 Open Access
Open Access
ARTICLE
Preoperative Feeding in Single Ventricle Neonates is Predictive of Shorter Time to Goal Feed
1
Department of Cardiovascular Surgery, Children’s National Heart Institute, The George Washington University School of
Medicine, Washington DC, USA
2
Department of Cardiology, Children’s National Heart Institute, The George Washington University School of Medicine,
Washington DC, USA
3
Department of Biostatistics, Children’s National Heart Institute, The George Washington University School of Medicine,
Washington DC, USA
* Corresponding Author: Sarah Clauss. Email:
Congenital Heart Disease 2022, 17(5), 505-518. https://doi.org/10.32604/chd.2022.021571
Received 21 March 2022; Accepted 30 May 2022; Issue published 06 September 2022
Abstract
Background: Patients with single ventricle anatomy are at increased risk of growth failure and malnutrition. Amongst cardiac centers, there is little standardization of feeding practices in this complex population. We hypothesized that initiation of our center’s preoperative feeding protocol would result in decreased gastrostomy tube (G-tube) use, decreased length of stay and would not result in increased Necrotizing Enterocolitis (NEC) rates. Methods: A single institution review of 52 patients who had undergone stage I single ventricle palliative repair was performed. Patient diagnoses were hypoplastic left heart syndrome (39%), atrioventricular canal (15%), and other (46%). Postoperative parameters such as time to goal feed and need for gastrostomy tube (G-tube) were compared among preoperatively fed and non-preoperatively fed groups. Time to goal feed was calculated as time from first postoperative enteral feed to goal volume of 100 mL/kg. Results: Of the 26 patients who met inclusion criteria for preoperative feeding, 22 patients (85%) were fed prior to surgery. Cox proportional hazard ratio revealed that age at surgery (p = 0.047) and being preoperatively fed (p = 0.001) were associated with reaching goal feed sooner. Multivariable analysis revealed that being preoperatively fed made a patient twice as likely to reach goal feed sooner (p = 0.047). Univariable logistic regression revealed that days on total parenteral nutrition (p = 0.018), length of hospitalization (p = 0.008), and time to 1st postoperative feed (p = 0.020) were significantly associated with higher odds of needing a G-tube postoperatively. Multivariable logistic regression did not show any predictors of postoperative G-tube usage. However, there was a trend towards lower G-tube usage in the preoperatively fed group. Conclusions: Implementing a standardized preoperative feeding protocol in single ventricle neonates can result in significantly shorter time to goal feed in preoperatively fed patients. It is beneficial for institutions to begin implementing standard feeding protocols to improve nutrition and growth outcomes.Graphic Abstract
Keywords
Although outcomes after single ventricle palliation procedures have improved over the years, newborns with single ventricle physiology continue to experience morbidities related to growth failure and feeding challenges [1]. Patients with single ventricle physiology are known to experience growth failure and malnutrition, which in turn, increases risk of infection, mortality and extended hospital stays [2–4]. The identifiable variables contributing to poor growth and nutritional status include chronically low oxygen saturations, tachypnea, venous congestion, inadequate energy intake, increased metabolic demands, gastrointestinal pathology and genetic and extracardiac anomalies [3,5–7].
Initial reports from The National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) showed great variation in care practices for single ventricle physiologies among multiple institutions [8,9]. This variation prompted many centers to refine current nutritional practices. Today, there is still a lack of consensus on the best practices for feeding these high-risk patients [3]. Although feeding protocols have been shown to elicit positive outcomes, bringing these protocols into practice remains a challenge, and can be influenced by personal bias and subjectivity [10].
Prior to 2017, Children’s National Hospital did not preoperatively feed neonates with single ventricle heart disease. In addition, our historic rate of gastrostomy tube (G-tube) placement was as high as 60% among infants with hypoplastic left heart syndrome (HLHS), noting that, our center did not discharge single ventricle interstage neonates with nasogastric tubes. A recent assessment of the NPC-QIC dataset similarly noted that 57% of 944 analyzed patients required a feeding tube at hospital discharge [11]. An analysis from the Pediatric Cardiac Critical Care Consortium (PC4) noted only half of patients assessed received preoperative feeds and almost half were discharged home with a feeding tube [12]. Current literature indicates that early enteral feeding promotes intestinal maturation, postoperative feeding tolerance, hemodynamics, respiratory status, wound healing, and shorter length of stay [13].
We theorized that a neonate who possesses the skills to eat by mouth preoperatively may resume their oral feeding faster postoperatively than those who never have fed. This resumption of feeds could result in shorter length of stay and decreased need for a G-tube. This theory is supported by Pickler et al. who evaluated the effects of the feeding experience on clinical outcomes and noted that preterm neonates who were given more oral feeding experience while under intensive care made a faster transition to full oral feeds, regardless of illness severity [14].
We hypothesized that the initiation of our center’s preoperative feeding protocol would result in decreased G-tube use, decreased length of stay and would not result in increased necrotizing enterocolitis (NEC) rates. We sought to analyze the outcomes of our newly implemented preoperative feeding protocol and determine what patient characteristics were associated with preoperative feeding and G-tube use.
Data for this single-center review were obtained directly from medical records in a prospective manner. A total of 60 neonatal patients were identified to have single ventricle physiology between August 2017 and December 2020. Eight patients were excluded because they did not undergo stage I surgical palliation, leaving 52 patients to be reviewed. August 2017 was used as the starting point, as it marked the initiation of a new feeding protocol at Children’s National Hospital. Approval from our Institutional Review Board was obtained, and a waiver of consent was granted. The study was conducted under Protocol P00010359.
Patients were required to meet specific criteria to follow the pre and postoperative feeding protocol established by our hospital (Appendix A). The protocol design was based heavily on review of current literature, citing the Toms et al article which analyzed preoperative feeding specifically in neonates with HLHS [15]. In addition, 9 protocols from other medium to large volume cardiac surgery centers were reviewed over personal correspondence [16–24]. The proposed protocol was evaluated and accepted by cardiac surgical and intensive care leadership, with input from a core group of cardiologists, registered dietitians, and nurse practitioners. All single ventricle neonates who underwent stage I palliation including Norwood, Blalock-Taussig shunt, Hybrid, pulmonary artery band/bands, and PDA stent were prospectively enrolled. Exclusion criteria for preoperative feeding included gestational age less than 37 weeks, intrauterine growth restriction or weight less than 2.5 kg, inotrope administration, lactate greater than or equal to 2.5, pH less than 7.3, or prostaglandin administration of greater than 0.05 mcg/kg/min. Exclusion criteria for postoperative feeding included high dose inotropic support, (dopamine ≥ 5 mcg/kg/min, milrinone > 0.5 mcg/kg/min, any dose of epinephrine), lactate greater than or equal to 2.5, pH less than 7.3, open chest and/or previous history of NEC. These exclusion criteria were based on literature review and consensus among our core group [15,23]. When available, human milk was offered first with standard infant formula as the secondary alternative.
Patient baseline characteristics of preoperatively fed versus non-fed patients were analyzed. Demographic data included race and ethnicity, birth weight, gestational age, diagnosis, age at surgery, genetic and/or non-cardiac anomaly. Preoperative and intraoperative clinical data included ventricular function, cardiac arrest, need for extracorporeal membrane oxygenation (ECMO), prostaglandin dependence, age at surgery and surgery type. Postoperative clinical events were reported and included length of hospital stay, time to goal feed, vocal cord paralysis, diaphragm paralysis, arrhythmia, cardiac arrest, ECMO, G-tube placement, NEC, and mortality. NEC was categorized by all stages I–III as referenced by Lee et al in their modified version of Bell’s Staging Criteria [25,26]. Episodes of NEC that occurred at any time point before or after stage I palliation, but prior to death or stage II palliation, were included. Time to full feed was defined as time from date of first enteral feed to date that patient reached a goal volume of 100 mL/kg.
Parameters affecting the need for postoperative G-tube were analyzed and included demographic and preoperative clinical factors such as race and ethnicity, ability to have preoperative feed, and types of first preoperative feed, whether human milk or formula. Time to first preoperative feed was recorded as time to either first enteral feed or oral feed, whichever came first. Intraoperative and postoperative factors included age at surgery, type of surgery, time to goal feed, days on total parenteral nutrition (TPN), arrhythmia, length of hospital stay, presence of decreased ventricular function at discharge, time to first postoperative enteral and oral feed, and mortality. Qualitative assessment of ventricular function was determined by single echocardiogram reader (SC) and was recorded as either mild, moderate, or severely depressed.
Categorical variables were presented as frequency and percentage. Chi-squared test or Fisher’s exact test were used when comparing categorical variables. Continuous variables were presented as means ± standard deviation when normally distributed, and median with interquartile range (IQR) when skewed. Two sample t-test was used when comparing normally distributed continuous variables and the Mann-Whitney test was used when comparing skewed continuous variables. Univariable logistic regression and Cox proportional hazard modeling were performed to examine associations between anatomical and surgical parameters on feeding outcomes. Variables with p-value < 0.05 were selected to perform multivariable analysis. Kaplan–Meier curve was used to analyze time to goal feed in preoperatively vs non-fed groups. Overall, 13.5% of data points were missing and were removed from analysis. A p-value of <0.05 was considered significant. Other qualitative variables related to barriers and deviations to the feeding protocol were identified. Statistical Analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA)
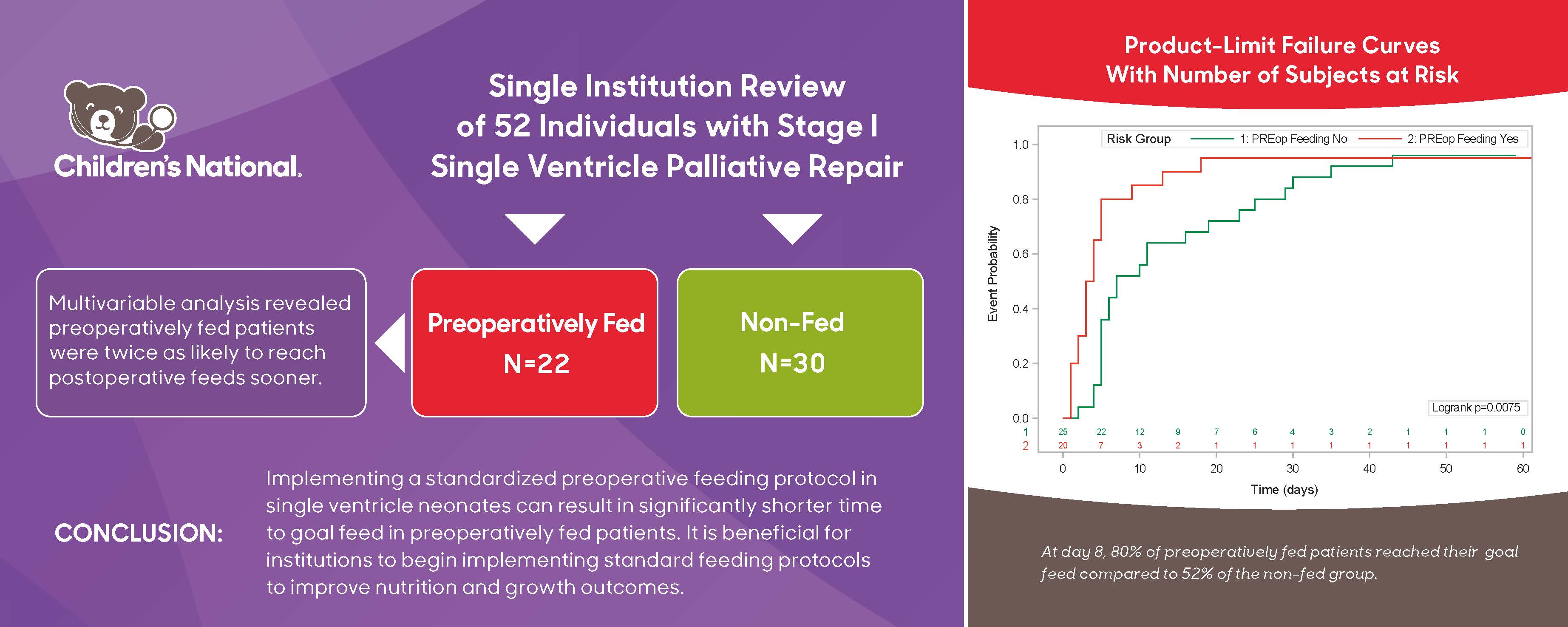
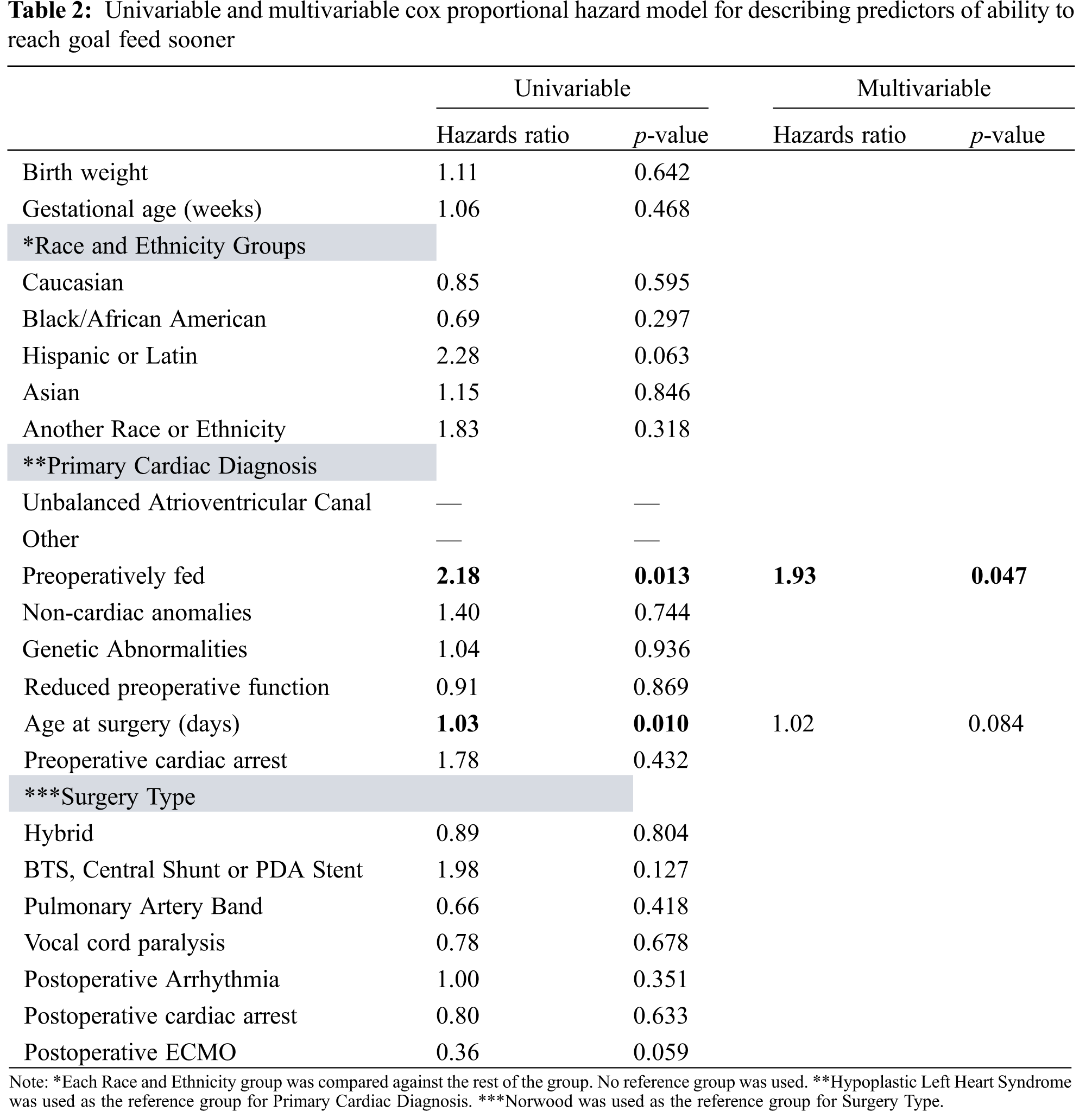
Among 52 patients with single ventricle lesions, 26 patients met all criteria for the institutional preoperative feeding protocol. Of the 26 who qualified, 22 patients were fed preoperatively. Those that deviated from the protocol were due to clinician preference (n = 2) as well as concern for clinical deterioration (n = 2). Table 1 summarizes baseline characteristics, including mortality, morphology, and surgical physiology, for all single ventricle patients. Overall, there were 20 HLHS, 8 unbalanced atrioventricular canal and 24 other diagnoses. Surgery types included 14 Norwood, 11 hybrid procedures, 18 shunts or PDA stents, and 9 pulmonary artery bands. Cardiac diagnosis (p = 0.005) and surgery type (p = 0.005) were both associated with being preoperatively fed. Of those in the preoperatively fed group, 6 (27%) were fed with human milk only, 9 (41%) were fed with formula only, and the remaining 7 (32%) were fed with both human milk and formula. All patients in the non-fed group were dependent on prostaglandin compared to 17 (77%) in the preoperatively fed group (p = 0.024). Mean age at surgery was greater in the preoperative fed group (p = 0.013). A shorter time to goal feed was observed in the preoperatively fed group (p = 0.001). By multivariable analysis, the sole predictor of reaching goal feed sooner was being preoperatively fed (Table 2).
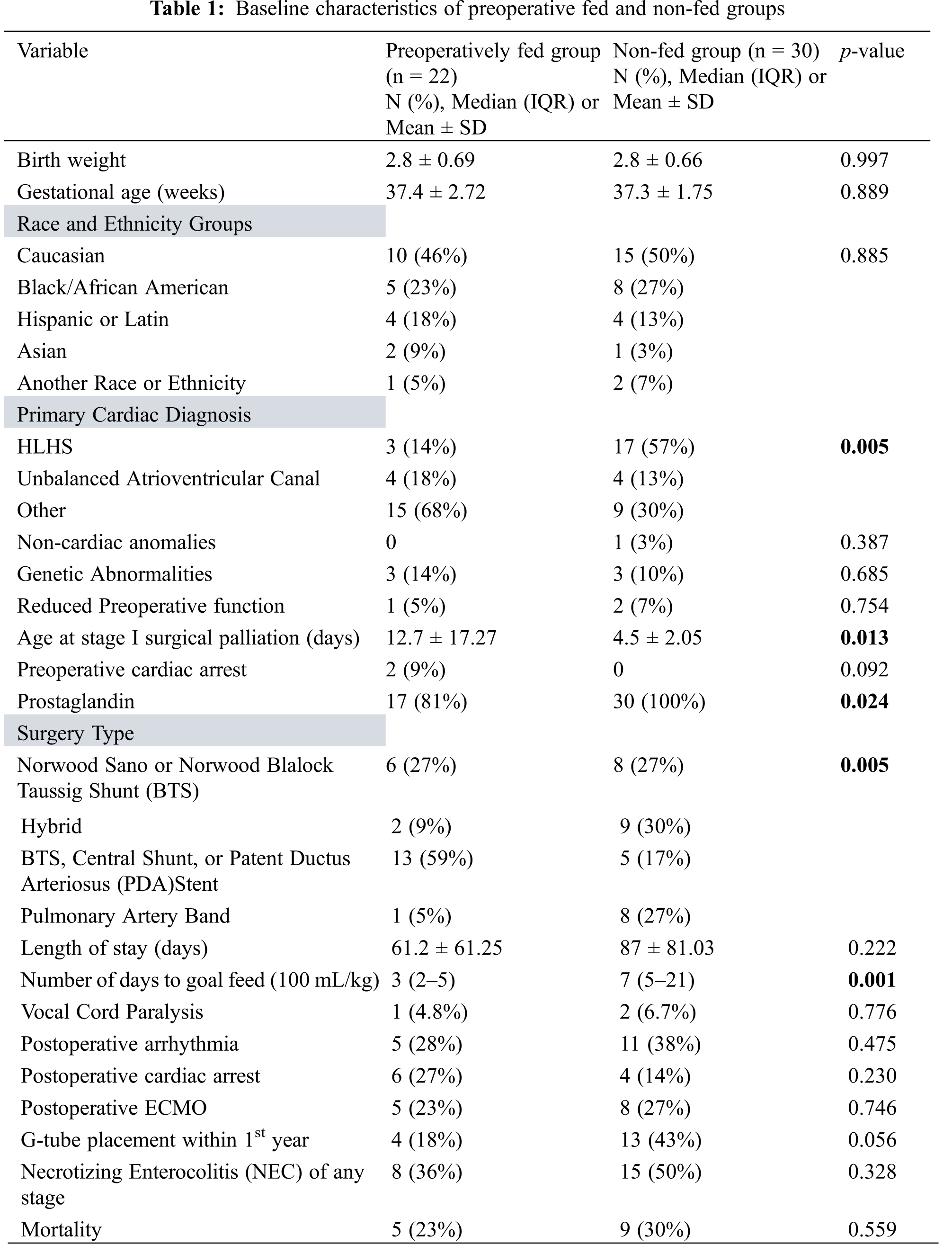
Throughout the hospital course, 15 of 29 patients (52%) in the non-fed group experienced at least one episode of NEC of any stage. Of these 15, only 2 had preoperative NEC, and both patients also had post-operative NEC. As categorized by modified Bell’s Staging Criteria, 8 out of these 15 patients had stage I NEC with a primary symptom of hematochezia, 4 had stage IIA, 2 experienced a combination of stage I and IIA episodes, and 1 patient experienced separate episodes of NEC stage I and IIB. Compared to the non-fed group, 8 of 22 patients (36%) in the preoperatively fed group experienced at least one episode of NEC of any stage (p = 0.328). Of those 8 patients, only 2 experienced preoperative NEC, and one of these patients went on to have 3 more episodes of postoperative NEC. Seven out of 8 patients had stage I NEC (isolated hematochezia), and one patient had stage IIA NEC. No patients in our review had stage III NEC.
A total of 13 (43%) patients in the non-fed group underwent G-tube placement vs. 4 (18%) patients in the preoperatively fed group (0.056). A total of 14 (27%) patients died postoperatively, 5 (23%) in the preoperatively fed group and 9 (30%) in the non-fed group (p = 0.559).
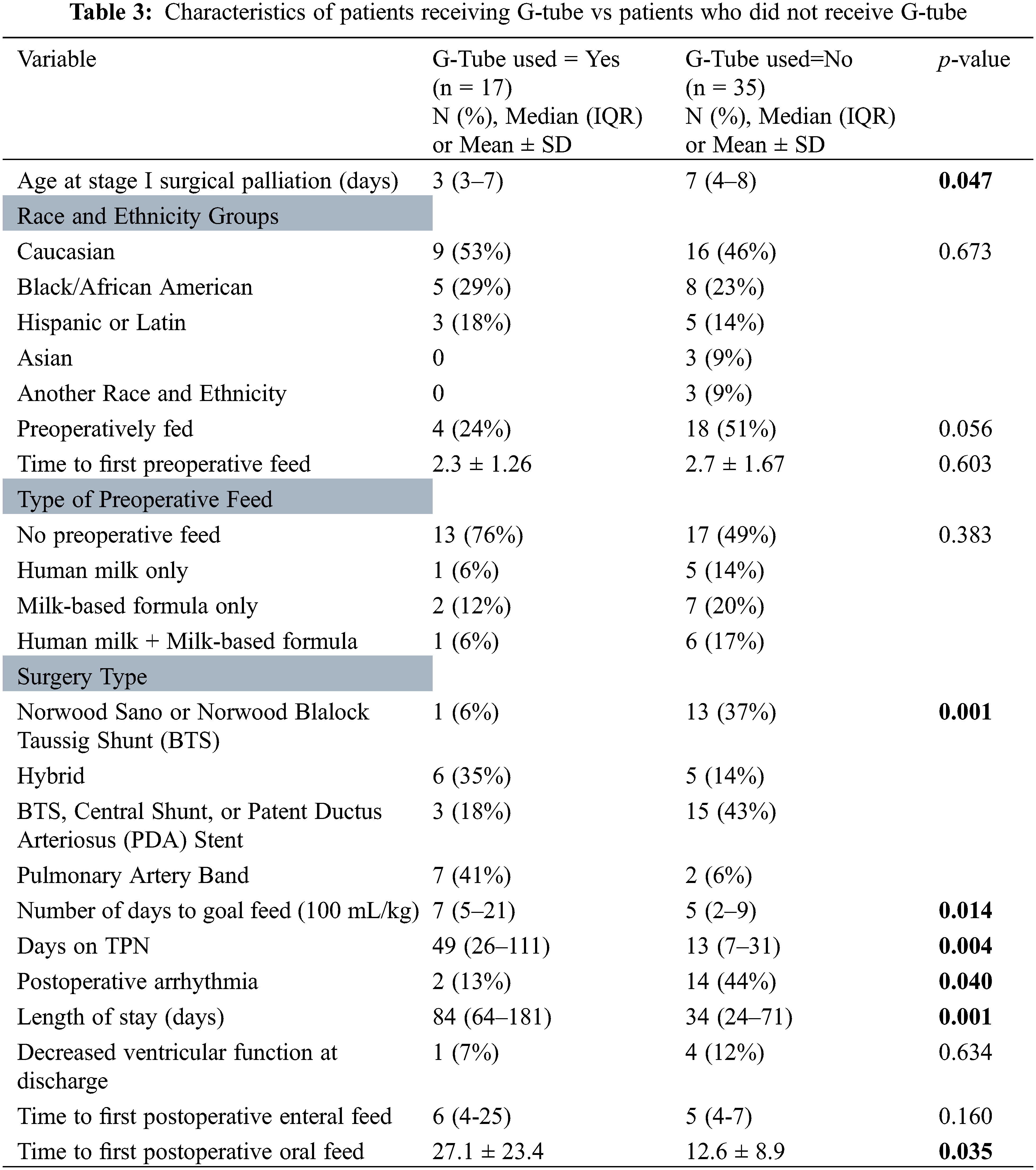
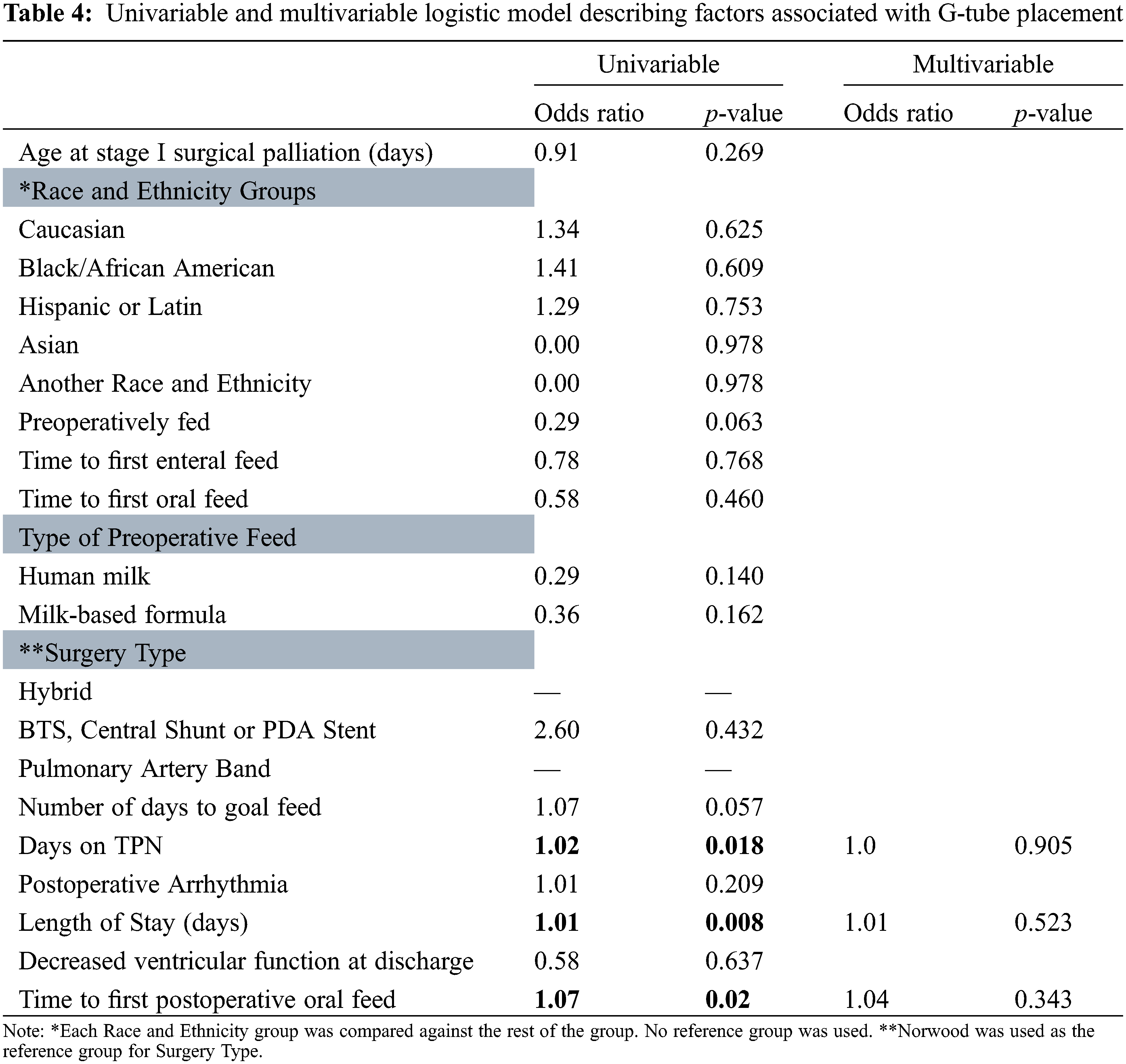
Table 3 characterizes the parameters predicting G-tube placement. Overall, there were 17 (33%) patients who required G-tube placement. Younger age at surgery, surgery type, longer amount of time to reach goal feed, longer amount of time on TPN, longer length of hospital stays, and longer time to reach first oral feed were all associated with requiring G-tube placement (p = 0.047, 0.001, 0.014, 0.004, 0.001, 0.035, respectively). Postoperative arrhythmia was associated with lower risk of needing a G-tube placed (p = 0.040). Of note, postoperative feeding routes included G-tube only (n = 4), Oral and G-tube (n = 10), Oral and NG tube (n = 1), Oral only (n = 26), and TPN (n = 11). Multivariable analysis revealed that there were not any independent predictors for placement of G-tube (Table 4).
A Kaplan Meier estimate of preoperatively fed patients reaching goal feed is illustrated in Fig. 1. At day 8, 80% of patients who were preoperatively fed had reached goal feed, compared to only 52% who were not preoperatively fed.
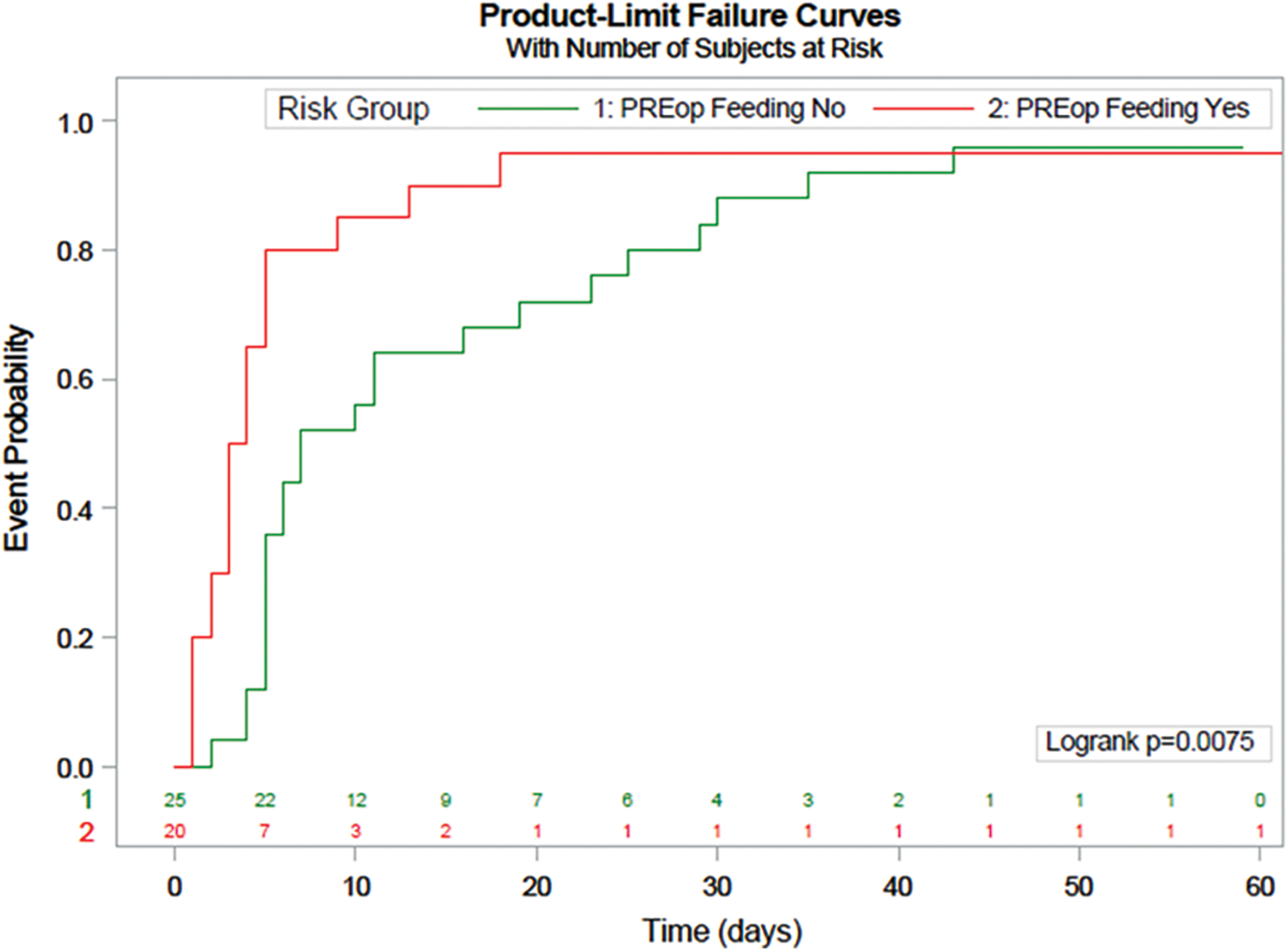
Figure 1: Kaplan Meier Estimate illustrating time it takes for patients to reach goal feed. Red line is preoperative fed group, green line is non-fed group
We found that the preoperative feeding of infants with single ventricle lesions was associated with significantly shorter times to goal feed. Although not significant, we also found lower median lengths of hospital stay and lower rates of G-Tube placement when patients were preoperatively fed. Amongst our patient population, 18% of preoperatively fed patients required a G-tube compared to 43% of those that were not preoperatively fed. Median length of stay was 61 days in the preoperatively fed group as opposed to 87 days in non-fed group, and these infants reached goal feeds at a faster rate when compared to their non-preoperatively fed counterparts. Length of stay may be affected by many different variables ranging from but not limited to a more complex postoperative course to potential complications of feeding advancement and tolerance.
Our study found that patients who were fed preoperatively exhibited no significant difference in NEC rates compared to patients who were not fed preoperatively. These results correlate with the study performed by Toms et al. which compared outcomes following preoperative feeds in neonates with HLHS [15]. Their group observed that those who were fed preoperative trophic feeds reached full feeds 8 days sooner than those not fed preoperatively, and they also did not find a difference in NEC rates between those who were fed and not fed preoperatively [15]. Other studies have shown that standardizing feeding protocols in patients with single ventricle conditions can result in a decreased rate of NEC [27]. These findings suggest that there is benefit associated with implementing preoperative feeding protocols in patients with single ventricle lesions.
In contrast, Beggs et al. showed no significant difference between the preoperative and non-fed groups in terms of length of hospital stay and time to full feed [28]. Kataria-Hale et al. also evaluated length of stay, feeding intolerance and NEC rates, and found no association with preoperative feeding [29]. These studies all include small sample sizes and observational methods, demonstrating the need for further studies [29].
Practitioners are often hesitant to initiate feeding due to concerns of preoperative NEC. While there is evidence of the benefits of early feeding in other neonatal populations, there is a clear lack of large studies in this specific population which further contributes to the apprehension to feed these infants. The Slicker et al review determined preoperative enteral feeding is safe for the infant with hypoplastic left heart syndrome who is hemodynamically stable with close monitoring [23]. It is important to note that delayed enteral feeding can contribute to cellular atrophy in the gut, pathologically abnormal increases in gut permeability, delayed postnatal intestinal development and maturation, and motility problems [30]. These complications increase the risk for bacterial translocation and impaired immune function and put the patient at risk for feeding intolerance, once feeds are initiated, by malabsorption of carbohydrates [23]. Early-fed infants have also been seen to tolerate full oral nutrition sooner, with fewer days of feeding intolerance, and shorter hospital stays [31].
Patients who are not able to be preoperatively fed are also at risk of delayed oral motor skills, further increasing the need for longer postoperative hospitalization, longer times to reach goal feed, and potentially increasing the risk of long-term dependence on tube feedings. While the recent study by Sagiv et al. did not find an association between preoperative feeding and an early achievement of tube-free feeding in the first year of life, they did find that preoperative feeding was safe and was not associated with an increase in NEC [11].
In those patients that do not meet the inclusion criteria as we described, it is worth evaluating different approaches to providing care for these non-fed patients, thus preventing negative outcomes. It is well understood that human milk contains anti-inflammatory and anti-microbial factors as well as cytokines that may promote the growth and development of the infant’s immune cells [32]. The practice of providing a mother’s own colostrum orally to the critically ill, preterm infant has been shown to be well tolerated and inexpensive with the potential to provide immunomodulatory protection [33]. The value of oropharyngeal colostrum immunotherapy has also been shown to reduce the number of days to achieve full enteral nutrition and may also be significantly beneficial when used in lieu of preoperative feeding [34]. One study by Coker-Bolt et al. found that patients with single ventricle circulation who were enrolled into an oral motor stimulation program had shorter lengths of hospitalization following cardiac surgery, and experienced a quicker time to full feed [35]. While Indramohan et al. [36] did not find a statistically significant difference in infants who received oral motor exercises on tube feeding at discharge, there was a clinical impact on hospital length of stay and tube feeding requirement. In addition to oral immune therapy, there are other non-nutritive practices that may provide benefits to the infant. It is suggested that simply holding the infant in breast-feeding position while the child sucks on a pacifier can create a nurturing feeding environment that will increase oral stimulation patterns and will help the child to indirectly develop feeding skills [37]. These studies provide future directions for research in complex patients who do not meet the inclusion criteria for preoperative feeding.
This study was limited by the relatively small, single-center sample size. A smaller sample size limited our ability to find correlations between some parameters and therefore they were not estimated due to quasi separation. The logistic model that was used to describe factors associated with G-tube placement had many predictors, which may have been another statistical limitation. This was an observational study, and therefore causal relationships cannot be made. The observational nature of the study also lends the possibility of inherent limitations related to missing data and data collection errors, either as a result of errors in actual data entry or the inconsistencies between multiple data coordinators pulling data from medical charts. Also, those in the non-fed group may have already been sicker and therefore excluded for preoperative feeding, although the parameters for exclusion seemed comparable among the preoperatively fed and non-fed groups. We would also note that although there were few deviations from the feeding protocol, they ultimately were a result of clinician preference.
Our center did see a reduction in G-Tube placement, decreased length of stay, and a significantly faster time to goal feed in the preoperatively fed group. There was no statistical difference in the NEC rates between the two groups. These findings support that with appropriate exclusion criteria, a preoperative feeding protocol can be safely initiated in neonates with complex single ventricle anatomy.
Authorship: All listed authors should have substantially contributed to the manuscript and have approved the final submitted version, which should include a description of each author’s specific work and contributorship. The authors confirm contribution to the paper as follows: study conception and design: S. Clauss, Y. d’Udekem; data collection: K. Kalinger, S. Davis; analysis and interpretation of results: J. Gai, A. Venna, Y. d’Udekem, S. Clauss; draft manuscript preparation: A. Venna, K. Kalinger, S. Davis. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: De-identified data will be available through email request to the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: Yves d’Udekem is a consultant with Actelion Pharmaceuticals. The other authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Cross, R. R., Harahsheh, A. S., McCarter, R., Martin, G. R. (2014). Identified mortality risk factors associated with presentation, initial hospitalisation, and interstage period for the Norwood operation in a multi-centre registry: A report from the National Pediatric Cardiology-Quality Improvement Collaborative. Cardiology in the Young, 24(2), 253–262. DOI 10.1017/S1047951113000127. [Google Scholar] [CrossRef]
2. Ghanayem, N. S., Allen, K. R., Tabbutt, S., Atz, A. M., Clabby, M. L. et al. (2012). Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. Journal of Thoracic and Cardiovascular Surgery, 144(4), 896–906. DOI 10.1016/j.jtcvs.2012.05.020. [Google Scholar] [CrossRef]
3. Clauss, S. B., Anderson, J. B., Lannon, C., Lihn, S., Beekman, R. H. et al. (2015). Quality improvement through collaboration: The national pediatric quality improvement collaborative initiative. Current Opinion in Pediatrics, 27(5), 555–562. DOI 10.1097/MOP.0000000000000263. [Google Scholar] [CrossRef]
4. Sano, S., Huang, S. C., Kasahara, S., Yoshizumi, K., Kotani, Y. et al. (2009). Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. Annals of Thoracic Surgery, 87(1), 178–186. DOI 10.1016/j.athoracsur.2008.08.027. [Google Scholar] [CrossRef]
5. Gaynor, J. W., Mahle, W. T., Cohen, M. I., Ittenbach, R. F., DeCampli, W. M. et al. (2002). Risk factors for mortality after the Norwood procedure. European Journal of Cardio-Thoracic Surgery, 22(1), 82–89. DOI 10.1016/S1010-7940(02)00198-7. [Google Scholar] [CrossRef]
6. Hehir, D. A., Cooper, D. S., Walters, E. M., Ghanayem, N. S. (2011). Feeding, growth, nutrition, and optimal interstage surveillance for infants with hypoplastic left heart syndrome. Cardiology in the Young, 21(S2), 59–64. DOI 10.1017/S1047951111001600. [Google Scholar] [CrossRef]
7. Rudd, N. A., Ghanayem, N. S., Hill, G. D., Lambert, L. M., Mussatto, K. A. et al. (2020). Interstage home monitoring for infants with single ventricle heart disease: Education and management: A scientific statement from the american heart association. Journal of the American Heart Association, 9(16), e014548. DOI 10.1161/JAHA.119.014548. [Google Scholar] [CrossRef]
8. Schidlow, D. N., Anderson, J. B., Klitzner, T. S., Beekman, R. H.3rd, Jenkins, K. J. et al. (2011). Variation in interstage outpatient care after the Norwood procedure: A report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenital Heart Disease, 6(2), 98–107. DOI 10.1111/j.1747-0803.2011.00509.x. [Google Scholar] [CrossRef]
9. Anderson, J. B., Iyer, S. B., Schidlow, D. N., Williams, R., Varadarajan, K. et al. (2012). Variation in growth of infants with a single ventricle. Journal of Pediatrics, 161(1), 16–21.e2103. DOI 10.1016/j.jpeds.2012.01.009. [Google Scholar] [CrossRef]
10. Furlong-Dillard, J., Neary, A., Marietta, J., Jones, C., Jeffers, G. et al. (2018). Evaluating the impact of a feeding protocol in neonates before and after biventricular cardiac surgery. Pediatric Quality & Safety, 3(3), e080. DOI 10.1097/pq9.0000000000000080. [Google Scholar] [CrossRef]
11. Sagiv, E., Tjoeng, Y. L., Davis, M., Keenan, E., Fogel, J. et al. (2022). Assessing the association between pre-operative feeding and the development of oral feeding skills in infants with single ventricle heart disease: An analysis of the NPC-QIC dataset. Pediatric Cardiology, 43(5), 1141–1155. DOI 10.1007/s00246-022-02837-9. [Google Scholar] [CrossRef]
12. Alten, J. A., Rhodes, L. A., Tabbutt, S., Cooper, D. S., Graham, E. M. et al. (2015). Perioperative feeding management of neonates with CHD: Analysis of the pediatric cardiac critical care consortium (PC4) registry. Cardiology in the Young, 25(8), 1593–1601. DOI 10.1017/S1047951115002474. [Google Scholar] [CrossRef]
13. Martini, S., Beghetti, I., Annunziata, M., Aceti, A., Galletti, S. et al. (2021). Enteral nutrition in term infants with congenital heart disease: Knowledge gaps and future directions to improve clinical practice. Nutrients, 13(3), 932. DOI 10.3390/nu13030932. [Google Scholar] [CrossRef]
14. Pickler, R. H., Best, A., Crosson, D. (2009). The effect of feeding experience on clinical outcomes in preterm infants. Journal of Perinatology, 29(2), 124–129. DOI 10.1038/jp.2008.140. [Google Scholar] [CrossRef]
15. Toms, R., Jackson, K. W., Dabal, R. J., Reebals, C. H., Alten, J. A. (2015). Preoperative trophic feeds in neonates with hypoplastic left heart syndrome. Congenital Heart Disease, 10(1), 36–42. DOI 10.1111/chd.12177. [Google Scholar] [CrossRef]
16. Braudis, N. J., Curley, M. A., Beaupre, K., Thomas, K. C., Hardiman, G. et al. (2009). Enteral feeding algorithm for infants with hypoplastic left heart syndrome poststage I palliation. Pediatric Critical Care Medicine, 10(4), 460–466. DOI 10.1097/PCC.0b013e318198b167. [Google Scholar] [CrossRef]
17. Becker, K. C., Hornik, C. P., Cotten, C. M., Clark, R. H., Hill, K. D. et al. (2015). Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. American Journal of Perinatology, 32(7), 633–638. DOI 10.1055/s-00000009. [Google Scholar] [CrossRef]
18. del Castillo, S. L., McCulley, M. E., Khemani, R. G., Jeffries, H. E., Thomas, D. W. et al. (2010). Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatric Critical Care Medicine, 11(3), 373–377. [Google Scholar]
19. Donnellan, A., Justice, L. (2016). Preoperative stabilization of infants with hypoplastic left heart syndrome before stage I palliation. Critical Care Nurse, 36(1), 52–59. DOI 10.4037/ccn2016461. [Google Scholar] [CrossRef]
20. Golbus, J. R., Wojcik, B. M., Charpie, J. R., Hirsch, J. C. (2011). Feeding complications in hypoplastic left heart syndrome after the Norwood procedure: A systematic review of the literature. Pediatric Cardiology, 32(4), 539–552. DOI 10.1007/s00246-011-9907-x. [Google Scholar] [CrossRef]
21. Jeffries, H. E., Wells, W. J., Starnes, V. A., Wetzel, R. C., Moromisato, D. Y. (2006). Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Annals of Thoracic Surgery, 81(3), 982–987. DOI 10.1016/j.athoracsur.2005.09.001. [Google Scholar] [CrossRef]
22. Jenkins, E. (2015). Feeding protocols for neonates with hypoplastic left heart syndrome: A review. AACN Advanced Critical Care, 26(3), 215–221. DOI 10.4037/NCI.0000000000000096. [Google Scholar] [CrossRef]
23. Slicker, J., Hehir, D. A., Horsley, M., Monczka, J., Stern, K. W. et al. (2013). Nutrition algorithms for infants with hypoplastic left heart syndrome; birth through the first interstage period. Congenital Heart Disease, 8(2), 89–102. DOI 10.1111/j.1747-0803.2012.00705.x. [Google Scholar] [CrossRef]
24. Willis, L., Thureen, P., Kaufman, J., Wymore, E., Skillman, H. et al. (2008). Enteral feeding in prostaglandin-dependent neonates: Is it a safe practice? Journal of Pediatrics, 153(6), 867–869. DOI 10.1016/j.jpeds.2008.04.074. [Google Scholar] [CrossRef]
25. Lee, J. S., Polin, R. A. (2003). Treatment and prevention of necrotizing enterocolitis. Seminars in Neonatology, 8(6), 449–459. DOI 10.1016/S1084-2756(03)00123-4. [Google Scholar] [CrossRef]
26. Gregory, K. E., Deforge, C. E., Natale, K. M., Phillips, M., van Marter, L. J. (2011). Necrotizing enterocolitis in the premature infant: neonatal nursing assessment, disease pathogenesis, and clinical presentation. Advances in Neonatal Care, 11(3), 155–166. DOI 10.1097/ANC.0b013e31821baaf4. [Google Scholar] [CrossRef]
27. Carpenito, K. R., Prusinski, R., Kirchner, K., Simsic, J., Miao, Y. et al. (2016). Results of a feeding protocol in patients undergoing the hybrid procedure. Pediatric Cardiology, 37(5), 852–859. DOI 10.1007/s00246-016-1359-x. [Google Scholar] [CrossRef]
28. Beggs, M. R., Joynt, C., Phillipos, E., Garcia Guerra, G., Larsen, B. M. K. (2015). Feeding practices and outcomes of infants undergoing the norwood procedure. Childhood Obesity and Nutrition, 7(6), 347–354. DOI 10.1177/1941406415615556. [Google Scholar] [CrossRef]
29. Kataria-Hale, J., Osborne, S. W., Hair, A., Hagan, J., Pammi, M. (2019). Preoperative feeds in ductal-dependent cardiac disease: A systematic review and meta-analysis. Hospital Pediatrics, 9(12), 998–1006. DOI 10.1542/hpeds.2019-0111. [Google Scholar] [CrossRef]
30. Neu, J. (2007). Gastrointestinal development and meeting the nutritional needs of premature infants. American Journal of Clinical Nutrition, 85(2), 629S–634S. DOI 10.1093/ajcn/85.2.629S. [Google Scholar] [CrossRef]
31. Berseth, C. L. (1992). Effect of early feeding on maturation of the preterm infant’s small intestine. Journal of Pediatrics, 120(6), 947–953. DOI 10.1016/S0022-3476(05)81969-9. [Google Scholar] [CrossRef]
32. Rodriguez, N. A., Meier, P. P., Groer, M. W., Zeller, J. M. (2009). Oropharyngeal administration of colostrum to extremely low birth weight infants: Theoretical perspectives. Journal of Perinatology, 29(1), 1–7. DOI 10.1038/jp.2008.130. [Google Scholar] [CrossRef]
33. Spatz, D. L. (2012). Innovations in the provision of human milk and breastfeeding for infants requiring intensive care. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 41(1), 138–143. DOI 10.1111/j.1552-6909.2011.01315.x. [Google Scholar] [CrossRef]
34. Xavier Ramos, M. S., Martins, C., Souza, E. S., Vieira, G. O., Gomes-Filho, I. S. et al. (2021). Oropharyngeal colostrum immunotherapy and nutrition in preterm newborns: Meta-analysis. Revista de Saude Publica, 55, 59. DOI 10.11606/s1518-8787.2021055003051. [Google Scholar] [CrossRef]
35. Coker-Bolt, P., Jarrard, C., Woodard, F., Merrill, P. (2013). The effects of oral motor stimulation on feeding behaviors of infants born with univentricle anatomy. Journal of Pediatric Nursing, 28(1), 64–71. DOI 10.1016/j.pedn.2012.03.024. [Google Scholar] [CrossRef]
36. Indramohan, G., Pedigo, T. P., Rostoker, N., Cambare, M., Grogan, T. et al. (2017). Identification of risk factors for poor feeding in infants with congenital heart disease and a novel approach to improve oral feeding. Journal of Pediatric Nursing, 35, 149–154. DOI 10.1016/j.pedn.2017.01.009. [Google Scholar] [CrossRef]
37. Butler, S. C., Huyler, K., Kaza, A., Rachwal, C. (2017). Filling a significant gap in the cardiac ICU: Implementation of individualised developmental care. Cardiology in the Young, 27(9), 1797–1806. DOI 10.1017/S1047951117001469. [Google Scholar] [CrossRef]
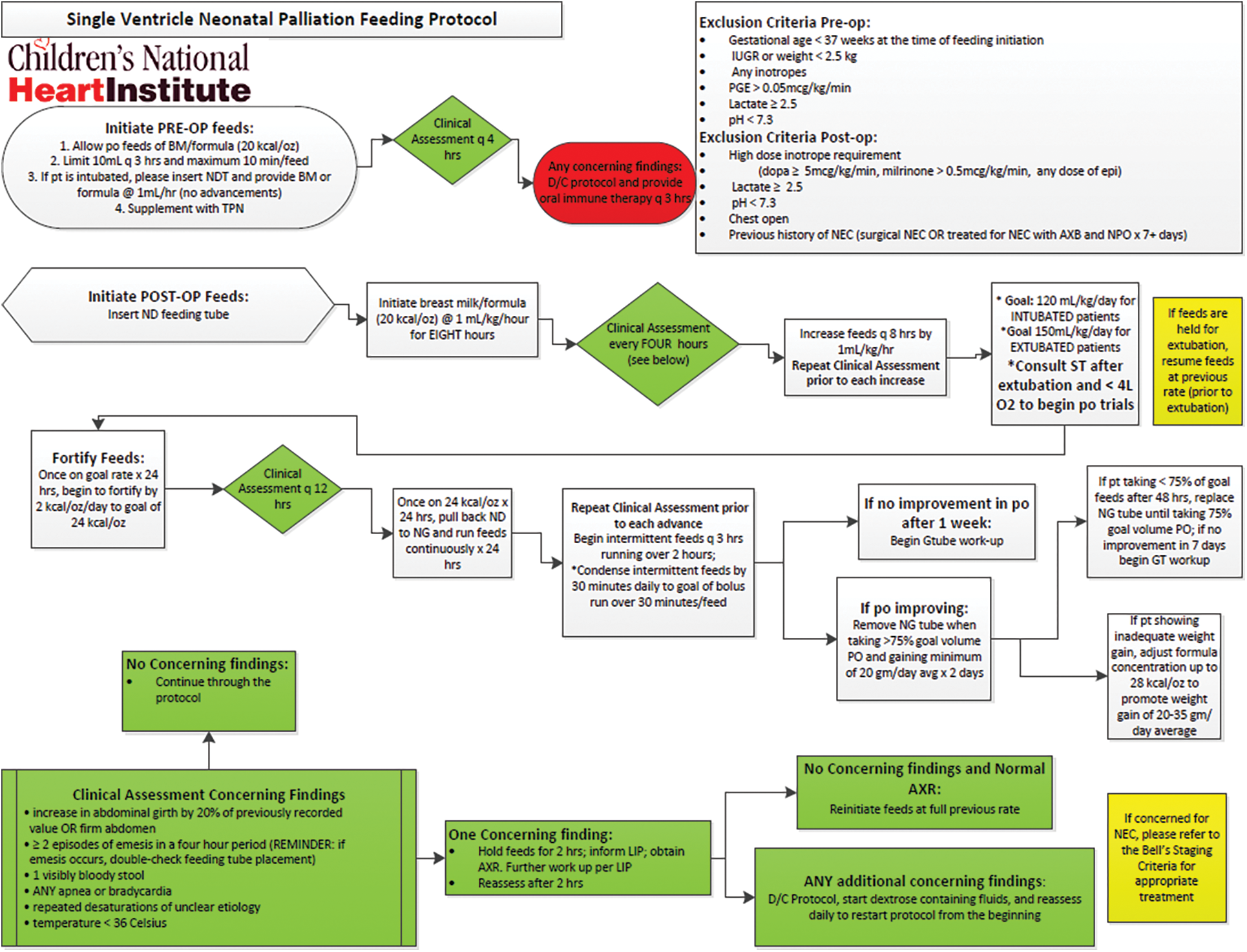
Appendix A: Single Ventricle Neonatal Palliation Feeding Protocol that has been used at Children’s National Hospital since 2017
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools