 Open Access
Open Access
CASE REPORT
A Rare Case of Transcatheter Closure of Both Inlet and Outlet of a Left Coronary Artery-to-Left Ventricular Fistula with Giant Coronary Artery Aneurysm
1
Department of Pediatric Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong
Academy of Medical Sciences, Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, China
2
Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong
Academy of Medical Sciences, Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, China
* Corresponding Author: Zhiwei Zhang. Email:
(This article belongs to the Special Issue: Nightmare Case Reports in Congenital Heart Disease)
Congenital Heart Disease 2022, 17(5), 541-549. https://doi.org/10.32604/chd.2022.024907
Received 13 June 2022; Accepted 08 July 2022; Issue published 06 September 2022
Abstract
A congenital coronary artery fistula (CCAF) combined with giant coronary aneurysm (CAA) is a rare congenital cardiac abnormality. We reported an 8-year-old patient who underwent transcatheter closure of both inlet and outlet of a proximal left coronary artery (LCA)-to-left ventricular (LV) fistula with CAA of 41 mm × 28 mm in diameter, during which acute occlusion of left anterior descending coronary artery (LAD) occurred immediately after device implantation at the inlet of fistula. We managed to prevent the patient from major adverse cardiac events by conservative therapy with dual antiplatelet agents instead of surgical removal of the device. The patient recovered well and had been follow-up for 2 years with no late complications reported.Graphic Abstract
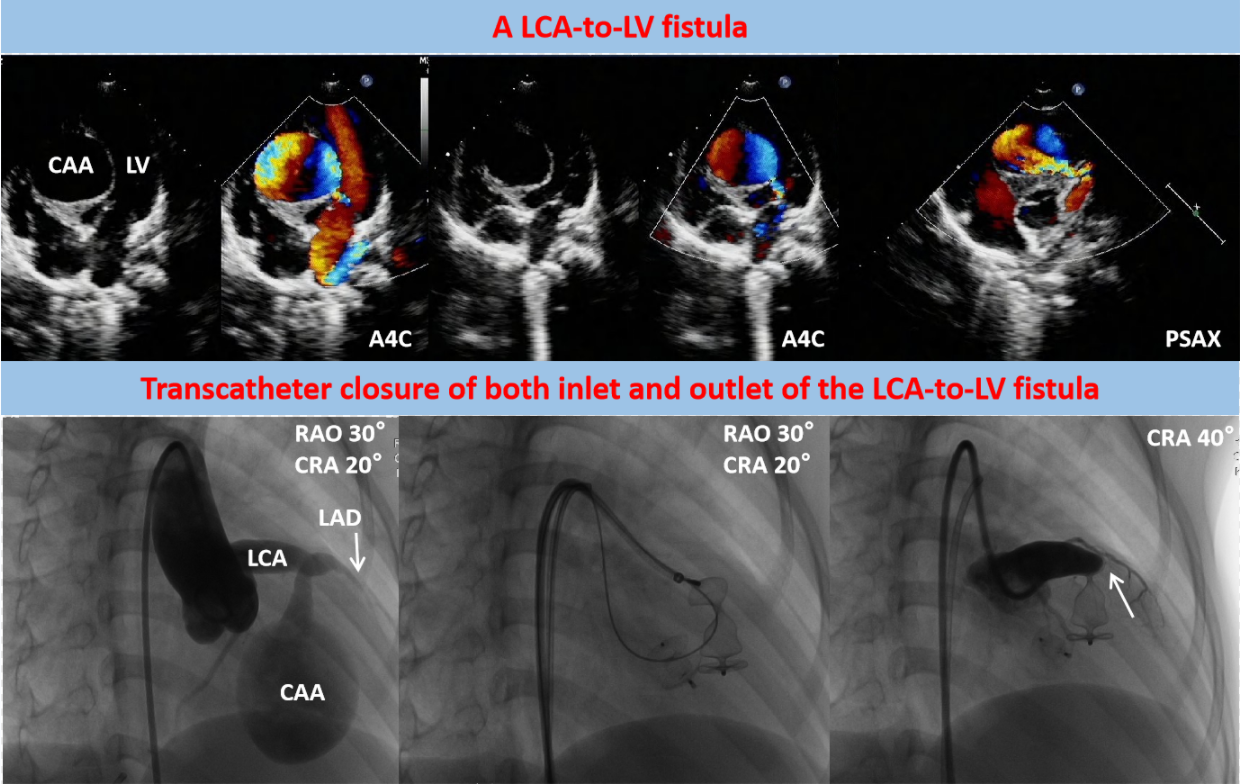
Keywords
A congenital coronary artery fistula (CCAF) is a rare cardiac malformation, accounting for 0.3% of patients with congenital heart disease and 0.06% of children undergoing echocardiography [1]. A giant coronary artery aneurysm (CAA), defined as an aneurysm larger than 20 mm, is an extremely uncommon disease, with reported prevalence of 5.9% in CCAFs [2]. CCAFs are usually asymptomatic early in life, especially when they are hemodynamically small shunts. Patients with large left-to-right shunts may develop congestive cardiac failure in infancy and occasionally in the neonatal period [3]. In older patients, symptoms may present such as dyspnea, angina of effort and occasionally arrhythmias [4]. Treatment is often advocated to reduce the risk of complications, including myocardial ischemia, infective endocarditis, thrombus formation, congestive heart failure, atrial and ventricular arrhythmias, and cardiac tamponade due to spontaneous rupture of a CAA [3–5].
Transcatheter intervention has been considered as an alternative therapy for a CCAF in recent years [6]. However, reported clinical experience regarding transcatheter closure of a CCAF with giant CAA have been very limited [7–9]. We present in this article, a rare case of transcatheter closure of both inlet and outlet of a proximal left coronary artery (LCA)-to-left ventricular (LV) fistula with giant CAA (41 mm × 28 mm in diameter). Although acute occlusion of left anterior descending coronary artery (LAD) occurred immediately after device implantation at the inlet of fistula, conservative treatment with dual antiplatelet agents for this challenging complication was successful, and the patient recovered well without any complications.
An 8-year-old girl (weight 16.5 kg) was referred to our department because of discovery of continuous cardiac murmur. On admission, her temperature was 36.8°C, and her blood pressure was 100/55 mmHg. Her heart rate was 90 bpm. Oxygen saturation was 100%. 3/VI continuous murmur could be heard at the 4th–5th intercostal space near the left sternal border. Electrocardiography (ECG) showed normal sinus rhythm.
Transthoracic echocardiography (TTE) revealed a severe dilation of the LCA ostium with a giant aneurysmal coronary artery located above the intraventricular septum (IVS) (Figs. 1A and 1B). The coronary artery was draining into LV cavity through an orifice with diameter of 4.0 mm. Color Doppler flow imaging detected a continuous double-phase turbulent spectrum at the orifice, suggesting the presence of an LCA-to-LV fistula (Figs. 1C and 1D). Since no sign of left ventricular overload was present, the shunt was considered small degree. Three-dimensional computed tomography (3D-CT) scan confirmed that the fistula was proximal type, originating from anterior interventricular branch of LCA and draining into LV cavity with giant CAA of 41 mm × 28 mm in diameter (Figs. 2A and 2B). Compression of both LV and right ventricular outflow tract (RVOT) by the giant CAA was seen (Figs. 2C and 2D). If left untreated, the patient would be in danger of aneurysm rupture, thrombus formation in the CAA, and heart failure. Taking all these factors into consideration, and to relieve compression of LV and RVOT, we decided to perform transcatheter closure of both the inlet and outlet of the fistula for the patient.
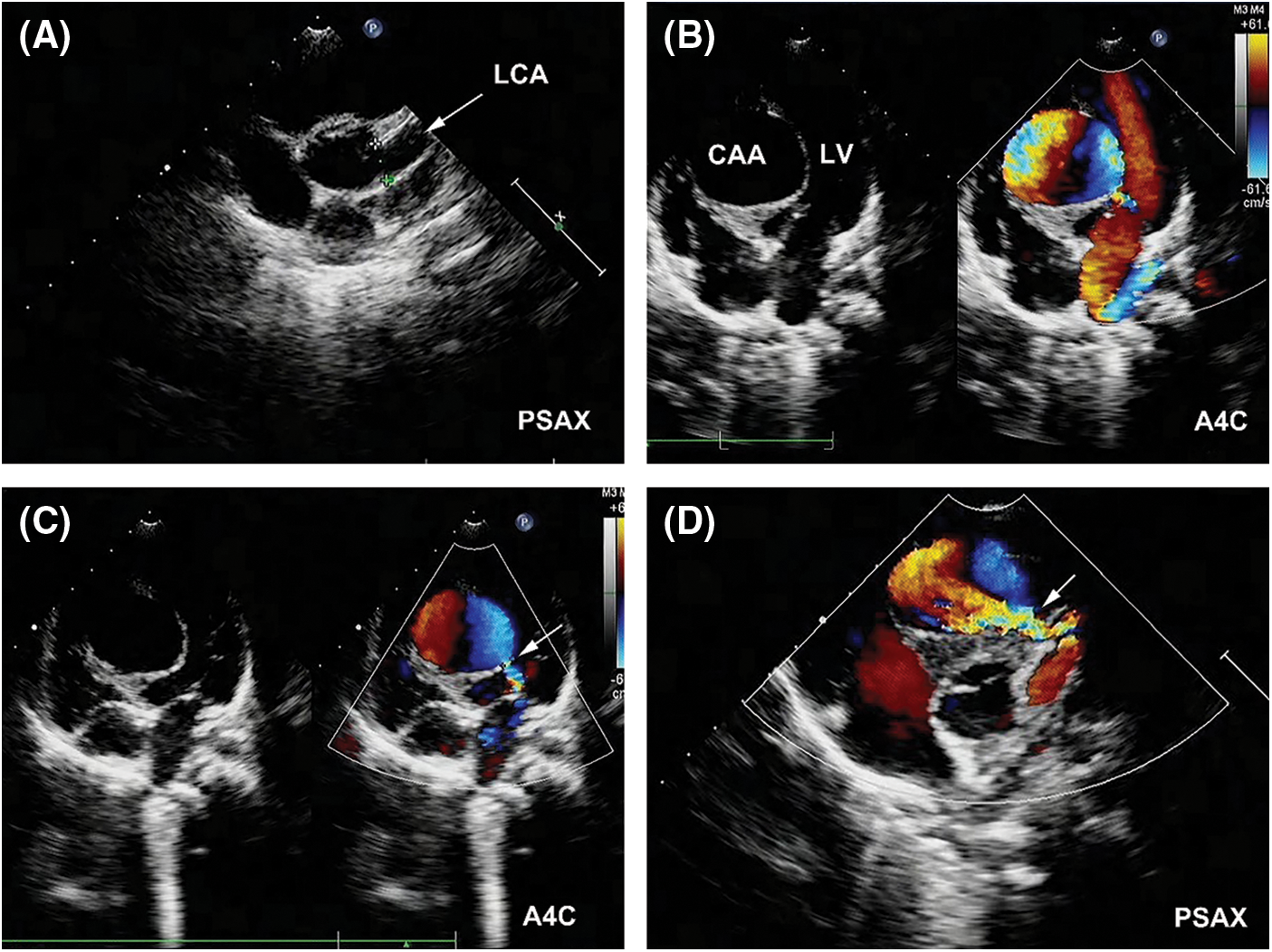
Figure 1: Pre-procedual TTE revealed a LCA-to-LV fistula with giant CAA. (A) The LCA (arrow) was significantly dilated in parasternal short-axis view (PSAX). (B) A giant CAA located above the IVS with compression of LV was demonstrated in apical four-chamber view (A4C). (C) The outlet of the fistula (arrow) in LV was detected (A4C). (D) The inlet of the fistula (arrow) from LCA was detected (PSAX)
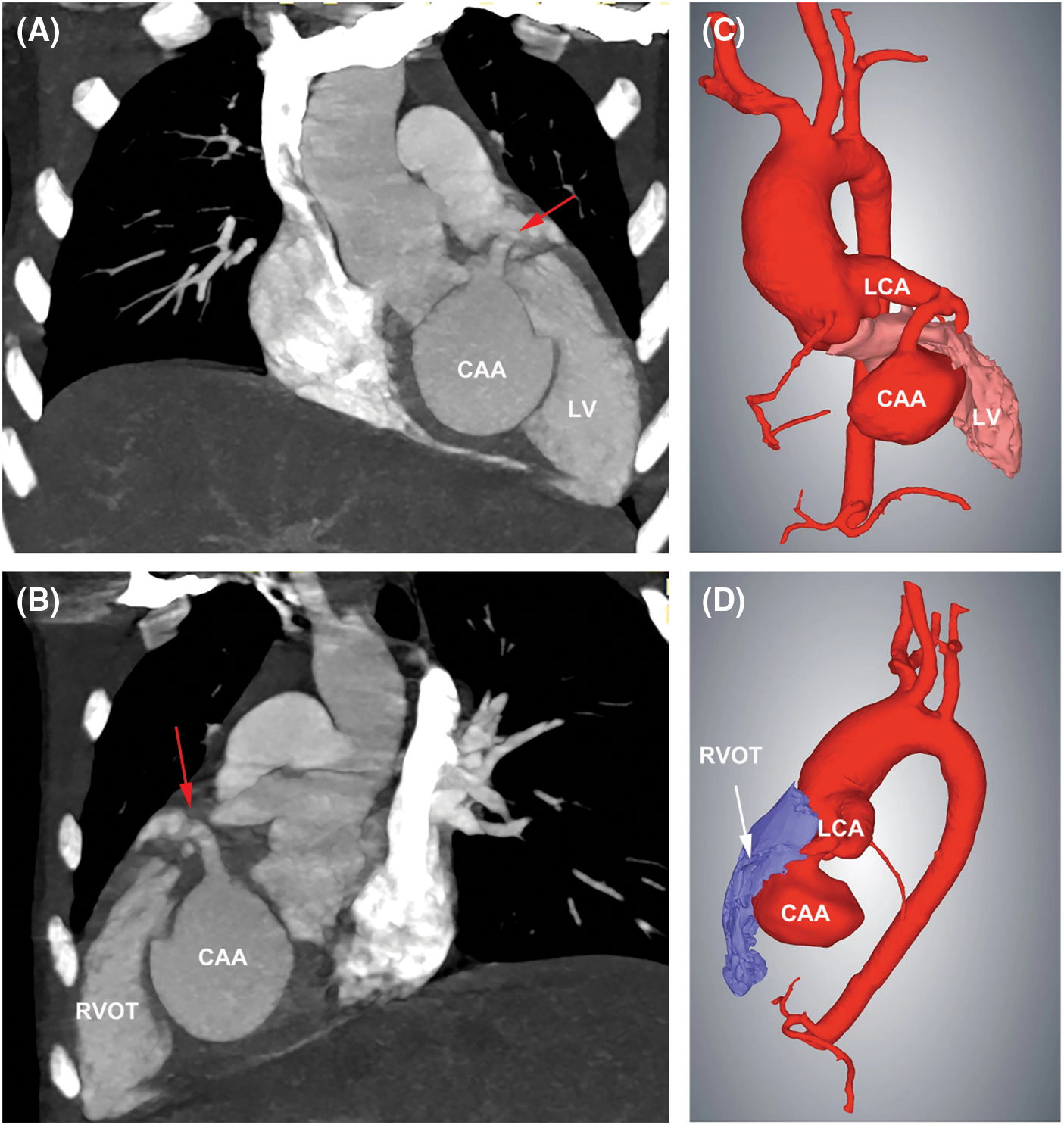
Figure 2: 3D-CT scan showed the LCA-to-LV fistula with giant CAA. (A) The fistula originated from LCA, and LV compression by the CAA was seen. Red arrow indicated the inlet of the fistula. (B) Compression of RVOT by the CAA was present. Red arrow indicated the inlet of the fistula. (C) 3D Reconstruction confirmed the compression of LV by the CAA. (D) 3D Reconstruction confirmed the compression of RVOT by the CAA
During the procedure, coronary angiography further confirmed the abnormalities in these structures and excluded any other coronary artery lesions (Fig. 3A). The diameters at the inlet and outlet of fistula were 7.2 and 3.5 mm, respectively. An arterial-arterial loop was created, and a 6-Fr sheath was delivered anterogradely through the aneurysm and reached to the drainage site. A 04-04 mm Amplatzer duct occluder II (ADO II, Abbott Structural Heart, Plymouth, MN, USA) and a 12 mm Amplatzer vascular plug II (AVP II, Abbott Structural Heart, Plymouth, MN, USA) were deployed at the outlet and inlet of fistula, respectively (Figs. 3B and 3C). After releasing the AVP II, repeated coronary angiography showed that distal LAD was occluded (Fig. 3D). However, there was no ST-T changes on 12-lead ECG during the procedure. Since the patient was at high risk of ventricular septal hematoma and left ventricular function impairment after incision of the aneurysm, we decided to keep the patient under conservative treatment instead of surgical removal of the device and ended the procedure.
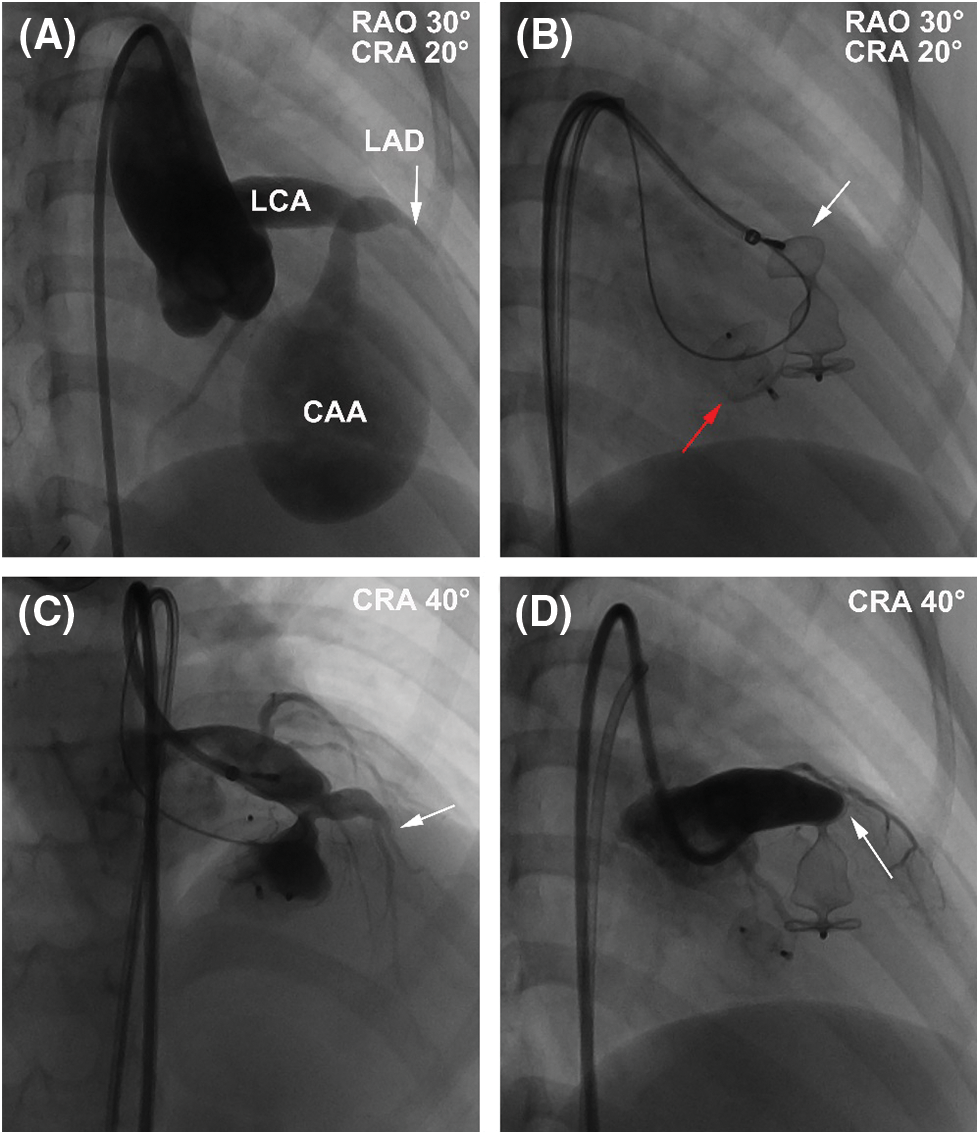
Figure 3: The procedure of transcatheter closure of the LCA-to-LV fistula with giant CAA. (A) RAO 30° and CRA 20° projection of left coronary angiography showed that the fistula originated from the anterior interventricular branch and drained to LV with an aneurysm of 41 mm × 28 mm in size. (B) A 04-04-mm ADO II (red arrow) was deployed at the outlet of fistula, and a 12-mm AVP II was deployed (white arrow) at the outlet of fistula at RAO 30° and CRA 20° projection. (C) CRA 40° projection of left coronary angiography showed that LAD (arrow) was visible before release of the AVP II. (D) CRA 40° projection of left coronary angiography showed that LAD was obstructed by the AVP II intermediately after release. White arrow indicated the occluded LAD
At postoperative day 1, the patient experienced elevation of cardiac troponin T (TnT, peak of 448 pg/mL; normal values < 14 pg/mL) and creatine kinase-MB (CK-MB, peak of 41.9 U/L; normal values < 24 U/L). Although no elevation of ST segment or ST-T changes was detected in ECG, her 24-h holter monitoring showed period of junctional escape rhythm and aberrant ventricular conduction, indicating potential myocardial ischemia (Figs. 4A and 4B). TTE demonstrated both devices were well located, without apparent residual shunt (Figs. 5A and 5B). Thrombus formation was detected within the CAA (Fig. 5C), and LCA ostium was still dilated (Fig. 5D). The patient was given dual antiplatelet therapy with aspirin (5 mg/kg) and clopidogrel (1 mg/kg). The remaining postoperative course was uneventful. Her ECG returned to normal sinus rhythm at postoperative day 2, and level of CK-MB returned to normal level (21 U/L). The patient recovered well and was discharged at postoperative day 7 post procedure without complications.
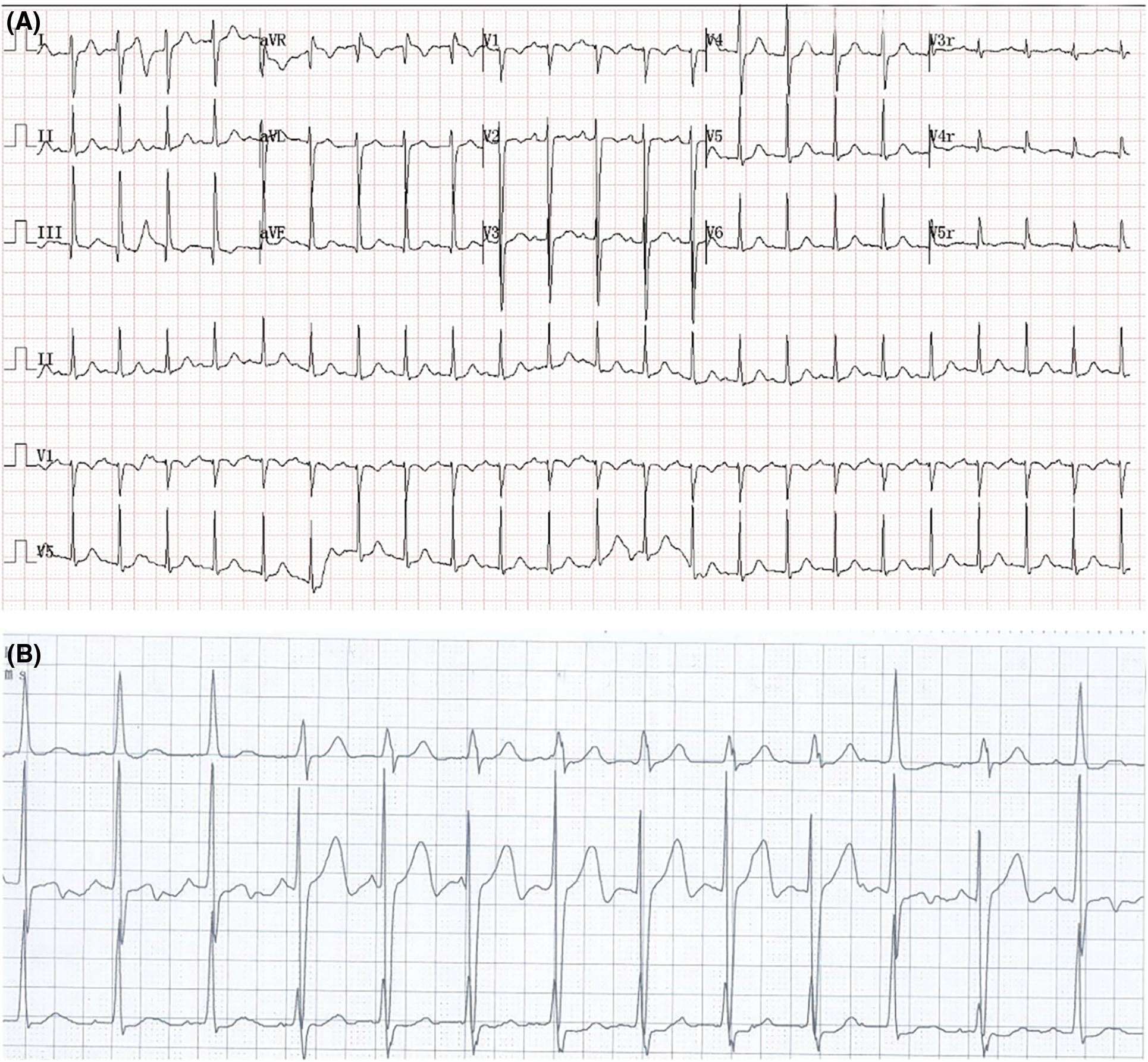
Figure 4: ECG results at postoperative day 1. (A) ECG showed sinus tachycardia. (B) 24-h holter monitoring showed period of junctional escape rhythm and aberrant ventricular conduction
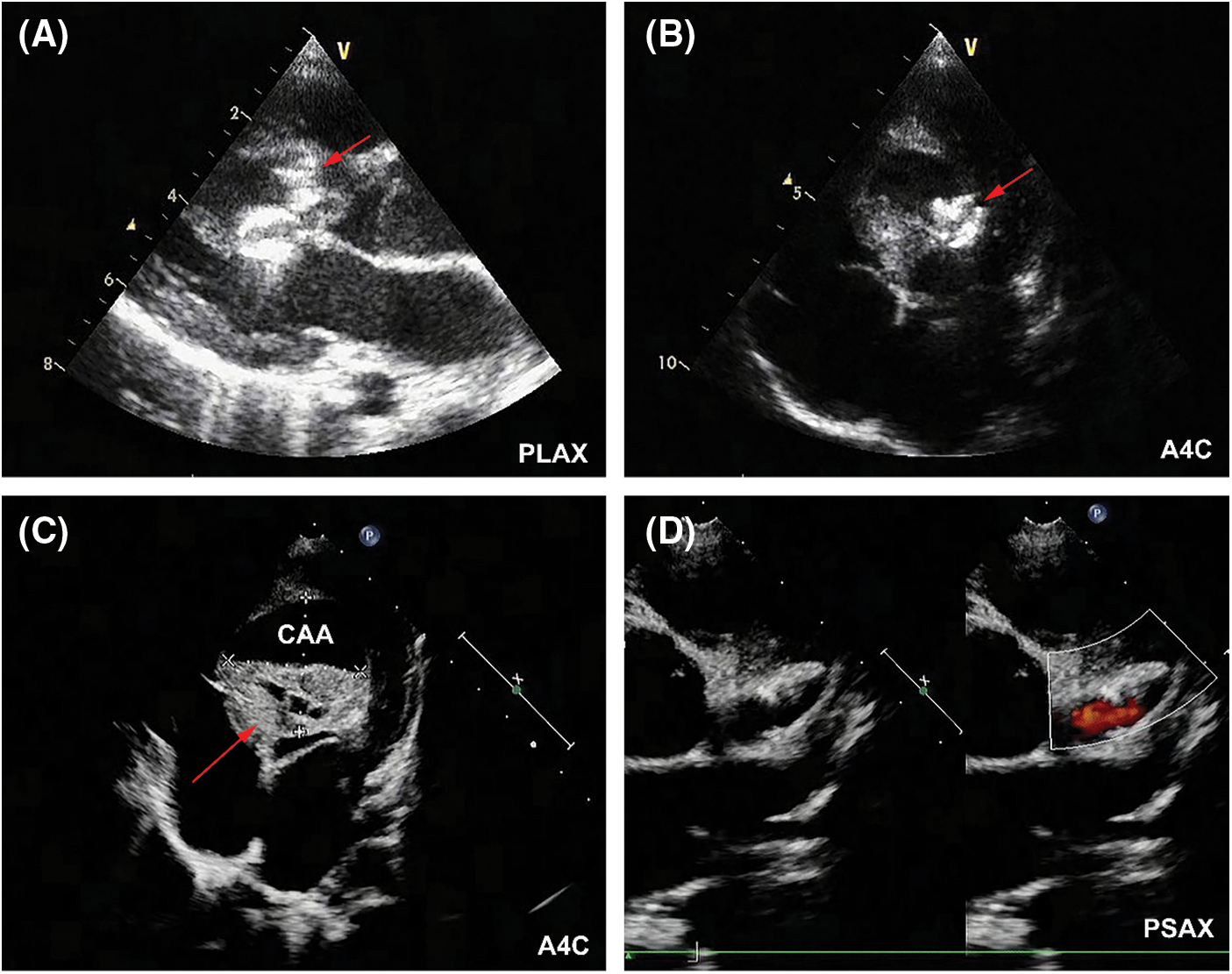
Figure 5: TTE results at postoperative day 1. (A) The AVP II (red arrow) was located at the inlet of fistula without residual shunt in parasternal long-axis view (PLAX). (B)The ADO II (red arrow) was located at the outlet of fistula without residual shunt (A4C). (C) Thrombus formation (red arrow) was detected within the CAA (A4C). (D) LCA was still significantly dilated (PSAX)
The patient was prescribed with dual antiplatelet therapy for a total of 6 months. Follow-up TTE at 2 years showed shrinkage size of CAA, and the two devices were in proper position without residual shunt (Figs. 6A and 6B). The diameter of LCA ostium has significantly reduced (Fig. 6C). LV function was normal (LV ejection fraction 62%), without signs of regional wall motion abnormality. Follow-up ECG at 2 years showed normal sinus rhythm (Fig. 6D). The patient was in good clinical conditions, without cardiovascular symptoms or signs of late complications such as myocardial infarction or thromboembolism.
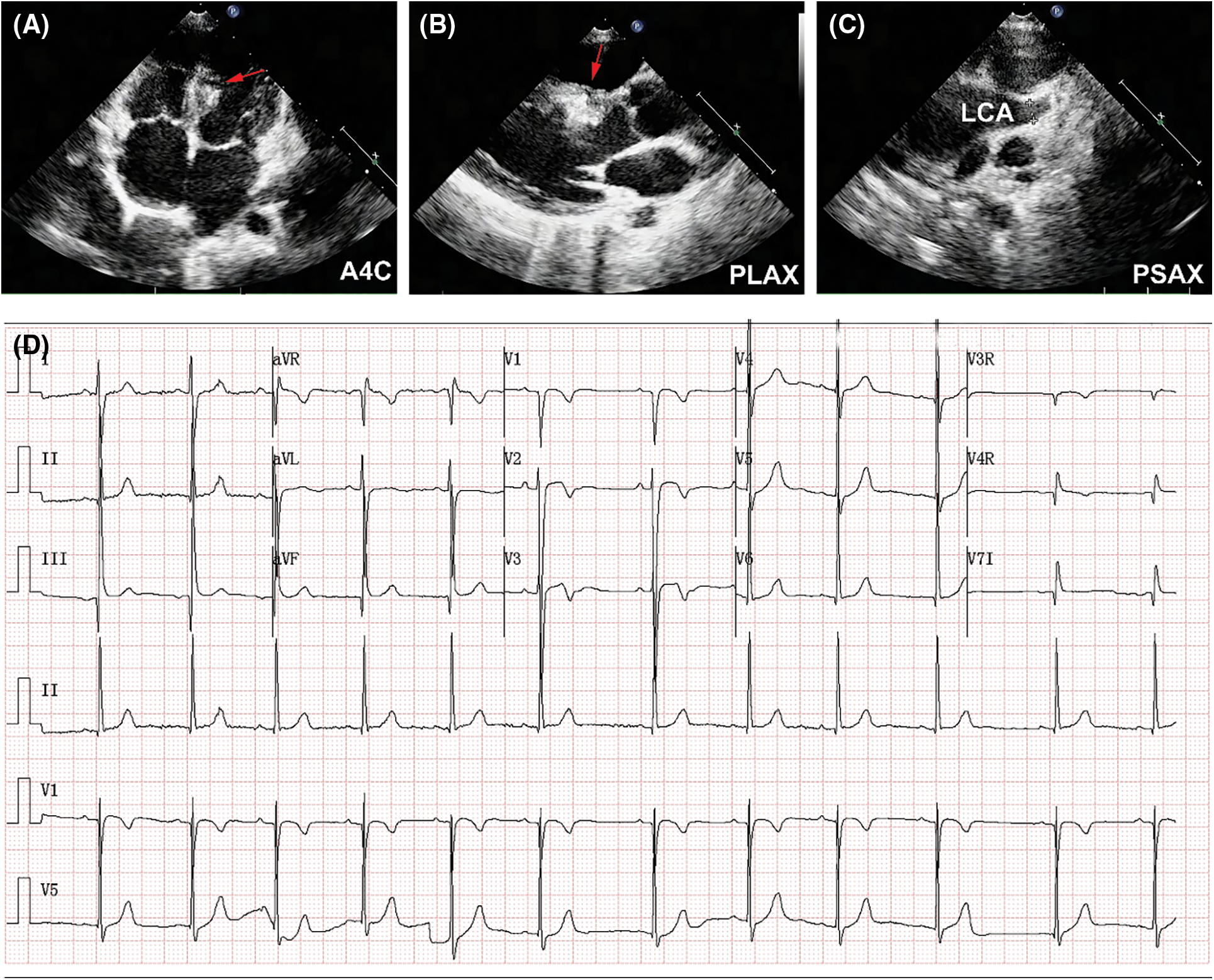
Figure 6: 2-year follow-up results. (A) Shrinkage size of CAA was demonstrated in TTE (A4C). Red arrow indicated the CAA. (B) Red arrow indicated the implanted device (PLAX). (C) Size reduction of LCA ostium was detected (PSAX). (D) ECG showed normal sinus rhythm
A CCAF draining into LV with giant CAA is an extremely rare pathology, and only a few treated cases have been reported in the literature [2,8]. Moreover, most of the fistulas with giant CAA originated from right coronary artery [10–14]. Li et al. [2] reported that CCAFs with giant CAA were diagnosed in 5 of 30,268 patients who underwent heart surgery, and only 1 of the fistulas originated from LCA. Because of the rare entity of this malformation, standard treatment strategies have not been established. In most cases, surgical closure of the fistula and incision of the aneurysm were applied [2,15].
For adults, atherosclerosis was found to be the most common cause (50%) of CAAs, followed by Kawasaki disease and congenital causes (17%) [16]. In children less than 5 years old, Kawasaki disease was an important etiology of CAAs [17]. In our case, the diagnosis of a CCAF with a CAA was based on the following reasons: (1) The CAA was confined to LCA, while Kawasaki disease usually affects more than 1 artery; (2) Coronary angiography revealed the existence of the CAA without concomitant stenosis in the affected coronary artery, while coronary artery stenosis was often seen in Kawasaki disease [18]; (3) The diagnosis of the fistula was confirmed to be originated from LCA and drained into LV by coronary angiography; (4) The patient had no symptoms of Kawasaki disease, such as fever, rash, extremity changes and conjunctivitis, or had a history of Kawasaki disease.
Wang et al. [7] reported that long-term outcome of transcatheter closure of CCAF draining to LV was satisfactory in patients without giant CAA. However, in patients with giant CAA, whether transcatheter closure was beneficial or not remained uncertain, mainly due to the fact that only 3 patients with giant CAA were included in the study [7]. In our case, the patient was at high risk of ventricular septal hematoma and left ventricular function impairment after surgical ligation of the fistula and incision of the aneurysm, because the fistula and CAA originated from the anterior interventricular branch which supplies the majority of IVS. Therefore, trancatheter closure of the fistula, rather than surgical procedure, was eventually performed in our case.
Gowda et al. [19] suggested that for proximal-type CCAFs, transcatheter closure of the fistula should be as close to the coronary origin as possible, and closure of the distal end at the same time, could contribute to the absence of thrombosis. For a proximal CCAF with CAA, although occlusion of the aneurysm distally could help avoid obstruction of the proximal feeding coronary artery, however, closure at the exist point of the aneurysm without closure at the entry point at the same time could lead to progressive aneurysm dilation and thrombus formation within the aneurysm [9,20]. Therefore, transcatheter closure of both the inlet and outlet of the fistula to exclude the aneurysm has been recommended to reduce the risk of complications such as aneurysm dilation, spontaneous rupture of aneurysm, and thrombosis in the dilated feeding coronary artery after closure [20,21].
In consistent with previous studies, we performed device closure in both inlet and outlet of the LCA-to-LV fistula. Follow-up UCG showed shrinkage size of giant CAA and dilated LCA, indicating that this treatment strategy was effective. After LAD was occluded, signs of myocardial injury were presented, including elevations of levels of TnT and CK-MB, and potential ischemic changes in holter monitoring. However, the patient was free from symptoms of myocardial infarction or severe myocardial ischemia, which may be due to the fact that blood supply of distal LAD has been significantly reduced because of coronary steal phenomenon caused by the proximal CCAF, and collateral blood supply from other coronary arteries prevented the relevant myocardium from ischemia. As a result, we considered that conservative treatment with dual antiplatelet agents may be a better and less harmful option in comparison to surgical procedure. The patient has been free from myocardial ischemic complications as yet. However, long-term follow-up was needed because she was still at high risk of potential complications related to the obstructed LAD and the low flow in the dilated LCA, such as coronary thrombosis and myocardial ischemia.
This case report illustrates that transcatheter closure of both inlet and outlet of the fistula might be a effective treatment strategy for a LCA-to-LV CCAF with giant CAA. Although acute LAD obstruction occurred immediately after device implantation, the patient was successfully treated with dual antiplatelet therapy without late complications. Moreover, optimal treatment approaches should be tailored individually, according to the distinct characteristics of affected coronary arteries and clinical presentation of patients with this type of CCAF.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: Zhiwei Zhang; Jian Zhuang; data collection: Yifan Li; analysis and interpretation of results: Yifan Li; Zewen Chen; draft manuscript preparation: Yifan Li, Zewen Chen. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All the procedures performed in our study involving human participants were approved by our institutional review board and were undertaken in accordance with the Declaration of Helsinki. Informed consent for publication was obtained from the patient.
Funding Statement: This work was supported by Guangdong Provincial Clinical Research Center for Cardiovascular Disease [Grant No. 2020B1111170011], National Key R&D Program of China [Grant No. 2016YFC1100305], and Shenzhen Sanming Medical Project of China [Grant No. SZSM201612057].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Latson, L. A. (2007). Coronary artery fistulas: How to manage them. Catheterization and Cardiovascular Interventions, 70(1), 110–116. DOI 10.1002/(ISSN)1522-726X. [Google Scholar] [CrossRef]
2. Li, D., Wu, Q., Sun, L., Song, Y., Wang, W. et al. (2005). Surgical treatment of giant coronary artery aneurysm. The Journal of Thoracic and Cardiovascular Surgery, 130(3), 817–821. DOI 10.1016/j.jtcvs.2005.04.004. [Google Scholar] [CrossRef]
3. Aggarwal, V., Mulukutla, V., Qureshi, A. M., Justino, H. (2018). Congenital coronary artery fistula: Presentation in the neonatal period and transcatheter closure. Congenital Heart Disease, 13(5), 782–787. DOI 10.1111/chd.12653. [Google Scholar] [CrossRef]
4. Qureshi, S. A. (2006). Coronary arterial fistulas. Orphanet Journal of Rare Diseases, 1(1), 51. DOI 10.1186/1750-1172-1-51. [Google Scholar] [CrossRef]
5. Ponthier, L., Brenot, P., Lambert, V., Petit, J., Riou, J. Y. et al. (2015). Closure of isolated congenital coronary artery fistula: Long-term outcomes and rate of re-intervention. Pediatric Cardiology, 36(8), 1728–1734. DOI 10.1007/s00246-015-1224-3. [Google Scholar] [CrossRef]
6. Feltes, T. F., Bacha, E., Beekman, R. H.Rd, Cheatham, J. P., Feinstein, J. A. et al. (2011). Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation, 123(22), 2607–2652. [Google Scholar]
7. Wang, X., Xu, L., Li, S., Liu, Q., Jin, J. et al. (2021). Transcatheter closure of coronary artery fistula draining into left ventricle: A long-term study. Revista Española de Cardiología (English Edition), 74(8), 721–723. DOI 10.1016/j.rec.2021.01.008. [Google Scholar] [CrossRef]
8. Pestana, G., Ribeiro, V., Sousa, C., Cruz, C., Vasconcelos, M. et al. (2018). Percutaneous closure of a fistulous giant coronary aneurysm. The Canadian Journal of Cardiology, 34(6), 812–813. DOI 10.1016/j.cjca.2018.01.088. [Google Scholar] [CrossRef]
9. Li, Y. F., Zhang, Z. W., Wang, S. S., Xie, Z. F., Zhang, X. et al. (2017). Transcatheter closure of congenital coronary artery fistulas with a giant coronary artery aneurysm in children: Experiences from a single center. Chinese Medical Journal, 130(16), 1919–1925. DOI 10.4103/0366-6999.211894. [Google Scholar] [CrossRef]
10. Nakamura, Y., Yutani, C., Imakita, M., Ishibashi-Ueda, H., Nishida, N. et al. (1998). A huge coronary aneurysm resulting from a coronary artery-to-left ventricle fistula. Internal Medicine, 37(4), 366–369. DOI 10.2169/internalmedicine.37.366. [Google Scholar] [CrossRef]
11. Sun, J. P., Yang, L., Zhao, Z., Zhang, X., Ma, G. (2019). A rare right coronary artery–left ventricular fistula with giant coronary artery and aneurysm. European Heart Journal–Cardiovascular Imaging, 20(5), 604. DOI 10.1093/ehjci/jey229. [Google Scholar] [CrossRef]
12. Diao, W., Shi, C., Liu, G., Liu, X. (2021). Coronary artery–left ventricular fistula with giant right coronary aneurysm: A case report and literature review. The Heart Surgery Forum, 24(3), E433–E436. DOI 10.1532/hsf.3777. [Google Scholar] [CrossRef]
13. Li, Y., Xie, M., Wang, X., Lv, Q., Yang, Y. et al. (2015). Two giant right coronary artery aneurysms with fistula to the left ventricle: Preliminary diagnosis by echocardiography. Echocardiography, 32(6), 1053–1055. DOI 10.1111/echo.12922. [Google Scholar] [CrossRef]
14. Yu, Y., Wang, Q., Li, C., Sun, J., Li, W. et al. (2022). Mysterious window: A right coronary artery-left ventricular fistula diagnosed by multiple imaging approaches. Echocardiography, 39(1), 118–121. DOI 10.1111/echo.15260. [Google Scholar] [CrossRef]
15. Wang, Y., Yang, Y., Xia, L., Ding, W., Ji, Q. et al. (2021). Surgical correction of coronary artery ectasia combining congenital coronary artery fistula. Congenital Heart Disease, 16(1), 95–106. DOI 10.32604/CHD.2021.014276. [Google Scholar] [CrossRef]
16. Devabhaktuni, S., Mercedes, A., Diep, J., Ahsan, C. (2016). Coronary artery ectasia-a review of current literature. Current Cardiology Reviews, 12(4), 318–323. DOI 10.2174/1573403X12666160504100159. [Google Scholar] [CrossRef]
17. Pham, V., Hemptinne, Q., Grinda, J. M., Duboc, D., Varenne, O. et al. (2020). Giant coronary aneurysms, from diagnosis to treatment: A literature review. Archives of Cardiovascular Diseases, 113(1), 59–69. DOI 10.1016/j.acvd.2019.10.008. [Google Scholar] [CrossRef]
18. Kawsara, A., Nunez, G. I., Alqahtani, F., Moreland, J., Rihal, C. S. et al. (2018). Management of coronary artery aneurysms. JACC: Cardiovascular Interventions, 11(13), 1211–1223. DOI 10.1016/j.jcin.2018.02.041. [Google Scholar] [CrossRef]
19. Gowda, S. T., Latson, L. A., Kutty, S., Prieto, L. R. (2011). Intermediate to long-term outcome following congenital coronary artery fistulae closure with focus on thrombus formation. The American Journal of Cardiology, 107(2), 302–308. DOI 10.1016/j.amjcard.2010.09.018. [Google Scholar] [CrossRef]
20. Promphan, W., Prachasilchai, P., Qureshi, S. A. (2015). Progressive aneurysmal dilation of coronary arterial fistula after transcatheter closure: Successfully treated by a second occlusion device. Cardiology in the Young, 25(4), 813–817. DOI 10.1017/S1047951114001310. [Google Scholar] [CrossRef]
21. Freund, J. E., Yuko-Jowi, C., Freund, M. W. (2015). Transcatheter embolization of a large aneurysm in a congenital coronary cameral fistula from the left coronary artery to the right ventricle. Catheterization and Cardiovascular Intervention, 85(3), 435–439. DOI 10.1002/ccd.25587. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools