 Open Access
Open Access
ARTICLE
A 20-Year Follow-up after the Fontan Operation in a Population with Hypoplastic Left Heart Syndrome
Department of Boston Children’s Hospital, Department of Anesthesiology Critical Care and Pain Medicine Division of Cardiac Anesthesia, Boston, 02115, USA
* Corresponding Author: Eleni P. Asimacopoulos. Email:
Congenital Heart Disease 2022, 17(5), 579-590. https://doi.org/10.32604/chd.2022.020334
Received 24 February 2022; Accepted 25 April 2022; Issue published 06 September 2022
Abstract
Background: Thromboembolic events are a cause of significant morbidity and mortality in the Fontan population. We previously reported on coagulation profile changes in a cohort of patients with hypoplastic left heart syndrome (HLHS) from Stage I through Fontan completion. In this report, we examine their clinical status, anticoagulation and incidence of thromboembolic events up to 20 years post Fontan. Methods: A retrospective chart review was conducted for twenty (20) surviving patients, from 1998 through December 2020. Patients who underwent orthotopic heart transplantation (OTx) were followed until their transplant. Patients who were found in the original study to have a factor VIII activity level >160%, were examined separately. Results: Most patients had follow-up within the last two years (2018–2020). Two patients underwent OTx and two patients died. Anticoagulation strategy was variable. Most patients were on aspirin monotherapy. There was a total of twelve thrombotic events (63.2%). These included six cerebrovascular accidents (two of which were fatal). Three out of the seven patients with elevated factor VIII activity from the original study had thromboembolic events (42.9%). Fontan complications were variable. Some degree of Fontan Associated Liver Disease was universal. Conclusions: This retrospective review of a group of single-ventricle patients post Fontan, illustrates the variability in anticoagulation therapy that exists in this population. A large proportion of patients suffered a significant thromboembolic event, including the patients with elevated factor VIII. Further investigation into the patients with elevated factor VIII may help determine whether a different antithrombotic strategy post Fontan would be beneficial.Keywords
Glossary of Abbreviations
| ALT | alanine aminotransferase |
| APC | atriopulmonary connection |
| AST | aspartate aminotransferase |
| BDG | bidirectional Glenn |
| CRT | cardiac resynchronization therapy |
| CVA | cerebrovascular accident |
| EC | extracardiac |
| FALD | Fontan Associated Liver Disease |
| HIT | heparin induced thrombocytopenia |
| HLHS | hypoplastic left heart syndrome |
| LT | lateral tunnel |
| LTFU | lost to follow up |
| mBTS | modified Blalock Taussig Shunt |
| NOAC | non vitamin K antagonist oral anticoagulant |
| NYHA | New York Heart Association |
| OTx | orthotopic heart transplantation |
| PLE | protein losing enteropathy |
| TIA | transient ischemic attack |
| TR | tricuspid regurgitation |
Operative survival and long-term outcomes for patients with single ventricle physiology undergoing the Fontan operation have markedly improved over the last few decades [1,2]. However, the Fontan circulation is still associated with several morbidities, resulting in decreased functional capacity and quality of life [1]. Thromboembolic events remain major contributing factors to morbidity and mortality in this patient population. The incidence of thrombosis and cerebrovascular accidents (CVA) can be as high as 19% in the Fontan population [1]. Characteristics which predispose to clot formation and are unique to the Fontan circulation compared to other types of congenital heart disease, include low flow with often elevated central venous pressure within the Fontan baffle and pulmonary circulation, and loss of the physiologic pulsatile circuit. Arrhythmia, ventricular dysfunction and hepatic disease also contribute to morbidity and mortality [2].
Conducted by Odegard et al. [3] in 1998–2009, a cohort of patients with hypoplastic left heart syndrome (HLHS) was followed in an original prospective, longitudinal study from birth until after the Fontan operation. The study evaluated the changes in coagulation profiles from before the Stage I palliation through Fontan completion. The study demonstrated significantly lower levels of both pro- and anti-coagulation factor levels when compared to age matched healthy controls, at all stages. After the Fontan procedure, there was a significant increase in factor VIII level, which could suggest a prothrombotic relationship in patients with Fontan physiology [3].
The purpose of this retrospective follow-up study of this population published in 2009 was to evaluate the clinical status, antithrombotic strategies and incidence of thrombotic events in the same group of patients with HLHS up to 20 years post the Fontan procedure.
In the original cohort, thirty-seven (37) neonates with the diagnosis of HLHS were enrolled and followed from 1998 to 2006, with the purpose of following the changes in coagulation profiles over time throughout stages of treatment [3–6]. Patients were excluded from the original study if they had other known congenital abnormalities or syndromes and had a pre-existing or known family history of hematologic disorders or coagulopathy. All patients underwent Stage I palliation with a modified Blalock Taussig Shunt (mBTS), Stage II with a bidirectional Glenn procedure (BDG), and the fenestrated lateral-tunnel Fontan procedure, and the study continued during the early period post the Fontan operation. A Gantt Chart of this cohort, including number of patients and age at surgery or investigation of coagulation profile, is shown in Fig. 1.
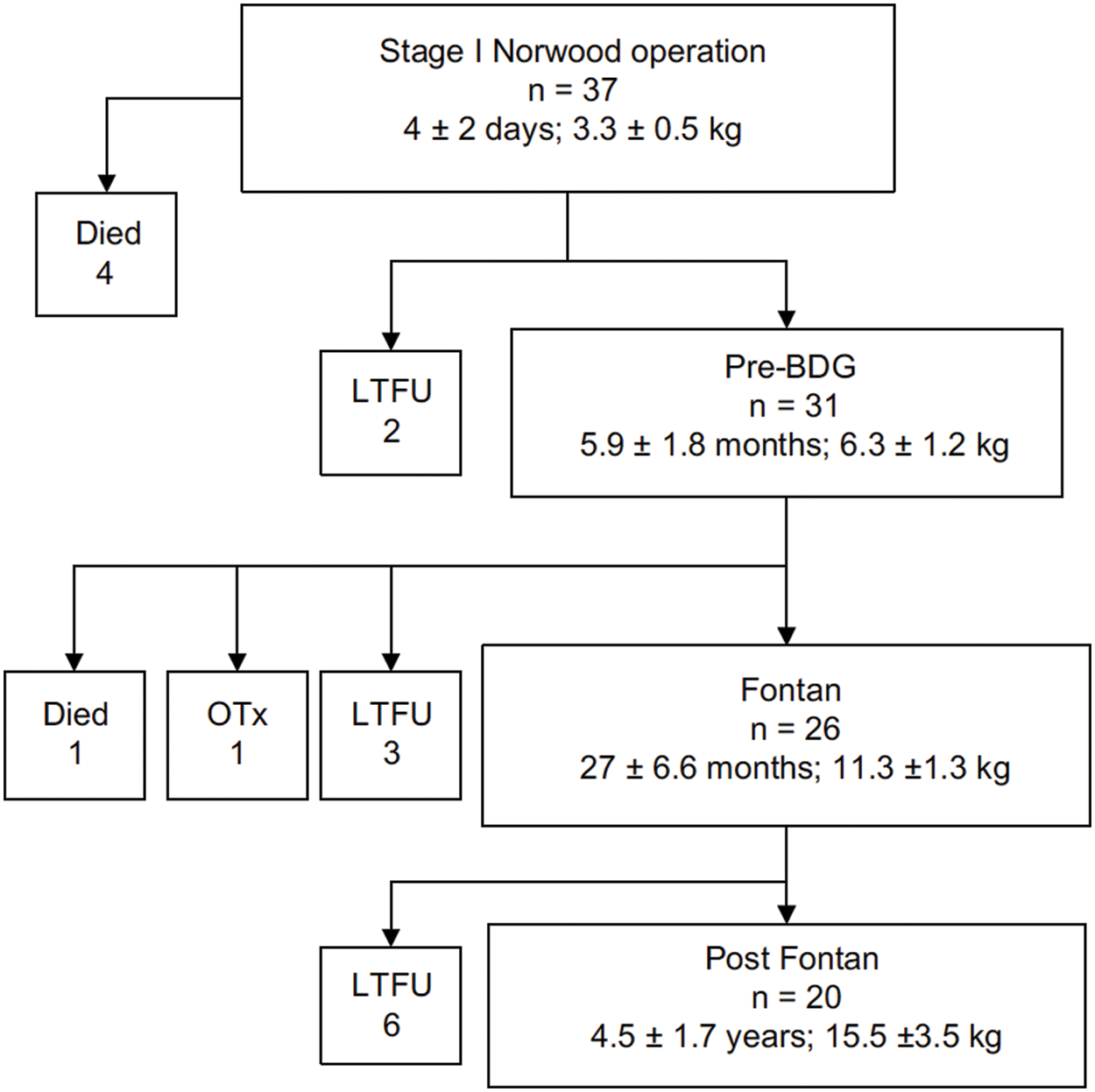
Figure 1: Number of original study patients from the study conducted by Odegard et al. [3], showing mean age and mean weight at each stage (Authors have obtained the copyright permission for this figure)
Notes: LTFU, lost to follow-up; BDG, bidirectional Glenn procedure; OTx, orthotopic heart transplant.
The twenty (20) surviving patients from the early post-Fontan group form the cohort of patients for this long term follow up. A retrospective electronic medical record review was conducted of these twenty patients. Chart reviews consisted of viewing clinical, procedural, and anesthetic notes. Chart reviews were conducted from the time of the Fontan completion through the most recent follow up visit, until the end of 2020 where available. In the early post-Fontan analysis, a group of patients were noted to have markedly elevated Factor VIII activity >160%, which could be a marker for risk of thrombus [4]. Those patients who had an elevated Factor VIII activity were examined separately. Patients who underwent orthotopic heart transplantation (OTx) were followed until their transplant and then exited the study.
Data was collected on the occurrence of thromboembolic events. These events were classified according to their location in the circulation: cerebrovascular (CVA or transient ischemic attack [TIA]), intracardiac thrombus and peripheral venous thrombus. Patient clinical status was documented based on clinician and parental assessment of their ability to carry out activities of daily living. Patients were symptomatically classified according to the New York Heart Association (NYHA) classification scale, with NYHA Class I being asymptomatic and NYHA IV symptoms at rest. Patients with ‘Failing Fontan’ symptoms (protein losing enteropathy [PLE] and plastic bronchitis) were automatically put into NYHA Class IV. Neurological and developmental status were collected based on clinician and parental assessment.
Antiplatelet and anticoagulation medications were documented for each patient. Compliance with this medication was noted based on provider documentation within the clinic note. Changes to regimens were also documented based on clinical events. Ventricular, atrioventricular valve, and semilunar valve function were assessed by 2-dimensional and Doppler echocardiographic examination for all patients at their follow up visits. Cardiac catheterization was not routinely conducted other than based on clinical indication. The availability of laboratory data was variable and included if important.
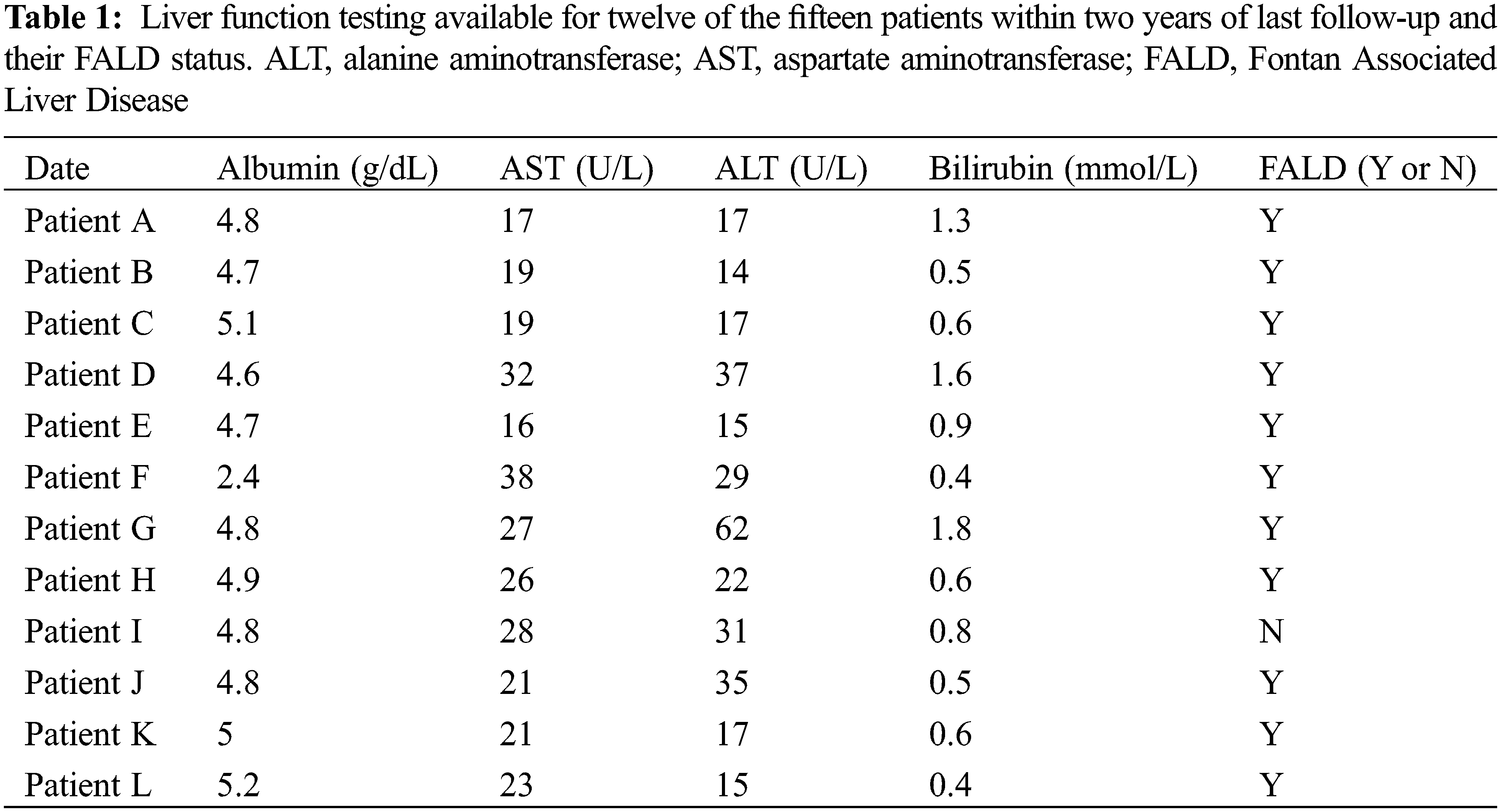
To assess for the impact of Fontan Associated Liver Disease (FALD), we also collected liver function testing values where available within two years of the last follow-up. The most common lab results were alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin and albumin. Gamma-glutamyl transferase and alkaline phosphatase were not tested for in most patients and therefore not included. Hepatic imaging, when performed, was also collected.
A Kaplan-Meier curve was constructed to estimate freedom from thromboembolic events during follow-up after Fontan completion. Numbers at risk are presented and 95% confidence bands were obtained using Greenwood’s formula. Statistical analyses were performed using Stata (version 16.1, Stata Corp LLC, College Station, Texas).
Of the twenty patients from the original study, two patients underwent OTx, two patients had died, and one out-of-state patient was lost to follow-up (LTFU). The remaining Fontan patients (n = 15) at the time of current follow up, had a mean age of 19.6 (±1.4 years) (Fig. 2). Two patients have been followed up in our cardiology clinics within the last three years (2016–2017), and the remaining 13 patients within the last year (2019–2020).
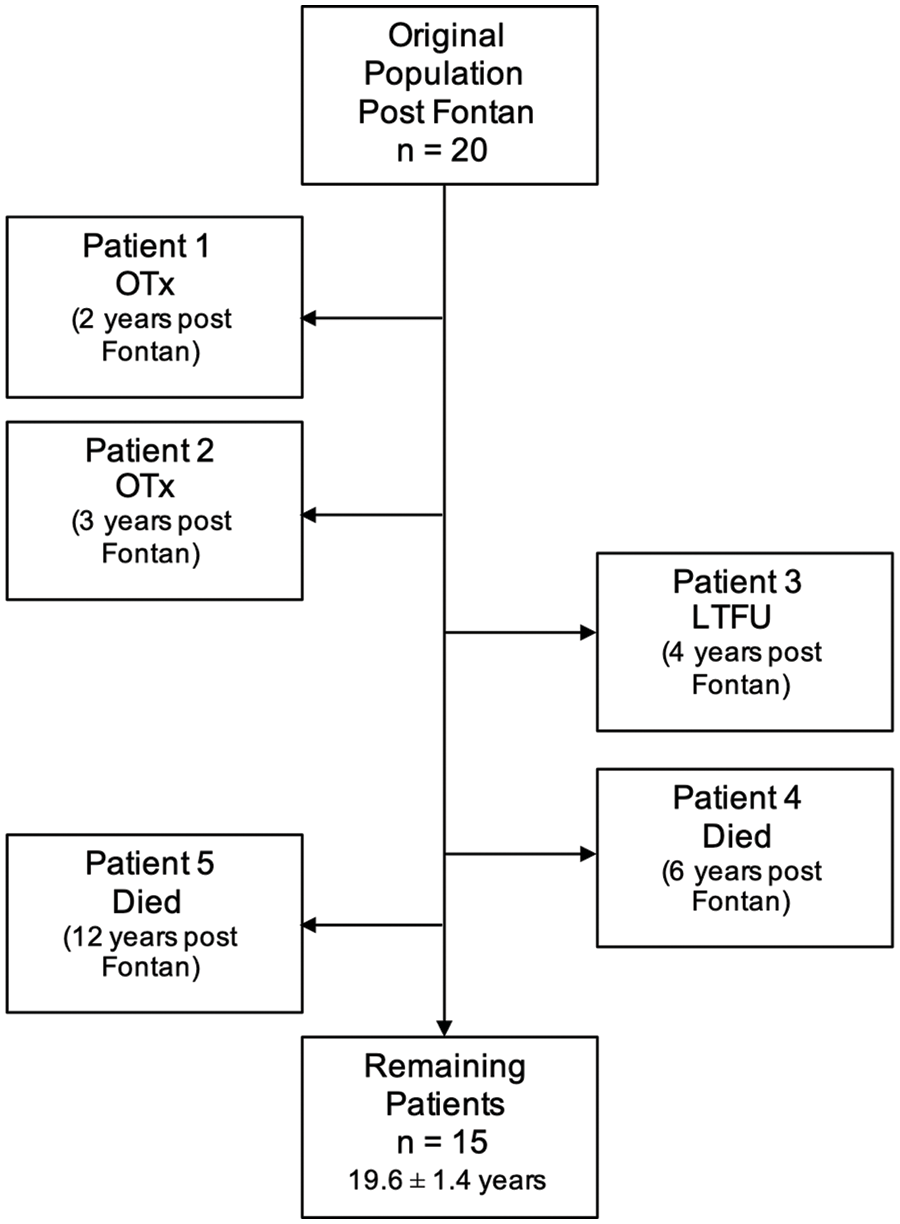
Figure 2: Current study patients showing the timeline of when patients died (n = 2), were transplanted (n = 2), or LTFU (n = 1). Mean age at last follow-up included
Of the patients who underwent transplantation, one patient had a transplant 27 months after the Fontan procedure because of ventricular failure. The second patient developed multiple complications after the Fontan procedure, including plastic bronchitis and intra-baffle thrombosis, and underwent transplantation 42 months after their Fontan procedure.
Of the 2 deaths, one patient developed severe protein losing enteropathy (PLE) and recurrent hemoptysis from arterio-pulmonary collateral (APC) vessels within 2 years of their Fontan procedure. In this setting, antiplatelet medication was discontinued. The patient ultimately had a catastrophic CVA, culminating in re-direction of care and death 7 years after their Fontan procedure; the cause of the CVA was not determined. The second patient who died also had developed severe PLE. Nine years after their Fontan procedure, the patient had an acute thalamic infarct from which the patient recovered. The patient was on antiplatelet therapy at the time of the infarct and Warfarin was added to the regimen after this. Unfortunately, 3 years later, the patient had a catastrophic CVA, culminating in re-direction of care and death; the cause of the CVA was not determined at the time.
Excluding the patient who was LTFU and for which no information was available, in the 19 patients post Fontan, there were 12 acute thromboembolic events in 10 patients (52.6%). Of these, 6 were cerebrovascular events (31.6%), five of which were CVAs and one of which was a TIA. One patient had two CVAs, and these were counted as separate events as they occurred 3 years apart from each other. There were 4 intracardiac clots (21.1%); 2 of the intracardiac clots were located within the Fontan baffle, the other two were located within the systemic ventricle. These four patients were all treated with an anticoagulant (warfarin or Apixaban). No interventions were performed. One patient had heparin-induced thrombocytopenia (HIT) immediately following their Fontan with minor digital tissue necrosis and nail bed loss. This patient had an episode concerning for digital thrombosis for which she was seen by a hematologist eight years later (Fig. 3).
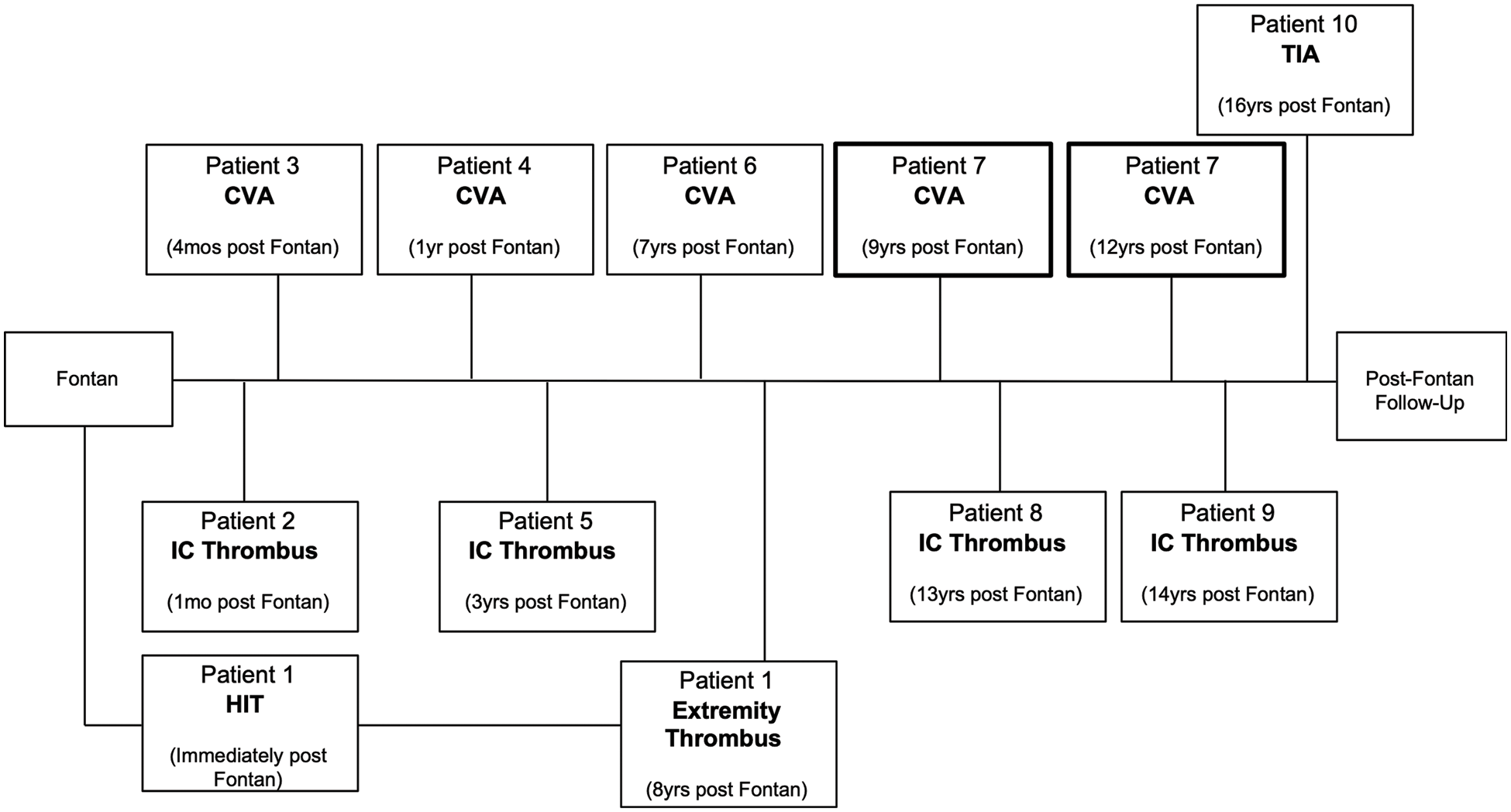
Figure 3: Timeline of thromboembolic events in the post Fontan group
Patients 1 and 7 had two independent thromboembolic events three years apart. Patient 7 (highlighted) had two CVAs. CVA, cerebrovascular accident; TIA, transient ischemic attack; HIT, heparin-induced thrombocytopenia.
The median time to thromboembolic event in this cohort was 7.5 years (range 1 month to 16 years). A freedom from event curve indicated that <50% of patients were free from thromboembolic event at 20-year follow-up (Fig. 4).
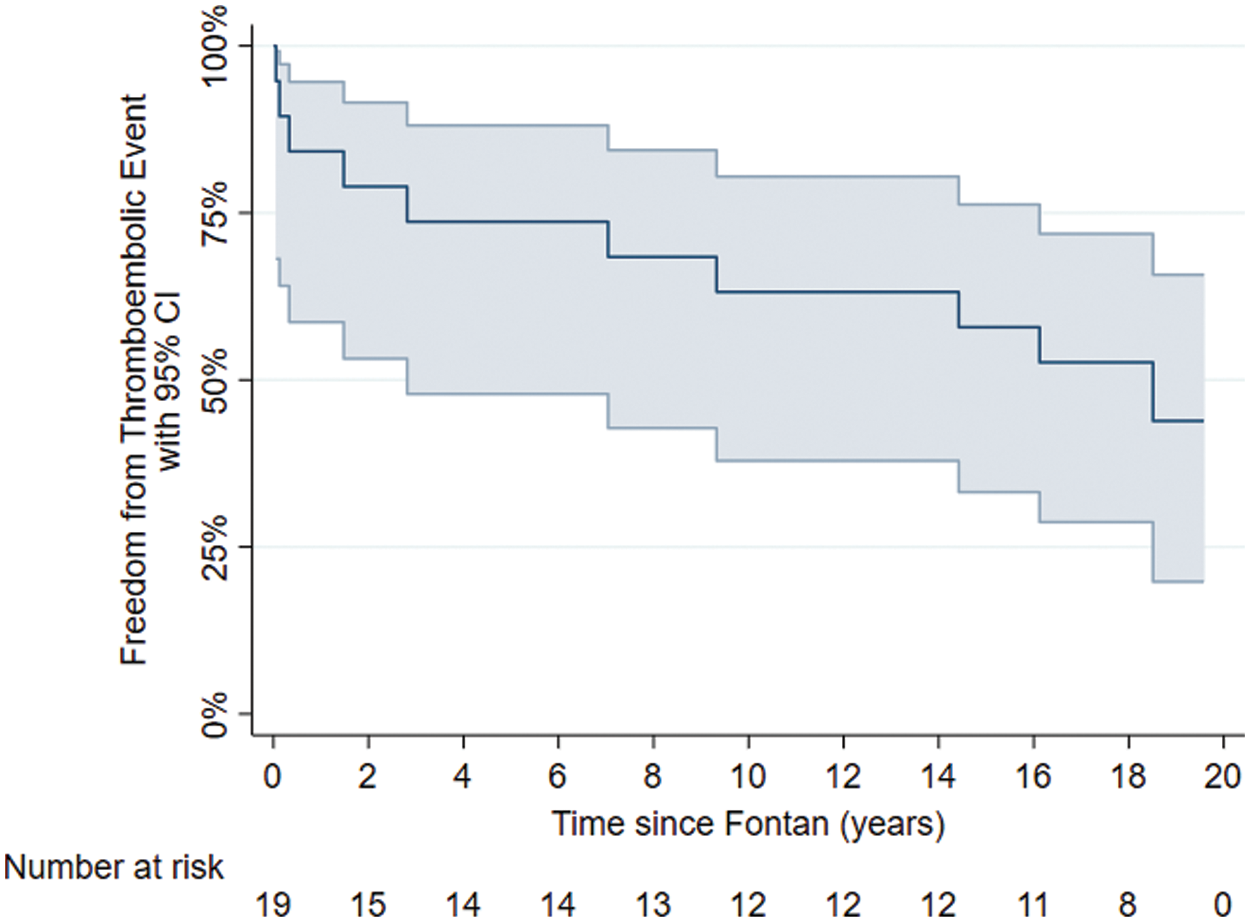
Figure 4: Freedom from thromboembolic events
In the original study of 37 patients, eight were found to have a factor VIII level >160% activity level [3]. One was lost to follow-up. Looking specifically at this group of seven patients, two of them had CVAs and one had intracardiac thrombus. This demonstrates an incidence of thrombotic event of 42.9% in patients with a factor VIII level >160%.
Treatment to prevent thrombus was at the discretions of each patient’s cardiologist and not according to a treatment protocol. Based on provider documentation for each patient clinical encounter, all of the patients in the study were compliant with their medications. At the last follow up, 13 of the 15 non-transplanted, living patients who were not lost to follow-up, were either receiving antiplatelet treatment with aspirin alone (n = 9), or combination with anticoagulation (2 aspirin and warfarin, and 2 aspirin and apixaban). One patient was on only warfarin and one patient was on apixaban only. Of the two patients who died, one was on aspirin and clopidogrel, and the other on aspirin, clopidogrel and warfarin (Fig. 5a). The anticoagulation treatment at the time in those patients who had a thrombotic event is shown in Fig. 5b.
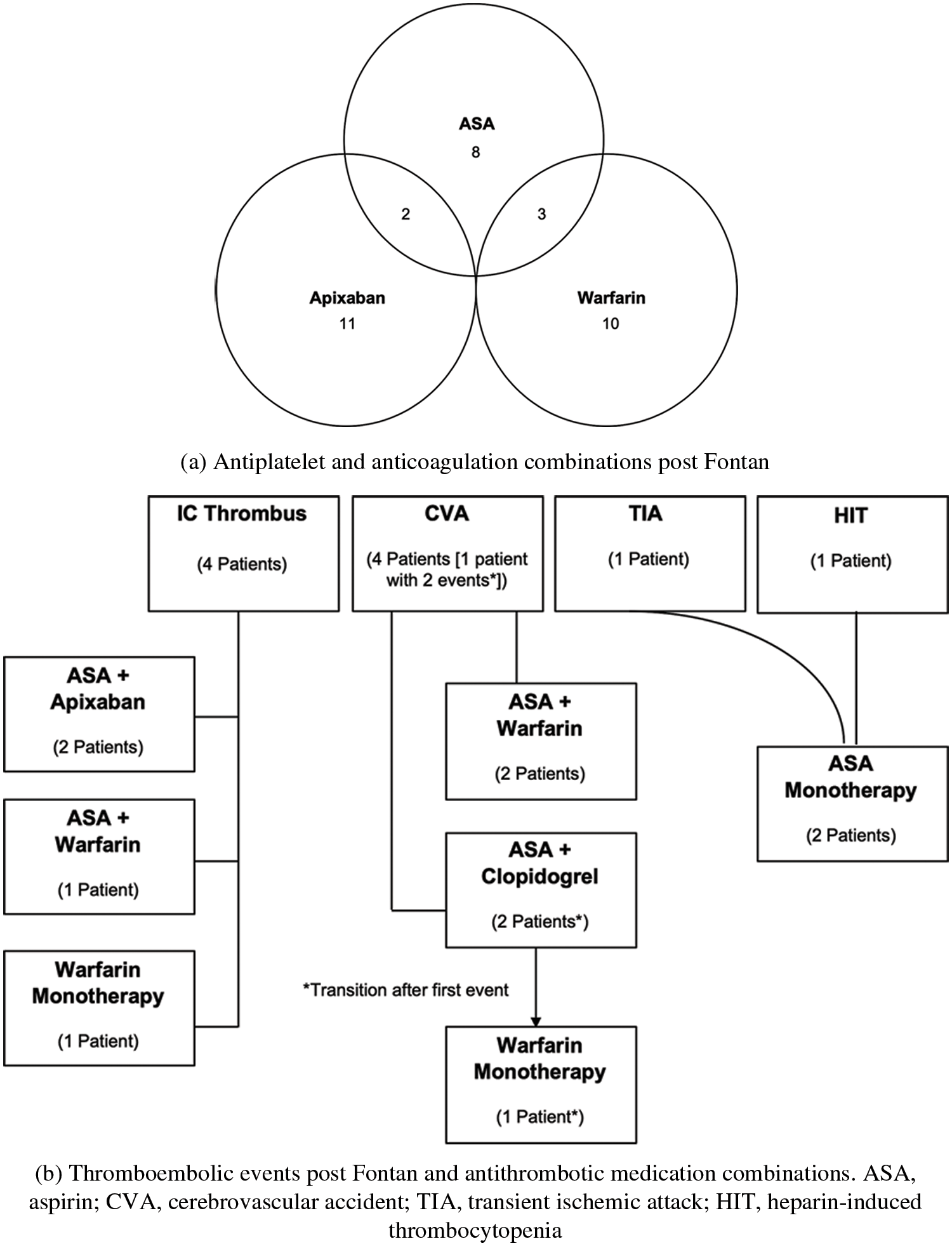
Figure 5: (a) Antiplatelet and anticoagulation combinations post Fontan, (b) Thromboembolic events post Fontan and antithrombotic medication combinations. ASA, aspirin; CVA, cerebrovascular accident; TIA, transient ischemic attack; HIT, heparin-induced thrombocytopenia
3.6 Functional and Neurologic Status
For the 15 non-transplanted, living patients who were not lost to follow-up, according to baseline functional level, thirteen would be classified as doing clinically well, NYHA Class I (86.7%). One patient has decreased exercise tolerance, NYHA Class II, and one has severe Fontan-related complications, NYHA Class IV. From a cognitive standpoint, eleven of the fifteen patients are eligible for (i.e., deferred college entry), attending or have finished higher education (73.3%) (college or university). Four out of the fifteen have developmental delay or behavioral and psychiatric issues (26.7%). Two of these four have had CVAs, one of which has more significant developmental delay and a seizure disorder. This patient had also suffered a prior CVA after the Stage I procedure. The other two of the four patients with developmental delay had intracardiac thrombus.
3.7 Fontan-Related Complications
Complications related to the Fontan procedure included PLE, plastic bronchitis, arrhythmia, pacemaker placement and FALD. The only patient with plastic bronchitis was transplanted. The two patients who died following their Fontan procedure had severe PLE. Of the remaining 15 patients, only one has severe PLE. One patient has had a significant arrhythmia burden requiring multiple electrophysiology studies and interventions. Four patients (26.7%) have permanent pacemakers, one of which needed conversion to cardiac resynchronization therapy (CRT).
FALD was present in fourteen of the fifteen patients remaining (93.3%). The one patient not diagnosed with FALD has not undergone specific imaging or testing. Overall, the degree of fibrosis was mild in most patients, with one patient having esophageal varices requiring periodic banding. Only one patient has had a liver biopsy. Liver function testing was available for twelve patients within two years of their last follow-up. In spite of the almost universal presence of FALD, laboratory testing was normal (Table 1).
Five out of the fifteen remaining patients had mild tricuspid regurgitation (TR). Three had mild-to-moderate TR. No patients had severe TR. Only three patients had mild and one patient had moderate right ventricular dysfunction. The remaining had either normal or low-normal function. Mild neo-aortic regurgitation was seen in only three patients.
In this follow-up to adulthood of a single-center cohort of patients with HLHS who were enrolled as newborns prior to Stage I palliation and followed through completion of the Fontan operation, 52.6% of patients experienced a thromboembolic event sometime after the Fontan operation. The intent at initial enrollment as newborns with HLHS was to describe the changes in anti- and pro-coagulants over the course of single ventricle palliation, and these studies reported significantly lower levels of both pro- and anti-coagulation factor levels when compared to age matched healthy controls, at all stages [3–6]. In some patients early after the Fontan procedure, a significant increase in factor VIII activity was noted which could be a potential pro-thrombotic risk [4], but on longer term follow up, these patients did not have a higher incidence of thrombus (42.9% incidence in the subgroup with previously elevated VIII activity vs. 66.6% in those who did not have an elevated VIII activity).
The short-term survival after the Fontan is reported as high as 95% [7]. However, for the specific patient population with HLHS, the longer-term outcomes indicate that only about 70% of patients survive to adulthood after the Fontan procedure [3]. In our initial cohort of 37 patients and excluding the 12 patients LTFU over the 20 years, transplant free survival of the Fontan circulation was 60% (15 of 25 patients). This cohort also experienced a number of known Fontan circulation–related complications in addition to the risk for thrombus. Three of 19 patients (15.8%) experienced severe PLE at some point since their Fontan operation. Of those patients with a Fontan circulation at the time of last follow up, 14 of 15 had FALD (93.3%), 5 of 15 patients (33.3%) had had an arrhythmia of some sort, 8 of 15 (53.3%) had at least mild TR and 4 of 14 (26.7%) had at least mild ventricular dysfunction. Despite this, we could classify 13 of 15 (86.7%) of patients with the Fontan circulation as NYHA functional Class I.
The risk for thrombus in our patient population is similar to the risk reported elsewhere. New information in this cohort indicated that the freedom from thromboembolic event was just under 50% at 20-year follow-up. It remains unclear how the risk for thrombus changes with age, and the potential contributing factors have been reported. These include the nature of the Fontan circulation with elevated central venous pressure and low flow states, and the potential for acquired pro-thrombotic states, such as with the development of FALD [8]. A recent study demonstrated progression to liver fibrosis in up to 43% of patients after thirty years with Fontan physiology [9]. The presence of subclinical liver alterations, often in the absence of elevated liver enzymes, are present from childhood [10,11] and in a series looking at liver biopsies from asymptomatic patients, changes were almost universally present [11,12]. There is no information relating FALD with changes in anticoagulation. Fourteen out of our fifteen patients in our follow-up group demonstrated some degree of liver pathology on imaging, including all 7 patients who had previously had an elevated Factor VIII activity. Out of the twelve patients in our cohort who had LFTs available, the values were normal in spite of coexisting pathology on imaging. While liver biopsy remains the gold standard for diagnosing FALD, and it has been reported that non-invasive imaging and cardiac catheterization data, specifically looking for Fontan baffle pressure elevation, may not correlate with liver biopsy findings [10]. There is no information relating changes in anti- and pro-coagulant activity or platelet function with liver biopsy findings, and this is an area for future investigation within our cohort.
With the elevated risk for thrombus after the Fontan operation, numerous articles have reported on strategies for managing anticoagulation and antiplatelet therapy. The American Heart Association Consensus Statement from 2019 [13] suggests that all patients post Fontan should receive antiplatelet therapy in the form of ASA, with the addition of anti-coagulation dependent upon individual risk factors for thrombosis. As noted in Figs. 5a and 5b, there were different strategies for anticoagulation and antiplatelet therapy in our cohort of patients, based on clinician preference predominantly. With the small cohort size on follow up and without laboratory testing, we were unable to determine whether a specific strategy was preferable.
Which medication, or combination of medications, to use in Fontan patients is debated [14,15]. While aspirin is commonly used, there is evidence for aspirin resistance, especially into adulthood [16]. In a multicenter retrospective study of Fontan patients from twelve North American Centers, Deshaies et al. [17] found that the risk of thromboembolic events was lower in those patients on antiplatelet therapy, and that anticoagulation therapy was not superior. Notably, one of the primary aims of this study by Deshaies was also to look at the differences in thromboembolic outcomes based on type of Fontan; atriopulmonary connection (APC), lateral tunnel (LT) and extracardiac (EC). APC was associated with the highest risk, and EC was superior to LT in terms of freedom from events [17]. In our study, all patients had a LT Fontan operation. There is limited information on the use of apixaban and other non-vitamin K antagonist oral anticoagulants (NOACs) in patients with congenital heart disease and are currently not recommended as first-line in the Fontan population [18]. Only two patients in our group were on apixaban.
The quality of life and psycho-social and emotional well-being are important longer-term outcomes [19]. Eleven out of the fifteen surviving patients in our cohort were either in college at follow-up or had the ability to go to college but had opted to wait. These eleven patients were noted to be physically active and independent. The remaining four patients had either significant developmental delay, as is the case for the two patients who suffered from CVAs. Two had milder developmental delay but difficulty with psychiatric and behavioral issues. These two patients also had complications related to their Fontan circulation, including PLE and arrhythmia. Similar to the Pediatric Heart Network Fontan Cohort [20], the large majority of our patients are in a somewhat ‘normal’ range for quality of life measures when compared to subjects free from CHD.
Over the course of our studies, 12 of the original cohort were LTFU to our institution because they lived out of state, and we have no information on whether these patients are alive or the current state of their Fontan circulation. Given the incidence of Fontan circulation-related complications in our cohort, it is important there be ongoing care and follow up, and as these patients reach adulthood, that there are seamless transitions of care.
There are limitations to this study, including that it is a retrospective chart review with a small sample size, which lead to limited ability to perform statistical analyses.
This retrospective review of a homogeneous cohort of patients with HLHS followed through staged palliations to adulthood shows the high incidence and risk for thrombus over time. Despite a high incidence of FALD, most patients had an acceptable quality of life and NYHA Class I functional status. Based on our review of the literature, we believe that our findings reflect what is seen in the general Fontan population. Given this, close surveillance of both FALD and changes in coagulation profiles over time is worthwhile for future investigation to potentially identify patients at higher risk and direct targeted therapy.
Authorship: All listed authors have substantially contributed to the manuscript and have approved the final submitted version. The authors confirm contribution to the paper as follows: study conception and design: Eleni P. Asimacopoulos and Kirsten C. Odegard; data collection: Eleni P. Asimacopoulos; analysis and interpretation of results: Eleni P. Asimacopoulos, Steven J. Staffa, Peter C. Laussen and Kirsten C. Odegard; draft manuscript preparation: Eleni P. Asimacopoulos, Steven J. Staffa, Peter C. Laussen and Kirsten C. Odegard. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study was approved by the Boston Children’s Hospital Institutional Review Board (Approval No. IRB-P00020852) and the patient consent was waived due to its retrospective nature.
Funding Statement: No separate funding was used in this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study. All co-authors have seen and agree with the contents of the manuscript.
References
1. Marshall, K. H., D’Udekem, Y., Sholler, G. F., Opotowsky, A. R., Costa, D. et al. (2020). Health-related quality of life in children, adolescents, and adults with a fontan circulation: A meta-analysis. Journal of the American Heart Association, 9(6), e014172. DOI 10.1161/JAHA.119.014172. [Google Scholar] [CrossRef]
2. Kutty, S., Jacobs, M. L., Thompson, W. R., Danford, D. A. (2020). Fontan circulation of the next generation: Why it’s necessary, what it might look like. Journal of the American Heart Association, 9(1), e013691. DOI 10.1161/JAHA.119.013691. [Google Scholar] [CrossRef]
3. Odegard, K. C., Zurakowski, D., DiNardo, J. A., Castro, R. A., McGowan, F. X. et al. (2009). Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through fontan completion. The Journal of Thoracic and Cardiovascular Surgery, 137(4), 934–941. DOI 10.1016/j.jtcvs.2008.09.031. [Google Scholar] [CrossRef]
4. Odegard, K. C., McGowan Jr, F. X., Zurakowski, D., Dinardo, J. A., Castro, R. A. et al. (2003). Procoagulant and anticoagulant factor abnormalities following the fontan procedure: Increased factor VIII may predispose to thrombosis. The Journal of Thoracic and Cardiovascular Surgery, 125(6), 1260–1267. DOI 10.1016/S0022-5223(02)73605-2. [Google Scholar] [CrossRef]
5. Odegard, K. C., McGowan Jr, F. X., Zurakowski, D., DiNardo, J. A., Castro, R. A. et al. (2002). Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the fontan procedure. The Annals of Thoracic Surgery, 73(6), 1770–1777. DOI 10.1016/S0003-4975(02)03580-4. [Google Scholar] [CrossRef]
6. Odegard, K. C., McGowan Jr, F. X., DiNardo, J. A., Castro, R. A., Zurakowski, D. et al. (2002). Coagulation abnormalities in patients with single-ventricle physiology precede the fontan procedure. The Journal of Thoracic and Cardiovascular Surgery, 123(3), 459–465. DOI 10.1067/mtc.2002.120010. [Google Scholar] [CrossRef]
7. Arunamata, A., Tacy, T. A., Kache, S., Mainwaring, R. D., Ma, M. et al. (2020). Recent outcomes of the extracardiac fontan procedure in patients with hypoplastic left heart syndrome. Annals of Pediatric Cardiology, 13(3), 186–193. DOI 10.4103/apc.APC_5_20. [Google Scholar] [CrossRef]
8. Firdouse, M., Agarwal, A., Chan, A. K., Mondal, T. (2014). Thrombosis and thromboembolic complications in fontan patients: A literature review. Clinical and Applied Thrombosis/Hemostasis, 20(5), 484–492. DOI 10.1177/1076029613520464. [Google Scholar] [CrossRef]
9. Pundi, K., Pundi, K. N., Kamath, P. S., Cetta, F., Li, Z. et al. (2016). Liver disease in patients after the Fontan operation. The American Journal of Cardiology, 117(3), 456–460. DOI 10.1016/j.amjcard.2015.11.014. [Google Scholar] [CrossRef]
10. Rathgeber, S. L., Guttman, O. R., Lee, A. F., Voss, C., Hemphill, N. M. et al. (2020). Fontan-associated liver disease: Spectrum of disease in children and adolescents. Journal of the American Heart Association, 9(1), e012529. DOI 10.1161/JAHA.119.012529. [Google Scholar] [CrossRef]
11. Gordon-Walker, T. T., Bove, K., Veldtman, G. (2019). Fontan-associated liver disease: A review. Journal of Cardiology, 74(3), 223–232. DOI 10.1016/j.jjcc.2019.02.016. [Google Scholar] [CrossRef]
12. Emamaullee, J., Zaidi, A. N., Schiano, T., Kahn, J., Valentino, P. L. et al. (2020). Fontan-associated liver disease: Screening, management, and transplant considerations. Circulation, 142(6), 591–604. DOI 10.1161/CIRCULATIONAHA.120.045597. [Google Scholar] [CrossRef]
13. Rychik, J., Atz, A. M., Celermajer, D. S., Deal, B. J., Gatzoulis, M. A. et al. (2019). Evaluation and management of the child and adult with fontan circulation: A scientific statement from the American Heart Association. Circulation, 140(6), e234–e284. DOI 10.1161/CIR.0000000000000696. [Google Scholar] [CrossRef]
14. Gnanappa, G. K., Celermajer, D. S., Sholler, G. F., Gentles, T., Winlaw, D. et al. (2017). The long-term management of children and adults with a fontan circulation: A systematic review and survey of current practice in Australia and New Zealand. Pediatric Cardiology, 38(1), 56–69. DOI 10.1007/s00246-016-1484-6. [Google Scholar] [CrossRef]
15. Viswanathan, S. (2016). Thromboembolism and anticoagulation after fontan surgery. Annals of Pediatric Cardiology, 9(3), 236–240. DOI 10.4103/0974-2069.189109. [Google Scholar] [CrossRef]
16. Chugh, R. (2019). The Fontan thromboprophylaxis dilemma: To give, or what not to give. Journal of the American College of Cardiology, 74(8), 1082–1085. DOI 10.1016/j.jacc.2019.07.021. [Google Scholar] [CrossRef]
17. Deshaies, C., Hamilton, R. M., Shohoudi, A., Trottier, H., Poirier, N. et al. (2019). Thromboembolic risk after atriopulmonary, lateral tunnel, and extracardiac conduit fontan surgery. Journal of the American College of Cardiology, 74(8), 1071–1081. DOI 10.1016/j.jacc.2019.06.051. [Google Scholar] [CrossRef]
18. Mongeon, F. P., Macle, L., Beauchesne, L. M., Bouma, B. J., Schwerzmann, M. et al. (2019). Non-vitamin K antagonist oral anticoagulants in adult congenital heart disease. The Canadian Journal of Cardiology, 35(12), 1686–1697. DOI 10.1016/j.cjca.2019.06.022. [Google Scholar] [CrossRef]
19. du Plessis, K., Peters, R., King, I., Robertson, K., Mackley, J. et al. (2018). “Will she live a long happy life?” parents’ concerns for their children with Fontan circulation. IJC Heart & Vasculature, 18, 65–70. DOI 10.1016/j.ijcha.2018.02.008. [Google Scholar] [CrossRef]
20. Atz, A. M., Zak, V., Mahony, L., Uzark, K., Shrader, P. et al. (2015). Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: The pediatric heart network fontan cohort. Congenital Heart Disease, 10(1), E30–E42. DOI 10.1111/chd.12193. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools