 Open Access
Open Access
ARTICLE
Evaluation of Biventricular Volume and Systolic Function in Children with Ventricular Septal Defect and Moderate to Severe Pulmonary Hypertension Using Real-Time Three-Dimensional Echocardiography
1
Children’s Heart Center, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College,
Huazhong University of Science & Technology, Wuhan, China
2
Department of Ultrasound, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College,
Huazhong University of Science & Technology, Wuhan, China
* Corresponding Author: Jun Gao. Email:
# These authors contributed equally
Congenital Heart Disease 2022, 17(6), 697-707. https://doi.org/10.32604/chd.2022.022648
Received 18 March 2022; Accepted 06 June 2022; Issue published 11 October 2022
Abstract
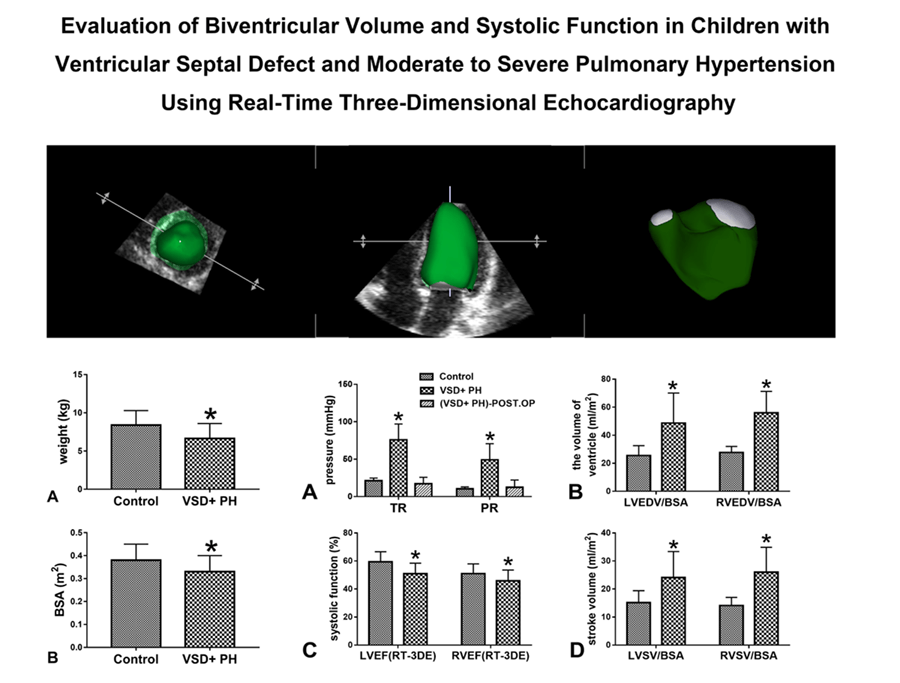
Background: Real-time three-dimensional echocardiography (RT-3DE) could obtain ventricular volume and ejection fraction rapidly and non-invasively without relying on ventricular morphology. This study aims to use RT-3DE to evaluate the changes in biventricular volume and systolic function in children with ventricular septal defect (VSD) and moderate to severe pulmonary hypertension (PH) before surgery. Methods: In this study 18 children with VSD and moderate to severe PH (VSD + PH Group) and 18 healthy children of the same age (Control Group) were recruited. Biventricular volume and systolic function were evaluated by RT-3DE. The measurements included: left and right ventricular volume indexed to body surface area (BSA), stroke volume (SV) indexed to BSA, and ejection fraction (EF). Results: The results showed left and right ventricular volume indexed to BSA and SV indexed to BSA were significantly increased in VSD + PH Group (VSD + PH Group vs. Control Group), LVEDV/BSA (ml/m2 ): 48.67 ± 21.46 vs. 25.59 ± 6.96, RVEDV/BSA (ml/m2 ): 55.98 ± 15.35 vs. 27.69 ± 4.37, LVSV/BSA (ml/m2 ): 24.08 ± 9.30 vs. 15.14 ± 4.29, RVSV/BSA (ml/m2 ): 26.02 ± 8.87 vs. 14.11 ± 2.89, (P < 0.05). While for EF in VSD + PH Group decreased (VSD + PH Group vs. Control Group), LVEF: 50.93 ± 7.50% vs. 59.38 ± 7.24%, RVEF: 45.84 ± 7.71% vs. 51.05 ± 6.90% (P < 0.05). Conclusion: In children with VSD and moderate to severe PH, increased biventricular volume and decreased systolic function were observed with RT-3DE, but biventricular systolic function remained within acceptable limits. The children in this study recovered well after surgery without serious perioperative complications, suggesting that biventricular systolic function may help facilitate the surgical decision-making process in children with VSD and moderate-tosevere PH.Graphic Abstract
Keywords
Ventricular septal defect (VSD) is one of the most common cardiac congenital malformations, accounting for almost 40% of all cardiac anomalies [1]. The main factors that determine the pathophysiological response of VSD are the size and direction of the shunt at the ventricular level and the degree of ventricular volume load. In patients with large VSD without pulmonary vascular disease, the increase in the volume load of the left atrium and left ventricle leads to dilatation of the left ventricle and eccentric left ventricular hypertrophy. The presence of related long-term pulmonary hypertension may eventually lead to right ventricular hypertrophy and dilation [2]. Therefore, in the large VSD, the ventricular morphology and pumping function will undergo greater changes.
In children with large VSD, the amount of left-to-right shunt at the ventricular level may initially be small due to high pulmonary vascular resistance in the early neonatal period. As the pulmonary vascular resistance decreases, the shunt flow from left to right increases. At the same time, the patient’s symptoms become more and more obvious due to excessive pulmonary blood flow. Interventricular shunting rises and the patient becomes increasingly symptomatic due to excessive pulmonary blood flow [3]. Children with large VSD and pulmonary hypertension (PH) usually have difficulty in breathing during the first few weeks of life and cannot grow and develop normally. In this case, surgery is usually recommended within 3 months after birth [2]. PH increases the risk of perioperative complications in children, prolongs the length of stay in the intensive care unit (ICU) and increases long-term mortality [4]. However, the current literature on ventricular size and function in children with VSD and PH is limited.
Two-dimensional (2D) and M-mode echocardiography are very important in the assessment of ventricular function, which are mainly measured based on geometric assumptions. The size and morphology of the ventricle in children with VSD and PH change, which is technically challenging [5]. Real-time three-dimensional echocardiography (RT-3DE) can directly measure the volume of the heart cavity and calculate the ejection fraction without relying on the geometry of the ventricular cavity, which can assess cardiac function in real time, non-invasively, quickly, and accurately. Therefore, this study aims to use RT-3DE to evaluate the changes in biventricular volume and systolic function in children with VSD and moderate-to-severe PH before surgery.
This study was approved by the Ethics Committee of Wuhan Children’s Hospital (Approval No. 2021R103-E01). Prospectively collect data from all participants for statistical analysis.
2.1.1 First Group: Healthy Volunteers (Control Group)
Prospectively collected healthy volunteers of the same age, a total of 21 cases were collected. The healthy volunteers were confirmed by clinical history, physical examination, electrocardiogram, and echocardiography.
2.1.2 Second Group: Children with VSD and Moderate-to-Severe PH (VSD + PH Group)
Prospective collection of children with VSD and moderate-to-severe PH, inclusion criteria: 1) Simple VSD or VSD + PFO (patent foramen ovale) (the shunt width of PFO ≤ 5mm); 2) The defect diameter of the ventricular septum ≥2/3 of the inner diameter of the aortic valve annulus; 3) Systolic pulmonary arterial pressure (SPAP) = right atrial pressure (approximately 5mmHg) + pressure gradient inferred from peak tricuspid regurgitation velocity. Mean pulmonary arterial pressure (mPAP) = right atrial pressure (approximately 5mmHg) + pressure gradient inferred from peak pulmonary regurgitation velocity in early diastole. SPAP ≥ 50mmHg and/or mPAP ≥ 40mmHg were diagnostic criteria to be moderate to severe PH. Exclusion criteria: 1) VSD accompanied by other cardiac structural malformations; 2) VSD with cardiomyopathy. A total of 23 children were collected.
All the selected children with VSD and moderate-to-severe PH underwent an echocardiography examination one day before surgery, and the relevant conventional and 3-dimensional ultrasound images were collected; postoperative echocardiography at the bedside was performed within 1–3 days after surgery to collect relevant conventional ultrasound image.
All echocardiograms were acquired by a single experienced cardiac sonographer. The study was conducted while the children were sleeping or quiet.
All preoperative echocardiographic studies were performed using the commercially available IE33 system (Philips Medical Systems, Andover, MA). Matrixarray transducers (S5-1 and S8-3) were used to obtain the routine M-mode, 2-dimensional and Doppler related parameters, and a dedicated 3-dimensional full sampling transducer (X3-1) was used for the 3-dimensional full volume data sets acquisition. Using the X3-1 probe for inspection to obtain a clear apical four-chamber image by adjusting the image depth, gain, etc., and ensure that the sampling sector covers the entire area of interest. Then, we activated the 3D full volume imaging mode and obtained 3-dimensional full-volume ultrasound images of consecutive complete cardiac cycles of at least four cycles for image storage. The acquired images were then stored in DICOM format and imported into a TomTec software (Image-Arena 4.6) (TomTec Imaging Systems, Munich, Germany) for off-line analysis.
All postoperative echocardiograms were performed at the bedside using a commercially available M9 system (Mindray Healthcare system, Shenzhen, China). Matrixarray transducers P10-4s were used to obtain conventional M-mode, 2-dimensional and Doppler related parameters.
2.3 RT-3DE Echocardiography Analysis
TomTec workstation LV-analysis and RV-Function software were used to analyze the left or right ventricular 3D volume database off-line respectively. LV-analysis: three standard sections of left ventricle including left ventricular apical four-chamber view, two-chamber view and three-chamber view were obtained by cutting and rotating the LV 3D database. The end-systolic and end-diastolic endocardial contours of left ventricle were delineated on the above three views (Figs. 1A and 1B). RV-Function: three orthogonal sections of right ventricle including right ventricular four-chamber view, short axis view, coronal view was obtained by cutting and rotating the RV 3D database. Firstly, on the four-chamber view determined the central point of mitral annulus, tricuspid annulus, and the apical level of the left ventricular cavity. Secondly the end-systolic and the end-diastolic endocardial contours of the above three sections of the right ventricle were delineated. Chordae tendineae, papillary muscles, and regulatory bundles were included in the delineation (Figs. 1C and 1D). Endocardial contours could be manually adjusted when necessary to optimize boundary position and tracking values were automatically calculated by the software. The left or right ventricular volume model were automatically generated by software and the volume-time curve was displayed by 2-dimensional coordinate diagram. Left and right ventricular end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF) values were also given.
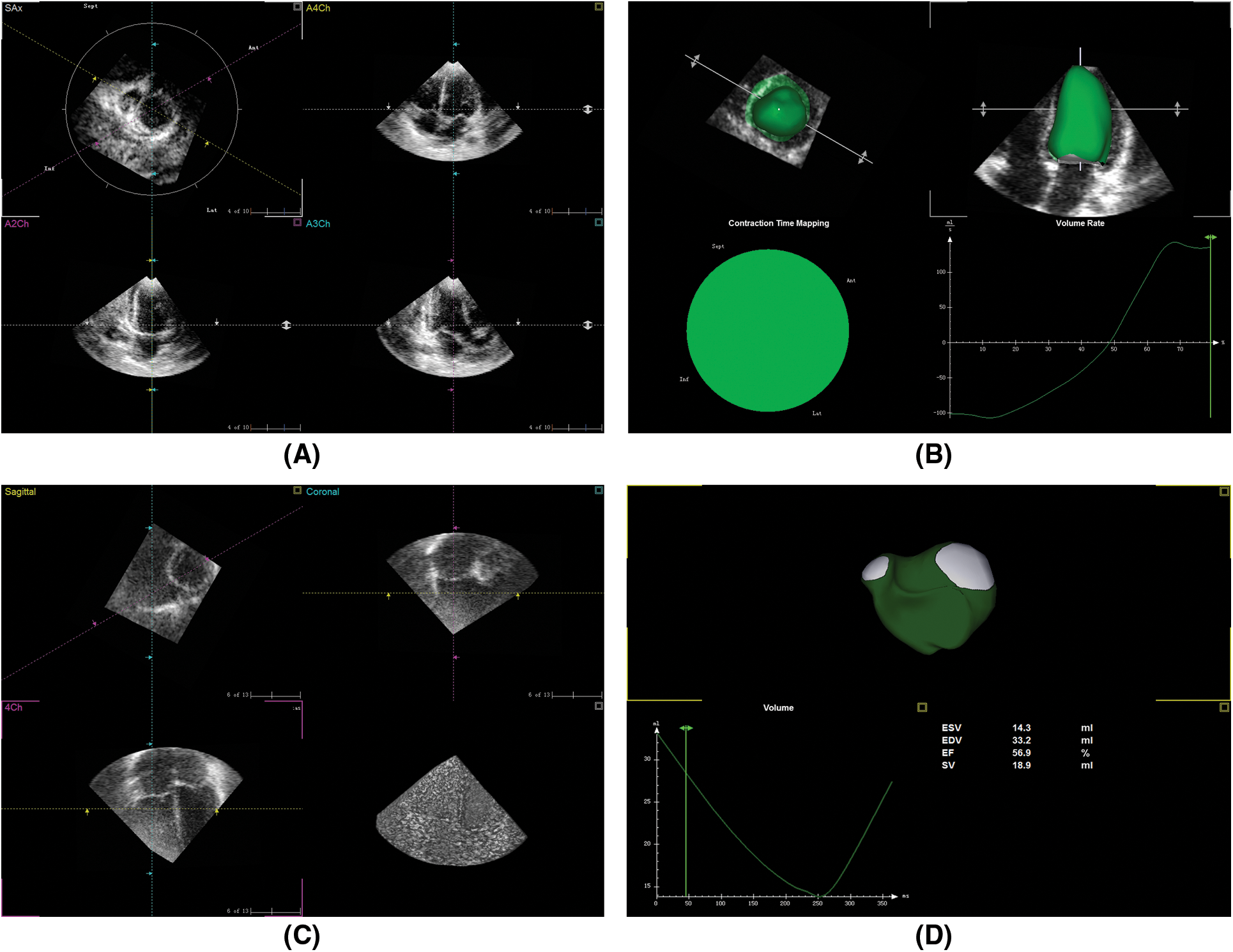
Figure 1: RT-3DE database analysis by TomTec
2.4 LV-Analysis and RV-Function Reproducibility
Twelve subjects were randomly selected from the study subjects to estimate the variability of the TomTec LV-analysis and RV-Function software. The 3-dimensional echocardiography measurements were performed by the two experienced cardiac sonographers successively to calculate the inter-observer variability, each blind to the other’s measurements. The other observer made a second measurement two weeks later to calculate the intra-observer variability. The reproducibility was estimated by the mean coefficient of variance (CV): the absolute value of the difference between the two measurements/the average value of the two measurements × 100%.
Statistical analyses were conducted using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA). All collected data were verified for completeness and were tested using the Kolmogorov-Smirnov test for normality. Continuous variables were presented as the mean ± standard deviation (SD) or median (interquartile range), and categorical variables were expressed as percentages. Continuous variables were compared using the independent sample t-test or Mann-Whitney U test, where appropriate. Chi-square test was used for the comparison of categorical variables. The Bland-Altman curve was used to test the inter- and intra-observer reproducibility of RT-3DE data. A P value < 0.05 was considered statistically significant.
Among the initially enrolled 44 subjects (23 VSD + moderate-to-severe PH patients, 21 controls). 5 VSD + moderate-to-severe PH patients and 3 controls were excluded due to insufficient RT-3DE imaging quality. 18 VSD + moderate-to-severe PH patients (12 females and 6 males), and 18 controls (10 females and 8 males) were included in the final analysis. Clinical characteristics of 18 VSD + moderate-to-severe PH patients and 18 controls are presented in Table 1. No significant difference was found between the groups in terms of clinical characteristics including age, sex, height, and heart rate (all P > 0.05). In contrast, the weight and body surface area (BSA) were significantly lower in VSD + PH Group as compared to Control Group (Fig. 2) (P < 0.05).
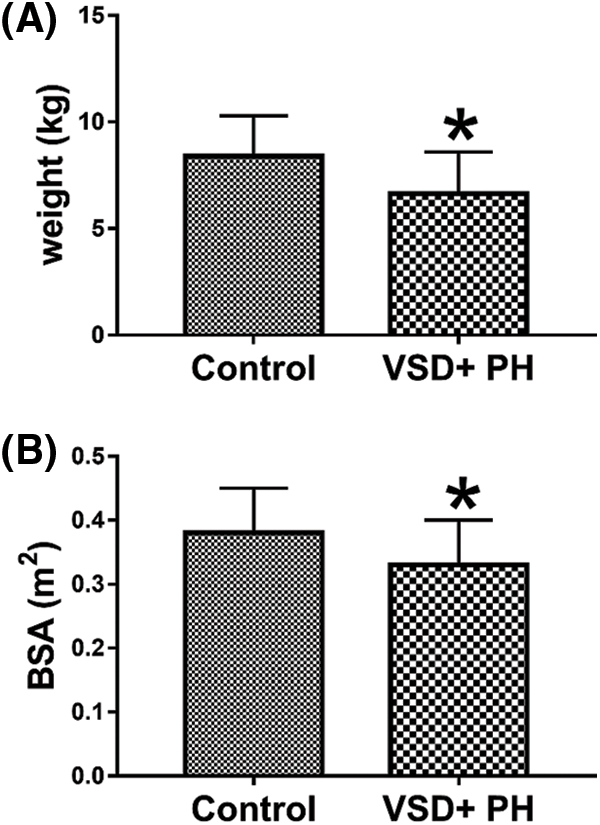
Figure 2: Comparison of weight and BSA of the normal group and the VSD + PH group. Asterisks indicate significant differences (P < 0.05)
3.2 Echocardiographic Characteristics
The echocardiographic parameters were summarized in Table 2. The ventricular septal defects in VSD + PH Group were medium to large. The proportion of children with bidirectional ventricular shunt was 83.33% (15/18 cases) in the study. SPAP ≥ 50mmHg and/or mPAP ≥ 40mmHg can be estimated from Fig. 3A. Compared with Control Group, the volume of left and right ventricle in VSD + PH Group were significantly higher (VSD + PH Group vs. Control Group), LVEDV/BSA: 48.67 ± 21.46ml/m2 vs. 25.59 ± 6.96ml/m2, RVEDV/BSA: 55.98 ± 15.35ml/m2 vs. 27.69 ± 4.37ml/m2 (Fig. 3B), while systolic function indexes: LVEF and RVEF decreased slightly (VSD + PH Group vs. Control Group), LVEF: 50.93 ± 7.50% vs. 59.38 ± 7.24%, RVEF: 45.84 ± 7.71% vs. 51.05 ± 6.90% (Fig. 3C). At the same time biventricular stroke volume indexed to BSA (LVSV/BSA, RVSV/BSA) in VSD + PH Group increased significantly (VSD + PH Group vs. Control Group), LVSV/BSA: 24.08 ± 9.30 ml/m2 vs. 15.14 ± 4.29 ml/m2, RVSV/BSA: 26.02 ± 8.87 ml/m2 vs. 14.11 ± 2.89 ml/m2 (Fig. 3D) (P < 0.05).
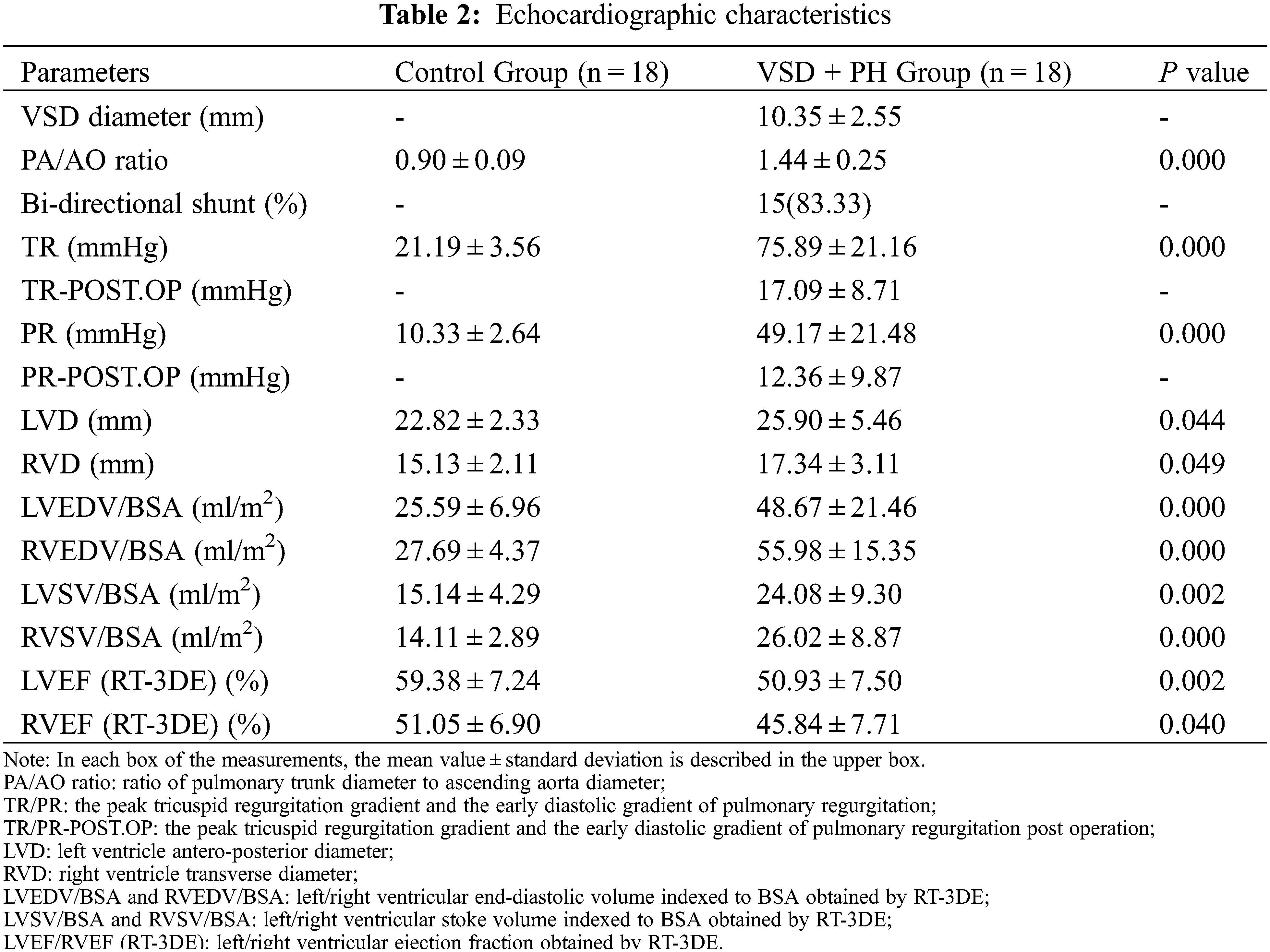
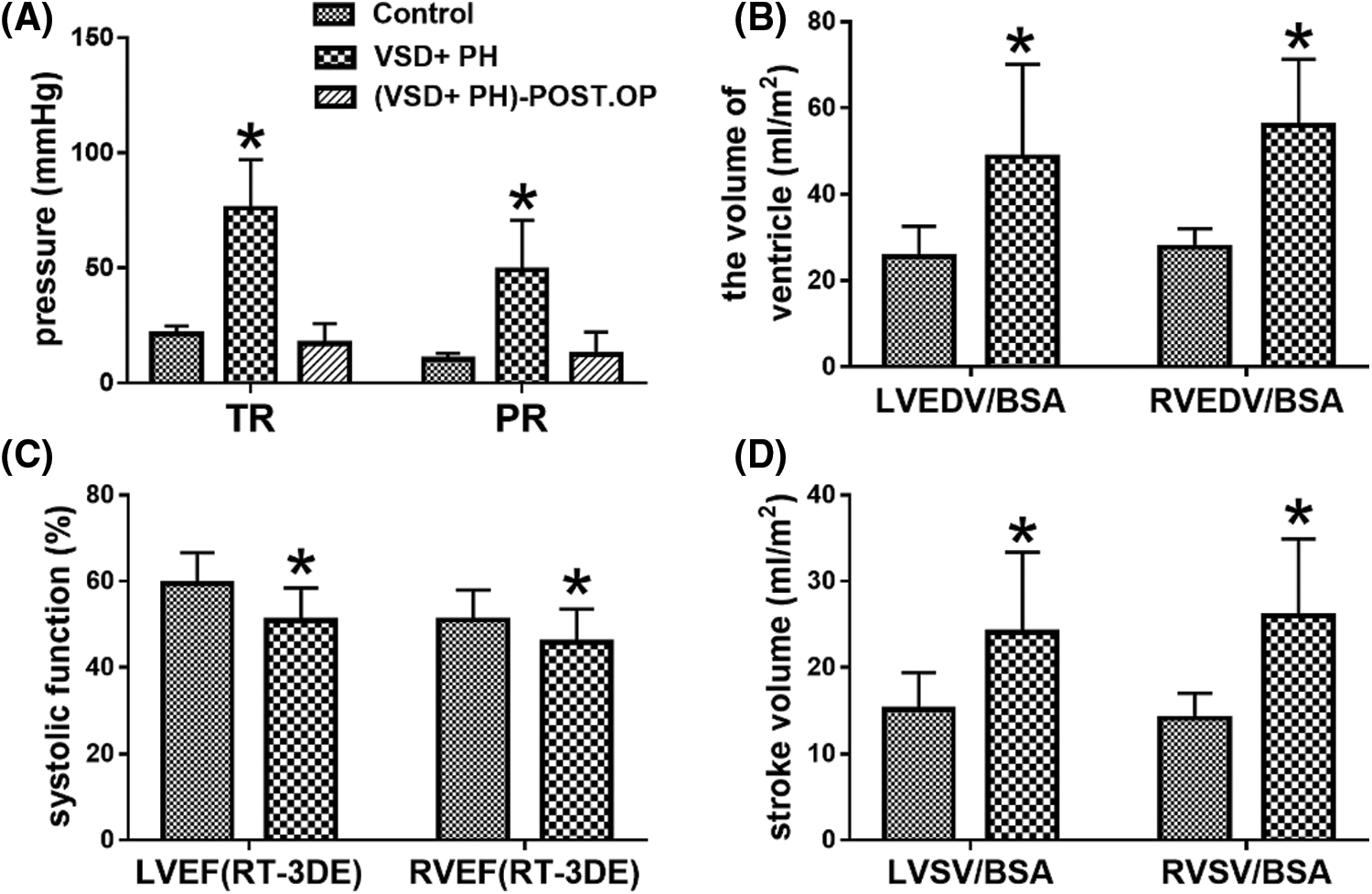
Figure 3: Comparison of some ultrasound data. Asterisks indicate significant differences (P < 0.05)
The Bland-Altman diagram shows a high degree of agreement within and between observers, with good repeatability (Figs. 4 and 5).
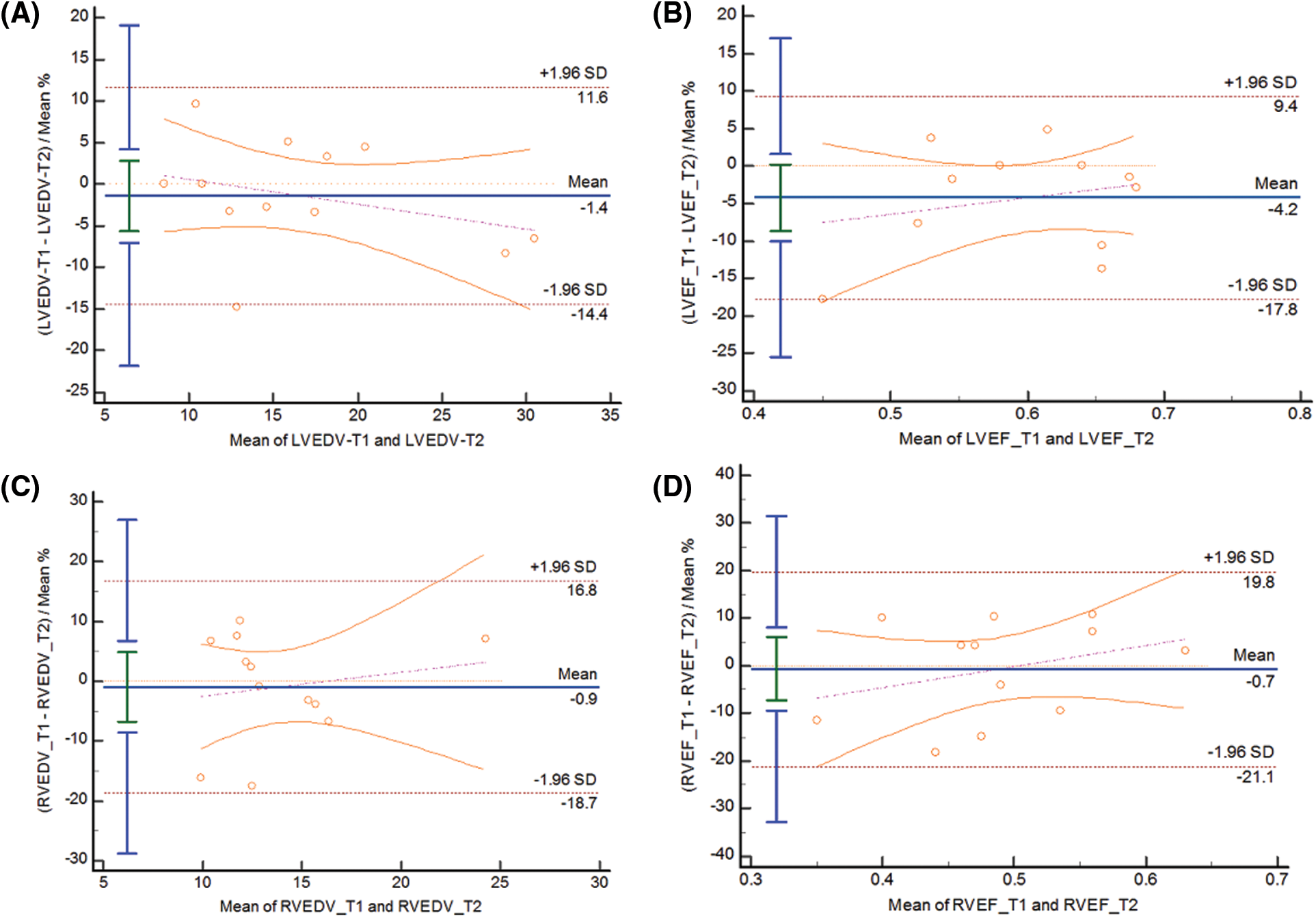
Figure 4: BA curve for intra-observer variability of LVEDV(A), LVEF(B), RVEDV(C), RVEF(D)
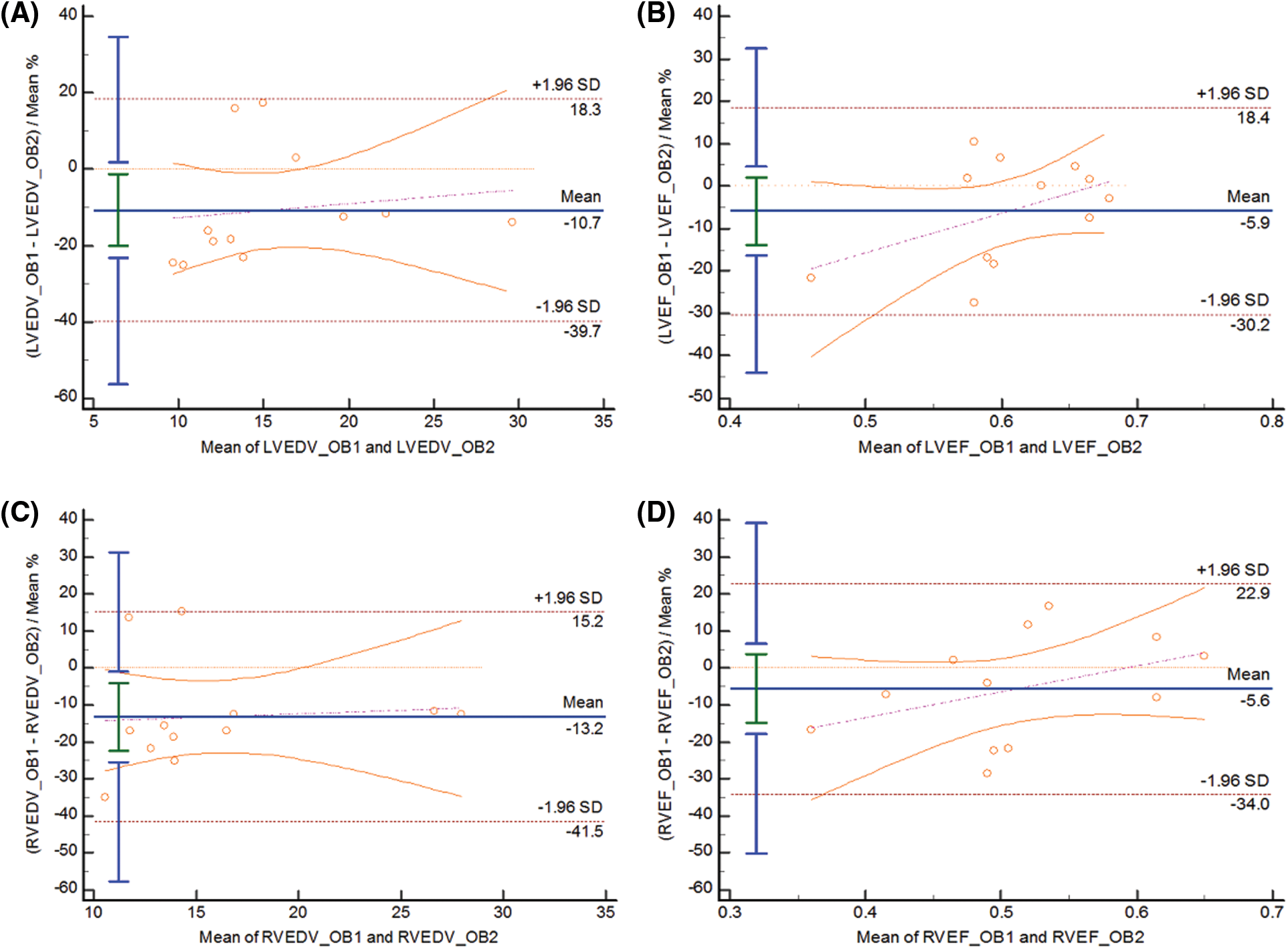
Figure 5: BA curve for inter-observer variability of LVEDV(A), LVEF(B), RVEDV(C), RVEF(D)
3.4 Clinical and Surgical Outcome
All 18 children of VSD + PH Group had rough breathing sounds in both lungs, wet rales were heard in 6 cases. Chest X-ray film showed 18 cases pulmonary congestion, 12 cases bronchiolitis pneumonia, and 3 cases lung consolidation. Although children in the VSD + PH Group had clinical symptoms of pulmonary congestion and cardiac insufficiency before surgery, all children recovered well after surgery.
Postoperative bedside echocardiography showed that the peak tricuspid regurgitation gradient and the early diastolic gradient of pulmonary regurgitation were significantly lower than those before surgery, which were 17.09 ± 8.71mmHg and 12.36 ± 9.87mmHg, respectively (Table 2 and Fig. 3A). The postoperative mechanical ventilation time of 18 children was 1–8 days, with an average of 3.56 ± 2.15 days, and the discharge time was 6–15 days, with an average of 10.17 ± 2.83 days.
Patients with cardiac defects which result in left-to-right shunting are at risk of developing PH, owing to the increased shear stress and circumferential stretch induced by increased pulmonary blood flow, which leads to endothelial dysfunction and progressive vascular remodeling and, thus, increased pulmonary vascular resistance [6]. For large VSD, there is a large left-to-right shunt at the ventricular level, leading to early increased pulmonary artery pressure, often with repeated pneumonia, congestive heart failure and growth retardation, and even life-threatening [7,8]. In this study, the growth and development of children in the VSD + PH Group had lower body weight and BSA than the Control Group (Fig. 2), and all had severe lung infections.
More and more reports indicate that early surgery is very effective in improving the prognosis of VSD combined with PH [2]. Early surgery can block the shunt and reduce the pulmonary blood flow, which is an effective means to prevent the transition from dynamic pulmonary hypertension to resistance pulmonary hypertension. Research by Rabinovitch et al. [9] showed that in 215 cases of congenital heart disease with PH, the average pulmonary artery pressure and/or pulmonary vascular resistance dropped to normal at 9 months after surgery, which was not related to the severity of intraoperative pulmonary vascular disease. Due to the immaturity of myocardial structure, function and metabolism, infants usually have poor tolerance to surgical trauma and ischemia. PH will also increase the risk of perioperative complications, prolong the length of ICU stay and increase long-term mortality [4]. Low cardiac output syndrome (LCOS) is one of the most serious complications of cardiac surgery. Preoperative assessment of cardiac function and selection of appropriate operation time are beneficial to reduce the occurrence of postoperative LCOS and arrhythmia [10–12]. Therefore, the timing of intervention in patients with VSD and PH is important, but it is often difficult to determine the optimal timing because of the lack of reliable data to guide decision-making.
Because of the morphological changes of the ventricle in children with VSD and moderate to severe PH, it is difficult to assume a single geometric shape. Conventional 2D echocardiography is difficult to measure ventricular systolic function, and 3D echocardiography addresses this problem. Previous studies have shown that RT-3DE has good correlation and consistency with angiography, MRI, and radionuclide ventricular angiography in the assessment of ventricular volume and EF [13,14]. Our study confirmed the feasibility of using RT-3DE to assess the bilateral ventricular volume and ejection fraction of children with VSD and moderate-to-severe PH. Our results showed that the left and right ventricular volume and stroke volume of children with VSD and moderate-to-severe PH increased, indicating that the bilateral ventricles were overloaded, and the ventricular pumping function was compensatory enhanced, the bilateral ventricles cannot fully pump the increased blood volume into the systemic and pulmonary circulation. RT-3DE showed that the ventricular systolic function of the VSD + PH Group before operation was lower than that of the Control Group, and the ventricle was enlarged. LVEF ≥ 50% is considered to have preserved ejection fraction [15], and the normal reference value of RVEF is 44% [16]. In our study, left ventricular systolic function (LVEF50.93 ± 7.50%) and right ventricular systolic function (RVEF45.84 ± 7.71%) in children with VSD + PH were still within an acceptable range. Recent studies have shown that RV volume and EF detected by RT-3DE are independent predictors of intermediate-high risk stratification in patients with PH [17], as well as independent predictors of prognosis [18]. An underfilling of the left heart rather than an intrinsic LV dysfunction is the mechanism behind the reduced longitudinal function [19]. Accurate assessment of biventricular systolic function may help to facilitate the decision-making process for surgery in children with VSD and moderate-to-severe PH. Considering that no patients with surgical failure or serious postoperative complications were included in the study, it is impossible to judge that the biventricular systolic function is the key to the timing of surgery.
Several limitations should be considered in interpreting the results of this investigation. First, a major limitation of our study was that the numbers were small, and the follow-up period was short. Thus, further study involving the large number of patients will be needed. Second, it is worth noting that several subjects failed to apply for LV-function and RV-Function software analysis successfully. The reason was that the real-time 3D volume database may be misaligned between images due to the limited frame frequency and the fast heart rate of infants. Finally, confined by RT-3DE image resolution, indistinct endocardial boundaries could also lead to overestimation or underestimation of true values when delineating endocardium.
In this study, we observed infants with VSD and moderate-to-severe PH with growth retardation and severe pulmonary infection, increased bilateral ventricular volume, stroke volume, and decreased EF, but the biventricular systolic function is still within an acceptable range. The children in this study recovered well after surgery without serious perioperative complications, suggesting that biventricular systolic function measured by RT-3DE may help facilitate the surgical decision-making process in children with VSD and moderate-to-severe PH. Further large-scale prospective studies are needed to determine the general significance of these findings.
Acknowledgement: Thanks to Professor Mingxing Xie of Tongji Medical College of Huazhong University of Science and Technology for his guidance and suggestions in this study.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: Huan Zhou and Jun Gao; data collection: Jin Kang, Xiaoyuan Feng, Li Zhou, Xia Xiao, Zhengliang Meng and Chengwen Guo; analysis and interpretation of results: Huan Zhou, Jin Kang and Jun Gao; draft manuscript preparation: Huan Zhou and Jun Gao. All authors reviewed the results and approved the final version of the manuscript.
Ethics Statement: This study was approved by the ethics committee of our hospital. Prospectively collect data from all participants for statistical analysis.
Funding Statement: Wuhan Health and Family Planning Commission Grant/Award (WX16D18).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Hoffman, J. I. (1995). Incidence of congenital heart disease: I. Postnatal incidence. Pediatric Cardiology, 16(3), 103–113. DOI 10.1007/BF00801907. [Google Scholar] [CrossRef]
2. Penny, D. J., Vick III, G. W. (2011). Ventricular septal defect. Lancet, 377(9771), 1103–1112. DOI 10.1016/S0140-6736(10)61339-6. [Google Scholar]
3. Rudolph, A. M. (1971). Circulatory adjustments after birth: Effects on ventricular septal defect. British Heart Journal, 33, 32–34. DOI 10.1136/hrt.33.Suppl.32. [Google Scholar] [CrossRef]
4. Barber, R. L., Fletcher, S. N. (2014). A review of echocardiography in anaesthetic and peri-operative practice. Part 1: Impact and utility. Anaesthesia, 69(7), 764–776. DOI 10.1111/anae.12663. [Google Scholar] [CrossRef]
5. Xing, Y., Chen, Y., Liu, Y., Kong, D., Yan, Y. et al. (2019). Evaluation of left atrial volume and function in patients with coronary slow flow phenomenon using real-time three-dimensional echocardiography. The International Journal of Cardiovascular Imaging, 35(12), 2197–2203. DOI 10.1007/s10554-019-01676-9. [Google Scholar] [CrossRef]
6. Gatzoulis, M. A., Alonso-Gonzalez, R., Beghetti, M. (2009). Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. European Respiratory Review, 18(113), 154–161. DOI 10.1183/09059180.00003309. [Google Scholar] [CrossRef]
7. Castaneda, A. R., Mayer Jr, J. E., Jonas, R. A., Lock, J. E., Wessel, D. L. et al. (1989). The neonate with critical congenital heart disease: Repair--a surgical challenge. The Journal of Thoracic and Cardiovascular Surgery, 98(5), 869–875. DOI 10.1016/S0022-5223(19)34265-5. [Google Scholar] [CrossRef]
8. Bhatt, M., Roth, S. J., Kumar, R. K., Gauvreau, K., Nair, S. G. et al. (2004). Management of infants with large, unrepaired ventricular septal defects and respiratory infection requiring mechanical ventilation. The Journal of Thoracic and Cardiovascular Surgery, 127(5), 1466–1473. DOI 10.1016/j.jtcvs.2003.11.030. [Google Scholar] [CrossRef]
9. Rabinovitch, M., Keane, J. F., Norwood, W. I., Castaneda, A. R., Reid, L. (1984). Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation, 69(4), 655–667. DOI 10.1161/01.CIR.69.4.655. [Google Scholar] [CrossRef]
10. Chin, K. M., Kim, N. H., Rubin, L. J. (2005). The right ventricle in pulmonary hypertension. Coronary Artery Disease, 16(1), 13–18. DOI 10.1097/00019501-200502000-00003. [Google Scholar] [CrossRef]
11. Humbert, M. (2007). The burden of pulmonary hypertension. The European Respiratory Journal, 30(1), 1–2. DOI 10.1183/09031936.00055407. [Google Scholar]
12. van Wolferen, S. A., van de Veerdonk, M. C., Mauritz, G. J., Jacobs, W., Marcus, J. T. et al. (2011). Clinically significant change in stroke volume in pulmonary hypertension. Chest, 139(5), 1003–1009. DOI 10.1378/chest.10-1066. [Google Scholar]
13. Niemann, P. S., Pinho, L., Balbach, T., Galuschky, C., Blankenhagen, M. et al. (2007). Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-tesla magnetic resonance imaging. Journal of the American College of Cardiology, 50(17), 1668–1676. DOI 10.1016/j.jacc.2007.07.031. [Google Scholar]
14. Johnson, T. R., Hoch, M., Huber, A., Römer, U., Reiser, M. F. et al. (2006). Quantifizierung der rechtsventrikulären funktion bei angeborenen herzfehlern: Korrelation von 3D-echokardiographie und MRT als sich ergänzende methoden [Quantification of right ventricular function in congenital heart disease: Correlation of 3D echocardiography and MRI as complementary methods]. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin, 178(10), 1014–1021. DOI 10.1055/s-2006-926945. [Google Scholar]
15. McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A. et al. (2021). 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal, 42(36), 3599–3726. DOI 10.1093/eurheartj/ehab368. [Google Scholar]
16. Ramani, G., Chen, W., Patel, S., Judy, J., Ton, V. K. (2019). Noninvasive assessment of right ventricular function in patients with pulmonary arterial hypertension and left ventricular assist device. Current Cardiology Reports, 21(8), 82. DOI 10.1007/s11886-019-1156-2. [Google Scholar]
17. Liu, B. Y., Wu, W. C., Zeng, Q. X., Liu, Z. H., Niu, L. L. et al. (2020). The value of three-dimensional echocardiography in risk stratification in pulmonary arterial hypertension: A cross-sectional study. The International Journal of Cardiovascular Imaging, 36(4), 577–584. DOI 10.1007/s10554-019-01743-1. [Google Scholar]
18. Moceri, P., Duchateau, N., Baudouy, D., Schouver, E. D., Leroy, S. et al. (2018). Three-dimensional right-ventricular regional deformation and survival in pulmonary hypertension. European Heart Journal-Cardiovascular Imaging, 19(4), 450–458. DOI 10.1093/ehjci/jex163. [Google Scholar]
19. Sjögren, H., Kjellström, B., Bredfelt, A., Steding-Ehrenborg, K., Rådegran, G. et al. (2021). Underfilling decreases left ventricular function in pulmonary arterial hypertension. The International Journal of Cardiovascular Imaging, 37(5), 1745–1755. DOI 10.1007/s10554-020-02143-6. [Google Scholar]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools