 Open Access
Open Access
ARTICLE
Exercise Catheterization for Hemodynamic Evaluation of Adults with Coarctation of the Aorta
1 Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota, USA
2 Department of Pediatric and Adolescent Medicine, Division of Pediatric Cardiology, Mayo Clinic, Rochester, Minnesota, USA
* Corresponding Author: William R. Miranda. Email:
Congenital Heart Disease 2022, 17(6), 605-615. https://doi.org/10.32604/chd.2022.023969
Received 20 May 2022; Accepted 20 June 2022; Issue published 11 October 2022
Abstract
Background: Coarctation of the aorta (CoA) is associated with a generalized arteriopathy and long-term complications despite repair. Data on invasive exercise hemodynamics in this population are lacking. Accordingly, we reviewed adults with CoA undergoing exercise catheterization to assess 1. hemodynamic profile; 2. feasibility for assessment of CoA severity. Methods: Twenty patients undergoing exercise cardiac catheterization (12 arm adduction and 8 supine cycle ergometry) at a quaternary care center between 2004 and 2021 were identified. Resting and exercise hemodynamic data were abstracted from the procedure logs. Results: Mean age was 43.6 ± 12.0 years. Eleven patients (55%) had resting pulmonary arterial wedge pressure (PAWP) >15 mmHg; among those undergoing arterial catheterization, left ventricular end-diastolic pressure was >15 mmHg in 63%. Eleven patients (55%) had pulmonary hypertension: 7 (35%) combined and 4 (20%) isolated post-capillary. At peak exercise, ΔPAWP/Δcardiac output (CO) ≥2 and Δmean pulmonary artery pressure/ΔCO ≥3 mmHg/l/min were found in 7 (78%) and 6 (67%) patients, respectively; the composite of exercise PAWP ≥25 mmHg or ΔPAWP/ΔCO >2 was seen in 12 (86%). CoA peak-to-peak gradients at baseline (n = 14) and during exercise (n = 9) were 12 (3–16) and 16 mmHg (9–28), respectively. Only 2 patients had an increase in CoA gradient to >20 mmHg with exercise. Conclusions: Diastolic dysfunction and pulmonary hypertension were highly prevalent, with exercise unmasking abnormal diastolic and pulmonary vascular reserve in some individuals. Most patients failed to show significant increases in CoA peak-to-peak gradients with exercise. Further studies are warranted to establish the best diagnostic method for CoA severity assessment.Keywords
Coarctation of the aorta (CoA) is associated with generalized arteriopathy that persists despite successful treatment/repair, and carries reduced survival [1–6]. The management of adults with CoA mostly relates to long-term complications following repair in childhood, although late diagnosis/intervention may occur [7,8]. Intrinsic aortic wall abnormalities and chronic pressure overload from mechanical obstruction result in impaired vascular compliance and endothelial dysfunction, which contribute to the development of arterial hypertension, premature coronary artery disease, cerebrovascular disease, and aortic aneurysms [9–11]. Ultimately, a maladaptive response of the left ventricle (LV) occurs, with myocyte hypertrophy and replacement fibrosis [12], alterations in myocardial viscoelastic properties, and, ultimately, diastolic and systolic dysfunction [13,14].
The need for re-intervention of repaired CoA has been reported in approximately 30% [3] but the definition of re-coarctation has varied in the literature. In addition, the challenges in hemodynamic assessment of CoA are well-known. This is reflected in the American College of Cardiology and the European Society of Cardiology guidelines for management of adult congenital heart disease, where indication for intervention is based on a combination of complications (e.g., hypertension), as well as hemodynamic and anatomic parameters [15,16]. Studies aimed at improving the diagnosis of CoA and re-coarctation have analyzed myocardial and hemodynamic response to exercise by non-invasive methods [17–20]. However, data on exercise invasive hemodynamic evaluation of pulmonary, systemic and ventricular pressures are lacking. Accordingly, we reviewed our experience of adults with CoA undergoing invasive rest and exercise hemodynamic evaluation. Our goals were: 1) to assess the hemodynamic profile at rest and under invasive exercise stress testing, 2) to analyze feasibility of this method for evaluation of CoA severity.
Adults with native and repaired CoA undergoing exercise cardiac catheterization at a quaternary care center between January 2004 and November 2021 were retrospectively identified using an electronical search tool. The study was approved by the Mayo Clinic Institutional Review Board (Mayo Clinic Adult Congenital Heart Disease Registry, IRB#20-007695) and only patients providing research authorization were included in the study.
Cardiac catheterization was performed in a fasting state under light sedation. Exercise was performed via supine cycle ergometry or arm adduction with 4-pound weights, as previously described [21]. Invasive blood pressure measurements in the ascending and descending aorta at baseline and maximal exercise were recorded to assess peak-to-peak gradient across the CoA at rest and its response to exercise. At baseline, LV diastolic dysfunction was defined as an end-diastolic pressure (LVEDP) >15 mmHg. Pulmonary arterial wedge pressure (PAWP) or direct left atrial pressure >15 mmHg was defined as elevated filling pressures. During exercise, a mean PAWP ≥25 mmHg or pulmonary artery pressure (mPAP) ≥40 mmHg were considered abnormal [22]. Precapillary pulmonary hypertension (PH) corresponded to resting mPAP ≥25 mmHg, PAWP ≤15 mmHg and pulmonary vascular resistance >3 Wood units, while those with post-capillary PH were subdivided into isolated postcapillary PH (defined as mPAP ≥25 mmHg, PAWP >15 mmHg and pulmonary vascular resistance ≤3 Wood units) and combined PH (mPAP ≥25 mmHg, PAWP >15 mmHg and pulmonary vascular resistance >3 Wood units) [23]. Pulmonary vascular resistance was calculated as (mPAP–PAWP or left atrial pressure)/pulmonary flow. Reported pressure measurements correspond to computer-generated averages over ≥5 consecutive cardiac cycles recorded during spontaneous breathing.
Pulmonary and systemic flows were calculated by the direct Fick method; alternatively, the indirect Fick or thermodilution methods were used. Cardiac output (CO) response to exercise was calculated as [ΔCO/(ΔVO2*0.006)] * 100; [24] a value ≥80% was defined as preserved CO reserve. ΔPAWP/ΔCO and ΔmPAP/ΔCO were assessed as measures of LV diastolic dysfunction and pulmonary vascular reserve; cut-off values of ≥2 and ≥3 mmHg/l/min, respectively, were defined as abnormal [22].
Continuous variables are presented as mean ± standard deviation or median (interquartile range) and nominal variables as counts (%). Comparisons between resting and exercise data were performed using paired Wilcoxon rank-sum test (non-parametric continuous variables). Statistical analysis was conducted with JMP for SAS V.14.1.0; p values < 0.05 were considered statistically significant.
The final cohort consisted of 20 patients with only 1 individual having unrepaired CoA. Demographic and clinical data are presented in Table 1. Mean age was 43.6 ± 12.0 years. All CoA were juxtaductal. Among those with repaired CoA, 18 (95%) had primary surgical repair while 1 (5%) had primary percutaneous intervention, with the median age at repair being 3 years (0–11). Fourteen patients (70%) required subsequent interventions [12 surgical (60%), 2 (10%) percutaneous] for re-coarctation. Associated congenital defects included aortic arch hypoplasia (10, 63%), bicuspid aortic valve (10, 53%), mitral valve anomalies (9, 45%), aortic valve/subaortic stenosis (4, 20%), atrial and ventricular septal defects (3, 15% each), and pulmonary valvular and supravalvular stenosis (1, 5%). Two patients (10%) had Turner syndrome. Coronary artery disease with >50% vessel obstruction was diagnosed in 4 patients (24%) by invasive angiography or cardiac computed tomography.
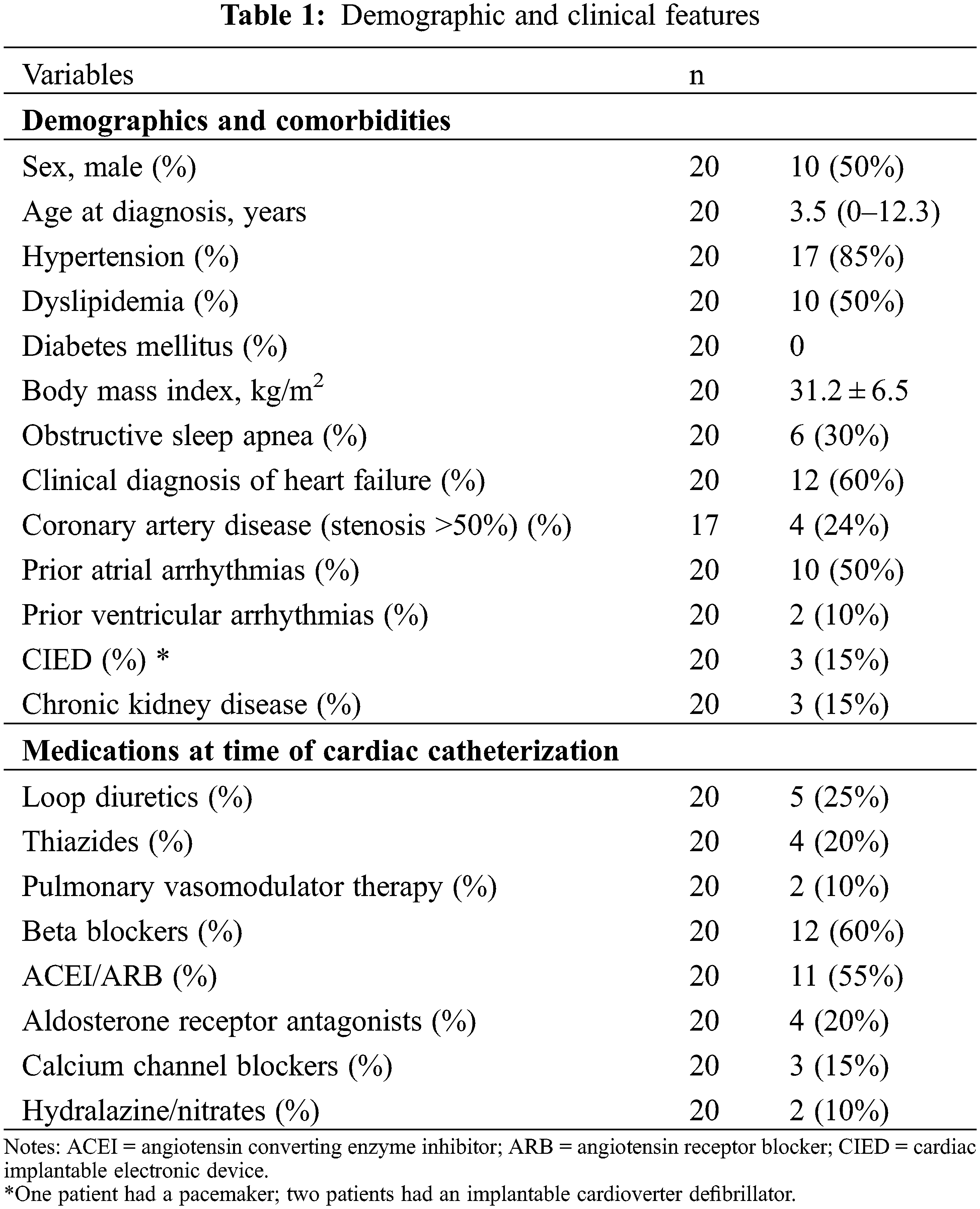
Four patients (20%) were in New York Heart Association functional class III–IV, while 4 were asymptomatic. Nine patients had pre-procedural outpatient upper and lower limb blood pressure measured; median systolic pressure gradient was 25 (15–31) mmHg. Detailed information of pre-procedural imaging is depicted in Table 2. Echocardiography was performed a median of 6 days (1–31) before catheterization. LV ejection fraction was >50% in all but 2 patients. Two patients had a small residual atrial septal defect (10%) and 1 (5%) had a small ventricular level shunt. An aortic or mitral valve prosthesis was present in 4 patients (20%) each (coexistent in 1). Ascending to descending aorta peak and mean gradients were 19 (13–30) and 13 (7–18) mmHg, respectively. The mean ratio of diameters between the aortic isthmus and the descending thoracic aorta at the diaphragm measured by cross-sectional imaging was 0.8 ± 0.2.
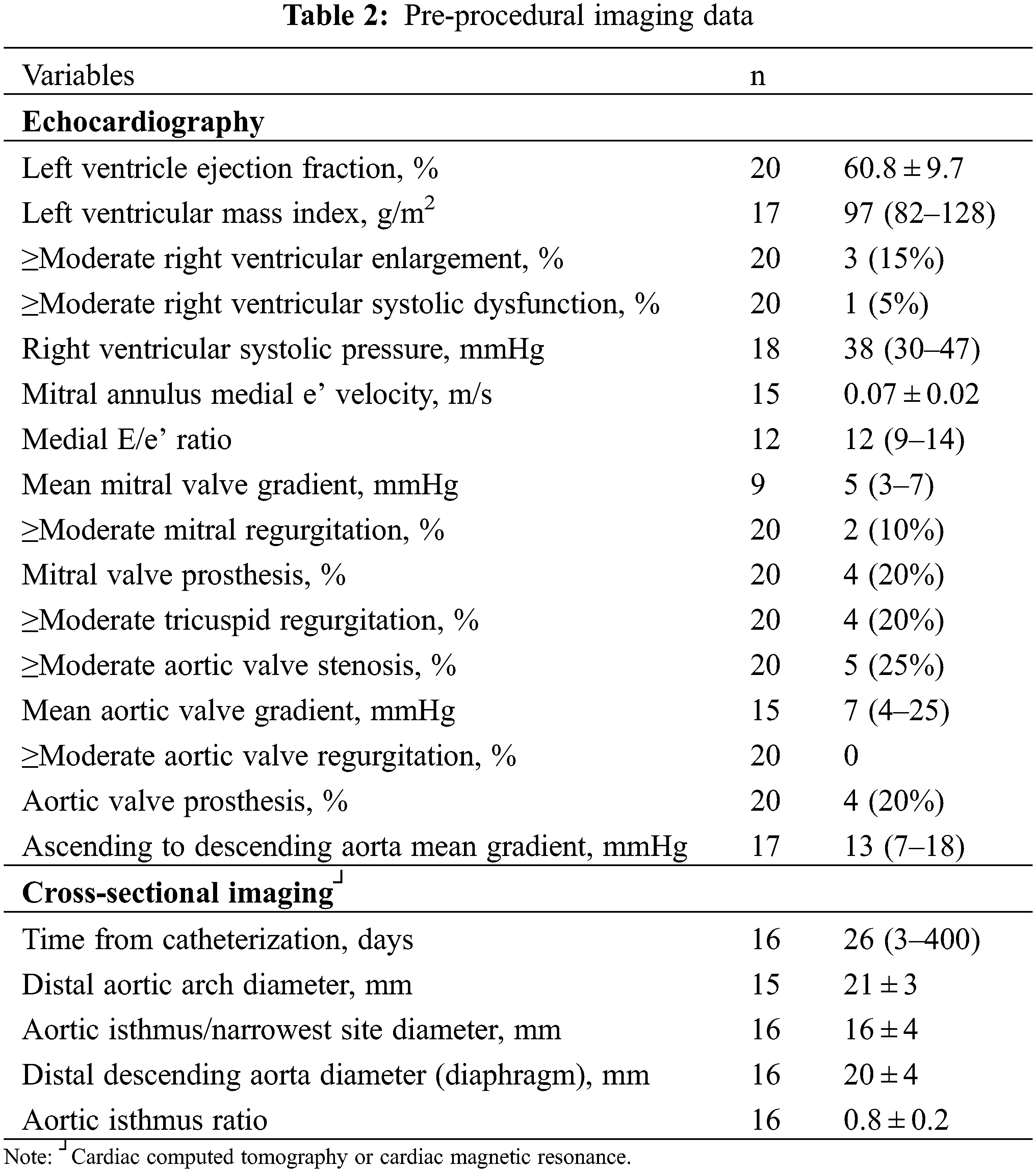
3.1 Resting and Exercise Invasive Hemodynamics
Detailed information about rest and exercise catheterization is shown in Table 3. Arterial catheterization was performed in 17 patients (85%) while venous catheterization was performed in all. The type of exercise was arm adduction in 12 cases (60%) and supine cycle ergometry in 8 (40%). Procedure indication was clinical deterioration in 9 patients (45%), need for additional hemodynamic evaluation following echocardiographic assessment in 8 (40%), preoperative evaluation for non-coarctation cardiac surgery in 2 (10%), and exercise-induced hypertension in 1 (5%).
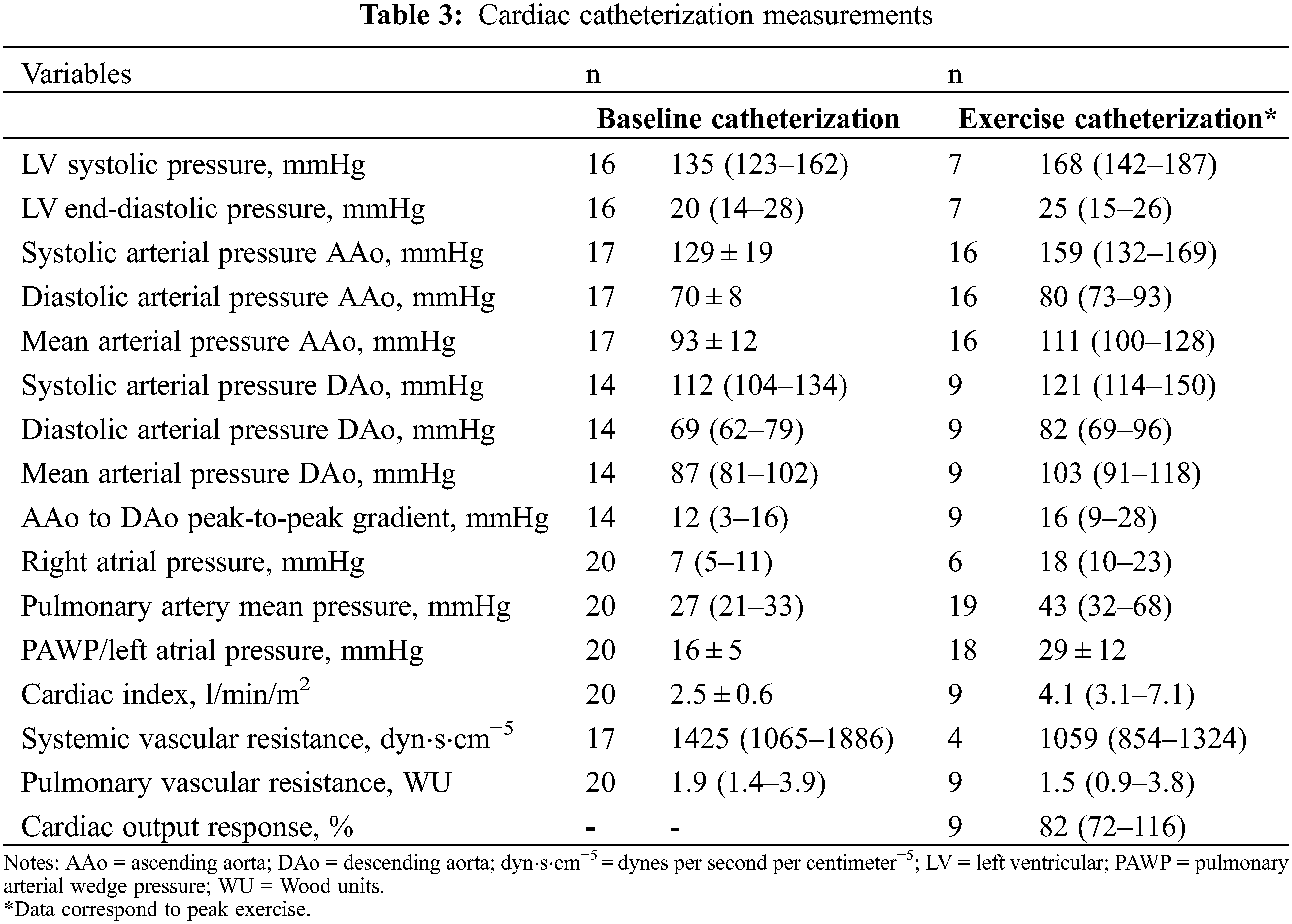
At rest, 10 patients (63%) had LVEDP >15 mmHg and 11 (55%) had PAWP >15 mmHg. Resting PH was present in 11 patients (55%): 7 (35%) combined and 4 (20%) isolated post-capillary. At peak exercise, 11 (61%) had PAWP ≥25 mmHg with 2 of them having normal resting PAWP. Thirteen individuals (68%) had an exercise mPAP ≥40 mmHg. Of 9 patients with CO measurement during exercise, normal cardiac output response was present in 6 (67%). ΔPAWP/ΔCO ≥2 and ΔmPAP/ΔCO ≥3 was found in 7 (78%) and 6 (67%), respectively. The composite variable of PAWP ≥25 mmHg or ΔPAWP/ΔCO >2 was present in 12 patients (86%) at peak exercise.
3.2 Assessment of Coarctation of the Aorta during Exercise
Peak-to-peak CoA gradient was measured at rest in 14 patients and during maximal exercise in 9. Comparison between rest and exercise peak-to-peak CoA gradients did not show statistically significant differences (12 [3–16] vs. 16 [9–28] mmHg, p = 0.4). All patients had resting peak-to-peak CoA gradients ≤ 20 mmHg. Two patients developed a gradient >20 mmHg with exercise (from 13 to 36 and from 20 to 64 mmHg); both undergoing cycle ergometer exercise. One patient had both arm exercise and dobutamine stress catheterization (maximal dose 10 mcg/kg/min); peak-to-peak gradient with exercise across the CoA was 17 mmHg with arm exercise and 36 mmHg with dobutamine.
Following catheterization, 7 patients (35%) underwent subsequent intervention due to re-coarctation. Their hemodynamic profile is presented in Table S1. Surgical repair with interposition grafting or extraanatomic bypass graft was performed in 2 patients each (13%); the remaining 3 cases were managed percutaneously (balloon angioplasty in 1 and coarctation stenting in 2). One additional patient underwent repair of coarctation site pseudoaneurysm. Four of the 7 patients with re-coarctation repair had post-intervention follow-up >6 months. Although improvement or stabilization of blood pressure control was seen early post-operatively, at mid-term all 4 patients had worsening hypertension with 3 needing intensification of their antihypertensive regimen. Of the 3 remaining patients in whom only early evaluation (<6 months post-intervention) was available, improvement in blood pressure control was observed in 2, while the remaining experienced no change in blood pressure readings.
To the best of our knowledge, this is the first study to assess rest and exercise invasive hemodynamics in adults with CoA. The main findings of the study are: (1) most patients demonstrated evidence of LV diastolic dysfunction/elevation in left filling pressures, particularly during exercise; (2) similarly, in this cohort, the prevalence of PH was high, with exercise unmasking underlying abnormal pulmonary vascular reserve; (3) most patients failed to show significant increases in CoA peak-to-peak gradients with arm exercise, and despite CoA re-intervention, multidrug antihypertensive treatment was still required.
Lifelong follow-up of patients with repaired CoA is mandatory due to long-term comorbidities and common need for re-intervention [4,25]. CoA represents the most frequent cause of hypertension in congenital heart disease, and it is associated with premature coronary artery disease and decreased life expectancy [1,3,12]. Symptomatic heart failure with preserved ejection fraction due to diastolic dysfunction with increased LV filling pressures is a common complication, especially in older patients [17,26]. Diastolic dysfunction has been well-reported in CoA by non-invasive methods, and results from a complex interplay of ventriculo-arterial uncoupling, intrinsic arteriopathy, LV hypertrophy, and additional congenital or acquired cardiovascular conditions [6,13,14]. Non-invasive exercise testing is recommended by the guidelines to assess exercise-induced hypertension and gradient response across the CoA and may also unmask abnormal myocardial reserve [15–18].
We present herein invasive hemodynamic evaluation in CoA, which is gold standard for diagnosis of diastolic dysfunction and PH. Our findings confirm previous non-invasive observations, demonstrating abnormal pulmonary vascular reserve and hemodynamic response to exercise in this population. In our cohort, LV diastolic dysfunction was highly prevalent and >50% had elevated PAWP at rest. Furthermore, exercise data showed high prevalence of impaired diastolic reserve and abnormal left heart compliance as shown by the composite of PAWP ≥25 mmHg or ΔPAWP/ΔCO >2 in 86%. These findings are of clinical importance, particularly in the evaluation of symptomatic patients late after CoA repair or those with noninvasive evidence of elevated pulmonary pressures. In addition, they provide further pathophysiologic basis for non-invasive observation of abnormal left atrial function [27] and the high burden of atrial arrythmias in this population [28].
Noteworthy, all patients with resting PH had concomitant elevated PAWP. These findings provide hemodynamic support to classifying patients with CoA and elevated pulmonary pressures as group II PH based on their anatomic substrate and expected pathophysiology [29]. Some individuals showed resting combined PH, consistent with pulmonary vascular remodeling, and evidence of reduced pulmonary vascular reserve during exercise was also common. Our group has recently reported on the prevalence of right heart dysfunction and PH in adults with CoA assessed by echocardiography and their association with increased risk for death and heart failure; similar findings have also been reported by others [30,31]. The findings of the present study do not only corroborate these noninvasive observations but underscore that the subpulmonary ventricle and the pulmonary vasculature should not be neglected in this population, despite CoA initially being a “left-sided” pathology [32].
Despite still being the ultimate diagnostic test, the invasive assessment of hemodynamics of native CoA and especially re-coarctation remains a clinical challenge. Gradients can be highly variable according to structural (presence of collateral vessels, for example) or physiologic (degree of sedation or need for general anesthesia) substrates. To mitigate this, some authors have studied the hemodynamic response to inotropes during cardiac catheterization. A recently published study in a younger CoA population (mean age at catheterization 27.3 ± 13.2 years) analyzed the use of epinephrine for gradient provocation prior to CoA intervention. In patients with low baseline CoA gradient but high gradients (>20 mmHg) after epinephrine administration, percutaneous intervention resulted in significant decrease in hypertension prevalence at mid-term follow-up [33]. In contrast, the subgroup without provocable gradient and therefore no CoA intervention, had worsening hypertension during follow-up, raising the question as to whether this was due to diagnostic failure of epinephrine stress testing, or rather to concomitant less modifiable factors. Studies have reported the use of isoproterenol [34,35] and dobutamine [36] as provocative maneuvers for assessment of CoA hemodynamics. The limitations of inotropic drugs for mimicking exercise are well-known and have been widely recognized in studies focused on stress testing for ischemic heart disease. Interestingly, in our cohort, 1 patient had both arm exercise and dobutamine stress catheterization, showing higher gradients during drug challenge. This patient did undergo subsequent intervention based on these findings without a long-term improvement in blood pressure control.
Invasive exercise evaluation might therefore represent a more physiologic test, better compared to noninvasive stress tests currently recommended by the guidelines. Most of our patients failed to show significant increases in blood pressure and CoA peak-to-peak gradients with exercise. Pressure gradient across an anatomic stenosis is determined by cross-sectional area and flow across the stenosis. One could argue that arm exercise could have been suboptimal for gradient provocation as flow augmentation in the descending aorta could have been insufficient with this form of exercise [20]. However, the fact that a high proportion of patients did have significant increases in intracardiac and pulmonary pressures at peak exercise with this type of exercise would argue against that, suggesting the lack of gradient provocation across the CoA to be indeed related to true absence of significant aortic obstruction. To support that hypothesis, of the 7 patients who underwent intervention after cardiac catheterization for “significant re-coarctation” by combination of anatomic, hemodynamic and clinical factors, all 4 with available long-term follow-up post-intervention had persistent hypertension. These patients might represent a more complex subgroup with severe diffuse arteriopathy, which limit the potential yield of CoA intervention [2,9,12,37].
The optimal method for the hemodynamic evaluation of re-coarctation remains to be elucidated and further studies are warranted to establish whether exercise and pharmacologic stress protocols at the time of catheterization could be used to unmask CoA severity. It is possible that using these provocative maneuvers, patients with unrepaired CoA and particularly re-coarctation could be categorized into 2 groups: those with true severe CoA who may benefit from invasive intervention [11,25], vs. those with a more complex phenotype but lacking major degrees of obstruction, where aggressive medical management, rather than CoA repair, may be of greater benefit [3,4,10].
Lastly, it should be noted that the only 2 patients with significant increase in CoA peak-to-peak gradients underwent exercise via supine cycle ergometer instead of arm adduction, highlighting the need to further investigate whether the diagnostic accuracy of these 2 types of exercise for CoA severity assessment is comparable. In addition, the “expected” gradients during exercise in those with and without anatomical obstruction need to be defined, as the cut-off of 20 mmHg used for resting gradients might not be applicable.
We acknowledge the small sample size and retrospective nature of the study. Patients referred for cardiac catheterization in current practice are typically more complex and this is reflected in the demographics of our cohort; thus, our results may not be fully applicable to all asymptomatic patients post-coarctation repair. For PH definition, we intentionally chose a mPAP cut-off value of ≥25 mmHg since our aim was to be more specific than sensitive. Peak-to-peak CoA gradient was not available in all cases; however, it was assessed in every case where there was concern of significant coarctation/re-coarctation. The type of exercise was chosen at the operator’s discretion, resulting in inherently heterogeneous results. Nevertheless, to the best of our knowledge, this is the first report of rest and exercise invasive hemodynamics in adults with CoA.
CoA is associated with significant vascular and myocardial disease at young ages. Prevalence of diastolic dysfunction and PH in our cohort was high, and exercise unmasked underlying abnormal diastolic and pulmonary vascular reserve. Most patients failed to show significant increases in CoA peak-to-peak gradients with exercise; further studies are warranted to establish the best diagnostic method to assess severity of CoA and select those patients who might benefit from CoA intervention.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: WRM, IMM; data collection: WRM, IMM; analysis and interpretation of results: WRM, IMM, CCJ; draft manuscript preparation: WRM, IMM. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data will be made available upon reasonable request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Brown, M. L., Burkhart, H. M., Connolly, H. M., Dearani, J. A., Cetta, F. et al. (2013). Coarctation of the aorta: Lifelong surveillance is mandatory following surgical repair. Journal of the American College of Cardiology, 62(11), 1020–1025. DOI 10.1016/j.jacc.2013.06.016. [Google Scholar] [CrossRef]
2. Choudhary, P., Canniffe, C., Jackson, D. J., Tanous, D., Walsh, K. et al. (2015). Late outcomes in adults with coarctation of the aorta. Heart, 101(15), 1190–1195. DOI 10.1136/heartjnl-2014-307035. [Google Scholar] [CrossRef]
3. Lee, M. G. Y., Babu-Narayan, S. V., Kempny, A., Uebing, A., Montanaro, C. et al. (2019). Long-term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart, 105(15), 1190–1196. DOI 10.1136/heartjnl-2018-314257. [Google Scholar] [CrossRef]
4. Dijkema, E. J., Leiner, T., Grotenhuis, H. B. (2017). Diagnosis, imaging and clinical management of aortic coarctation. Heart, 103(15), 1148–1155. DOI 10.1136/heartjnl-2017-311173. [Google Scholar] [CrossRef]
5. Shone, J. D., Sellers, R. D., Anderson, R. C., Adams Jr, P., Lillehei, C. W. et al. (1963). The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. American Journal of Cardiology, 11, 714–725. DOI 10.1016/0002-9149(63)90098-5. [Google Scholar] [CrossRef]
6. Jain, C. C., Warnes, C. A., Egbe, A. C., Cetta, F., DuBrock, H. M. et al. (2020). Hemodynamics in adults with the shone complex. American Journal of Cardiology, 130, 137–142. DOI 10.1016/j.amjcard.2020.06.024. [Google Scholar] [CrossRef]
7. Ungerleider, R. M., Pasquali, S. K., Welke, K. F., Wallace, A. S., Ootaki, Y. et al. (2013). Contemporary patterns of surgery and outcomes for aortic coarctation: An analysis of the society of thoracic surgeons congenital heart surgery database. The Journal of Thoracic and Cardiovascular Surgery, 145(1), 150–158. DOI 10.1016/j.jtcvs.2012.09.053. [Google Scholar] [CrossRef]
8. Connolly, H. M., Huston, 3rd, J., Brown Jr, R. D., Warnes, C. A., Ammash, N. M. et al. (2003). Intracranial aneurysms in patients with coarctation of the aorta: A prospective magnetic resonance angiographic study of 100 patients. Mayo Clinic Proceedings, 78(12), 1491–1499. DOI 10.4065/78.12.1491. [Google Scholar] [CrossRef]
9. Hager, A., Kanz, S., Kaemmerer, H., Schreiber, C., Hess, J. (2007). Coarctation long-term assessment (COALASignificance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. The Journal of Thoracic and Cardiovascular Surgery, 134(3), 738–745. DOI 10.1016/j.jtcvs.2007.04.027. [Google Scholar] [CrossRef]
10. Morgan, G. J., Lee, K. J., Chaturvedi, R., Bradley, T. J., Mertens, L. et al. (2013). Systemic blood pressure after stent management for arch coarctation implications for clinical care. JACC: Cardiovascular Interventions, 6(2), 192–201. DOI 10.1016/j.jcin.2012.10.009. [Google Scholar] [CrossRef]
11. Forbes, T. J., Kim, D. W., Du, W., Turner, D. R., Holzer, R. et al. (2011). Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: An observational study by the CCISC (Congenital cardiovascular interventional study consortium). Journal of the American College of Cardiology, 58(25), 2664–2674. DOI 10.1016/j.jacc.2011.08.053. [Google Scholar] [CrossRef]
12. de Divitiis, M., Pilla, C., Kattenhorn, M., Donald, A., Zadinello, M. et al. (2003). Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta. Journal of the American College of Cardiology, 41(12), 2259–2265. DOI 10.1016/s0735-1097(03)00480-7. [Google Scholar] [CrossRef]
13. Lombardi, K. C., Northrup, V., McNamara, R. L., Sugeng, L., Weismann, C. G. (2013). Aortic stiffness and left ventricular diastolic function in children following early repair of aortic coarctation. American Journal of Cardiology, 112(11), 1828–1833. DOI 10.1016/j.amjcard.2013.07.052. [Google Scholar] [CrossRef]
14. Egbe, A. C., Miranda, W. R., Connolly, H. M., Borlaug, B. A. (2021). Coarctation of aorta is associated with left ventricular stiffness, left atrial dysfunction and pulmonary hypertension. American Heart Journal, 241, 50–58. DOI 10.1016/j.ahj.2021.07.005. [Google Scholar] [CrossRef]
15. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2020). 2020 ESC guidelines for the management of adult congenital heart disease: The task force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPCInternational Society for Adult Congenital Heart Disease (ISACHD). European Heart Journal, 42(6), 563–645. DOI 10.1093/eurheartj/ehaa554. [Google Scholar] [CrossRef]
16. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation, 139(14), e698–e800. DOI 10.1161/CIR.0000000000000603. [Google Scholar] [CrossRef]
17. Chen, C. K., Cifra, B., Morgan, G. J., Sarkola, T., Slorach, C. et al. (2016). Left ventricular myocardial and hemodynamic response to exercise in young patients after endovascular stenting for aortic coarctation. The Journal of the American Society of Echocardiography, 29(3), 237–246. DOI 10.1016/j.echo.2015.11.017. [Google Scholar] [CrossRef]
18. Yogeswaran, V., Connolly, H. M., Al-Otaibi, M., Ammash, N. M., Warnes, C. A. et al. (2018). Prognostic role of hypertensive response to exercise in patients with repaired coarctation of aorta. Canadian Journal of Cardiology, 34(5), 676–682. DOI 10.1016/j.cjca.2018.02.004. [Google Scholar] [CrossRef]
19. Egbe, A. C., Miranda, W. R., Devara, J., Iftikhar, M., Shaik, L. et al. (2021). Effect of combined ventricular-arterial stiffening on exercise hemodynamics in adults with repaired coarctation of aorta. CJC Open, 3(5), 603–608. DOI 10.1016/j.cjco.2021.01.001. [Google Scholar] [CrossRef]
20. Markel, H., Rocchini, A. P., Beekman, R. H., Martin, J., Palmisano, J. et al. (1986). Exercise-induced hypertension after repair of coarctation of the aorta: Arm versus leg exercise. Journal of the American College of Cardiology, 8(1), 165–171. DOI 10.1016/s0735-1097(86)80108-5. [Google Scholar] [CrossRef]
21. Borlaug, B. A., Nishimura, R. A., Sorajja, P., Lam, C. S., Redfield, M. M. (2010). Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation: Heart Failure, 3(5), 588–595. DOI 10.1161/circheartfailure.109.930701. [Google Scholar] [CrossRef]
22. Jain, C. C., Borlaug, B. A. (2020). Performance and interpretation of invasive hemodynamic exercise testing. Chest, 158(5), 2119–2129. DOI 10.1016/j.chest.2020.05.552. [Google Scholar] [CrossRef]
23. Galiè, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I. et al. (2016). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERSEndorsed by: Association for European Paediatric and Congenital Cardiology (AEPCInternational Society for Heart and Lung Transplantation (ISHLT). European Heart Journal, 37(1), 67–119. DOI 10.1093/eurheartj/ehv317. [Google Scholar] [CrossRef]
24. Chomsky, D. B., Lang, C. C., Rayos, G. H., Shyr, Y., Yeoh, T. K. et al. (1996). Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation, 94(12), 3176–3183. DOI 10.1161/01.cir.94.12.3176. [Google Scholar] [CrossRef]
25. Holzer, R., Qureshi, S., Ghasemi, A., Vincent, J., Sievert, H. et al. (2010). Stenting of aortic coarctation: Acute, intermediate, and long-term results of a prospective multi-institutional registry--congenital Cardiovascular interventional study consortium (CCISC). Catheterization and Cardiovascular Interventions, 76(4), 553–563. DOI 10.1002/ccd.22587. [Google Scholar] [CrossRef]
26. Rinnström, D., Dellborg, M., Thilén, U., Sörensson, P., Nielsen, N. E. et al. (2016). Left ventricular hypertrophy in adults with previous repair of coarctation of the aorta; association with systolic blood pressure in the high normal range. International Journal of Cardiology, 218, 59–64. DOI 10.1016/j.ijcard.2016.05.033. [Google Scholar] [CrossRef]
27. Egbe, A. C., Miranda, W. R., Oh, J. K., Connolly, H. M. (2021). Prognostic implications of left heart diastolic dysfunction in adults with coarctation of aorta. European Heart Journal Cardiovasc Imaging, 22(11), 1332–1340. DOI 10.1093/ehjci/jeab165. [Google Scholar] [CrossRef]
28. Kirsh, J. A., Walsh, E. P., Triedman, J. K. (2002). Prevalence of and risk factors for atrial fibrillation and intra-atrial reentrant tachycardia among patients with congenital heart disease. American Journal of Cardiology, 90(3), 338–340. DOI 10.1016/s0002-9149(02)02480-3. [Google Scholar] [CrossRef]
29. Rosenzweig, E. B., Abman, S. H., Adatia, I., Beghetti, M., Bonnet, D. et al. (2019). Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. European Respiratory Journal, 53(1), DOI 10.1183/13993003.01916-2018. [Google Scholar] [CrossRef]
30. Egbe, A. C., Miranda, W. R., Jain, C. C., Connolly, H. M. (2021). Right heart dysfunction in adults with coarctation of aorta: Prevalence and prognostic implications. Circulation: Cardiovascular Imaging, 14(12), 1100–1108. DOI 10.1161/CIRCIMAGING.121.013075. [Google Scholar] [CrossRef]
31. Oliver, J. M., Gallego, P., Gonzalez, A. E., Sanchez-Recalde, A., Bret, M. et al. (2014). Pulmonary hypertension in young adults with repaired coarctation of the aorta: An unrecognised factor associated with premature mortality and heart failure. Internation Journal of Cardiology, 174(2), 324–329. DOI 10.1016/j.ijcard.2014.04.060. [Google Scholar] [CrossRef]
32. Friedberg, M. K. (2021). Aortic coarctation is right out of left field: The impact of pulmonary hypertension and right ventricular dysfunction on clinical outcomes. Circulation. Cardiovascular Imaging, 14(12), 1109–1111. DOI 10.1161/CIRCIMAGING.121.013740. [Google Scholar] [CrossRef]
33. Meijs, T. A., Krings, G. J., Saad, A., Molenschot, M. M. C., Doevendans, P. A. et al. (2020). Epinephrine stress testing during cardiac catheterization in patients with aortic coarctation. American Heart Journal, 225, 78–87. DOI 10.1016/j.ahj.2020.05.007. [Google Scholar] [CrossRef]
34. Patel, N. D., Sullivan, P. M., Takao, C. M., Badran, S., Ahdoot, J. et al. (2018). Unmasking the borderline coarctation: The utility of isoproterenol in the paediatric cardiac catheterisation laboratory. Cardiology in the Young, 28(6), 804–810. DOI 10.1017/S1047951118000239. [Google Scholar] [CrossRef]
35. Kim, K. S., Eryu, Y., Asakai, H., Hayashi, T., Kaneko, M. et al. (2012). Isoproterenol stress test during catheterization of patients with coarctation of the aorta. Pediatrics International, 54(4), 461–464. DOI 10.1111/j.1442-200X.2012.03572.x. [Google Scholar] [CrossRef]
36. Banaszak, P., Szkutnik, M., Kusa, J., Banaszak, B., Białkowski, J. (2009). Utility of the dobutamine stress echocardiography in the evaluation of the effects of a surgical repair of aortic coarctation in children. Cardiology Journal, 16(1), 20–25. [Google Scholar]
37. Ou, P., Bonnet, D., Auriacombe, L., Pedroni, E., Balleux, F. et al. (2004). Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. European Heart Journal, 25(20), 1853–1859. DOI 10.1016/j.ehj.2004.07.021. [Google Scholar] [CrossRef]
6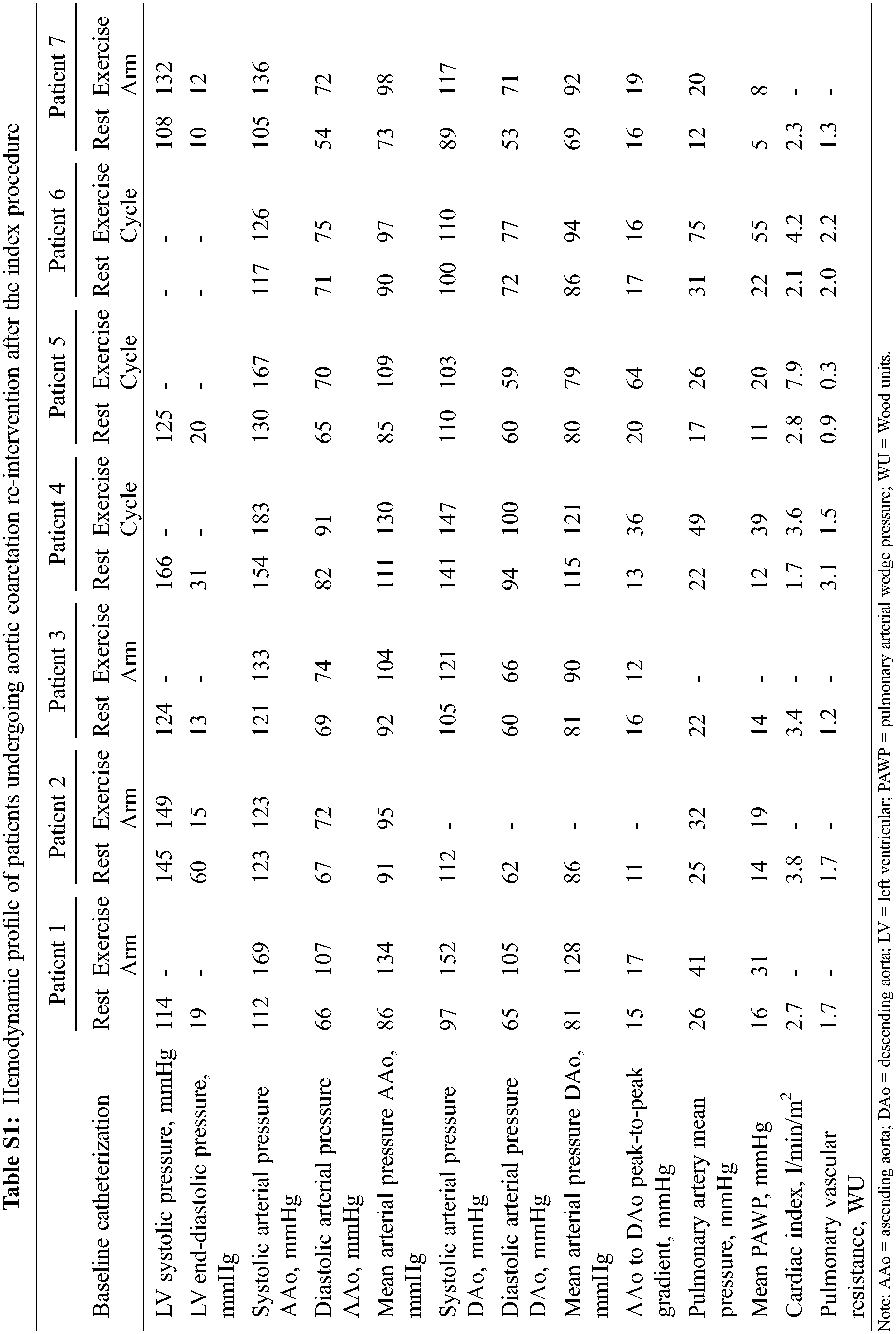
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools