 Open Access
Open Access
ARTICLE
Incidence and Related Risk Factors of Junctional Ectopic Tachycardia in Infants after Cardiac Surgery for Congenital Heart Disease
1
Department of Pediatrics, Congenital Heart Disease Center, Division of Pediatric Cardiology, Severance Cardiovascular Hospital,
Yonsei University College of Medicine, Seoul, Korea
2
Department of Pediatrics, Yonsei University Wonju College of Medicine, Wonju, Korea
3
Department of Thoracic and Cardiovascular Surgery, Division of Cardiovascular Surgery, Congenital Heart Disease Center,
Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea
4
Department of Pediatrics and Biomedical Engineering, School of Medicine, Emory University, Atlanta, USA
* Corresponding Author: Nam Kyun Kim. Email:
# These authors equally contributed to this work as first author
Congenital Heart Disease 2022, 17(5), 569-578. https://doi.org/10.32604/chd.2022.018436
Received 24 October 2021; Accepted 11 April 2022; Issue published 06 September 2022
Abstract
Objective: Junctional ectopic tachycardia is common after cardiac surgery for congenital heart disease. However, its incidence and related risk factors in infants after cardiac surgery are not well known. The objective of this study was to determine the overall incidence and related risk factors for junctional ectopic tachycardia in neonates and infants. Methods: We enrolled a total of 271 patients aged <1 year who underwent open cardiac surgery at Severance Cardiovascular Hospital from January 2018 to December 2020. Exclusion criteria were immediate postoperative mortality, other arrhythmias detected in the perioperative period, and prematurity. Result: The overall incidence of junctional ectopic tachycardia was 12.9%. The logistic regression analysis revealed that longer cardiopulmonary bypass time, surgery involving atrioventricular node stretching, and the presence of early repolarization on preoperative electrocardiography increased the risk of junctional ectopic tachycardia. Patients with junctional ectopic tachycardia had longer intubation time and intensive care unit stay. Conclusion: Junctional ectopic tachycardia is a common arrhythmia after cardiac surgery for congenital heart disease in infants. Occasionally, infants developing junctional ectopic tachycardia after cardiac surgery have specific preoperative electrocardiography findings. The risk factors for junctional ectopic tachycardia were associated not only with surgical procedural factors but also with preoperative electrocardiographic parameters.Keywords
Junctional ectopic tachycardia (JET) is common after cardiac surgery for congenital heart disease. JET is a type of narrow QRS complex tachycardia originating from the atrioventricular (AV) node or proximal His bundle, usually with AV dissociation [1] .The overall incidence of JET after congenital heart surgery ranges from 1.4% to 14.3% in children, according to previous studies [1–4]. Patients who have JET during the postoperative period show increased morbidity, such as longer intubation time and increased intensive care unit (ICU) stay [2,5–7]. Moreover, a previous study showed that JET following cardiac surgery was associated with higher mortality [2]. Postoperative JET management includes sedation, low body temperature, correction of electrolyte abnormalities, and reduction in inotropic drug doses. In some cases, overdrive atrial pacing and pharmacotherapy, such as with amiodarone, may be necessary, and in rare cases, extracorporeal membrane oxygenation support is required [8–10]. Associated cardiac surgery include Tetralogy of Fallot (TOF) (14%–37%), repair of anomalous pulmonary venous return (36%), arterial switch operation (23%), surgery to correct AV canal defects (10%–21%), the Norwood procedure (20%), and interrupted aortic arch repair (17%) [11]. Heterotaxy syndrome with a risk of arrhythmia has been reported to be associated with JET [12–14]. Several studies have reported possible risk factors for JET, such as younger age, longer cardiopulmonary bypass (CPB) time, longer aortic cross-clamp time, electrolyte disturbances, and use of inotropic agents [2–4,6,15]. However, the incidence and related risk factors of JET in neonates and infants after cardiac surgery are not well known. Therefore, this study aimed to determine the overall incidence and risk factors of JET in this group of patients.
2.1 Patients and Data Collection
We enrolled patients under 1 year of age who underwent open cardiac surgery at Severance Cardiovascular Hospital from January 2018 to December 2020. These patients underwent surgical repair of a congenital cardiac lesion using CPB with deep hypothermic circulatory arrest. In the case of Blalock–Taussig shunt surgery, extracorporeal membrane oxygenation was used instead of CPB. The postoperative period was defined as up to 30 days after operation or until hospital discharge, whichever was the earliest. The exclusion criteria were immediate postoperative mortality, other arrhythmias detected during the preoperative period, and prematurity. One patient with postoperative ventricular tachycardia unrelated to JET was excluded. Baseline characteristics and information regarding surgical procedure, duration of cross-clamp time, and CPB time were retrospectively obtained from medical records. The Aristotle scale was used to assess the complexity level of the surgical procedures. The basic and total scores on the Aristotle scale were obtained from the electronic database. We analyzed the intubation duration, length of ICU stay, and total hospitalization stay as postoperative outcomes. Cardiopulmonary resuscitation (CPR), survival at day 30, and use of inotropic agents, such as dopamine, dobutamine, milrinone, epinephrine, and/or norepinephrine, were also examined. We calculated the inotropic score at the time of arrhythmia onset using the following formula: vasoactive inotropic score (VIS) = (dopamine dose * 1) + (dobutamine dose * 1) + (adrenaline dose * 100) + (noradrenaline dose * 100) + (milrinone dose * 10). The drug doses were presented in μg/kg/min [16,17].
2.2 Arrhythmia Diagnosis and Management
The 12-lead electrocardiogram findings were retrospectively evaluated by pediatric electrophysiologists (N, K, and K). JET was defined as narrow QRS complex tachycardia with AV dissociation or AV association with retrograde P waves. For patients with A postoperative bundle branch block, electrocardiography (ECG) revealed JET with wide QRS complex tachycardia. Based on the preoperative ECG data, we identified an early repolarization pattern (ERP), which is a known risk factor for arrhythmia. Referring to Pieroni et al. [18], the diagnostic criterion for ERP was defined as an elevation of ≥0.1 mV in the J-point or ST segment, with notching or slurring in at least two inferior (II, III, and aVF) and/or lateral leads (I, aVL, and V4–6; Supplementary Fig. 1). According to our institution s treatment protocol, amiodarone was used as the first-line antiarrhythmic drug for hemodynamically compromised JET. Amiodarone was administered as a 5 mg/kg loading infusion for 0.5 h, followed by a continuous infusion of 10–20 mg/kg/day. Conservative treatments, including surface cooling, sedation, correction of abnormal electrolytes, atrial pacing, and, if possible, decrease in inotropic doses were used. We corrected the postoperative electrolyte imbalance to maintain serum K level > 4.0 mmol/L, ionized Ca level > 1.2 mmol/L, and serum Mg level > 1.5 mmol/L.
Data are presented as frequency (percentage) or as mean (range). Patients with and without JET were compared for baseline-and procedure-related characteristics and outcomes using the chi-square test for categorical variables and independent t-test for continuous variables. Factors that could affect JET development were analyzed using binary logistic regression. Statistical significance was set at P < 0.05. Factors with a high correlation with each other were excluded from the analysis. Results are expressed as odds ratio (OR) with 95% confidence intervals (CI). All analyses were performed using SPSS 25.0 for Windows (IBM, Chicago, Illinois, USA).
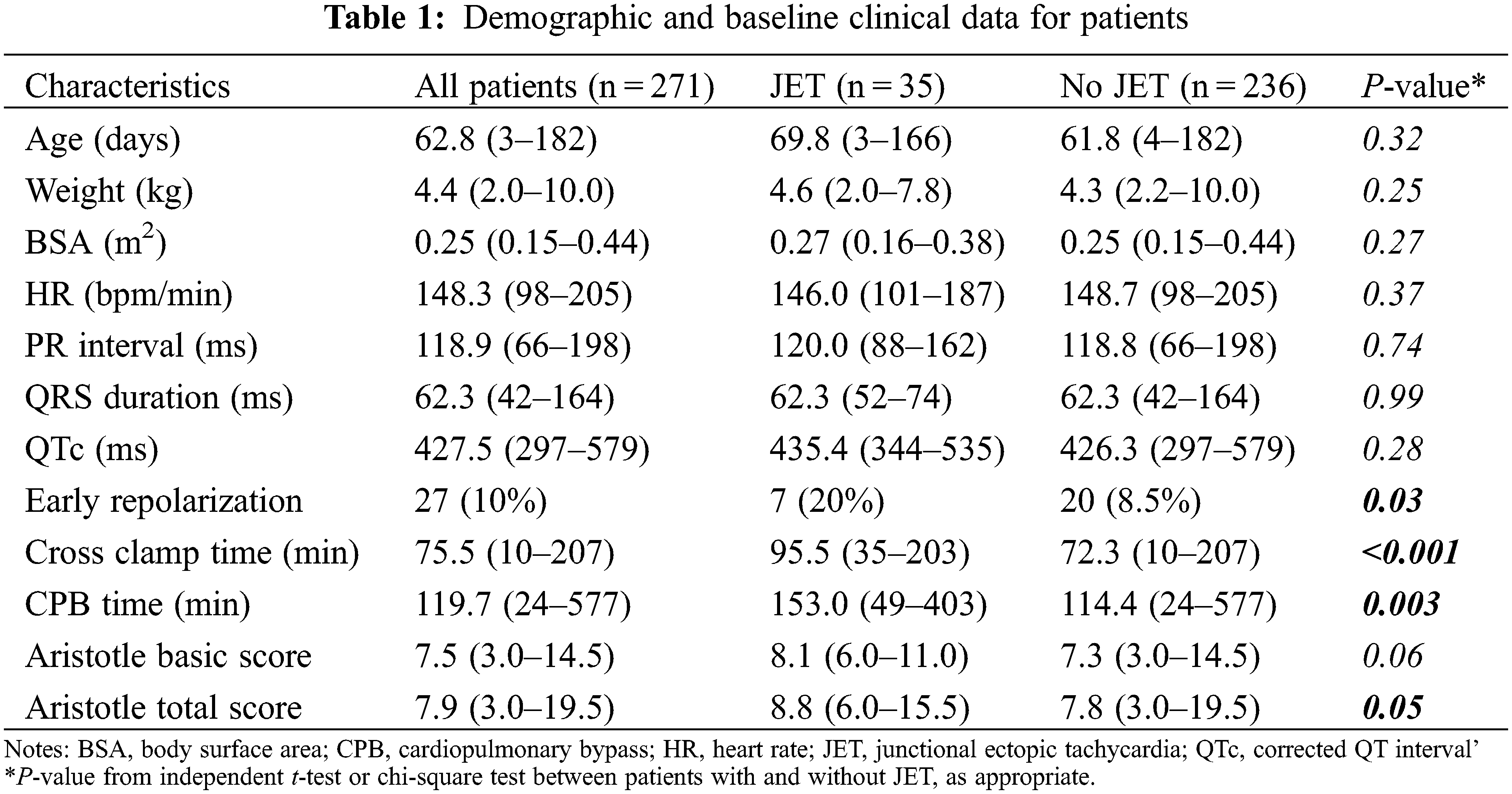
3.1 Baseline Characteristics of the Enrolled Patients
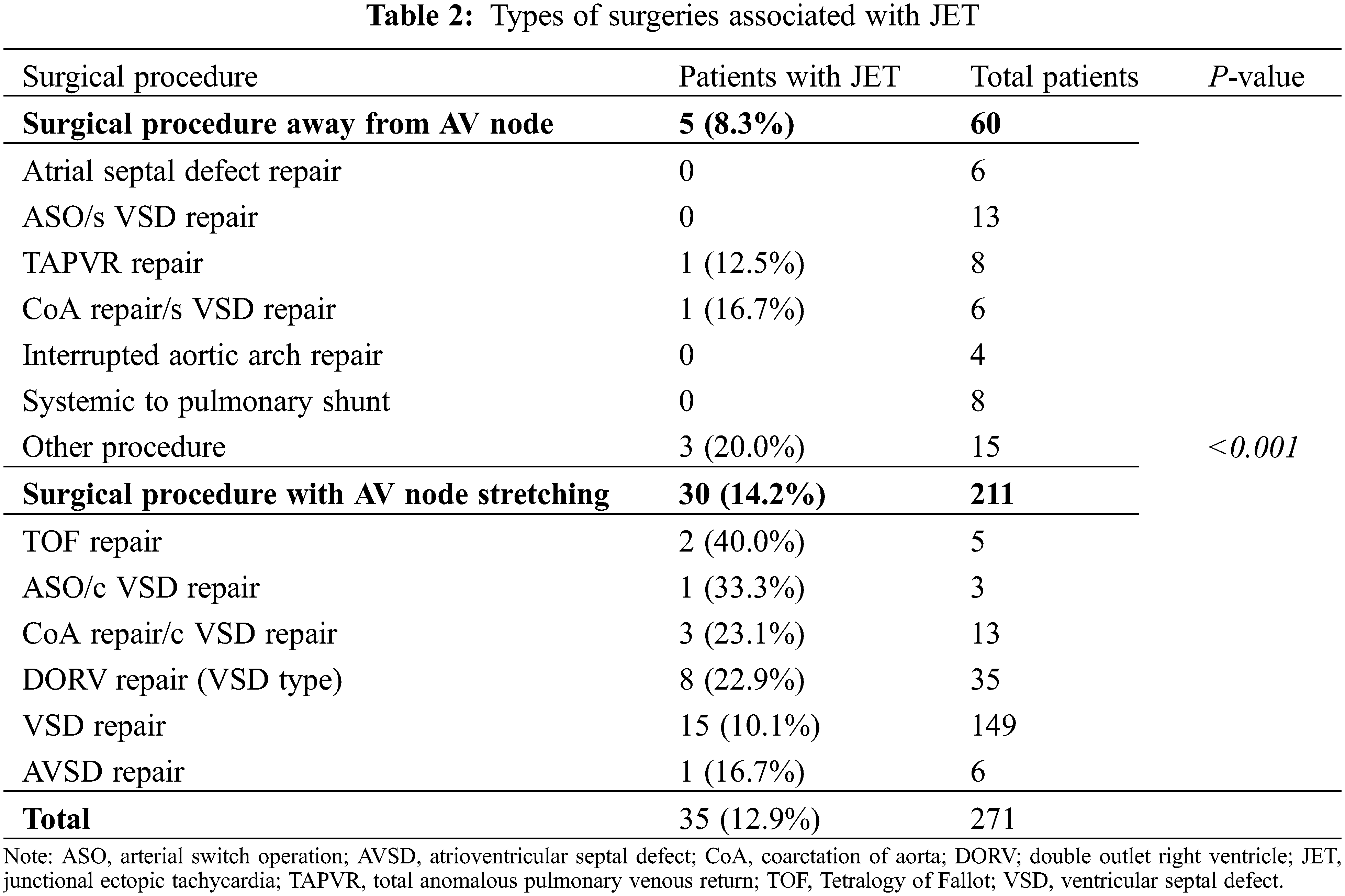
A total of 271 patients aged under 1 year were enrolled in the study. We identified 35 patients with JET during the postoperative period. Thus, the overall incidence of JET was 12.9% (35/271; Table 1). Baseline characteristics, such as patient age, weight, and body surface area, were not significantly different between the patient with JET and without JET groups. Postoperative and preoperative (data not shown) ECG parameters, including heart rate, PR interval, QRS duration, and QTc interval, were not significantly different between both the groups. However, preoperative early repolarization was more prevalent in patients with JET than in those without JET (20% vs. 8.5%, P = 0.03). Cross-clamp time (95.5 vs. 72.3 min, P < 0.001) and CPB time (153.0 vs. 114.4 min, P = 0.003) were significantly longer in the JET group than in those without JET. The Aristotle total score was also higher in patients with JET (8.8 vs. 7.8, P = 0.05). Table 2 shows the occurrence of JET according to the type of surgical procedure. The incidence of JET was significantly higher during the surgery for AV node stretching (P < 0.001). These conditions included TOF and its variants (40.0%), coarctation repair with ventricular septal defect (VSD) closure (23.1%), and VSD-type double outlet right ventricle (DORV) (22.9%) (Table 2).
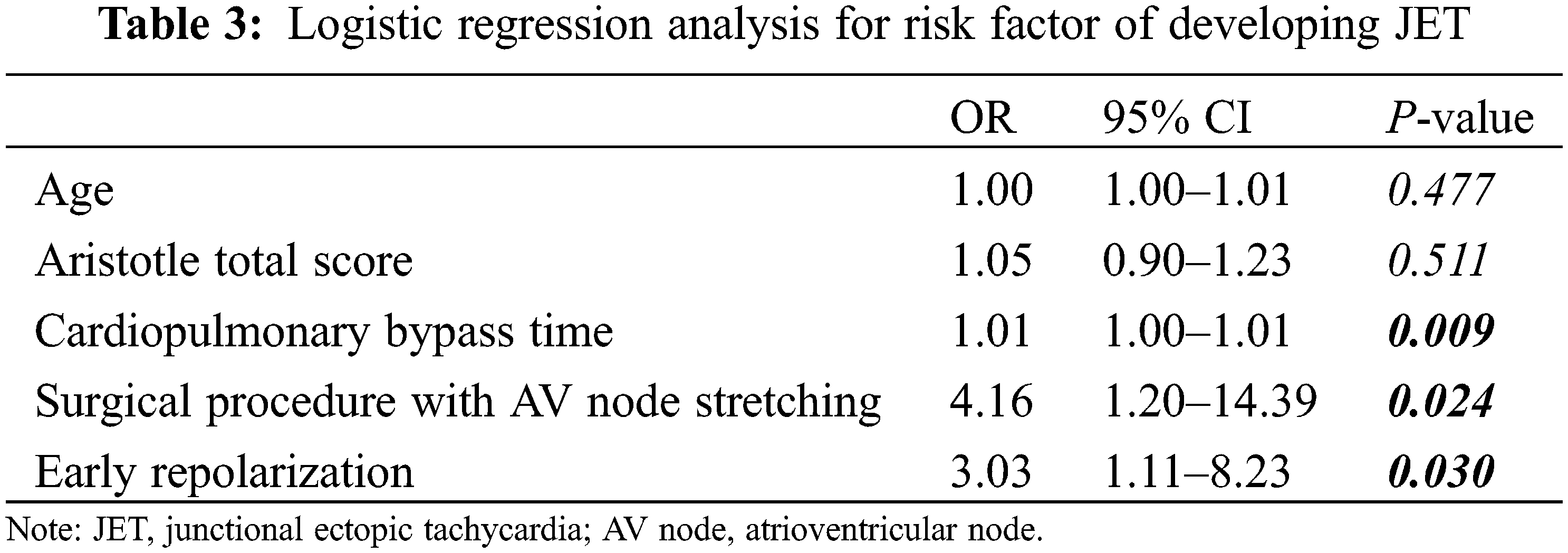
Binary logistic regression analysis revealed that the type of surgery involving the AV node was a strong independent risk factor for JET (OR = 4.16; 95% CI: 1.20–14.39, P < 0.024), followed by preoperative early repolarization (OR = 3.03; 95% CI: 1.11–8.23, P < 0.030; Table 3). CPB time was a weak predictive risk factor for JET. Young age and the Aristotle total score were not related to JET development. The Aristotle basic score and cross-clamp time were excluded as risk factors in the logistic analysis because of their strong relationship with the Aristotle total score and CPB time, respectively.
All JET events started during the first 24 h after heart surgery. JET onset time ranged from 0.6 to 20.7 h, with mean ± SD of 8.8 ± 6.37 h. The mean VIS at the time of JET onset was 13.6 μg/kg/min. In our study, there was no electrolyte imbalance at the time of JET.
3.3 Outcomes in Neonates and Infants with Postoperative JET
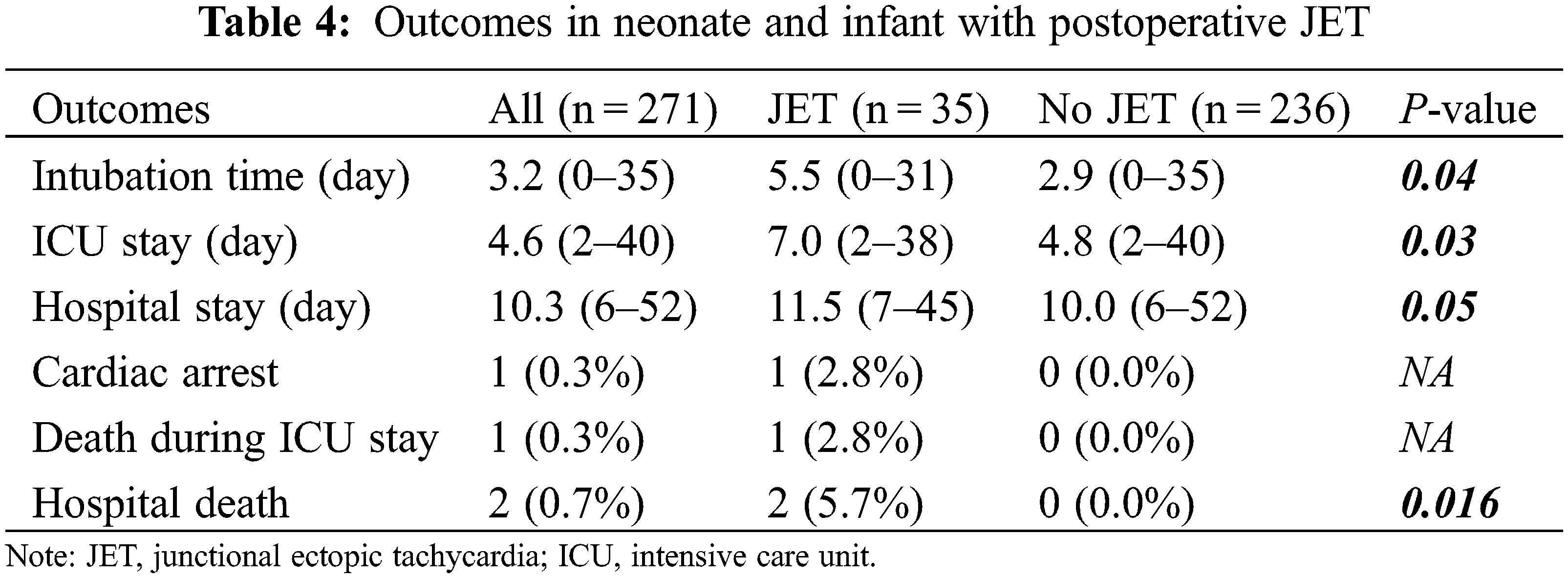
Patients with JET had longer intubation time (5.5 vs. 2.9 days, P = 0.035), length of ICU stay (7.0 vs. 4.8 days, P = 0.03), and total hospital stay (11.5 vs. 10.0 days, P = 0.05; Table 4). Ten patients with JET were treated with conservative maneuvers, without any complications noted. Twenty-five (71.4%) patients were treated using amiodarone with conservative treatment. Two patients with JET (2.8%) died, which was a significant result compared that noted in patients without JET (P = 0.016). Among these, one patient with low cardiac output syndrome died with ventricular fibrillation (VF) despite CPR, followed by extracorporeal life support therapy. Another patient had an 18-p deletion syndrome and died from recurrent pneumonia unrelated to JET.
JET is the most common type of tachyarrhythmia occurring in the postoperative period after congenital heart surgery, with an incidence ranging from 1.4% to 14.3% [1–4]. Our study showed an overall incidence of 12.9% for JET, which is comparable with the findings of a previous study on neonates and infants aged under 1 year [1].
In our study, low body weight was not a risk factor for JET, which is inconsistent with a previous study finding [19]. We included only newborns and infants aged under 1 year, and the weight gain in children with congenital heart defects was slow. Therefore, the weight difference among patients was insufficient to show statistical differences.
For congenital heart defect factors, our study showed a relatively higher incidence of JET in patients with TOF, coarctation of the aorta with VSD, and VSD-type DORV. These heart diseases are related to surgery in the vicinity of the AV node. These findings are consistent with those of previous studies that reported an increased incidence of postoperative JET after surgery that can damage the AV node or bundle through muscle resection or excessive traction [6,13,20–22]. The postoperative JET after atrial septal defect or coarctation of the aorta repair, which does not directly include the AV nodal area, may have been caused by other mechanisms, such as intraoperative hypoperfusion or ischemia.
For surgical factors in our study, longer CPB time was a risk factor for JET, which is consistent with that reported in previous studies [1,3,7,22]. The basic and total scores on the Aristotle scale are complex stratification instruments for pediatric cardiac surgical procedures [23–25]. Unlike findings of previous studies [7,26], a high score on the Aristotle scale was not associated with JET development in our study. This means that the type of surgery performed close to the AV node had a more significant effect on the occurrence of JET than the complexity of the surgery.
For electrophysiological factors in our study, we found a strong association between preoperative early repolarization and JET. In addition, the high prevalence of ERP in patients with and without JET was consistent with the findings of previous reports of a higher prevalence of early repolarization in the pediatric population than adults [27,28]. In a meta-analysis, ERP was associated with an increased risk and a low-to-intermediate absolute incidence rate of death from arrhythmia [29]. Experimental studies have suggested that J-point elevation is related to increased transmural heterogeneity of ventricular repolarization, which could be related to VF [30]. However, the relationship between ERP and VF in our study is less clear because of the small number of cases of VF. Reentry may lead to reciprocal tachycardia, including atrial, junctional, and AV tachycardia. Favorable conditions of reentry include increased myocardial mass, decreased conduction velocity, and accelerated repolarization [31]. Evidence for proving a direct correlation between ERP and JET is limited, despite this classical concept. We cannot conclude whether ERP directly caused postoperative JET from this single-center retrospective study. However, some studies have shown that CPB surgery can change electrophysiological properties, including myocardial repolarization and QTc interval in children [32,33]. In particular, infants have unique calcium transients in the atrial myocytes [34]. Therefore, further studies on the relevance of the repolarization change in postoperative JET are required.
Other factors that could affect the development of JET include electrolyte abnormalities and inotropic drug use [15,35]. In our study, some patients developed electrolyte imbalance immediately after surgery, but we aggressively corrected postoperative electrolyte imbalance. When an electrolyte abnormality was reviewed in patients with JET, it was not found at the time of JET onset. Because of the frequently adjusted dose of inotropic dugs immediately after surgery, the time point for comparing the inotropic score in patients without JET was difficult to determine; therefore, VIS was not compared between the groups in our study. However, the VIS of patients with JET in this study was similar to that reported in other studies [36,37]. Further studies on the use of inotropic drugs as risk factors for JET are required.
Our study showed that JET is related to longer intubation time, length of ICU stay, total hospital stay, and hospital death, as shown in previous studies [1,2,5]. In our study, two patients with JET died, which was significant number of patients. However, it is difficult to explain the direct association between JET and morality because the cause of death was not related to JET but was associated with other underlying morbidities. Although there have been several previous reports on the association between JET and mortality, the mortality-related postoperative JET showed a decreased incidence in our study compared to that shown in previous reports, which is thought to be due to the improved management of JET [4,15,35,38–40]. Postoperative JET is transient and treatable, but its treatment is often challenging. In the case of JET that is not controlled by primary treatment, such as sedation, cooling, and correcting electrolyte abnormalities, pharmacotherapy must be used. Amiodarone is the most widely used drug [15,41–43]. Recently, there have been many reports of successful conversion to the sinus rhythm with ivabradine as an adjuvant to amiodarone or a first-line agent in pediatric patients with postoperative JET [15,44–47]. However, there are insufficient data on the use of ivabradine in pediatric patients. In addition, the parenteral formulation of ivabradine has not been approved by the Food and Drug Administration; therefore, its oral formulation has limitations, in that it can be affected by absorption or bioavailability, particularly in neonates [46]. Further studies on the availability and efficacy of ivabradine in pediatric patients in the management of postoperative JET are required.
This study has several limitations. First, this study had a retrospective design. Although our practices conformed to institutional protocols, the surgical or postoperative procedure could not be standardized. In addition, we did not include detailed information, including clinical features, biochemical measurements (such as oxygen saturation) or blood gas analysis. Second, a few patients whose sternum was left open were evaluated using limb-lead ECG alone. Third, laboratory results, such as NT-proBNP or troponin-T levels, were not analyzed. Finally, the association between surgical procedural factors and JET could differ depending on the era and institution in which the surgery was performed.
Despite these limitations, our study identified preoperative ERP as a strong risk factor for JET in neonates and infants after congenital heart surgery. This finding should be evaluated in a multicenter prospective study to clarify the underlying mechanism.
JET is a common type of arrhythmia that occurs after cardiac surgery for congenital heart disease in infants. It is mostly manageable and has a benign course but is associated with increased morbidity, as assessed by the longer intubation time, length of ICU stay, and total hospital stay. Clinicians should be aware of postoperative JET if the patient is in a high-risk group. Our results suggest that risk factors for JET include multifactorial conditions. Surgery in the vicinity of the AV node was strongly correlated with JET. Preoperative ERP had a significant association with the development of JET in our study compared to that reported in previous studies. Surgery should be performed as gently as possible, particularly in the vicinity of the conduction system. In addition, patients who undergo surgery involving the AV node and/or preoperative ERP should be monitored carefully for the occurrence of JET after surgery and treated appropriately. Through risk stratification, further research on optimal management to prevent and control JET at an early stage is required.
Authorship: The authors confirm contribution to the paper as follows: data collection, draft manuscript preparation, critical revision of the article: Jae Hee Seol; analysis and interpretation of results, data collection, draft manuscript, data collection: Se Yong Jung; analysis and interpretation of results, study conception and design, data collection: Jae Young Choi; study conception and design, data collection: Han Ki Park; study conception and design, data collection: Young Hwan Park; study conception and design, critical revision of the article: Nam Kyun Kim. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The Institutional Review Board of Severance Hospital approved this study (Study Approval No. 4-2019-0259). The requirement for informed consent was waived due to the study’s retrospective nature.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study
References
1. Zampi, J. D., Hirsch, J. C., Gurney, J. G., Donohue, J. E., Yu, S. et al. (2012). Junctional ectopic tachycardia after infant heart surgery: Incidence and outcomes. Pediatric Cardiology, 33(8), 1362–1369. DOI 10.1007/s00246-012-0348-y. [Google Scholar] [CrossRef]
2. Andreasen, J. B., Johnsen, S. P., Ravn, H. B. (2008). Junctional ectopic tachycardia after surgery for congenital heart disease in children. Intensive Care Medicine, 34(5), 895–902. DOI 10.1007/s00134-007-0987-2. [Google Scholar] [CrossRef]
3. Batra, A. S., Chun, D. S., Johnson, T. R., Maldonado, E. M., Kashyap, B. A. et al. (2006). A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatric Cardiology, 27(1), 51–55. DOI 10.1007/s00246-005-0992-6. [Google Scholar] [CrossRef]
4. Makhoul, M., Oster, M., Fischbach, P., Das, S., Deshpande, S. (2013). Junctional ectopic tachycardia after congenital heart surgery in the current surgical era. Pediatric Cardiology, 34(2), 370–374. DOI 10.1007/s00246-012-0465-7. [Google Scholar] [CrossRef]
5. Dodge-Khatami, A., Miller, O. I., Anderson, R. H., Gil-Jaurena, J. M., Goldman, A. P. et al. (2002). Impact of junctional ectopic tachycardia on postoperative morbidity following repair of congenital heart defects. European Journal of Cardio-Thoracic Surgery, 21(2), 255–259. DOI 10.1016/S1010-7940(01)01089-2. [Google Scholar] [CrossRef]
6. Mildh, L., Hiippala, A., Rautiainen, P., Pettilä, V., Sairanen, H. et al. (2011). Junctional ectopic tachycardia after surgery for congenital heart disease: Incidence, risk factors and outcome. European Journal of Cardio-Thoracic Surgery, 39(1), 75–80. DOI 10.1016/j.ejcts.2010.04.002. [Google Scholar] [CrossRef]
7. Rekawek, J., Kansy, A., Miszczak-Knecht, M., Manowska, M., Bieganowska, K. et al. (2007). Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. The Journal of Thoracic and Cardiovascular Surgery, 133(4), 900–904. DOI 10.1016/j.jtcvs.2006.12.011. [Google Scholar] [CrossRef]
8. Catton, K. G., Peterson, J. K. (2020). Junctional ectopic tachycardia: Recognition and modern management strategies. Critical Care Nurse, 40(1), 46–55. DOI 10.4037/ccn2020793. [Google Scholar] [CrossRef]
9. Dyamenahalli, U., Tuzcu, V., Fontenot, E., Papagiannis, J., Jaquiss, R. D. et al. (2012). Extracorporeal membrane oxygenation support for intractable primary arrhythmias and complete congenital heart block in newborns and infants: Short-term and medium-term outcomes. Pediatric Critical Care Medicine, 13(1), 47–52. DOI 10.1097/PCC.0b013e3182196cb1. [Google Scholar] [CrossRef]
10. Haas, N. A., Plumpton, K., Justo, R., Jalali, H., Pohlner, P. (2004). Postoperative junctional ectopic tachycardia (JET). Zeitschrift fur Kardiologie, 93(5), 371–380. DOI 10.1007/s00392-004-0067-3. [Google Scholar] [CrossRef]
11. Kean, A. C., Hazle, M., LaPage, M. J., Bromberg, B. I. (2015). Junctional tachycardia: Congenital, acquired, postoperative. In: Dick II, M. (Ed.Clinical cardiac electrophysiology in the young, pp. 157–169. New York, NY: Springer. DOI 10.1007/978-1-4939-2739-5_11. [Google Scholar] [CrossRef]
12. Bae, E. J., Noh, C. I., Choi, J. Y., Yun, Y. S., Kim, W. H. et al. (2005). Twin AV node and induced supraventricular tachycardia in Fontan palliation patients. Pacing and Clinical Electrophysiology, 28(2), 126–134. DOI 10.1111/j.1540-8159.2005.09450.x. [Google Scholar] [CrossRef]
13. Kylat, R. I., Samson, R. A. (2019). Junctional ectopic tachycardia in infants and children. Journal of Arrhythmia, 36(1), 59–66. DOI 10.1002/joa3.12282. [Google Scholar] [CrossRef]
14. Ozawa, Y., Asakai, H., Shiraga, K., Shindo, T., Hirata, Y. et al. (2019). Cardiac rhythm disturbances in heterotaxy syndrome. Pediatric Cardiology, 40(5), 909–913. DOI 10.1007/s00246-019-02087-2. [Google Scholar] [CrossRef]
15. Sasikumar, N., Kumar, R. K., Balaji, S. (2021). Diagnosis and management of junctional ectopic tachycardia in children. Annals of Pediatric Cardiology, 14(3), 372–381. DOI 10.4103/apc.apc_35_21. [Google Scholar] [CrossRef]
16. Davidson, J., Tong, S., Hancock, H., Hauck, A., da Cruz, E. et al. (2012). Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Medicine, 38(7), 1184–1190. DOI 10.1007/s00134-012-2544-x. [Google Scholar] [CrossRef]
17. Gaies, M. G., Gurney, J. G., Yen, A. H., Napoli, M. L., Gajarski, R. J. et al. (2010). Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric Critical Care Medicine, 11(2), 234–238. DOI 10.1097/PCC.0b013e3181b806fc. [Google Scholar] [CrossRef]
18. Pieroni, M., Bellocci, F., Crea, F. (2008). Sudden cardiac arrest associated with early repolarization. The New England Journal of Medicine, 359(7), 761–762. DOI 10.1056/NEJMc081272. [Google Scholar] [CrossRef]
19. Pfammatter, J. P., Wagner, B., Berdat, P., Bachmann, D. C., Pavlovic, M. et al. (2002). Procedural factors associated with early postoperative arrhythmias after repair of congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery, 123(2), 258–262. DOI 10.1067/mtc.2002.119701. [Google Scholar] [CrossRef]
20. Dodge-Khatami, A., Miller, O. I., Anderson, R. H., Goldman, A. P., Gil-Jaurena, J. M. et al. (2002). Surgical substrates of postoperative junctional ectopic tachycardia in congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery, 123(4), 624–630. DOI 10.1067/mtc.2002.121046. [Google Scholar] [CrossRef]
21. Paech, C., Dähnert, I., Kostelka, M., Mende, M., Gebauer, R. (2015). Association of temporary complete AV block and junctional ectopic tachycardia after surgery for congenital heart disease. Annals of Pediatric Cardiology, 8(1), 14–19. [Google Scholar]
22. Paluszek, C., Brenner, P., Pichlmaier, M., Haas, N. A., Dalla-Pozza, R. et al. (2019). Risk factors and outcome of post fallot repair junctional ectopic tachycardia (JET). World Journal for Pediatric & Congenital Heart Surgery, 10(1), 50–57. [Google Scholar]
23. Jacobs, J. P., Jacobs, M. L., Lacour-Gayet, F. G., Jenkins, K. J., Gauvreau, K. et al. (2009). Stratification of complexity improves the utility and accuracy of outcomes analysis in a multi-institutional congenital heart surgery database: Application of the risk adjustment in congenital heart surgery (RACHS-1) and aristotle systems in the society of thoracic surgeons (STS) congenital heart surgery database. Pediatric Cardiology, 30(8), 1117–1130. [Google Scholar]
24. Jacobs, M. L., Jacobs, J. P., Jenkins, K. J., Gauvreau, K., Clarke, D. R. et al. (2008). Stratification of complexity: The risk adjustment for congenital heart surgery-1 method and the aristotle complexity score--past, present, and future. Cardiology in the Young, 18(S2), 163–168. [Google Scholar]
25. Manrique, A. M., Arroyo, M., Lin, Y., El Khoudary, S. R., Colvin, E. et al. (2010). Magnesium supplementation during cardiopulmonary bypass to prevent junctional ectopic tachycardia after pediatric cardiac surgery: A randomized controlled study. The Journal of Thoracic and Cardiovascular Surgery, 139(1), 162–169.e2. [Google Scholar]
26. Sahu, M. K., Das, A., Siddharth, B., Talwar, S., Singh, S. P. et al. (2018). Arrhythmias in children in early postoperative period after cardiac surgery. World Journal for Pediatric & Congenital Heart Surgery, 9(1), 38–46. [Google Scholar]
27. McCorquodale, A., Poulton, R., Hendry, J., Norrish, G., Field, E. et al. (2017). High prevalence of early repolarization in the paediatric relatives of sudden arrhythmic death syndrome victims and in normal controls. Europace, 19(8), 1385–1391. [Google Scholar]
28. Sinner, M. F., Reinhard, W., Müller, M., Beckmann, B. M., Martens, E. et al. (2010). Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: A population-based prospective cohort study (MONICA/KORA). PLoS Medicine, 7(7), e1000314. [Google Scholar]
29. Wu, S. H., Lin, X. X., Cheng, Y. J., Qiang, C. C., Zhang, J. (2013). Early repolarization pattern and risk for arrhythmia death: A meta-analysis. Journal of the American College of Cardiology, 61(6), 645–650. [Google Scholar]
30. Abe, A., Ikeda, T., Tsukada, T., Ishiguro, H., Miwa, Y. et al. (2010). Circadian variation of late potentials in idiopathic ventricular fibrillation associated with J waves: Insights into alternative pathophysiology and risk stratification. Heart Rhythm, 7(5), 675–682. [Google Scholar]
31. Olsson, S. B. (1984). Pathophysiology of re-entrant dysrhythmias. European Heart Journal, 5(suppl_B), 19–23. DOI 10.1093/eurheartj/5.suppl_B.19. [Google Scholar] [CrossRef]
32. Han, C. W., Woo, S. B., Choi, J. Y., Jung, J. W., Park, Y. H. et al. (2013). QTc prolongation after ventricular septal defect repair in infants. Korean Circulation Journal, 43(12), 825–829. DOI 10.4070/kcj.2013.43.12.825. [Google Scholar] [CrossRef]
33. Aburawi, E. H., Souid, A. K., Liuba, P., Zoubeidi, T., Pesonen, E. (2013). Early changes in myocardial repolarization and coronary perfusion after cardiopulmonary bypass surgery for ASD repair in children. BMC Cardiovascular Disorders, 13, 67. DOI 10.1186/1471-2261-13-67. [Google Scholar] [CrossRef]
34. Wagner, M. B., Wang, Y., Kumar, R., Tipparaju, S. M., Joyner, R. W. (2005). Calcium transients in infant human atrial myocytes. Pediatric Research, 57(1), 28–34. DOI 10.1203/01.PDR.0000148066.34743.10. [Google Scholar] [CrossRef]
35. Clark, B. C., Berger, J. T., Berul, C. I., Jonas, R. A., Kaltman, J. R. et al. (2018). Risk factors for development of ectopic atrial tachycardia in post-operative congenital heart disease. Pediatric Cardiology, 39(3), 459–465. DOI 10.1007/s00246-017-1773-8. [Google Scholar] [CrossRef]
36. El Amrousy, D. M., Elshmaa, N. S., El-Kashlan, M., Hassan, S., Elsanosy, M. et al. (2017). Efficacy of prophylactic dexmedetomidine in preventing postoperative junctional ectopic tachycardia after pediatric cardiac surgery. Journal of the American Heart Association, 6(3), e004780. DOI 10.1161/JAHA.116.004780. [Google Scholar] [CrossRef]
37. Ismail, M. F., Arafat, A. A., Hamouda, T. E., El Tantawy, A. E., Edrees, A. et al. (2018). Junctional ectopic tachycardia following tetralogy of fallot repair in children under 2 years. Journal of Cardiothoracic Surgery, 13(1), 60. DOI 10.1186/s13019-018-0749-y. [Google Scholar] [CrossRef]
38. Tharakan, J. A., Sukulal, K. (2014). Post cardiac surgery junctional ectopic tachycardia: A ‘hit and run’ tachyarrhythmia as yet unchecked. Annals of Pediatric Cardiology, 7(1), 25–28. DOI 10.4103/0974-2069.126545. [Google Scholar] [CrossRef]
39. Hoffman, T. M., Bush, D. M., Wernovsky, G., Cohen, M. I., Wieand, T. S. et al. (2002). Postoperative junctional ectopic tachycardia in children: Incidence, risk factors, and treatment. The Annals of Thoracic Surgery, 74(5), 1607–1611. DOI 10.1016/S0003-4975(02)04014-6. [Google Scholar] [CrossRef]
40. Case, C. L., Gillette, P. C. (1993). Automatic atrial and junctional tachycardias in the pediatric patient: Strategies for diagnosis and management. Pacing and Clinical Electrophysiology, 16(6), 1323–1335. DOI 10.1111/j.1540-8159.1993.tb01719.x. [Google Scholar] [CrossRef]
41. Kovacikova, L., Hakacova, N., Dobos, D., Skrak, P., Zahorec, M. (2009). Amiodarone as a first-line therapy for postoperative junctional ectopic tachycardia. The Annals of Thoracic Surgery, 88(2), 616–622. DOI 10.1016/j.athoracsur.2009.04.088. [Google Scholar] [CrossRef]
42. Plumpton, K., Justo, R., Haas, N. (2005). Amiodarone for post-operative junctional ectopic tachycardia. Cardiology in the Young, 15(1), 13–18. DOI 10.1017/S1047951105000041. [Google Scholar] [CrossRef]
43. Saul, J. P., Scott, W. A., Brown, S., Marantz, P., Acevedo, V. et al. (2005). Intravenous amiodarone for incessant tachyarrhythmias in children: A randomized, double-blind, antiarrhythmic drug trial. Circulation, 112(22), 3470–3477. DOI 10.1161/CIRCULATIONAHA.105.534149. [Google Scholar] [CrossRef]
44. Krishna, M. R., Kunde, M. F., Kumar, R. K., Balaji, S. (2019). Ivabradine in post-operative junctional ectopic tachycardia (JETBreaking new ground. Pediatric Cardiology, 40(6), 1284–1288. DOI 10.1007/s00246-019-02149-5. [Google Scholar] [CrossRef]
45. Kumar, V., Kumar, G., Joshi, S., Sharma, V. (2017). Ivabradine for junctional ectopic tachycardia in post congenital heart surgery. Indian Heart Journal, 69(5), 666–667. DOI 10.1016/j.ihj.2017.09.007. [Google Scholar] [CrossRef]
46. Kumar, V., Kumar, G., Tiwari, N., Joshi, S., Sharma, V. et al. (2019). Ivabradine as an adjunct for refractory junctional ectopic tachycardia following pediatric cardiac surgery: A preliminary study. World Journal for Pediatric & Congenital Heart Surgery, 10(6), 709–714. DOI 10.1177/2150135119876600. [Google Scholar] [CrossRef]
47. Lim, J., Mok, Y. H., Loh, Y. J., Tan, T. H., Lee, J. H. (2017). The impact of time to rate control of junctional ectopic tachycardia after congenital heart surgery. World Journal for Pediatric & Congenital Heart Surgery, 8(6), 685–690. DOI 10.1177/2150135117732544. [Google Scholar] [CrossRef]
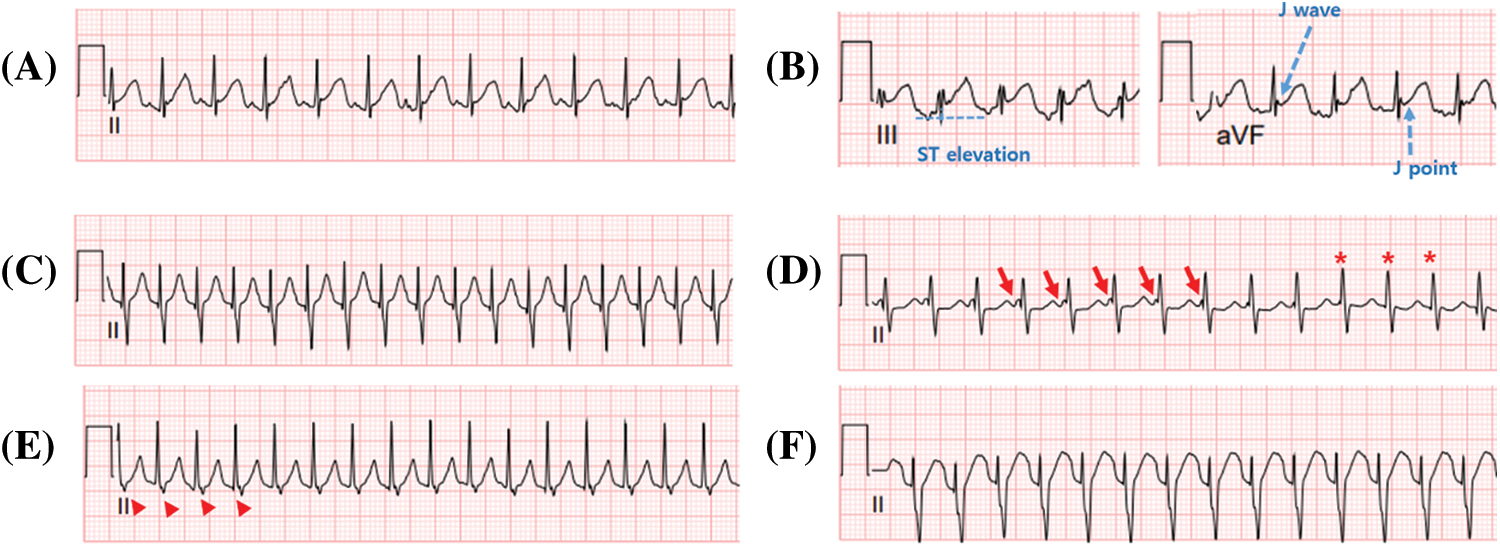
Supplementary Figure 1: Representative electrocardiography showing early repolarization and junctional ectopic tachycardia. A, B. Early repolarization electrocardiography showing the J wave, J-point, and ST elevation ≥ 1 mm. C–F. Electrocardiographs showing various characteristics of junctional ectopic tachycardia. C. Narrow QRS complex tachycardia without the P wave. D. Narrow QRS complex tachycardia with atrioventricular dissociation. Arrows indicate P waves, and the asterisk indicates that the P wave is hidden in the QRS wave. E. Narrow QRS complex tachycardia with the retrograde P wave. The arrowhead indicates the retrograde P wave and short RP interval. F. Wide QRS complex tachycardia with the right bundle branch block. All electrocardiographs were recorded at 25 mm/s with a voltage gain setting of 10 mm/mV.
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools