

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.022274
ARTICLE
Mortality and Long-Term Outcome of Neonates with Congenital Heart Disease and Acute Perinatal Stroke: A Population-Based Case-Control Study
1First Department of Pediatrics, Semmelweis University, MTA Centre of Excellence, Budapest, Hungary
2Department of Pediatrics, Saint John Hospital and North-Buda Unified Hospitals, Budapest, Hungary
3Epiconsult BioMedical Consulting and Medical Communications Agency, Dover, USA
4Department of Epidemiology, Johns Hopkins University, Baltimore, USA
5Department of Rehabilitation, Saint John Hospital and North-Buda Unified Hospitals, Budapest, Hungary
6Institute of Mathematics and Base Sciences, Hungarian University of Agriculture and Life Sciences, Gödöllő, Hungary
7Gottsegen National Cardiovascular Center, Budapest, Hungary
8Children’s Hospital Los Angeles, University of Southern California, Los Angeles, USA
*Corresponding Author: Eszter Vojcek. Email: vojcek.eszter@med.semmelweis-univ.hu
Received: 07 March 2022; Accepted: 25 May 2022
Abstract: Objective: Neonates with congenital heart disease (CHD) and perinatal stroke have high mortality and survivors are at risk for poor long-term neurodevelopmental outcome. The aim of this study was to assess the risk factors and outcome of neonates with both CHD and MRI-confirmed perinatal stroke (Study Group) and compare those to the risk factors and outcome of infants matched for CHD without stroke (Control-1) and of infants matched for MRI-confirmed stroke without CHD (Control-2). Methods: We conducted a population-based case-control study enrolling 28 term neonates with CHD and MRI-confirmed acute perinatal stroke born between 2007–2017 in the Central-Hungarian Region. Each of the control groups included 56 infants. The Bayley Scales of Infant Development-II, the Brunet-Lézine test and the Binet Intelligence scales-V were used for neurodevelopmental follow-up at a median age of 61 months. Results: Mortality was highest in the Study Group (25% compared to 5% and 2%, respectively, p = 0.001). Adverse neurodevelopmental outcome was prevalent in the Study (53%) and Control-2 Groups (52%, p = 0.03). Significantly different parameters among the three groups included Apgar scores, mode of delivery, gestational age at birth, cardiac interventions and twin pregnancy. In a multivariable regression analysis adjusted for clinically relevant parameters, patients in the Study Group had significantly higher odds for mortality compared to patients in the Control-1 Group (OR: 6.5 95% CI: 1.1–39.4). Conclusions: Neonates with perinatal stroke and CHD are at a higher risk for dying compared to neonates with CHD without stroke. In addition, the stroke-associated direct insult to the brain likely plays an important role in the development of neurodevelopmental morbidity in these patients.
Keywords: Congenital heart disease; perinatal stroke; neurodevelopmental outcome; mortality
Nomenclature
| CHD | Congenital heart disease |
| OR | Odds ratio |
| NA | Not applicable |
| MCA | Middle cerebral artery |
| TGA | Transposition of the great arteries |
| ASD | Atrial septum defect |
| VSD | Ventricular septum defect |
| SD | Standard deviation |
| CI | Confidence interval |
Perinatal stroke is an important cause of acquired brain injury in neonates causing substantial long-term morbidity. Neurological deficits in survivors of perinatal stroke include cognitive impairment [1], hemiparetic cerebral palsy in childhood [2,3], language delay, epilepsy, and/or behavioral disorders often manifesting only at school age [4]. Therefore, perinatal stroke constitutes a considerable burden on affected children, their families and society [5].
Congenital heart disease (CHD), especially cyanotic and/or complex CHD is one of the major risk factors for perinatal stroke [6,7]. The birth prevalence of CHD is 8.2 per 1000 live births in Europe [8]. Among infants with perinatal stroke, the prevalence of congenital or acquired heart disease is 10% to 29% [9,10] depending on the inclusion of infants with an isolated patent foramen ovale. Infants with CHD have multiple risk factors for stroke including right-to-left shunting, cardiac interventions, and embolism [7].
Motor and global developmental delay occurs more frequently in infants who present with CHD compared to the unaffected population [11]. This observation holds true despite significant advances in the diagnosis and treatment of children with CHD [11]. In neonates with CHD, multiple risk factors are thought to be involved in the development of learning, cognitive and attention deficit. These include reduced brain growth during fetal life [12–14], reduced ability to tolerate the stress associated with the transition from fetal to postnatal life [15], cardiac surgical procedures especially those requiring cardiopulmonary bypass in the immediate postnatal period [16] and other, patient- and environment-related factors, such as genetic syndromes, prematurity, and socioeconomic status [15,17,18]. It is tempting to speculate that the improved rate of survival in neonates with complex CHD requiring cardiac surgery also plays a role in this phenomenon [19,20].
Little is known about the long-term neurodevelopmental outcome of neonates with perinatal stroke [21–23], and for neonates with perinatal stroke and CHD the data are especially scant [24]. Therefore, we aimed to describe the patient characteristics of neonates with CHD and perinatal stroke (Study Group) and compare their outcome to that of neonates with CHD without perinatal stroke (Control-1) and neonates with perinatal stroke without CHD (Control-2). The primary goal of the study was to investigate whether perinatal stroke in conjunction with CHD increases mortality. The secondary aim was to compare the long-term neurodevelopment outcome of the Study Group patients to the infants in the control groups.
We designed a population-based case-control study of term (37–41 weeks’ gestation) and near-term (35–36 weeks’ gestation) neonates with CHD and perinatal stroke (Study Group) born between the 1st of January 2007 and the 31st of December 2017 in Central-Hungary. Cardiac interventions were performed in a single center (Gottsegen National Cardiovascular Center, Budapest, Hungary). Neonates with acute arterial ischemic stroke and acute hemorrhagic stroke confirmed by MRI prior to 28 days of postnatal life were included in the Study and Control-2 Groups. Congenital heart disease was defined as a defect in the structure of the heart or great vessels that was present at birth [25]. We enrolled (1) term or near-term neonates (>35 weeks’ gestation) up to 28 days of postnatal age, (2) patients with perinatal stroke without any other brain abnormality on brain MRI (Study and Control-2 Groups), and (3) patients with CHD in the Study and Control-1 Groups. Neonates with CHD matching the cardiac diagnosis of the study infants but without perinatal stroke (Control-1) and subjects with perinatal stroke but without CHD (Control-2) were used as controls. Controls were recruited in a 1:2 ratio. Patient selection is shown in Appendices A.1, A.2, A.3. Infants with encephalitis, tumor, non-accidental brain injury, presumed perinatal stroke, border-zone injuries secondary to asphyxia, extra-axial hemorrhage without parenchymal bleeding as well as bilateral preterm brain injury were excluded [26]. To decrease the heterogeneity of the patients enrolled, neonates with cerebral sinovenous thrombosis were not included. Patients with isolated ductus arteriosus with or without a patent foramen ovale were also excluded from the Study and Control-1 Groups.
The Institutional Review Board of the Hungarian Medical Research Council approved the study (19934–4/2018/EKU). Informed verbal parental consent was obtained to recruit patients into the study and perform the neurodevelopmental assessments.
Clinical information was extracted from chart reviews. Baseline clinical characteristics, stroke types and the frequencies of cardiac interventions/open-heart surgeries of patients in the Study Group and Control Groups are summarized in Table 1.
Brain imaging was carried out using 3 T Philips Achieva and 3 T Philips Ingenia MR scanners (Philips Medical Systems, Best, The Netherlands) at a single center, in the Department of Neuroradiology, Medical Imaging Center, Semmelweis University, Budapest, Hungary. The scanning protocol included diffusion-weighted imaging, apparent diffusion coefficient map, conventional T1- and T2-weighted imaging, T2*- and susceptibility-weighted imaging. Finally, in selected cases MR spectroscopy and/or MR angiography was added, as appropriate. Radiologists trained and experienced in neonatal brain MRI evaluated the scans. Classification was based on the shape, extent and localization of the affected brain compartment supplied by the given arteries [2,13]. Each stroke was assigned to one of the most predominant arterial territory or to the affected brain compartment as described by Govaert et al. [26]. Due to the relatively small number of patients involved in this study, arterial ischemic strokes were dichotomized to be localized within the territory of the middle cerebral artery (MCA) or to the anterior cerebral artery/posterior cerebral artery. Based on their largest diameter, strokes were also subcategorized as small (<3 cm) or large (≥3 cm) strokes (Table 1).
2.4 Neurodevelopmental Outcome
Neurodevelopmental outcome was assessed during routine follow-up appointments or follow-up appointments arranged for the purposes of this study in 2018. We used follow-up data from 18 months until 12 years of age. Adverse outcome was defined if any one or more of the following sequelae occurred: Cerebral palsy, cognitive deficit, behavioral problems, epilepsy, language delay, visual field defect or hearing loss. Developmental tests used during the follow-up visits are described in detail in the Appendix B [27–31].
Echocardiography was performed by pediatric cardiologists in 92 neonates based on the clinical symptoms. The echocardiography findings were described as normal or CHD. Based on findings of echocardiography and MR angiography, malformations of the heart were further subcategorized to specific cardiac diagnoses (Table 2).
Descriptive statistics included frequencies and percentages for categorical variables and means and standard deviations (SD) for continuous variables. Fisher’s exact tests for categorical and one-way ANOVA for continuous variables were performed to assess associations. Multivariable logistic regression analysis was fitted to ascertain the effect of the Study Group and Control-1 and Control-2 Groups on the likelihood that a patient would die, while controlling for other clinically relevant variables revealed by the Fisher’s exact tests. Dummy variables were introduced to categorize patients into the Study and Control-1 and Control-2 Groups. Factors found to be clinically relevant by the Fisher’s exact tests included Apgar score at 1 min, mode of delivery, gestational age at birth, cardiac interventions, and twin pregnancy. The model was checked according to the Hosmer and Lemeshow’s goodness of fit test. All statistical tests were two-sided, and p-values of < 0.05 were considered to represent statistical significance. Statistical analyses were performed using IBM SPSS Statistics (Version 25, IBM Corp. Armonk, NY, USA).
A total of 1400 MR images obtained prior to 28 days postnatal life were reviewed. Altogether 225 patients were diagnosed with acute perinatal stroke in Central-Hungary over the 11-year study period yielding a disease incidence of 1 per 1400 live births. Echocardiography confirmed CHD in the 28 patients (Table 2), resulting in an incidence of neonates with CHD and perinatal stroke of 1 per 11,000.
Comparison of the baseline characteristics of the Study Group and the Control-1 and Control-2 Groups revealed several statistically significant differences (Table 1). Neonates with CHD only (Control-1 Group) were born at a significantly younger gestational age than the neonates in the Study and Control-2 Groups (p = 0.003). In addition, neonates in the Control-1 group were born via C-section at a higher rate than infants in the Study and Control-2 Groups (89% vs. 39% and 48%, p < 0.001). The occurrence of cardiac interventions including Rashkind septostomy or open-heart surgery in the neonatal period was significantly higher among subjects in the Study Group compared to the Control-1 Group (68% vs. 38%, p = 0.01) (Table 1). There was no statistically significant difference in the frequency of stroke types between the Study and Control-2 Groups (Table 1).
Comparison of the baseline characteristics of the Study Group and the Control-1 and Control-2 Groups revealed several statistically significant differences (Table 1). Neonates with CHD only (Control-1 Group) were born at a significantly younger gestational age than the neonates in the Study and Control-2 Groups (p = 0.003). In addition, neonates in the Control-1 group were born via C-section at a higher rate than infants in the Study and Control-2 Groups (89% vs. 39% and 48%, p < 0.001). The occurrence of cardiac interventions including Rashkind septostomy or open-heart surgery in the neonatal period was significantly higher among subjects in the Study Group compared to the Control-1 Group (68% vs. 38%, p = 0.01) (Table 1). There was no statistically significant difference in the frequency of stroke types between the Study and Control-2 Groups (Table 1).
3.2 Long-Term Outcome of Neonates with Heart Disease and/or Stroke
After excluding patients who died in the neonatal period, long-term neurodevelopmental outcome data were available in 19 (90%) of the 21 surviving Study Group patients (CHD and perinatal stroke), 38 (72%) of the 53 surviving infants in the Control-1 Group (CHD without perinatal stroke) and 52 (95%) of the 55 surviving subjects in the Control-2 Group (perinatal stroke without CHD). The last follow-up visit took place at a median age of 61 months [range 18–144 months]. Neonates who were lost to follow-up did not differ regarding the type of stroke or CHD from the subjects completing neurodevelopmental follow-up.
Compared to the control groups, mortality was highest in neonates with CHD and perinatal stroke (25%, p = 0.001). Of note is that mortality was the lowest in patients with stroke without CHD (Control-2 Group, 2%). Data on neurodevelopmental outcome after 18 months of age revealed that normal outcome was most prevalent in the surviving patients of the Control-1 Group (CHD only, 74%) compared to the patients in the Study (47%) or Control-2 Groups (48%, p = 0.03). Accordingly, abnormal neurodevelopmental outcome was higher in the Study (53%) and Control-2 Groups (52%) compared to the subjects in the Control-1 Group (26%, p = 0.03) (Table 3). The rate of cerebral palsy was highest among patients with perinatal stroke only (Control-2), while the rate of cognitive impairment and epilepsy was highest in the Study Group. Altogether, six children (32%) in the Study Group experienced at least 1 seizure after the neonatal period. However, after excluding one patient with febrile seizures and another one who was initially diagnosed with epilepsy but was later discontinued to take antiepileptic medication and remained seizure-free for more than one year without treatment, the rate of active epilepsy in the Study Group was 21% at the time of the last neurodevelopmental follow-up (Table 3).
Finally, in the Study Group, we could not identify any difference among the risk factors in subjects who died compared to those who survived, and among those with normal outcome compared to those with adverse outcome (data not shown).
3.3 Risk Factors Associated with Mortality
Multivariable regression analysis was fitted to investigate the risk of mortality in the Study and Control-1 and Control-2 Groups, while adjusting for clinically relevant parameters, including gestational age at birth, Apgar score at 1 min, vaginal birth, twin pregnancy and cardiac interventions. First, Control-1 Group was selected as a reference variable (Table 4) and then we repeated the analysis using Control-2 Group as a reference variable.
Of the predictor variables, patients in the Study Group had 6.5 times higher odds for mortality compared to patients in the Control-1 Group (OR: 6.5 95% CI: 1.1–39.4) while adjusting for all the other relevant potentially confounding clinical parameters.
Then we used the Control-2 Group as the reference variable in the multivariable logistic regression model adjusted for the clinically relevant parameters. In this model, the risk of mortality was not increased in the Study Group compared to the Control-2 Group. Additionally, no other parameters were found to be significantly different in the logistic regression models (data not shown).
This is a population-based cohort study conducted in term and near-term neonates with aims to assess the impact of the co-occurrence of CHD and perinatal stroke on mortality and long-term neurodevelopmental outcome. In line with that data in the literature [32], the average annual incidence of perinatal stroke was 1 in 1400 live births in the present study. The average annual incidence of CHD and perinatal stroke in our population was 1 in 11,000 live births. Therefore, using the birth prevalence of CHD in Europe [8], approximately one in 90 neonates with CHD is also expected to present with perinatal stroke. This finding underscores the need of awareness for the risk of stroke in infants with CHD. The finding that 12% of the neonates with perinatal stroke had CHD as well, lends further support to this notion. Finally, two-thirds of the patients were diagnosed with cyanotic CHD giving further support for the increased propensity to brain injury seen in children with cyanotic CHD.
The uniqueness of the present study is that it compares mortality, clinical characteristics and neurodevelopmental outcome of neonates with CHD and perinatal stroke (Study Group) to neonates with CHD without perinatal stroke (Control-1 Group) and patients with perinatal stroke without CHD (Control-2 Group). The rate of adverse outcome of neonates with perinatal stroke and CHD was similar to that in the study by Cheng et al. with a median follow-up of 15.3 months [20]. However, in addition to investigating the perinatal risk factors in neonates with CHD and perinatal stroke, we followed a high proportion of infants for at least 18 months and with a median follow-up age of 61 months. The rate of adverse neurodevelopmental outcome in the Control-1 and Control-2 Groups was also similar to that described in previously published studies [24,33,34].
Nevertheless, we have found significant differences in gestational age at birth, Apgar scores, the number of twin pregnancies, mode of delivery and the frequencies of cardiac interventions between the Study and the Control Group patients. After adjusting for clinically relevant risk factors, mortality remained significantly higher in neonates with CHD and perinatal stroke compared to the Control-1 Group patients (CHD only).
The rate of abnormal neurodevelopmental outcome was higher in patients affected by perinatal stroke irrespective of the presence or absence of coexisting CHD: 53% in the Study and 52% in the Control-2 Groups compared to 26% in patients with CHD only (Control-1 Group) (p = 0.03). Thus, normal neurodevelopmental outcome was most prevalent in patients without perinatal stroke. This finding suggests that the stroke-associated direct insult to the brain plays an exceptionally important role in the development of neurodevelopmental morbidity. However, given our finding that a higher proportion of neonates with CHD undergoing surgery develop stroke, the possibility of sequentiality also arises in the context of CHD and perinatal stroke. Indeed, studies examining the risk of open-heart surgery or Rashkind septostomy in neonates with CHD found both procedures to be independent risk factors for stroke [35–37]. Our findings that the rate of these procedures was significantly higher in patients of the Study than Control-1 Group and that half of the strokes occurred after surgery, lend further support to this notion in the literature [36,37].
The rate of C-section was significantly higher in subjects with CHD only compared to neonates with CHD and perinatal stroke and with perinatal stroke without CHD (89% vs. 39% and 48%, respectively, p < 0.001). The reason for this finding is unclear, but the high percentage of multiple gestations, the lower gestational age and the prenatal diagnosis of CHD in most the patients in the Control-1 Group likely played a role in influencing the obstetric management. Although C-section may offer some protection from the occurrence of neurological deficits [21], it remains unclear whether the higher C-section rate in Control-1 Group patients contributed to the absence of stroke and the higher rate of normal neurodevelopmental outcome in this patient population.
Mortality in infants with perinatal stroke varies between 2% and 25% depending on the pathomechanism of stroke and other clinical risk factors [38]. While mortality in neonates with CHD is around 4% [39], mortality described in the literature for children with both CHD and stroke may be as high as 33% [20]. Along with these lines, we have also found that mortality was significantly higher in patients with perinatal stroke and CHD (25%) compared to neonates with CHD alone (5%, OR: 6.5 95% CI: 1.1–39.4). Intensive care procedures and especially extracorporeal membrane oxygenation and ventricular assist device are associated with increased mortality in neonates with CHD and stroke [7,20]. Additionally, in previous studies, use of inotropes, the number of cardiac procedures and parenchymal hemorrhage were also associated with mortality in patients with CHD and stroke [20]. The novelty of our study is that after adjusting for neonates with matching CHD or matching strokes only, as well as to the relevant clinical risk factors, mortality was still significantly higher in neonates with CHD and stroke compared to patients with CHD only (Control-1). Therefore, although the reasons of the increased mortality of neonates with CHD and stroke are incompletely understood, it is tempting to speculate that the coexistence of multiple clinical risk factors synergistically contributes to the increased mortality in this patient population.
In agreement with previously published studies, one-third of the patients with perinatal stroke developed later cerebral palsy [34]. In our study, the finding that the rate of cerebral palsy was only 16% among neonates with perinatal stroke and CHD (Study Group) must be interpretated with caution. Due to the high mortality (25%) in the Study Group, subjects with worse prognosis including higher rate of cerebral palsy might have passed away in the neonatal period. On the other hand, we hypothesize that the cumulative effect of delayed microstructural development in combination with multiple ischemic/hemorrhagic events in infants with CHD and perinatal stroke contributed to the higher rate of epilepsy and cognitive deficit [40]. This finding is in agreement with recent data showing that in children with perioperative neonatal brain injuries, cognitive outcome is worse at school age [41].
Our study has limitations to be considered. First, similarly to other studies on this subject, our study was limited by the small sample size. Hence, the number of variables tested in the multivariable model was limited. However, since the co-occurrence of CHD and perinatal stroke is rare, observational studies on a modest sample size remain an avenue for hypothesis generation. Additionally, we attempted to counterbalance the effect of the limited number of variables tested in the multivariable model by using two control groups: One with matching CHD and the other one with matching perinatal strokes. Secondly, as the scarcity of perinatal stroke makes it difficult to collect data from a single center, we used the data from all Neonatal Intensive Care Units in Budapest, Hungary. While involving several centers might affect the completeness and consistency of the data collected, in our case this limitation was likely mitigated by the high level of harmonization provided by the universal health care system in Hungary. Moreover, the rate of long-term follow-up was lowest in patients with CHD without perinatal stroke (Control-1 Group). As for motor impairment, we only studied cerebral palsy since it is considered as the most important and uniformly diagnosable motor impairment among patients with perinatal stroke. Finally, due to the limited number of patients involved in the Study Group, we were unable to specifically address long-term outcome of subgroups assigned to specific territorial involvement of brain regions affected by the stroke. Nevertheless, this limitation is not unique to our study since another investigation could also not identify specific perinatal cerebral findings associated with poor neurodevelopmental outcome in infants with CHD and stroke [41]. The authors instead suggested identifying brain lesions based on etiology instead of the imaging pattern to predict neurodevelopmental outcome [41]. On the other hand, in previous studies on larger patient population, we showed that in neonates with arterial ischemic stroke, the main MCA stroke, multiple strokes, the involvement of the corticospinal tract and the presence of infection/inflammation were independent predictors of adverse outcome [34], while in neonates with hemorrhagic stroke, the parietal lobe hemorrhage, the involvement of the thalamus/basal ganglia, hemorrhage affecting multiple lobes and seizures on admission were associated with adverse outcome [42].
Strengths of the study include the enrollment of patients with perinatal stroke confirmed by brain MRI. A high proportion of infants also had long-term follow-up, performed between 18 months and early school age, allowing for the documentation of long-term neurodevelopmental outcome. Longitudinal follow-up of neurodevelopmental sequelae in patients with perinatal brain injury is important to assess speech and other higher cognitive functions that only emerge later in childhood [4]. Finally, by using two control groups, we adjusted the multivariable regression analysis to most of the clinically relevant risk factors influencing mortality and the long-term outcome of neonates with CHD and perinatal stroke.
In conclusion, patients with CHD and perinatal stroke are at a significantly higher risk for dying compared to infants with CHD only. In addition, the rate of neurodevelopmental morbidity is higher in neonates with perinatal stroke with or without CHD. This finding suggests that the stroke-associated direct insult to the brain plays an important role in the development of neurodevelopmental morbidity in these patients. Further studies with larger sample size are needed to evaluate the relationship between mortality, neurodevelopmental outcome and clinical risk factors in patients with CHD and perinatal stroke to improve the clinical decision-making process and enhance our ability to provide individualized prediction models and thus parental counseling.
Authorship: The authors confirm contribution to the paper as follows: Study conception and design: E. Vojcek, V. A. Gyarmathy, I. Seri; data collection: E. Vojcek; follow-up: R. Graf; cardiac interventions: L. Ablonczy, Z. Prodan, analysis and interpretation of results: A. M. Laszlo; draft manuscript preparation: E. Vojcek, V. A. Gyarmathy, I. Seri. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data: The data that support the findings of this study are available from the corresponding author, E.V., upon reasonable request.
Funding Statement: Financial support for this work (E.V.) was provided by the New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund (ÚNKP-21–3-II-SE-5) (https://www.unkp.hu) and by the Semmelweis University (EFOP-3.6.3.-VEKOP-16–2017–00009) (https://semmelweis.hu/phd/2021/11/09/).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kolk, A., Ennok, M., Laugesaar, R., Kaldoja, M. L., Talvik, T. (2011). Long-term cognitive outcomes after pediatric stroke. Pediatric Neurology, 44(2), 101–109. DOI 10.1016/j.pediatrneurol.2010.08.012. [Google Scholar] [CrossRef]
2. Mercuri, E., Rutherford, M., Cowan, F., Pennock, J., Counsell, S. et al. (1999). Early prognostic indicators of outcome in infants with neonatal cerebral infarction: A clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics, 103(1), 39–46. DOI 10.1542/peds.103.1.39. [Google Scholar] [CrossRef]
3. Wagenaar, N., Martinez-Biarge, M., van der Aa, N. E., van Haastert, I. C., Groenendaal, F. et al. (2018). Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics, 142(3), e20174164. DOI 10.1542/peds.2017-4164. [Google Scholar] [CrossRef]
4. Westmacott, R., MacGregor, D., Askalan, R., deVeber, G. (2009). Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke, 40(6), 2012–2019. DOI 10.1161/STROKEAHA.108.533976. [Google Scholar] [CrossRef]
5. Ferriero, D. M., Fullerton, H. J., Bernard, T. J., Billinghurst, L., Daniels, S. R. et al. (2019). Management of stroke in neonates and children: A scientific statement from the American heart association/Americain stroke association. Stroke, 50(3), e51–e96. DOI 10.1161/STR.0000000000000183. [Google Scholar] [CrossRef]
6. Dunbar, M., Kirton, A. (2018). Perinatal stroke: Mechanisms, management, and outcomes of early cerebrovascular brain injury. The Lancet Child & Adolescent Health, 2(9), 666–676. DOI 10.1016/S2352-4642(18)30173-1. [Google Scholar] [CrossRef]
7. Sinclair, A. J., Fox, C. K., Ichord, R. N., Almond, C. S., Bernard, T. J. et al. (2015). Stroke in children with cardiac disease: Report from the international pediatric stroke study group symposium. Pediatric Neurology, 52(1), 5–15. DOI 10.1016/j.pediatrneurol.2014.09.016. [Google Scholar] [CrossRef]
8. van der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/j.jacc.2011.08.025. [Google Scholar] [CrossRef]
9. Ganesan, V., Prengler, M., McShane, M. A., Wade, M. A., Kirkham, F. J. (2003). Investigation of risk factors in children with arterial ischemic stroke. Annals of Neurology, 53, 167–173. DOI 10.1002/ana.10423. [Google Scholar] [CrossRef]
10. Barnes, C., Newall, F., Furmedge, J., Mackay, M., Monagle, P. (2004). Arterial ischemic stroke in children. Journal of Paediatrics and Child Health, 40, 384–387. DOI 10.1111/j.1440-1754.2004.00407.x. [Google Scholar] [CrossRef]
11. Limperopoulos, C., Majnemer, A., Shevell, M. I., Rohlicek, C., Rosenblatt, B. et al. (2002). Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. Journal of Pediatrics, 141(1), 51–58. DOI 10.1067/mpd.2002.125227. [Google Scholar] [CrossRef]
12. Hayek, C., Rajagopalan, V., Meouchy, J., Votava-Smith, J., Miller, D. et al. (2020). Ventricular and total brain volumes in infants with congenital heart disease: A longitudinal study. Journal of Perinatology, 40(9), 1383–1388. DOI 10.1038/s41372-020-0711-4. [Google Scholar] [CrossRef]
13. von Rhein, M., Buchmann, A., Hagmann, C., Huber, R., Klaver, P. et al. (2013). Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain, 137(1), 268–276. DOI 10.1093/brain/awt322. [Google Scholar] [CrossRef]
14. Claessens, N. H. P., Khalili, N., Isgum, I., Ter Heide, H., Steenhuis, T. J. et al. (2019). Brain and CSF volumes in fetuses and neonates with antenatal diagnosis of critical congenital heart disease: A longitudinal MRI study. American Journal of Neuroradiology, 40(5), 885–891. DOI 10.3174/ajnr.A6021. [Google Scholar] [CrossRef]
15. Gaynor, W. J. (2014). The encephalopathy of congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery, 148(5), 1790–1791. DOI 10.1016/j.jtcvs.2014.09.061. [Google Scholar] [CrossRef]
16. Wotherspoon, J. M., Eagleson, K. J., Gilmore, L., Auld, B., Hirst, A. et al. (2020). Neurodevelopmental and health-related quality-of-life outcomes in adolescence after surgery for congenital heart disease in infancy. Developmental Medicine of Child Neurology, 62(2), 2014–2220. DOI 10.1111/dmcn.14251. [Google Scholar] [CrossRef]
17. Gaynor, W. J., Wernovsky, G., Jarvik, G. P., Bernbaum, J., Gerdes, M. et al. (2007). Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery, 133(5), 1344–1353. DOI 10.1016/j.jtcvs.2006.10.087. [Google Scholar] [CrossRef]
18. Wei, D., Azen, C., Bhombal, S., Hastings, L., Paquette, L. (2015). Congenital heart disease in low-birth-weight infants: Effects of small for gestational age (SGA) status and maturity on postoperative outcomes. Pediatric Cardiology, 36(1), 1–7. DOI 10.1007/s00246-014-0954-y. [Google Scholar] [CrossRef]
19. Hoffman, J. L., Mack, G. K., Minich, L., Benedict, S. L., Heywood, M. et al. (2011). Failure to impact prevalence of arterial ischemic stroke in pediatric cardiac patients over three decades. Congenital Heart Disease, 6(3), 211–218. DOI 10.1111/j.1747-0803.2011.00510.x. [Google Scholar] [CrossRef]
20. Cheng, H. H., Rajagopal, S., McDavitt, E., Wigmore, D., Williams, K. et al. (2016). Stroke in acquired and congenital heart disease patients and Its relationship to hospital mortality and lasting neurologic deficits. Pediatric Critical Care Medicine, 17(10), 976–983. DOI 10.1097/PCC.0000000000000902. [Google Scholar] [CrossRef]
21. Kirton, A., Armstrong-Wells, J., Chang, T., deVeber, G., Rivkin, M. J. et al. (2011). Symptomatic neonatal arterial ischemic stroke: The international pediatric stroke study. Pediatrics, 128(6), e1402–e1410. DOI 10.1542/peds.2011-1148. [Google Scholar] [CrossRef]
22. Nelson, K. B., Lynch, J. K. (2004). Stroke in newborn infants. The Lancet Neurology, 3(3), 150–8. DOI 10.1016/S1474-4422(04)00679-9. [Google Scholar] [CrossRef]
23. Chabrier, S., Peyric, E., Drutel, L., Deron, J., Kossorotoff, M. et al. (2016). Multimodal outcome at 7 years of Age after neonatal arterial ischemic stroke. The Journal of Pediatrics, 172, 156–161.e3 DOI 10.1016/j.jpeds.2016.01.069. [Google Scholar] [CrossRef]
24. Majnemer, A., Limperopoulos, C., Shevell, M., Rosenblatt, B., Rohlicek, C. et al. (2006). Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. The Journal of Pediatrics, 148(1), 72–77. DOI 10.1016/j.jpeds.2005.08.036. [Google Scholar] [CrossRef]
25. Limperopoulos, C., Majnemer, A., Shevell, M. I., Rosenblatt, B., Rohlicek, C. et al. (1999). Neurologic status of newborns with congenital heart defects before open-heart surgery. Pediatrics, 103(2), 402–408. DOI 10.1542/peds.103.2.402. [Google Scholar] [CrossRef]
26. Govaert, P., Ramenghi, L., Taal, R., de Vries, L., Deveber, G. (2009). Diagnosis of perinatal stroke I: Definitions, differential diagnosis and registration. Acta Paediatrica, 98(10), 1556–1567. DOI 10.1111/j.1651-2227.2009.01461.x. [Google Scholar] [CrossRef]
27. Bayley, N. (1993). BSID-II: Bayley scales of infant development (Second Edition). San Antonio: Hartcourt Brace & Company. [Google Scholar]
28. Brunet, O., Lézine, I., Josse, D. (1997). Brunet-lézine révisé : Échelle de développement psychomoteur de la première enfance: Manuel BLR-C. Issy-Les-Moulineaux (FranceEtablissements d’Applications Psychotechniques. [Google Scholar]
29. Roid, G. H. (2003). Stanford binet intelligence scales (5th Ed.). Itasca, IL: Riverside Publishing. [Google Scholar]
30. Palisano, R., Rosenbaum, P., Walter, S., Russell, D., Wood, E. et al. (1997). Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology, 39(4), 214–223. DOI 10.1111/j.1469-8749.1997.tb07414.x. [Google Scholar] [CrossRef]
31. Berg, A. T., Berkovic, S. F., Brodie, M. J., Buchhalter, J., Cross, J. H. et al. (2010). Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia, 51(4), 676–685. DOI 10.1111/j.1528-1167.2010.02522.x. [Google Scholar] [CrossRef]
32. Dunbar, M., Mineyko, A., Hill, M., Hodge, J., Floer, A. et al. (2020). Population based birth prevalence of disease-specific perinatal stroke. Pediatrics, 146(5), e2020013201. DOI 10.1542/peds.2020-013201. [Google Scholar] [CrossRef]
33. Bradley, S. M., Lipkin, P. H., Newburger, J. W., Peacock, G., Gerdes, M. et al. (2012). Neurodevlopmental outcomes in children with congenital heart disease: Evaluation and management. Circulation, 126, 1143–1172. DOI 10.1161/CIR.0b013e318265ee8a. [Google Scholar] [CrossRef]
34. Vojcek, E., Jermendy, A., Laszlo, A. M., Graf, R., Rudas, G. et al. (2021). The role of brain territorial involvement and infection/inflammation in the long-term outcome of neonatal arterial ischemic stroke: A population-based cohort study. Early Human Development, 158, 105393. DOI 10.1016/j.earlhumdev.2021.105393. [Google Scholar] [CrossRef]
35. Dimitropoulos, A., McQuillen, P. S., Sethi, V., Moosa, A., Chau, V. et al. (2013). Brain injury and development in newborns with critical congenital heart disease. Neurology, 81(3), 241–248. DOI 10.1212/WNL.0b013e31829bfdcf. [Google Scholar] [CrossRef]
36. Domi, T., Edgell, D. S., McCrindle, B. W., Williams, W. G., Chan, A. K. et al. (2008). Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics, 122(6), 1292–1298. DOI 10.1542/peds.2007-1459. [Google Scholar] [CrossRef]
37. Block, A. J., McQuillen, P. S., Chau, V., Glass, H., Poskitt, K. J. et al. (2010). Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery, 140(3), 550–557. DOI 10.1016/j.jtcvs.2010.03.035. [Google Scholar] [CrossRef]
38. Lynch, J. K., Nelson, K. B. (2001). Epidemiology of perinatal stroke. Current Opinion in Pediatrics, 13(6), 499–505. DOI 10.1097/00008480-200112000-00002. [Google Scholar] [CrossRef]
39. Yeh, S. J., Chen, H. C., Lu, C. W., Wang, J. K., Huang, L. M. et al. (2013). Prevalence, mortality, and the disease burden of pediatric congenital heart disease in Taiwan. Pediatrics and Neonatology, 54(2), 113–118. DOI 10.1016/j.pedneo.2012.11.010. [Google Scholar] [CrossRef]
40. Claessens, N. H. P., Chau, V., de Vries, L. S., Jansen, N. J. G., Au-Young, S. H. et al. (2019). Brain injury in infants with critical congenital heart disease: Insights from two clinical cohorts with different practice approaches. Journal of Pediatrics, 215, 75–82.E2. DOI 10.1016/j.jpeds.2019.07.017. [Google Scholar] [CrossRef]
41. Claessens, N. H. P., Algra, S. O., Ouwehand, T. L., Jansen, N. J. G., Schappin, R. et al. (2018). Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Developmental Medicine of Child Neurology, 60(10), 1052–1058. DOI 10.1111/dmcn.13747. [Google Scholar] [CrossRef]
42. Vojcek, E., Graf, R., Laszlo, A. M., Gyebnar, G., Seri, I. (2022). Long-term neurodevelopmental outcome of neonates born at term with perinatal haemorrhagic stroke: A population-based study. Developmental Medicine of Child Neurology. DOI 10.1111/dmcn.15149. [Google Scholar] [CrossRef]
Appendix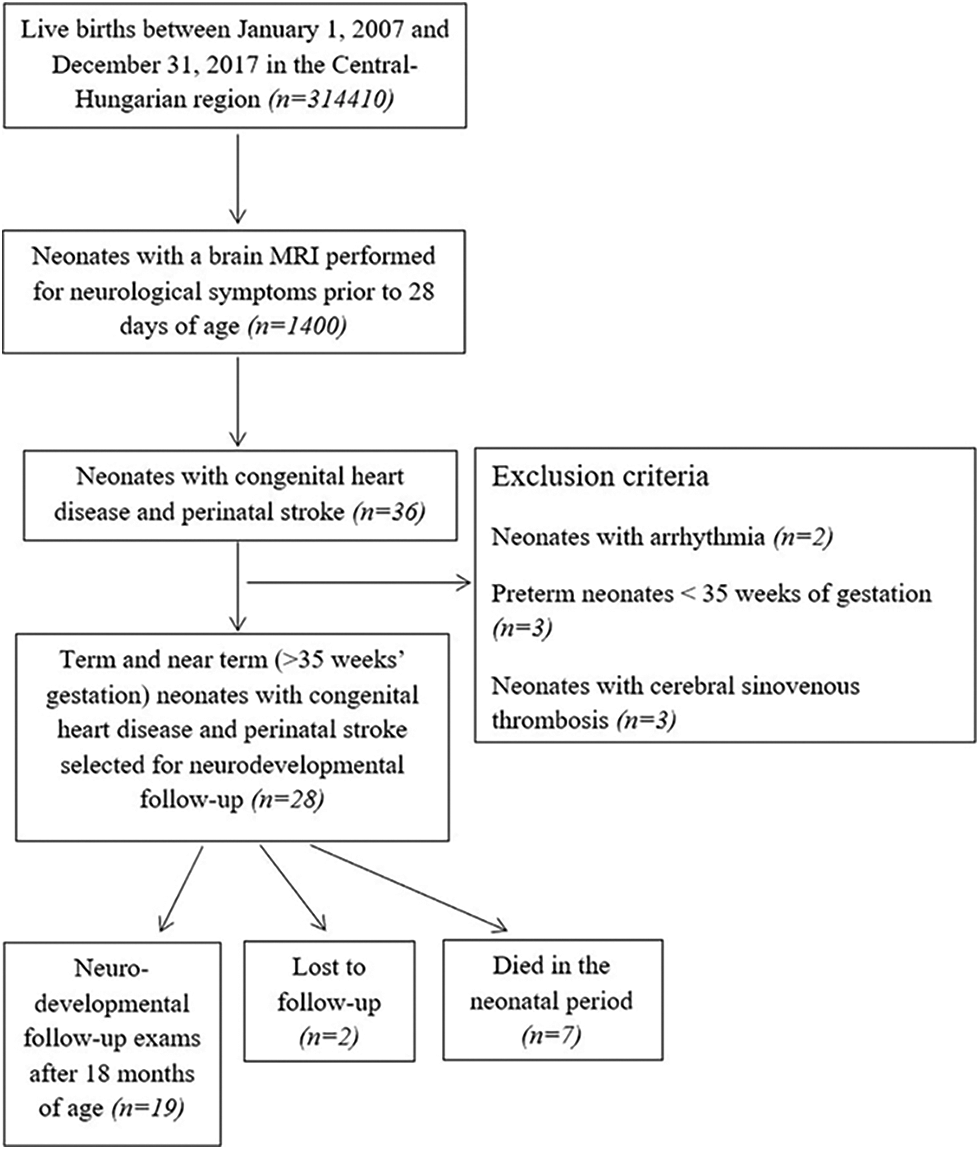
Appendix A.1: Patient selection and inclusion and exclusion criteria for neonates with congenital heart disease and acute perinatal stroke (Study Group)
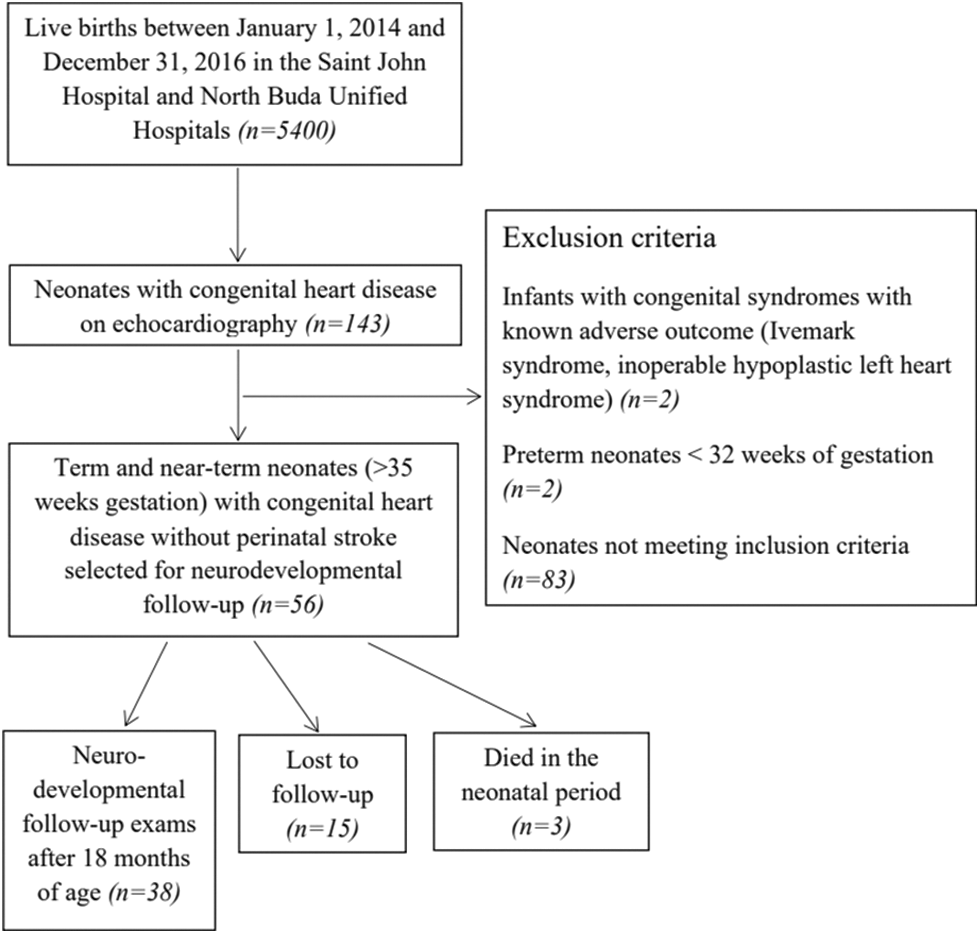
Appendix A.2: Patient selection and inclusion and exclusion criteria for neonates with congenital heart disease without acute perinatal stroke (Control-1 Group)
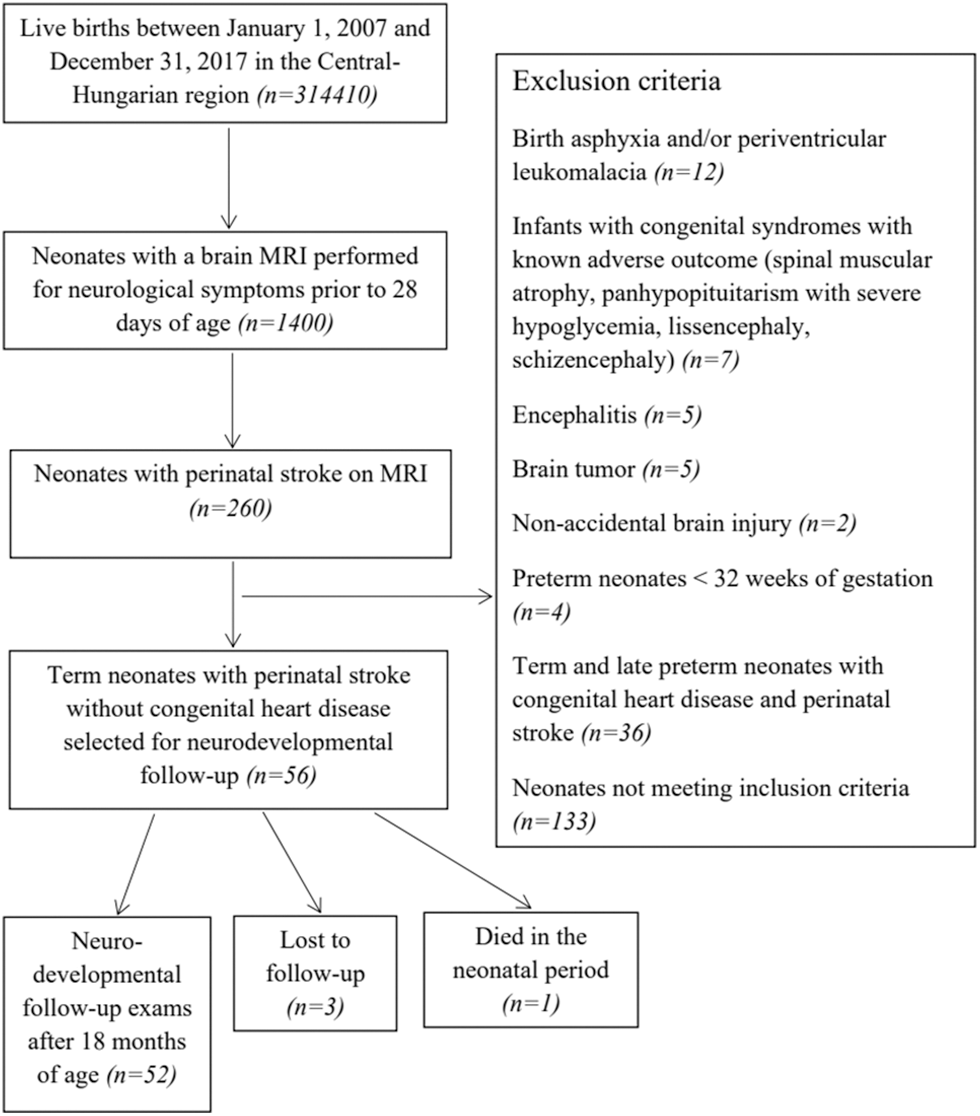
Appendix A.3: Patient selection and inclusion and exclusion criteria for neonates with acute perinatal stroke without congenital heart disease (Control-2 Group)
Appendix B: Neurodevelopmental tests used during the follow-up visits
In children up to 3 years of age, we used the Bayley Scales of Infant Development, Second Edition (BSID-II) mental and psychomotor developmental indices or by the revised Brunet-Lézine scale (global developmental quotient score and sub-scores). Beyond 3 years of age, the Stanford-Binet Intelligence Scales (Fifth Edition) was used to assess cognitive development. Moderate-severe impairment was classified as Stanford-Binet IQ score of <55, Revised Brunet-Lézine Developmental Quotient (DQ) score of <70 and Bayley-II Mental Developmental Index score of <70, while mild cognitive impairment was classified as Stanford-Binet IQ score of 79–55, Revised Brunet-Lézine DQ score of 70–84 and Bayley-II Mental Developmental Index score of 70–85. No cognitive impairment was defined as Stanford-Binet IQ score of ≤80, revised Brunet-Lézine DQ score of ≤85 and Bayley-II Mental Developmental Index score of <85.
Cerebral palsy was diagnosed by using the criteria of the European Cerebral Palsy Surveillance Network. Motor impairment was further classified with the Gross Motor Function Classification Scale (GMFCS). Behavioral problems were considered only after 3 years of age and clinical psychologists or pediatric psychiatrists established the diagnosis. The International League Against Epilepsy Commission on Classification and Terminology was used for classification of generalized and focal seizures and epileptic syndromes. Visual field defects were diagnosed by pediatric ophthalmologists. To assess visual-motor functioning, the Bender-Gestalt test was performed. Fine motor impairment was classified according to the Santucci classification. Language delay was defined as a language score of <1 standard deviation (SD) on BSID-II or on the revised Brunet-Lézine scale, or as a diagnosis of speech and/or language disorder. Hearing loss was defined as a need for hearing aid or reduced auditory evoked potentials.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |