 Open Access
Open Access
CASE REPORT
Appropriate Heart Rate in a Patient with Repaired Tetralogy of Fallot
1 Department of Transitional Medicine, Division of Congenital Heart Disease, Shizuoka General Hospital, Shizuoka, Japan
2 Congenital Heart Disease Center, Nara Medical University, Kashihara, Japan
3 Department of Cardiology, Shizuoka General Hospital, Shizuoka, Japan
4 Department of Clinical Engineering, Shizuoka General Hospital, Shizuoka, Japan
* Corresponding Author: Aya Miyazaki. Email:
Congenital Heart Disease 2022, 17(6), 647-652. https://doi.org/10.32604/chd.2022.021837
Received 08 April 2022; Accepted 28 June 2022; Issue published 11 October 2022
Abstract
Appropriate heart rate in a failing pulmonary ventricle remains unknown, particularly in congenital heart disease with unique hemodynamics. A 71-year-old male with repaired tetralogy of Fallot and a pacemaker for a sinus node dysfunction suffered from heart failure symptoms with preserved left ventricular function. Simply changing the pacemaker’s lower rate from 60 to 75 bpm, New York Heart Association classification improved from III to II, and hemodynamic parameters drastically improved. We regarded this case as informative. Appropriate heart rate could be higher in congenital patients with failing right and non-failing left ventricles than in adults with malfunctioning LV.Graphic Abstract
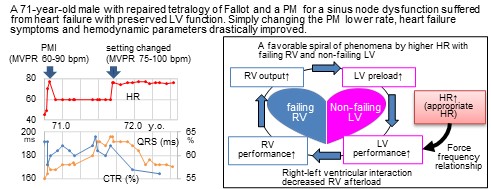
Keywords
Appropriate heart rate (HR) remains unclear in congenital heart disease, particularly when symptoms of heart failure progress. Heart failure is often because of the failing RV rather than the LV. We have experienced a patient in whom the HR setting was quite effective. The indicative case is herein described.
A male patient, 71-year-old, presented severe heart failure symptoms (New York Heart Association (NYHA) classification; III), hypoxemia (percutaneous oxygen saturation (SpO2); 84%–92% in room air), and marked exercise intolerance (could not reach the bathroom without a break at home). He previously underwent intracardiac repair for tetralogy of Fallot at 26 years old. A small atrial septal defect was observed. On trans-thoracic and trans-esophageal echocardiography, the hole was shunting right-to-left continuously without the Valsalva maneuver. The tricuspid valve was severely regurgitant. The right atrium was significantly dilated, and the interventricular septum was shifted leftward in diastole. The left ventricular (LV) function seemed preserved (LV end-diastolic diameter; 44 mm, LV ejection fraction; 57%) (Fig. 1).
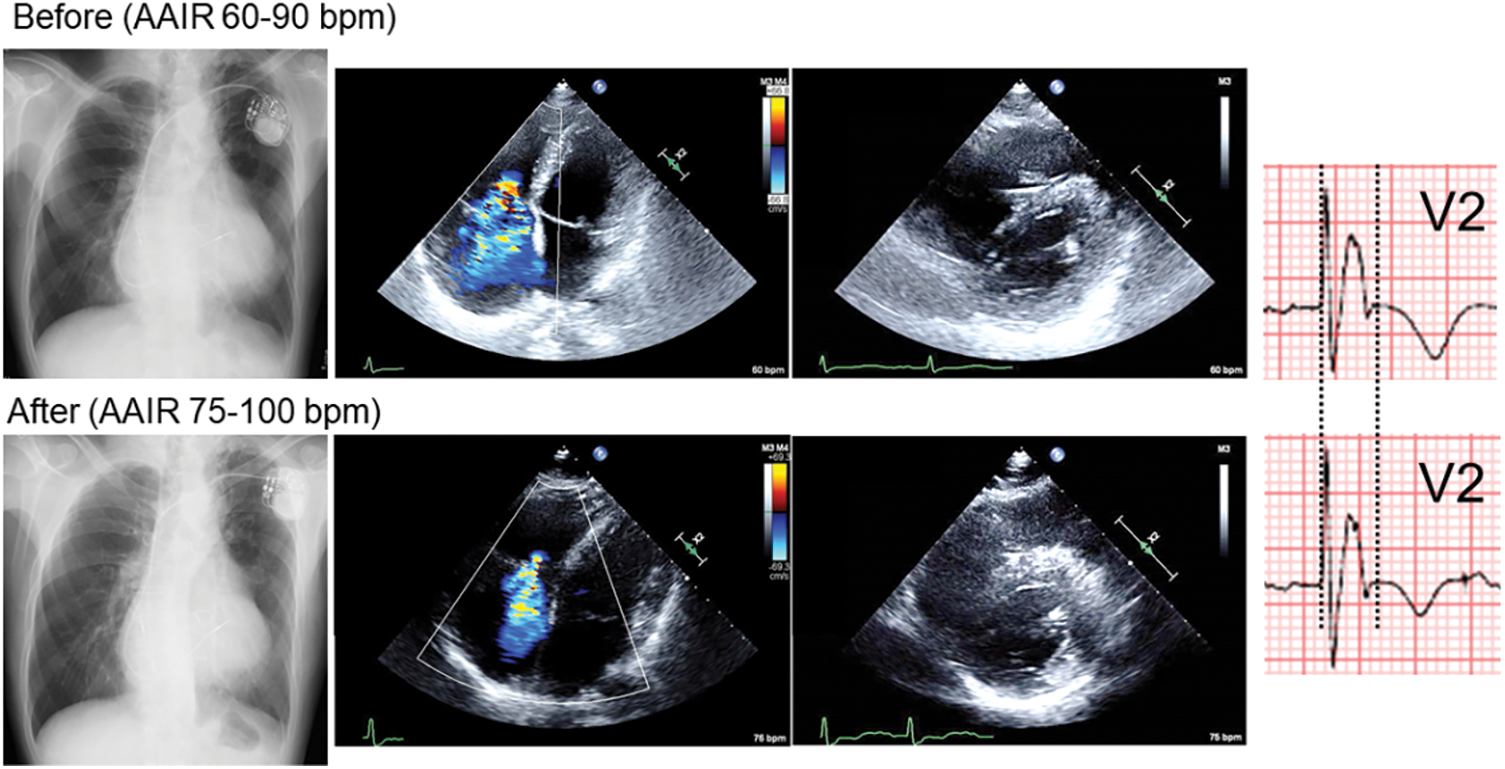
Figure 1: Chest X-ray, 4-chamber and short-axis echocardiography, and electrocardiogram in V2 before and 4 months (Chest X-ray, Echocardiography) or 6 months (electrocardiogram) after changing pacemaker lower rate from 60 to 75 bpm
He had undergone catheter ablations three times, between 61 and 66 years old, for intra-atrial reentrant tachycardia and atrial fibrillation. A dual-chamber pacemaker (Azure XT DR®, Medtronic, Minneapolis, MN, USA) was implanted for sinus nodal dysfunction at 70 years old (Fig. 2). At that time, the pacemaker was set for AAIR with managed ventricular pacing 60–90 bpm: the atrial and ventricular pacing burden being 91% and 2%, respectively (Fig. 3).
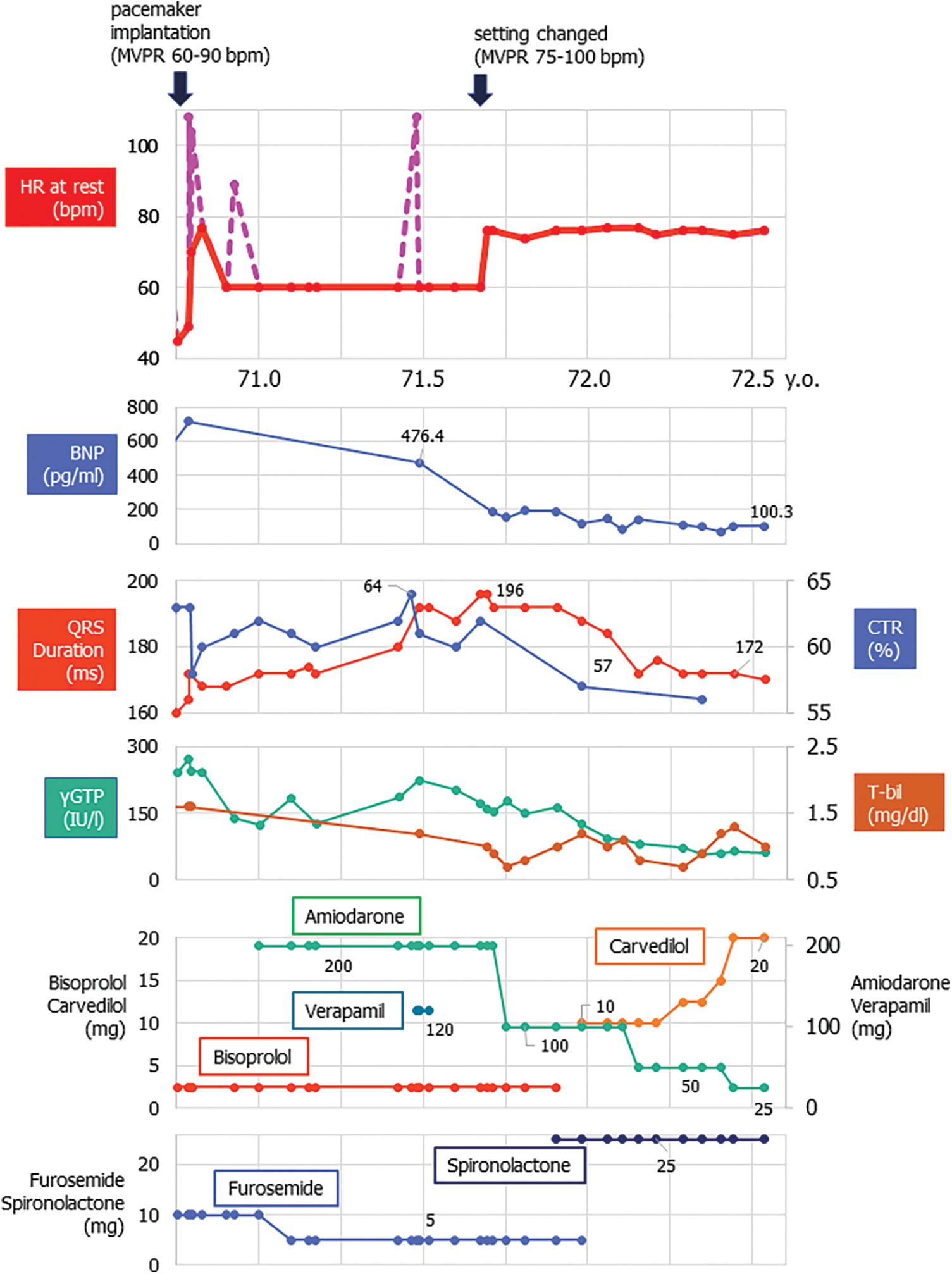
Figure 2: Clinical course in a 71-year-old patient with repaired tetralogy of Fallot following pacemaker implantation
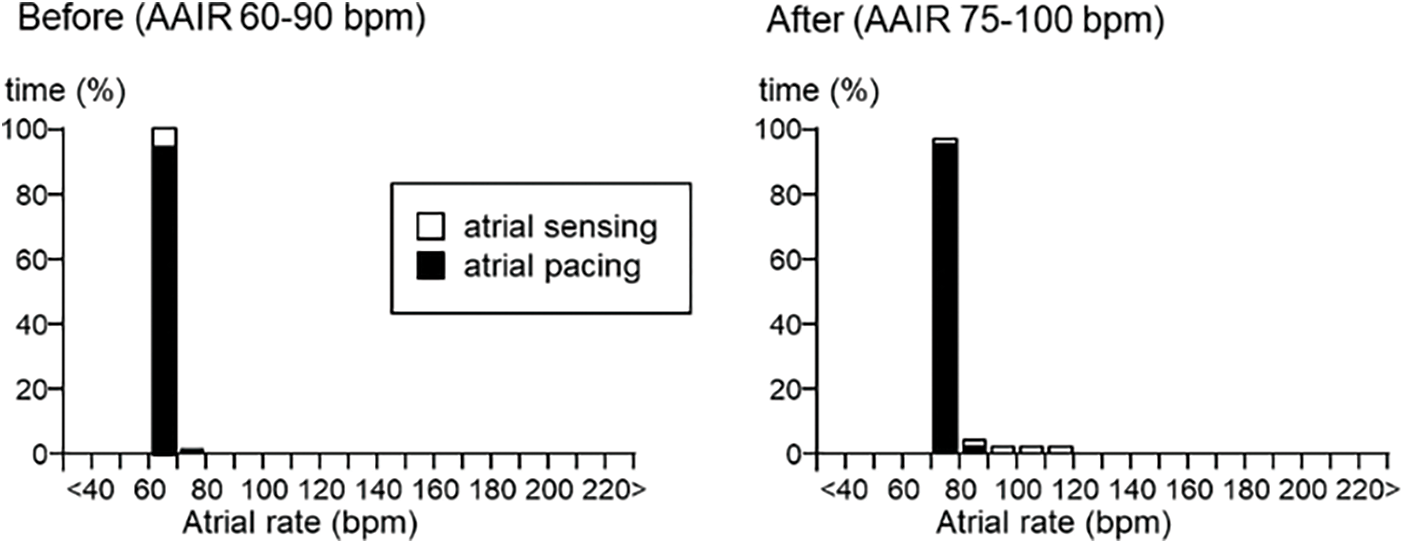
Figure 3: Pacemaker atrial rate histogram before and after changing pacemaker setting (AAIR with managed ventricular pacing)
Note: Before: atrial and ventricular pacing burden 91% and 2%, respectively.
After: atrial and ventricular pacing burden 97.9% and <0.1%, respectively.
Left black arrow showed the timing of pacemaker implantation with the setting of MVPR 60–90 bpm. Right black arrow showed the timing of changing the pacemaker setting to MVPR 75–100 bpm. Upper graph showed the resting heart rate (HR). Red line showed the HR during sinus rhythm or during atrial pacing. Pink dot pink line indicated the one during atrial tachycardia.
BNP; brain natriuretic peptide, CTR; cardiothoracic ratio by X-ray, γGPT; gamma-glutamyltransferase, T-bil; total bilirubin.
After the recent episode of heart failure, we changed the setting of managed ventricular pacing up to 75–100 bpm. Also, home oxygenation therapy was commenced. Eventually, his heart failure symptoms gradually improved (down to NYHA class II) on the same dose of anti-heart failure drugs (Fig. 3). SpO2 came up to 91%–97% in room air. He now enjoys taking a walk (6 min walk test; 384 m with room air). Atrial tachyarrhythmias are under control with a smaller dose of amiodarone. The serum level of the brain natriuretic peptide, cardiothoracic ratio on chest X-ray, and QRS duration on electrocardiogram changed favorably (Figs. 1 and 2). Tricuspid regurgitation and diastolic leftward shift of the ventricular septum improved (Fig. 1).
Five months after pacing modification, we performed the catheterization to evaluate hemodynamics with changing pacing rate. Central venous pressure decreased with stable LV end-systolic pressure in higher atrial pacing (Fig. 4).
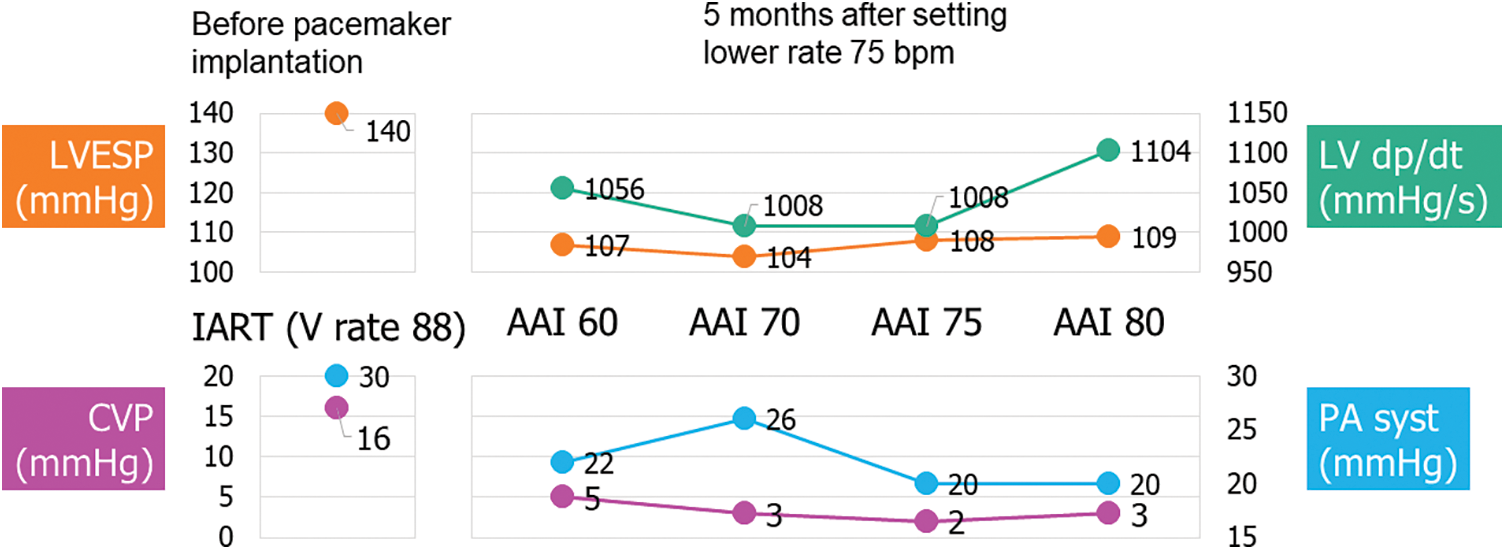
Figure 4: Pressure measurement at catheterization before pacemaker implantation and 5 months after changing pacemaker lower rate up to 75 bpm
Note: Before: Catheterization at 68 years old, during intra-atrial reentrant tachycardia (IART). Pulmonary resistance 4.7 WUm2.
After: Pulmonary resistance 4.1 WUm2.
CVP; central venous pressure, LVEDP; left ventricular end-systolic pressure, PA syst; systolic pulmonary pressure.
A higher HR at rest is known to be unfavorable for cardiovascular events either in the general population or in patients with heart failure [1]. In the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) [2], the placebo group showed that patients with higher HR had augmented risks of cardiovascular death and hospital admission for heart failure. In the ivabradine group, patients with HR < 60 bpm on the treatment had fewer primary composite endpoint events than those with higher HR. Accordingly, the recent guidelines recommend decreasing HR in patients who suffer from heart failure with reduced ejection fraction (HFrEF) and whose heart rate is over 70 or 75 bpm at rest [3,4].
The circumstance was rather different in our present patient; a higher HR setting appeared appropriate. We regarded our experience as informative when considering appropriate HR in congenital heart disease with dysfunction of the RV, the pulmonary ventricle.
Right-left ventricular interaction is an important factor when the RV is failing. The RV wraps around the LV partly in its short axis, and LV contraction contributes its kinetics to the 20%–40% of RV mechanical work [5,6]. In a canine study, RV pressure rose as LV pressure was elevated by a partial constriction of the aorta [7]. A good performance of the LV contributes to the improvement of RV performance.
When the LV is well-functioning, higher HR increases LV performance up to 160–180 bpm according to the force-frequency relationship [8]. This should consequently turn out beneficial for RV performance on the basis of direct ventricular interaction. RV afterload decreases with lower left atrial pressure. This indirect and hemodynamical effect allows the LV to act even better with increased preload from the pulmonary circulation. Thus, higher HR causes a favorable spiral of phenomena (Fig. 5). A key of the story is probably the presence of a non-failing LV. That sounds the essence of difference between the adult HFrEF patients and those with congenital heart disease. Setting a higher HR might not work even in patients with congenital heart disease in whom the LV was malfunctioning.
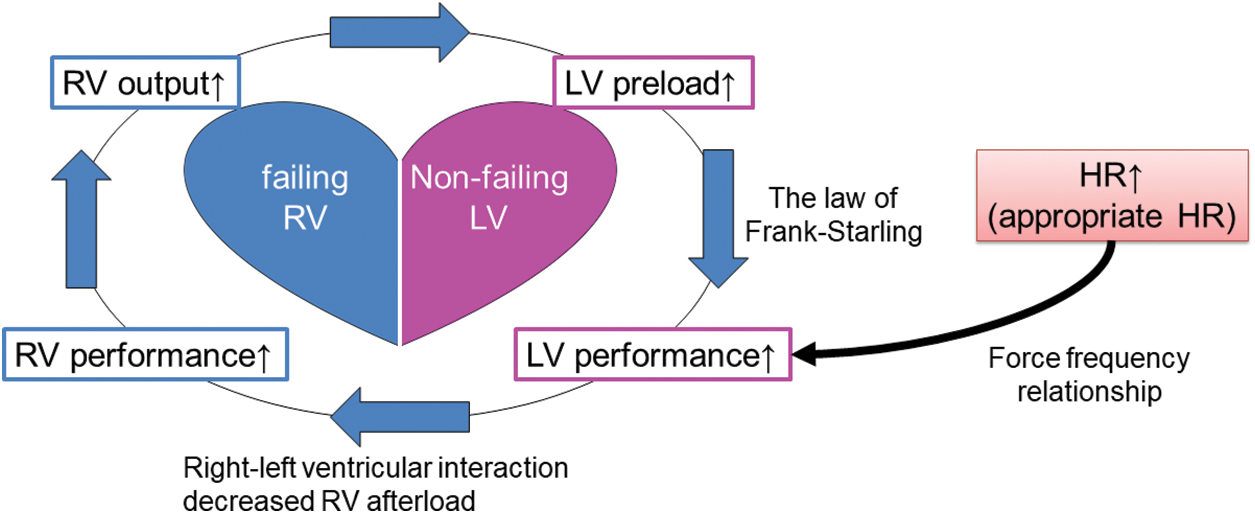
Figure 5: Hemodynamic consequence related to higher heart rate with failing right and non-failing left ventricles
Note: RV; right ventricle, LV; left ventricle, HR; heart rate.
As far as we know, only a solitary report described the relationship between HR and the pulmonary RV performance in repaired tetralogy of Fallot [9]; right ventricular end diastolic volume index (RVEDVI) was larger with a lower HR. As HR decreased by 1 bpm, RVEDVI increased by 1.09 ml/m2.
Another point we should raise is the fact that HR was controlled with a pacing system. This is fundamentally different from the SHIFT study. As is well known, repaired tetralogy of Fallot shows a pattern of right bundle branch block quite frequently. Together with the site of ventricular pacing, the pattern of ventricular contraction is abnormal. The overall LV contraction may not coordinate well by pacing with higher HR. When determining optimal HR in repaired tetralogy with heart failure, multiple factors should be taken into account. In our present patient with sinus node dysfunction and good atrioventricular conduction, setting the pacemaker at lower rate 75 bpm was of a great help.
Heart failure improved in a patient with repaired tetralogy of Fallot by setting the pacemaker at a relatively high heart rate. Optimal HR in a general heart failure population occasionally seems inapplicable for congenital heart disease, particularly when the LV is functioning reasonably well, and heart failure is on the RV side.
Authorships: The authors confirm contribution to the paper as follows: study conception and design: A. Miyazaki. Author; data collection: A. Miyazaki, Y. Takeuchi, J. Tomida, Y. Fujimoto, N. Mitsushita, A. Ikai; analysis and interpretation of results: A. Miyazaki, H. Uemura, Y. Ono; draft manuscript preparation: A. Miyazaki, H. Uemura. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national guidelines on human experimentation (Japan) and with the Helsinki Declaration of 1975 (as revised in 2013). This type of retrospective case report was determined not to require approval by the ethics committee. Informed Consent was obtained from the subject involved in the study.
Acknowledgement: The authors would like to thank all medical staff involved in treating this patient.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare they have no conflicts of interest to report regarding the present study.
References
1. Custodis, F., Schirmer, S. H., Baumhäkel, M., Heusch, G., Böhm, M. et al. (2010). Vascular pathophysiology in response to increased heart rate. Journal of the American College of Cardiology, 56(24), 1973–1983. DOI 10.1016/j.jacc.2010.09.014. [Google Scholar] [CrossRef]
2. Böhm, M., Swedberg, K., Komajda, M., Borer, J. S., Ford, I. et al. (2010). Heart rate as a risk factor in chronic heart failure (SHIFTThe association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet, 376(9744), 886–894. DOI 10.1016/S0140-6736(10)61259-7. [Google Scholar] [CrossRef]
3. McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A. et al. (2021). 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal, 42(36), 3599–3726. DOI 10.1093/eurheartj/ehab368. [Google Scholar] [CrossRef]
4. Tsutsui, H., Ide, T., Ito, H., Kihara, Y., Kinugawa, K. et al. (2021). JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circulation Journal, 85(12), 2252–2291. DOI 10.1253/circj.CJ-21-0431. [Google Scholar] [CrossRef]
5. Konstam, M. A., Kiernan, M. S., Bernstein, D., Bozkurt, B., Jacob, M. et al. (2018). Evaluation and management of right-sided heart failure: A scientific statement from the American Heart Association. Circulation, 137(20), e578–e622. DOI 10.1161/CIR.0000000000000560. [Google Scholar] [CrossRef]
6. Sheehan, F., Redington, A. (2008). The right ventricle: Anatomy, physiology and clinical imaging. Heart, 94(11), 1510–1515. DOI 10.1136/hrt.2007.132779. [Google Scholar] [CrossRef]
7. Santamore, W. P., Dell’Italia, L. J. (1998). Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Progress in Cardiovascular Diseases, 40(4), 289–308. DOI 10.1016/S0033-0620(98)80049-2. [Google Scholar] [CrossRef]
8. Mulieri, L. A., Leavitt, B. J., Martin, B. J., Haeberle, J. R., Alpert, N. R. (1993). Myocardial force-frequency defect in mitral regurgitation heart failure is reversed by forskolin. Circulation, 88(6), 2700–2704. DOI 10.1161/01.CIR.88.6.2700. [Google Scholar] [CrossRef]
9. Jolley, M., Hickey, K., Annese, D., Gauvreau, K., Geva, T. et al. (2015). Resting heart rate influences right ventricular volume in repaired tetralogy of Fallot. Pediatric Cardiology, 36(4), 813–820. DOI 10.1007/s00246-014-1088-y. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools