 Open Access
Open Access
REVIEW
The Relationship between T-Wave Alternans and Adverse Cardiac Events in Patients with Congenital Long QT Syndrome: A Systematic Review and Meta-Analysis
1
School of Clinical Medicine, Tsinghua University, Beijing, 100084, China
2
Department of Cardiology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing,
102218, China
* Corresponding Author: Ping Zhang. Email:
# These authors contributed equally to this work (First author)
Congenital Heart Disease 2022, 17(5), 557-567. https://doi.org/10.32604/CHD.2021.017292
Received 29 April 2021; Accepted 18 June 2021; Issue published 06 September 2022
Abstract
Background: T-wave alternans (TWA) is a risk factor of ventricular arrhythmias or sudden cardiac death (SCD) in patients with ischemic cardiomyopathy. Nevertheless, the relationship between TWA and adverse cardiac events (ACE) in patients with congenital long QT syndrome (LQT) remains controversial. Methods: A systematic electronic search of PubMed, Embase and the Cochrane Library was conducted from database inception dates to 28 April 2021 and assessed the relationship between TWA and ACE in patients with LQTS. Sub-group analysis evaluated the association between microvolt TWA (MTWA) and ACE in different monitoring models and ECGlead numbers. Results: A pooled analysis of seven studies of 625 patients with LQTS showed that TWA was significantly associated with ACE (OR 3.16, 95%CI 1.86–5.37, P < 0.001). Advanced analysis showed that macroscopic TWA was significantly related to ACE (OR 6.01, 95%CI 2.96–12.21, P < 0.001), while MTWA did not (OR 0.92, 95%CI 0.37–2.30, P = 0.85). Sub-group analysis showed that MTWA recorded in 24 h continuous ECG (OR 6.79, 95%CI 0.80–57.75, P = 0.08) might have a stronger association with ACE than recorded in stress ECG (OR 0.28, 95%CI 0.07–1.10, P = 0.07). No difference was observed between MTWA measured in multi-lead ECG and limited ECG leads (P = 0.15). Conclusions: Macroscopic TWA was significantly related to ACE in patients with LQTS. In terms of MTWA, MTWA recorded in 24 h continuous ECG might have a stronger association with ACE than stress ECG, but still deserves further evaluation.Keywords
Congenital long QT syndrome (LQTS) is a potentially lethal cardiac channelopathy, characterized by delayed ventricular repolarization and abnormal spatiotemporal heterogeneity of repolarization, both of which generate a vulnerable myocardial substrate promoting Torsade de Pointes (Tdp), and predispose to sudden cardiac death (SCD) [1–3]. Now, one of the greatest challenges for the management of LQTS patients is to assess the individual risk of adverse cardiac events (ACE). Uncertainty about how to identify the high-risk individuals may lead to overtreatment of lower-risk patients or failure to protect the higher-risk ones [4]. A predictor to aid in the individual’s risk stratification of ACE would be most welcome.
T-wave alternans (TWA), which reflects spatiotemporal heterogeneity of repolarization, manifesting a beat-to-beat fluctuation in the morphology, amplitude and polarity of the T-wave or ST-segment, can be divided into macroscopic TWA and microvolt TWA (MTWA). Both macroscopic TWA and MTWA have long been recognized and closely linked to life-threatening ventricular arrhythmias [5,6]. Studies and meta-analyses have indicated that both macroscopic TWA and MTWA are risk factors of ventricular arrhythmias or SCD in patients with ischemic cardiomyopathy (ICM) [7–10]. However, controversial results were obtained in patients with congenital LQTS [11–17]. To further clarify the relationship between TWA and ACE in patients with LQTS, we performed a meta-analysis of currently available medical literature.
Our systematic review and meta-analysis is performed in accordance with the guidelines from the Preferred Reporting Items of Systematic Review and Meta-Analysis (PRISMA) [18] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) [19]. A systematic electronic search of PubMed, Embase and the Cochrane Library was conducted from database inception dates to 28 April 2021, without language restriction. The search strategies were as follows: ((long QT.) OR (LQTS)) AND (((T-wave alternans) OR (T wave alternans)) OR (TWA)) in PubMed; ‘long qt syndrome’ AND ‘t wave alternans’ in EMBASE; “long QT syndrome” in All Text AND “T wave alternans” in All Text in Cochrane Library. The reference lists of trials, systematic review and meta-analyses were also manually screened for additional studies that fit our inclusion criteria.
2.2 Inclusion Criteria and Exclusion Criteria
The inclusion criteria were: (1) LQTS were diagnosed by diagnostic criteria (Schwartz score ≥ 4) and/or cardiac channel gene screening according to guidelines [2,20]; (2) TWA had been measured; (3) endpoints included Tdp, syncope, cardiac arrest or SCD. Studies were excluded if less than 10 patients enrolled.
After removing duplicates, two authors (YY and L.T.T.) independently screened the title and abstract, and the candidate list of articles was confirmed by reviewing the full text of the remaining studies based on predefined inclusion criteria. We used a predefined standard data-extraction form to collect information by one author (YY) and verified by another author (L.T.T.). The information for each trial included: study design, enrolled population, sample size, types of TWA, method for TWA monitoring, ECG-leads for TWA measurement, method for MTWA measurement, patients with β-blocker therapy, patients with TWA, and endpoints (Tdp, syncope, cardiac arrest or SCD).
Macroscopic TWA positive was based on the presence of beat-to-beat changes in configuration and polarity of T waves recorded during stable sinus rhythm on at least two leads of any available 12-lead ECG [11]. M.T.W.A. could be detected and analyzed with special instruments, which was categorized into negative, positive and indeterminate. In the time-domain Modified Moving Average (MMA) method, peak TWA ≥ 42 μV in the 12 leads with HR < 120 bpm were determined positive, otherwise, negative [12,13]. The frequency domain (SM) method was used in three studies [14–16]. Schmitt’ research defined MTWA as positive when sustained alternans was present at rest or at an onset HR < 110 bpm; MTWA was defined as negative if the criteria for positivity were not met and at least 1 min of artifact-free data without significant alternans was identified while the HR was maintained at either a level <105 bpm or within 5 bpm of the maximum HR; otherwise, indeterminate [14]. In Kannampuzha’s study [16], MTWA tests were defined as positive if MTWA appeared at or below a patient-specific onset HR threshold of 110 bpm, if the MTWA appeared with a magnitude of over 1.9 µV and had an alternans ratio (to noise) over 3, and if the MTWA was sustained for at least a minute during the time that the heart remained over the HR threshold; The MTWA test was negative if significant alternans was not elicited until after the HR ≥ 105 bpm, and it did not meet criteria for being positive; otherwise, indeterminate. While in Nemec’s study, MTWA tests were defined as positive if MTWA (power at the alternans frequency > 4 µV2 and ratio of the alternans frequency power to mean noise band power > 4) was recorded at HR < 150 bpm. Otherwise, negative [15].
The highest MTWA was recorded in precordial leads (V1–V6), and most frequently in lead V2 (43.8%), a single ECG lead detected ≤ 63.6% of MTWA, whereas the combined leads (V2–V5) detected 100% of MTWA [13]. In our meta-analysis, MTWA measured leads including at least leads V2–V5 was defined as multi-lead ECG, otherwise limited lead ECG.
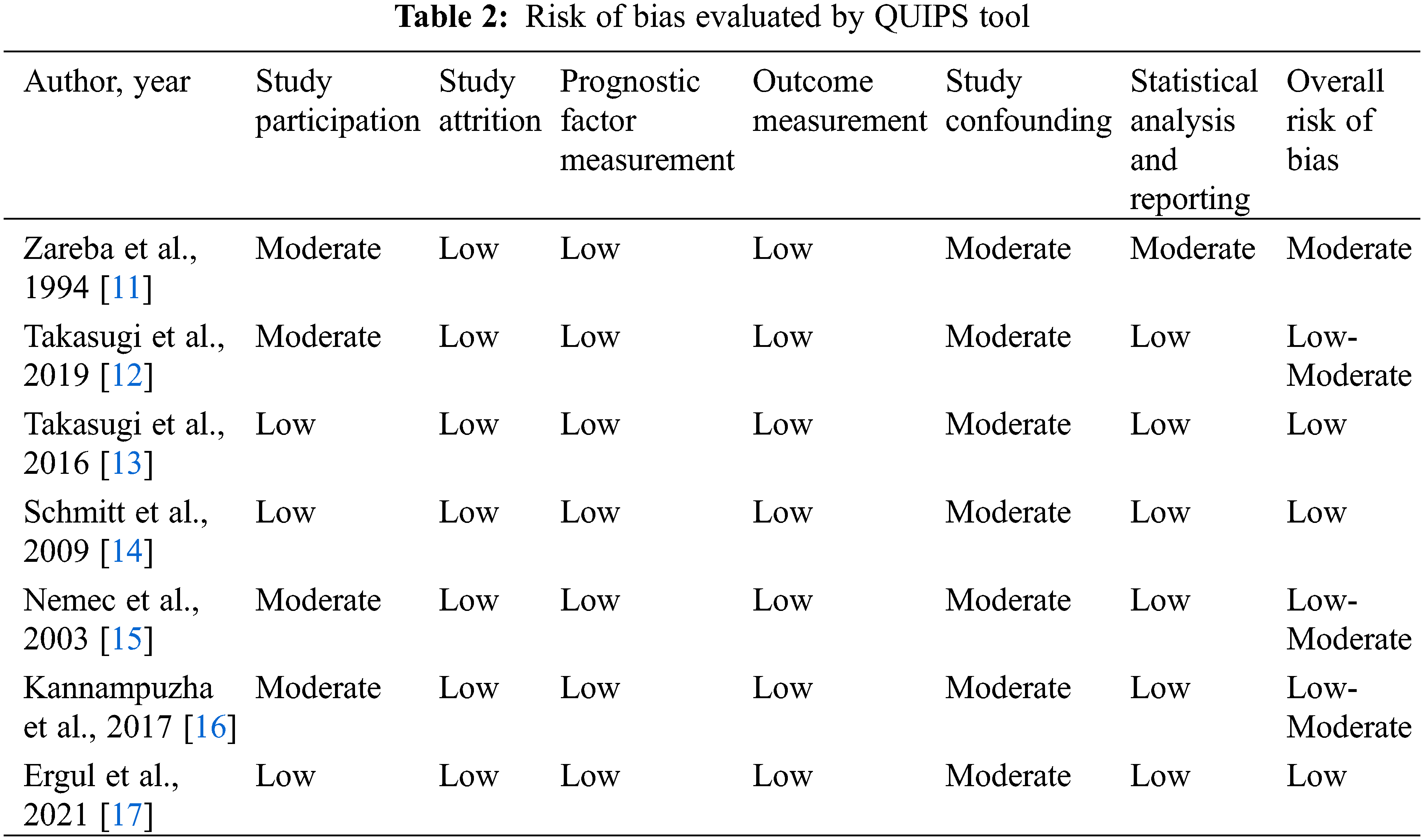
We used the Quality in Prognosis Studies (QUIPS) tool [21,22] to assess the individual study quality and risk of bias. QUIPS tool covered six aspects, including study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting, which were important in observational prognostic research. The final quality assessment of included articles was with a full agreement between all authors.
Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) was used for all data analyses. We used fixed-effects meta-analysis to pool data. Heterogeneity across each meta-analysis was evaluated by I2; values around 25%~50%, 50%~75% and >75% represented mild, moderate and severe heterogeneity, respectively. P < 0.05 was considered statistically significant.
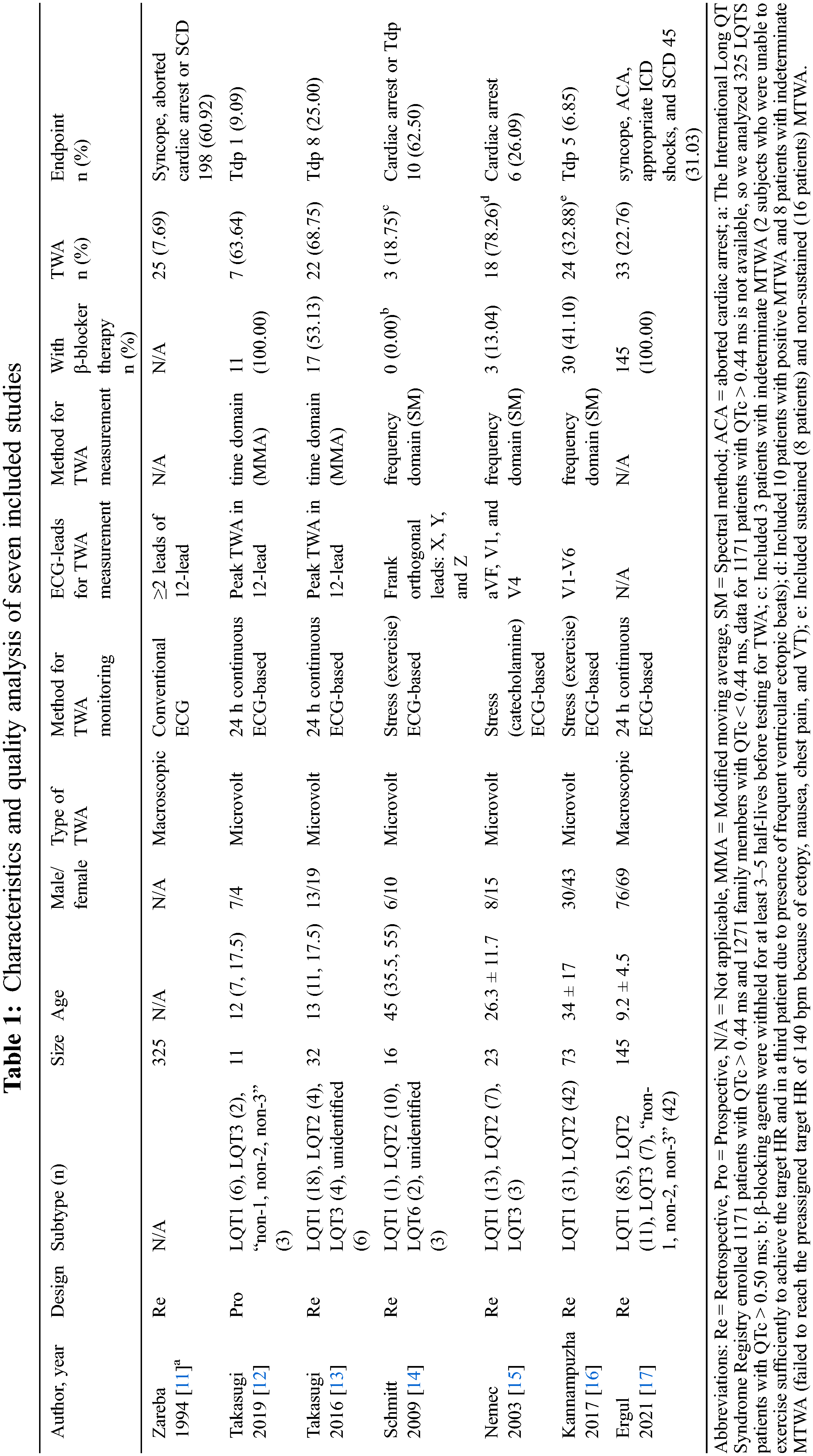
Two authors independently screened titles and abstracts of 353 citations yielded from electronic search. After removing the duplicate and irrelevant articles, seven studies met the predefined inclusion criteria and were included in our meta-analysis (Fig. 1). The characteristics of included studies were summarized in Table 1. Among these studies, five studies [12–16] focused on the relationship between MTWA and ACE (Tdp, syncope, aborted cardiac arrest or SCD), and only two studies [11,17] analyzed the relationship between macroscopic TWA and ACE (Tdp or cardiac arrest). On the other hand, four [11–13,17] discussed TWA without stress (transient 12-lead ECG or 24 h continuous ECG), and three [14–16] with exercise or catecholamine. Finally, a total of 625 patients were included, 273 (43.68%) suffered ACE, 132 (21.12%) had non-negative TWA, and 58 (43.94%) suffered ACE in patients with non-negative TWA. The main age ranged from 9 to 45 years old.

Figure 1: Flowchart of literature search and reports selection
3.2 Risk of Bias Evaluated by QUIPS Tool
As shown in Table 2, the overall risk of bias in three studies [13,14,17] was judged to be low, and one [11] was judged to be moderate, three were judged between low-moderate [12,15,16]. The most important components of bias were introduced by limited or absent adjustment for confounders (study confounding), poorly described and unrepresentative study participation (study participation), and the use of statistical models not correcting for individual and group differences in follow-up time (statistical analysis and reporting).
3.3 Analysis of the Relationship between TWA and ACE
To investigate the relationship between TWA and ACE, we pooled all data from seven studies, including 625 patients to perform analysis (Fig. 2). Compared to TWA negative group, non-negative TWA was significantly associated with ACE (OR 3.16, 95%CI 1.86–5.37, P < 0.001), as shown in Fig. 2A. To further evaluate the relationship between macroscopic TWA/MTWA and ACE respectively, we performed advanced analysis by the type of TWA. Results showed that macroscopic TWA was significantly related to ACE (OR 6.01, 95%CI 2.96–12.21, P < 0.001), as shown in Fig. 2B, while MTWA was not (OR 0.92, 95%CI 0.37–2.30, P = 0.85), as shown in Fig. 2C.

Figure 2: Forest plot showing the relationship between TWA and ACE. A: The relationship between TWA (macroscopic and microvolt) and ACE, TWA non-negative group included patients with macroscopic TWA positive, MTWA positive and MTWA indeterminate; B: The relationship between macroscopic TWA and ACE; C: The relationship between MTWA and ACE, MTWA non-negative group included patients with MTWA positive and MTWA indeterminate
3.4 Sub-Group Analysis of MTWA
To further determine the relationship between MTWA and ACE, an advanced sub-group analysis was performed. As shown in Fig. 3A, MTWA recorded in 24 h continuous ECG tended to associate with ACE, but did not achieve statistical significance (OR 6.79, 95%CI 0.80–57.75, P = 0.08), while MTWA recorded in stress ECG did not (OR 0.28, 95%CI 0.07–1.10, P = 0.07). As shown in Fig. 3B, no difference was observed between MTWA measured in multi-lead ECG and limited ECG leads (P = 0.15).

Figure 3: Sub-group analysis of MTWA. A: Sub-group analysis of MTWA recorded in 24 h continuous ECG or stress ECG; B: Sub-group analysis of MTWA measured in multi-lead ECG or limited ECG leads
Our meta-analysis is the first attempt to determine the relationship between TWA and ACE in patients with LQTS. In the present study, we found that the incidence of ACE (Tdp, syncope, aborted cardiac arrest or SCD) in patients with LQTS was relatively high (up to 43.68%), while the proportion of patients with non-negative TWA was relatively low (21.12%). Indeed, our current meta-analysis demonstrated that macroscopic TWA was significantly related to ACE (OR 6.01, 95%CI 2.96–12.21, P < 0.001). In terms of MTWA, MTWA recorded in 24 h continuous ECG (OR 6.79, 95%CI 0.80–57.75, P= 0.08) might have a stronger association with ACE than stress ECG (OR 0.28, 95%CI 0.07–1.10, P = 0.07). No difference was observed between MTWA measured in multi-lead ECG and limited ECG leads (P = 0.15). Hence, macroscopic TWA and MTWA recorded in 24 h continuous ECG have a strong association with ACE in patients with LQTS but still deserve further evaluation.
The prevalence of LQTS might be approximately 1:2000 among whites [23]. In the International Long QT Syndrome Registry, 21.50% (525/2442) experienced a major cardiac event during routine registry follow-up [11]. In our study, its proportion was up to 43.68%. The detection rate of TWA in patients with LQTS ranged from 7.69% (25 of 325 patients) in standard 12-lead ECG to 68.75% (22 of 32 patients) in 24 h continuous ECG [11,13], and 78.26% (18 of 23 patients) in stress ECG [15]. However, the proportion of patients with non-negative TWA was only 21.12% in our analysis. Several possible reasons might explain the TWA underestimation. First, β-blocker significantly reduced both peak TWA level and frequency of TWA [12,13], while some patients enrolled in our meta-analysis were treating with β-blockers, which is the main cause of TWA underestimation. Second, TWA was a variable and transient occurrence, which may be underestimated in 12-lead ECGs [11,24]. Third, MTWA were often undetectable in common techniques. Thus, two contemporary techniques, including the frequency-domain SM method and the time-domain MMA method, should be widely used in clinical studies [5,11,24]. Fourth, the highest MTWA levels were recorded in precordial leads (V1–V6), and most frequently in lead V2 (43.8%), a single ECG lead detected ≤63.6% of MTWA, whereas the combined leads (V2–V5) detected 100% of MTWA [13]. Thus, the proportion of MTWA may be underestimated, despite during exercise testing. Given the above, 24 h continuous 12-lead ECGs measured in multi-lead should be used in LQTS patients before β-blockers treatment to detect TWA.
Several meta-analyses have tried to demonstrate the correlation between TWA and ACE in patients with ICM, post-MI, ischemic congestive heart failure (CHF), nonischemic CHF, and nonischemic dilated cardiomyopathy (DCM) [9,25–27]. These meta-analyses concluded a similar result that TWA is greatly valuable for the prediction of ACE, both MTWA and macroscopic TWA. However, the relationship between TWA and ACE in patients with LQTS is still unknown. Here we showed that macroscopic TWA had a strong association with ACE, but MTWA did not, which is inconsistent with previous reports [9,25–27]. In our meta-analysis, sub-group analysis for MTWA included five studies with 155 patients, and small sample sizes make the results unstable. Also, a different standard for MTWA monitoring, measurement, and the definition of MTWA positive increased the heterogeneity between included studies. In addition, drug intervention, like β-blockers and mexiletine, may reduce the occurrence of ACE and the detection of MTWA [12,13,17]. What’s more, most of our included studies were retrospective cohort, which has an inherent limitation in the quality of evidence. Therefore, there is an urgent need for prospective, multicenter, large-scale trials to assess the relationship between TWA and ACE in patients with LQTS, especially MTWA.
MTWA observed at HR < 110 bpm in the states of exercise or catecholamine-provoked is considered pathological [14–16]. Our meta-analysis showed that MTWA recorded in 24 h continuous ECG rather than in stress ECG might have an association with ACE, which is inconsistent with the Finnish Cardiovascular Study (FINCAVAS) [28]. In FINCAVAS, the prognostic power of TWA during exercise is superior to pre-exercise or post-exercise; the study enrolled low-risk populations with coronary heart disease, palpitation or post-myocardial infarction (MI), in which exercise aggravates myocardial ischemia [28]. In our meta-analysis, LQT2 and LQTS3 account for 55.36% (62/112) of the patients in the stress ECG group; it has been reported that only 13% of cardiac events occurred during exercise in patients with LQTS2 and LQTS3 [29]. Moreover, only 427 of 1325 (32.2%) LQTS patients experienced their first cardiac events during acute arousal caused by exercise, swimming, emotion, or noise [30]. Therefore, the relationship between MTWA in stress ECG and ACE should be evaluated separately among different types of LQTS. Moreover, in FINCAVAS, during exercise, hazard ratios (HRs) of total mortality and cardiovascular mortality were significantly higher when MTWA ≥ 50 μV, and MTWA ≥ 90 μV yield maximum HRs for total and cardiovascular death, so the cut-off point of MTWA in exercise or catecholamine-provoked should be redefined [28]. Lastly, MTWA in stress ECG was measured in limited ECG leads, which may be underestimated, despite during exercise testing. So, the relationship between MTWA in stress ECG and ACE needs a more detailed assessment.
The highest MTWA levels were recorded in precordial leads (V1–V6), and most frequently in lead V2 (43.8%), a single ECG lead detected ≤63.6% of MTWA, whereas the combined leads (V2–V5) detected 100% of MTWA [13]. By comparing the relationship between MTWA measured in multi-lead ECG and limited-lead ECG, our sub-group analysis showed that no difference was observed between MTWA measured in multi-lead ECG and limited ECG leads. Our analysis included three studies [12,13,16] that measured MTWA in multi-lead ECG (at least leads V2–V5) using the time-domain MMA method, while two studies [14,15] measured MTWA in limited leads ECG using frequency-domain SM, which may account for the most of our result. In addition, a limited sample directly affected the stability of the results. So, the difference between MTWA measured in multi-lead ECG and limited leads ECG should be confirmed in a larger sample using the same method, time-domain MMA method or frequency-domain SM.
Given the nature of our study as a systematic review and meta-analysis, our analyses are limited by the reported data in the original reports [22]. The limitations of our meta-analysis are as follows. First, for the low prevalence of LQTS, studies with small sample sizes evaluated the relationship between TWA and ACE, but didn’t adjust confounding factors, so whether TWA makes a significant independent contribution to the risk of cardiac events or depend on other confounding factors, as showing in the International Long QT Syndrome Registry, macroscopic TWA contributed to increased risk of a major cardiac event primarily related to the length of QTc [11], remains unknown. Second, a different standard for MTWA monitoring, measurement, and the definition of MTWA positive increased the heterogeneity between included studies. Third, a considerable proportion of patients initiated antiarrhythmic drugs (β-blocker and mexiletine) before the ECG test, which may affect the occurrence of ACE and the detection of TWA [12,31,32]. Fourth, most of our included studies were retrospective cohort, which is inherently (but not necessarily) limited the quality of evidence [22]. Given the above, there is an urgent need for prospective, multicenter, large-scale trials to assess the relationship between TWA and ACE in patients with LQTS, especially MTWA.
Our current meta-analysis demonstrated that macroscopic TWA was significantly related to ACE in patients with LQTS. In terms of MTWA, MTWA recorded in 24 h continuous ECG might have a stronger association with ACE than stress ECG but still deserves further evaluation.
Funding Statement: This work was supported by Beijing Municipal Science & Technology Commission (No. Z191100006619007) and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20190902) of Professor Ping Zhang.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. JCS Joint Working Group (2012). Guidelines for risks and prevention of sudden cardiac death (JCS 2010Digest version. Circulation Journal, 76(2), 489–507. [Google Scholar]
2. Priori, S. G., Wilde, A. A., Horie, M., Cho, Y., Behr, E. R. et al. (2013). HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm, 10(12), 1932–1963. [Google Scholar]
3. Sugrue, A., Rohatgi, R. K., Noseworthy, P. A., Kremen, V., Bos, J. M. et al. (2017). Architectural T-wave analysis and identification of on-therapy breakthrough arrhythmic risk in Type 1 and Type 2 Long-QT syndrome. Circulation Arrhythmia & Electrophysiology, 10(11), e005648. [Google Scholar]
4. Kaufman, E. S., Deschenes, I. (2017). T-wave morphology analysis to detect high risk in Long-QT syndrome. Circulation Arrhythmia & Electrophysiology, 10(11), e005920. [Google Scholar]
5. Verrier, R. L., Klingenheben, T., Malik, M., El-Sherif, N., Exner, D. V. et al. (2011). Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility--Consensus guideline by International Society for Holter and Noninvasive Electrocardiology. Journal of the American College of Cardiology, 58(13), 1309–1324. [Google Scholar]
6. European Heart Rhythm, A., Heart Rhythm, S., Zipes, D. P., Camm, A. J., Borggrefe, M. et al. (2006). ACC/AHA/ESC, 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Journal of the American College of Cardiology, 48(5), e247–346. [Google Scholar]
7. Costantini, O., Hohnloser, S. H., Kirk, M. M., Lerman, B. B., Baker, J. H. et al. (2009). The ABCD (Alternans before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. Journal of the American College of Cardiology, 53(6), 471–479. [Google Scholar]
8. Chow, T., Kereiakes, D. J., Bartone, C., Booth, T., Schloss, E. J. et al. (2006). Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. Journal of the American College of Cardiology, 47(9), 1820–1827. [Google Scholar]
9. Chen, Z., Shi, Y., Hou, X., Xu, S., Zou, J. (2013). microvolt T-wave alternans for risk stratification of cardiac events in ischemic cardiomyopathy: A meta-analysis. International Journal of Cardiology, 167(5), 2061–2065. [Google Scholar]
10. Ikeda, T., Saito, H., Tanno, K., Shimizu, H., Ozawa, Y. (2002). T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. American Journal of Cardiology, 89(1), 79–82. [Google Scholar]
11. Zareba, W., Moss, A. J., le Cessie, S., Hall, W. J. (1994). T wave alternans in idiopathic long QT syndrome. Journal of the American College of Cardiology, 23(7), 1541–1546. [Google Scholar]
12. Takasugi, N., Takasugi, M., Goto, H., Kuwahara, T., Kawasaki, M. et al. (2019). Effect of beta-blockade on quantitative microvolt T-wave alternans in 24-hour continuous 12-lead ECG recordings in patients with long QT syndrome. Ann Noninvasive Electrocardiol, 24(4), e12640. [Google Scholar]
13. Takasugi, N., Goto, H., Takasugi, M., Verrier, R. L., Kuwahara, T. et al. (2016). Prevalence of Microvolt T-wave alternans in patients with long QT syndrome and its association with torsade de pointes. Circulation: Arrhythmia and Electrophysiology, 9(2), e003206. [Google Scholar]
14. Schmitt, J., Baumann, S., Klingenheben, T., Richter, S., Duray, G. et al. (2009). Assessment of microvolt T-wave alternans in high-risk patients with the congenital long-QT syndrome. Annals of Noninvasive Electrocardiology, 14(4), 340–345. [Google Scholar]
15. Nemec, J., Ackerman, M. J., Tester, D. J., Hejlik, J., Shen, W. K. (2003). Catecholamine-provoked microvoltage T wave alternans in genotyped long QT syndrome. Pacing and Clinical Electrophysiology, 26(8), 1660–1667. [Google Scholar]
16. Kannampuzha, J. A., Sengodan, P., Avula, S., White, B., Ganocy, S. J. et al. (2018). Non-sustained microvolt level T-wave alternans in congenital long QT syndrome types 1 and 2. Journal of Electrocardiology, 51(2), 303–308. [Google Scholar]
17. Ergul, Y., Tunca Sahin, G., Kafali, H. C., Ozturk, E., Ozgur, S. et al. (2021). Clinical and genetic characteristics and course of congenital long QT syndrome in children: A nine-year single-center experience. Anatolian Journal of Cardiology, 25(4), 250–257. [Google Scholar]
18. Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–W64. [Google Scholar]
19. Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D. et al. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA, 283(15), 2008–2012. [Google Scholar]
20. Liu, J. F., Jons, C., Moss, A. J., McNitt, S., Peterson, D. R. et al. (2011). Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long QT syndrome. Journal of the American College of Cardiology, 57(8), 941–950. [Google Scholar]
21. Hayden, J. A., van der Windt, D. A.,Cartwright, J. L., Cote, P., Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Annals of Internal Medicine, 158(4), 280–286. [Google Scholar]
22. Bosman, L. P., Sammani, A., James, C. A., Cadrin-Tourigny, J., Calkins, H. et al. (2018). Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: A systematic review and meta-analysis. Heart Rhythm, 15(7), 1097–1107. [Google Scholar]
23. Schwartz, P. J., Stramba-Badiale, M., Crotti, L., Pedrazzini, M., Besana, A. et al. (2009). Prevalence of the congenital long-QT syndrome. Circulation, 120(18), 1761–1767. [Google Scholar]
24. Cho, M. S., Nam, G. B., Kim, Y. G., Hwang, K. W., Kim, Y. R. et al. (2015). Electrocardiographic predictors of bradycardia-induced torsades de pointes in patients with acquired atrioventricular block. Heart Rhythm, 12(3), 498–505. [Google Scholar]
25. Gehi, A. K., Stein, R. H., Metz, L. D., Gomes, J. A. (2005). Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: A meta-analysis. Journal of the American College of Cardiology, 46(1), 75–82. [Google Scholar]
26. Gupta, A., Hoang, D. D., Karliner, L., Tice, J. A., Heidenreich, P. et al. (2012). Ability of Microvolt T-wave alternans to modify risk assessment of ventricular tachyarrhythmic events: A meta-analysis. American Heart Journal, 163(3), 354–364. [Google Scholar]
27. De Ferrari, G. M., Sanzo, A. (2009). T-wave alternans in risk stratification of patients with nonischemic dilated cardiomyopathy: Can it help to better select candidates for ICD implantation? Heart Rhythm, 6(3 Suppl), S29–S35. [Google Scholar]
28. Minkkinen, M., Kahonen, M., Viik, J., Nikus, K., Lehtimaki, T. et al. (2009). Enhanced predictive power of quantitative TWA during routine exercise testing in the Finnish Cardiovascular study. Journal of Cardiovascular Electrophysiology, 20(4), 408–415. [Google Scholar]
29. Schwartz, P. J., Priori, S. G., Spazzolini, C., Moss, A. J., Vincent, G. M. et al. (2001). Genotype-phenotype correlation in the long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation, 103(1), 89–95. [Google Scholar]
30. Ali, R. H., Zareba, W., Moss, A. J., Schwartz, P. J., Benhorin, J. et al. (2000). Clinical and genetic variables associated with acute arousal and nonarousal-related cardiac events among subjects with long QT syndrome. American Journal of Cardiology, 85(4), 457–461. [Google Scholar]
31. Klingenheben, T., Gronefeld, G., Li, Y. G., Hohnloser, S. H. (2001). Effect of metoprolol and d,l-sotalol on microvolt-level T-wave alternans. Results of a prospective, double-blind, randomized study. Journal of the American College of Cardiology, 38(7), 2013–2019. [Google Scholar]
32. Rashba, E. J., Cooklin, M., MacMurdy, K., Kavesh, N., Kirk, M. et al. (2002). Effects of selective autonomic blockade on T-wave alternans in humans. Circulation, 105(7), 837–842. [Google Scholar]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools