the coronary artery [5]. It should be pointed out that in our Case 1 who had persistent heart dysfunction, the hematoma presented as one of the largest in all the 5 cases. It is sensible that the larger the hematoma is, the higher the chance that the myocardial perfusion or hemodynamics may be affected. Though it could not be confirmed, the persistent heart dysfunction may be an outcome of impaired myocardial perfusion and ischemia.
Today TEE during operation is a useful tool for evaluating immediate surgical results in patients with congenital heart defects [21,22]. At our institution, we routinely use TEE for all congenital cardiac surgery procedures in patients whose weights are greater than 5 kg for evaluation of preoperative diagnostic accuracy, and postoperative results as well. According to our experience and also reported previously [23], it has been a highly useful and safe tool that helps to achieve the best results for our patients, including identifying an IVSH following pediatric heart repair in the operating room in a timely fashion as IVSH has the tendency to enlarge [24].
Management is dictated by initial presentation. Electrocardiograms are mandatory initially to ensure no atrioventricular block and as the hematoma resolves we feel it is unlikely to result in delayed atrioventricular nodal issues. According to size of the hematoma and hemodynamics, the management of IVSH can be conservative with serial echocardiography monitoring or conservative therapy [4–7,10,11,25], surgical evacuation [2,8,25], or multiple intraoperative needle aspirations or puncture [3] or even extracorporeal membrane oxygenation as a bridging strategy to manage severe hemodynamic instability while awaiting resolution of the hematoma [14]. In our experience, the majority of the patient seemed largely tolerant with the IVSH as shown on TEE without signs of outflow obstruction/compression or ventricular wall dyskinesia. If the patient presented with ventricular dysfunction upon occurrence of IVSH, re-run of CPB to drain IVSH may be necessary. In our series, the majority of the patients were managed conservatively, and the hematoma absorbed completely with satisfactory outcomes except for Case 1 who had been presenting with continuous left ventricular dysfunction. For Case 1, there was no sign suggesting ventricular dysfunction under TEE in the operating room. It is not clear whether myocardial impairment occurred soon after the formation of the hematoma or later as the hematoma potentially developed or enlarged as no serial daily echocardiograms were performed following the operation. Only one received surgical evacuation of the hematoma as judged by the surgeon. At our institution, we generally do not use aspirin or any other anticoagulant after VSD repair. This may have also contributed to regression of the hematomas. As for the hematoma size change over time, only Case 2 showed temporary increase. There was actually no difference between this patient and the other patient in terms of operation and perioperative management including medications/anticoagulants used. We reckon this may have been attributed to interobserver variance as different doctors performed echocardiograms or different echocardiographic views, on which the sizes were measured, were used.
Interventricular septum hematoma is a rare compilation following congenital cardiac repair and seem to happen in infants more frequently. Evaluation of the interventricular septum following congenital cardiac repair is imperative. Most cases can be managed successfully on a case-by-case basis. Surgical intervention to drain the hematoma may be necessary in selected cases.
Author Contributions: XD and YH conceived and designed the study, performed the analysis, interpreted results and drafted the manuscript. JD performed the analysis, interpreted results. XY, PH, JL, GY, XD enrolled subjects, provided clinical examinations and surgery, acquired laboratory data and prepared the database for the analysis. YH performed echocardiography of all the patients involved in the study. All authors read and approved the final version of the manuscript.
Data Availability Statement: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013) and has been approved by the Institutional Review Board of Hunan Children’s Hospital. (HCHLL-2022-40).
Funding Statement: This project was supported by the Natural Science Foundation of Hunan Province, China (Grant No. 2019JJ80106).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. van der Bom, T., Zomer, A. C., Zwinderman, A. H., Meijboom, F. J., Bouma, B. J. et al. (2011). The changing epidemiology of congenital heart disease. Nature Reviews Cardiology, 8(1), 50–60. DOI 10.1038/nrcardio.2010.166. [Google Scholar] [CrossRef]
2. Drago, M., Butera, G., Giamberti, A., Lucente, M., Frigiola, A. (2005). Interventricular septal hematoma in ventricular septal defect patch closure. The Annals of Thoracic Surgery, 79(5), 1764–1765. DOI 10.1016/j.athoracsur.2003.10.123. [Google Scholar] [CrossRef]
3. Padalino, M. A., Speggiorin, S., Pittarello, D., Milanesi, O., Stellin, G. (2007). Unexpected interventricular septal hematoma after ventricular septal defect closure: Intraoperative echocardiographic early detection. European Journal of Echocardiography, 8(5), 395–397. DOI 10.1016/j.euje.2006.06.001. [Google Scholar] [CrossRef]
4. Bailey, F. J., Jivanji, S. G., Kostolny, M. (2017). Postoperative interventricular septal haematoma following tetralogy of Fallot repair and perimembranous ventricular septal defect repair. Interactive CardioVascular and Thoracic Surgery, 24(2), 296–298. DOI 10.1093/icvts/ivw337. [Google Scholar] [CrossRef]
5. Eyileten, Z., Aliyev, A., Ciftci, O., Ucar, T., Odek, C. et al. (2013). An extremely rare complication of congenital heart surgery: Interventricular septal hematoma. The Turkish Journal of Pediatrics, 55(6), 662–664. [Google Scholar]
6. Jacobs, S., Rega, F., Vlasselaers, D., Gewillig, M., Meyns, B. (2010). Dealing with a septal hematoma after switch operation with ventricular septal defect closure. Heart Surgery Forum, 13(4), E263–264. DOI 10.1532/HSF98.20091171. [Google Scholar] [CrossRef]
7. Jensen, R., Burg, P., Anderson, C., Garabedian, C., Garabedian, H. et al. (2007). Postoperative ventricular septal hematoma: Natural history of two pediatric cases. The Journal of Thoracic and Cardiovascular Surgery, 133(6), 1651–1652. DOI 10.1016/j.jtcvs.2007.01.052. [Google Scholar] [CrossRef]
8. Mart, C. R., Kaza, A. K. (2011). Postoperative dissecting ventricular septal hematoma: Recognition and treatment. ISRN Pediatrics, 2011(534940), 1–3. DOI 10.5402/2011/534940. [Google Scholar] [CrossRef]
9. Patel, M. D., Goldstein, B. S., Kral Kollars, C. A. (2018). Diagnosis and observational management of a postoperative interventricular septal hematoma in a pediatric patient. CASE, 2(5), 228–230. DOI 10.1016/j.case.2018.04.001. [Google Scholar] [CrossRef]
10. Yamazawa, H., Takeda, A., Nakajima, H., Tachibana, T., Aoki, M. (2017). Interventricular septal hematoma following repair of a ventricular septal defect. Journal of Cardiac Surgery, 32(6), 390–393. DOI 10.1111/jocs.13145. [Google Scholar] [CrossRef]
11. Abdelghani, M. S., Chapra, A., Abed, H., Al-Qahtani, A., Alkindi, F. (2021). Interventricular septal hematoma: A rare complication of retrograde chronic total occlusion intervention. Heart Views, 22(3), 201–205. DOI 10.4103/HEARTVIEWS.HEARTVIEWS_203_20. [Google Scholar] [CrossRef]
12. Yoneyama, F., Matsubara, M., Sakamoto, H., Hiramatsu, Y. (2017). Interventricular septal hematoma associated with congenital heart surgery: A case report and literature review. The Journal of Thoracic and Cardiovascular Surgery, 153(4), e55–e57. DOI 10.1016/j.jtcvs.2016.10.053. [Google Scholar] [CrossRef]
13. Zhuang, J., Chen, J. M., Huang, X. (2008). Interventricular septal dissecting haematoma. European Heart Journal, 29(20), 2488. DOI 10.1093/eurheartj/ehn182. [Google Scholar] [CrossRef]
14. Jegatheeswaran, A., Cohen, M. S., Gaynor, J. W., Mascio, C. E., Spray, T. L. et al. (2020). Extracorporeal membrane oxygenation as a novel management strategy for interventricular septal hematoma following ventricular septal defect repair. The Journal of Thoracic and Cardiovascular Surgery, 159(5), 1936–1940. DOI 10.1016/j.jtcvs.2019.09.150. [Google Scholar] [CrossRef]
15. Vargas-Barron, J., Roldan, F. J., Romero-Cardenas, A., Molina-Carrion, M., Vazquez-Antona, C. A. et al. (2009). Dissecting intramyocardial hematoma: Clinical presentation, pathophysiology, outcomes and delineation by echocardiography. Echocardiography, 26(3), 254–261. DOI 10.1111/j.1540-8175.2008.00804.x. [Google Scholar] [CrossRef]
16. James, T. N., Burch, G. E. (1958). Blood supply of the human interventricular septum. Circulation, 17(3), 391–396. DOI 10.1161/01.cir.17.3.391. [Google Scholar] [CrossRef]
17. Hosseinpour, A. R., Anderson, R. H., Ho, S. Y. (2001). The anatomy of the septal perforating arteries in normal and congenitally malformed hearts. The Journal of Thoracic and Cardiovascular Surgery, 121(6), 1046–1052. DOI 10.1067/mtc.2001.113604. [Google Scholar] [CrossRef]
18. Tejero-Trujeque, R. (2000). High-pressure water jet injuries: A surgical emergency. Journal of Wound Care, 9(4), 175–179. DOI 10.12968/jowc.2000.9.4.25976. [Google Scholar] [CrossRef]
19. Sampson, C. S. (2008). High-pressure water injection injury. International Journal of Emergency Medicine, 1(2), 151–154. DOI 10.1007/s12245-008-0026-2. [Google Scholar] [CrossRef]
20. De Gennaro, L., Brunetti, N. D., Ramunni, G., Buquicchio, F., Corriero, F. et al. (2010). Septal rupture with right ventricular wall dissecting haematoma communicating with left ventricle after inferior myocardial infarction. European Journal of Echocardiography, 11(6), 477–481. DOI 10.1093/ejechocard/jep236. [Google Scholar] [CrossRef]
21. Bettex, D. A., Pretre, R., Jenni, R., Schmid, E. R. (2005). Cost-effectiveness of routine intraoperative transesophageal echocardiography in pediatric cardiac surgery: A 10-year experience. Anesthesia & Analgesia, 100(5), 1271–1275. DOI 10.1213/01.ANE.0000149594.81543.F0. [Google Scholar] [CrossRef]
22. American Society of Anesthesiologists and the of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. (2010). Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology, 112(5), 1084–1096. DOI 10.1097/ALN.0b013e3181c51e90. [Google Scholar] [CrossRef]
23. Dieleman, J. M., Myles, P. S., Bulfone, L., Younie, S., van Zaane, B. et al. (2020). Cost-effectiveness of routine transoesophageal echocardiography during cardiac surgery: A discrete-event simulation study. British Journal of Anaesthesia, 124(2), 136–145. DOI 10.1016/j.bja.2019.10.023. [Google Scholar] [CrossRef]
24. Haranal, M., Hew, C. C., Dillon, J. J. (2019). Myocardial rupture secondary to ventricular septal hematoma: A case report and review of contemporary literature. World Journal for Pediatric and Congenital Heart Surgery, 10(6), 793–795. DOI 10.1177/2150135119872202. [Google Scholar] [CrossRef]
25. Zhu, J., Liu, H., Zhang, J., Feng, X., Wu, S. et al. (2013). Interventricular septal hematoma after congenital cardiac surgery. The Annals of Thoracic Surgery, 95(6), 2171–2173. DOI 10.1016/j.athoracsur.2012.10.077. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools