

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.021545
ARTICLE
Congenital Coronary Artery Fistula in Children: A Review of 28 Cases with Clinical and Imaging Outcomes
1Division of Pediatric Cardiology, Department of Pediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
2Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
*Corresponding Author: Chodchanok Vijarnsorn. Email: cvijarnsorn@yahoo.com
Received: 20 February 2022; Accepted: 05 June 2022
Abstract: Background: Congenital coronary artery fistula (CCAF) is a rare anomaly. Treatment strategies tend to close the defect with a symptomatic and significant shunt, primarily based on expert consensus and case series. Results for long-term follow-up in children are limited Methods: We conducted a retrospective study to assess clinical and imaging outcomes of children with CCAF at Siriraj Hospital, Thailand during 2000–2020. Patients with single ventricle were excluded. Treatment strategies [surgical closure (SC), and percutaneous closure (PC)] were classified and the clinical outcomes at the follow-up in 2021, including coronary thrombosis, myocardial ischemia, and the results of cardiovascular imaging were reviewed. Results: Twenty-eight children with CCAF were included in the study. The median age at diagnosis was 2.5 years (2 days–18 years). Presenting symptoms were audible murmur (82%) and heart failure (35%). Most of fistulae arose from the right coronary artery (12/28) and exited at the right atrium (11/28). In recent visits (0.5–14 years follow-up), six patients with asymptomatic small CCAF were managed by watchful follow-up without complications. PC was primarily treated in 11 children: 7 underwent successful procedures; 1 had a residual shunt and required re-intervention; 1 had ischemic symptoms immediately after the procedure with left coronary occlusion that required device removal plus SC and 2 were technically unable to place the device, requiring SC. Four patients were waiting for interventions (1 PC and 3 SC). Cardiovascular imaging surveillance that followed closure demonstrated asymptomatic thrombus formation in three patients (1 PC and 2 SC). No mortality presented. Conclusion: CCAF with significant shunt is indicated to close either SC or PC. Ischemic events are rare but have been reported after closure. In addition, thrombus formation should be watched for post-intervention. Surveillance with cardiovascular imaging is recommended after defect closure (ideally 1–5 years post closure), or at interval follow-ups in patients with symptoms to evaluate possible recanalization, thrombus, or ischemia. Life-long clinical and echocardiographic follow-up is warranted. Watchful follow-up is acceptable for hemodynamically insignificant fistula without complication in the series.
Keywords: Congenital coronary artery fistula; pediatrics; surgical closure; transcatheter closure; thrombosis
Congenital coronary artery fistula (CCAF) is a rare form of congenital heart disease. It is defined as a direct connection of the coronary artery to an ending vessel (coronary artery fistula) or chamber (coronary cameral fistula). The prevalence of CAF is 0.002% in the general population, 0.4% among all cardiac malformations, and up to 0.9% in patients undergoing cardiovascular computerized tomography (CCT) for cardiac or non-cardiac purposes [1–5]. Moderate to large CCAF can lead to significant left to right shunting from the involved coronary artery to the exit structure, resulting in heart failure, myocardial steal, and ischemia. The presence of symptoms, complications, and a significant shunt are the main indications for percutaneous closure (PC) or surgical closure (SC) [5–10]. Post-closure sequelae, such as residual leakage, thrombosis with or without myocardial infarction, and coronary stenosis are important concerns [1,8,11,12], and watchful waiting in asymptomatic patients with small shunting has been accepted in the adult guidelines [6,10]. Cardiovascular imaging such as CCT and cardiovascular magnetic resonance (CMR) is widely used to evaluate high-risk coronary anomalies and for surveillance follow-ups [5,6,10,13]. To date, definite guidelines for the treatment, timing of interventions, and recommendations for long-term follow-up in children have not been established. At Siriraj Hospital, therapeutic interventions for children with CCAF have been performed, based on patient symptoms, shunt size, age, fistulous anatomy, associated cardiac anomalies, and underlying disease with an awareness of the risk-benefits and the limited resources. CCT and CMR have been used for diagnostic evaluations and follow-ups whenever possible. In this report, the authors aim to assess the clinical outcomes and results of imaging at follow-up for pediatric patients with CCAF who were treated at our medical center for the last two decades.
This retrospective study was approved by the Siriraj Institutional Review Board, Faculty of Medicine, Siriraj Hospital, Mahidol University (Study number 787/2563 (EC2)) and informed consent from patients was waived, though a specific process was required to ensure protection of the subjects’ confidentiality. All research methods were performed in accordance with Good Clinical Practice (GCP) guidelines and regulations. The Trial Registration Number is: TCTR20210602001.
Data from pediatric patients diagnosed with CCAF between January 01, 2000, and December 31, 2020, was reviewed. Patients with complex congenital anomalies such as pulmonary atresia with intact ventricular septum, or single ventricle were excluded from the study. Patient baseline characteristics included gender, age at diagnosis and treatment, presenting symptoms (cardiac murmur, congestive heart failure, or chest pain), ischemic evidence from 12-channel electrocardiography (ECG), and left ventricular ejection fraction (LVEF) from transthoracic echocardiography (TTE) at diagnosis. Fistula anatomy and associated cardiac condition were explored from TTE, CCT, CMR, or coronary angiography. Size of CCAF was classified as small, medium, or large if the fistula diameter was <1, 1–2, or >2-times the largest diameter of the coronary vessel not feeding the coronary fistula, as previously described [5]. Treatment strategies, including surgical closure (SC), percutaneous closure (PC), and conservative watchful follow-up were classified. The management of individual patients was discussed in pediatric cardiology and cardiovascular thoracic surgery conferences prior to proceeding with a closure. PC is preferred in patients with CCAF who have the proximal type, single exit site and non-tortuous fistula. If CCAF anatomy was not suitable for PC, a surgical approach was chosen. Asymptomatic small CCAF received a watchful follow-up. The PC technique via the transarterial or transvenous approach to the CAF origin and SC under general anesthesia and cardiopulmonary bypass were performed as standard protocols as reported in the literature [5,14,15].
Long-term clinical and imaging outcomes were examined, including major adverse cardiac events (MACE) and mortality, using either the current hospital’s databases or phone contact at the patient’s most recent follow-up visit on November 30, 2021. An MACE was defined as having a cardiovascular-related illness including coronary occlusion, heart failure, cardiac tamponade, clinical or imaging evidence of coronary thrombosis, and myocardial ischemia (MI) by either CMR, CCT, or coronary angiography (CAG), requiring coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI) following treatment.
Statistical analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA). Demographics and treatment data were presented as frequencies with percentages for the categorical variables and mean ± SD or median with a range for continuous variables.
The study was comprised of 28 children, with demographics and characteristics of CCAF shown in Table 1. The age at diagnosis and age at treatment ranged from 2 days to 18 years (median of 2.5 years) and 1 month to 15 years (median of 5 years), respectively. Females predominated (57%). The common presentation in this series was cardiac murmur (82%), while heart failure was reported in 35% of the patients. Of the 28 patients, only 5 had a decreased NYHA FC to II. Isolated CCAF was identified in 15 patients and associated simple congenital heart disease was noted in 13 patients (46%), which included ventricular septal defect, atrial septal defect, partial atrioventricular canal (PAVC), patent ductus arteriosus, bicuspid aortic valve, tetralogy of Fallot, and pulmonary atresia with ventricular septal defect (PA/VSD). The type of CCAF was mostly proximal (n = 25), and most of these were of a single opening with a diameter ranging from 1 to 10.9 mm (median of 4.9 mm). Eighteen patients had significantly enlarged terminal openings, which required closure. Complex fistulous structures were observed such as two openings (2 cases), tortuosity (8 cases), aneurysm (1 case), and single coronary artery (2 cases). The most common origin and drainage sites for the fistulae were RCA (n = 12) and RA (n = 11), respectively. All patients had preserved LV function and no ischemic pattern on ECG.
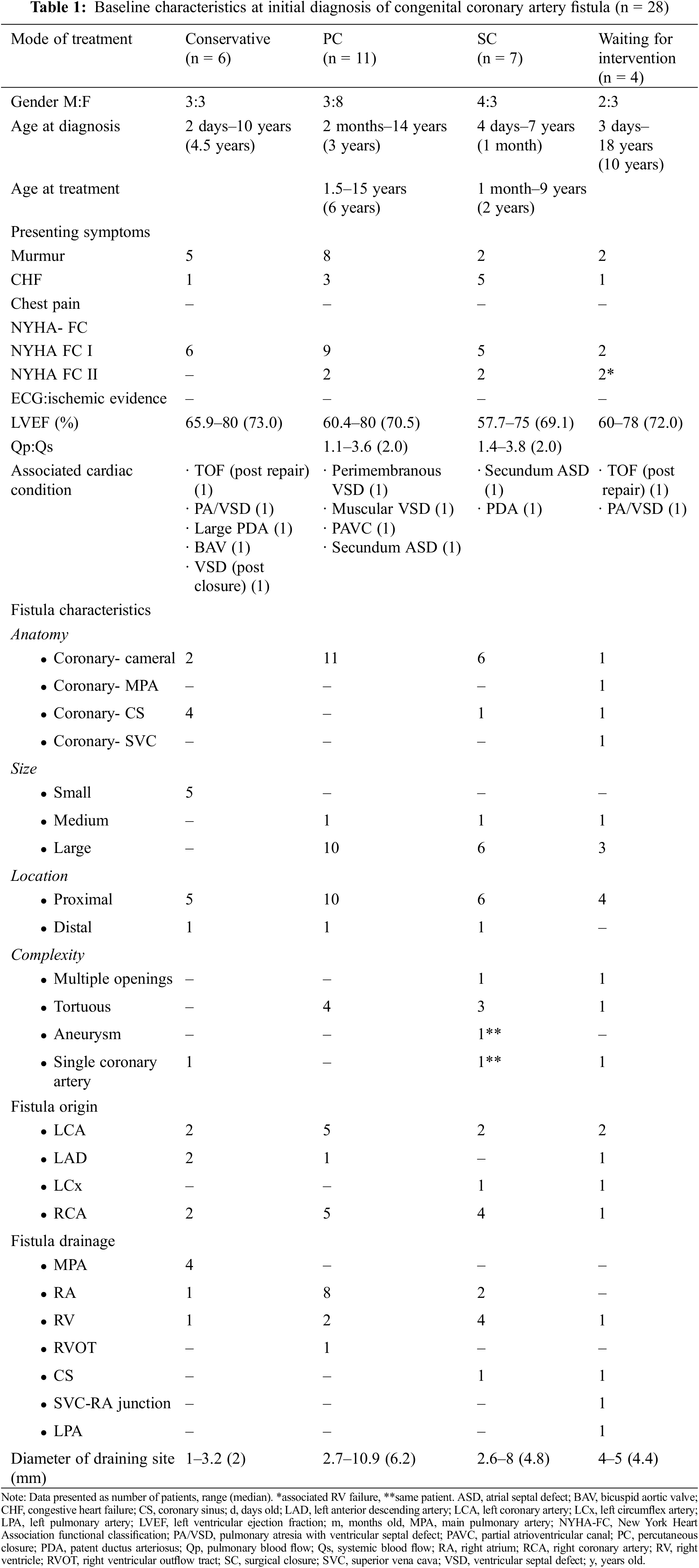
Eleven and seven patients who had significant increased pulmonary blood flow (Qp) underwent percutaneous closure (PC) and surgical closure (SC), respectively. Complex fistula such as multiple drainage sites and aneurysm formation were sent to surgery as the primary treatment (Table 1). Four patients were on a waiting list for intervention and six patients had watchful follow-up. No death was reported in any of the treatment groups (Fig. 1).
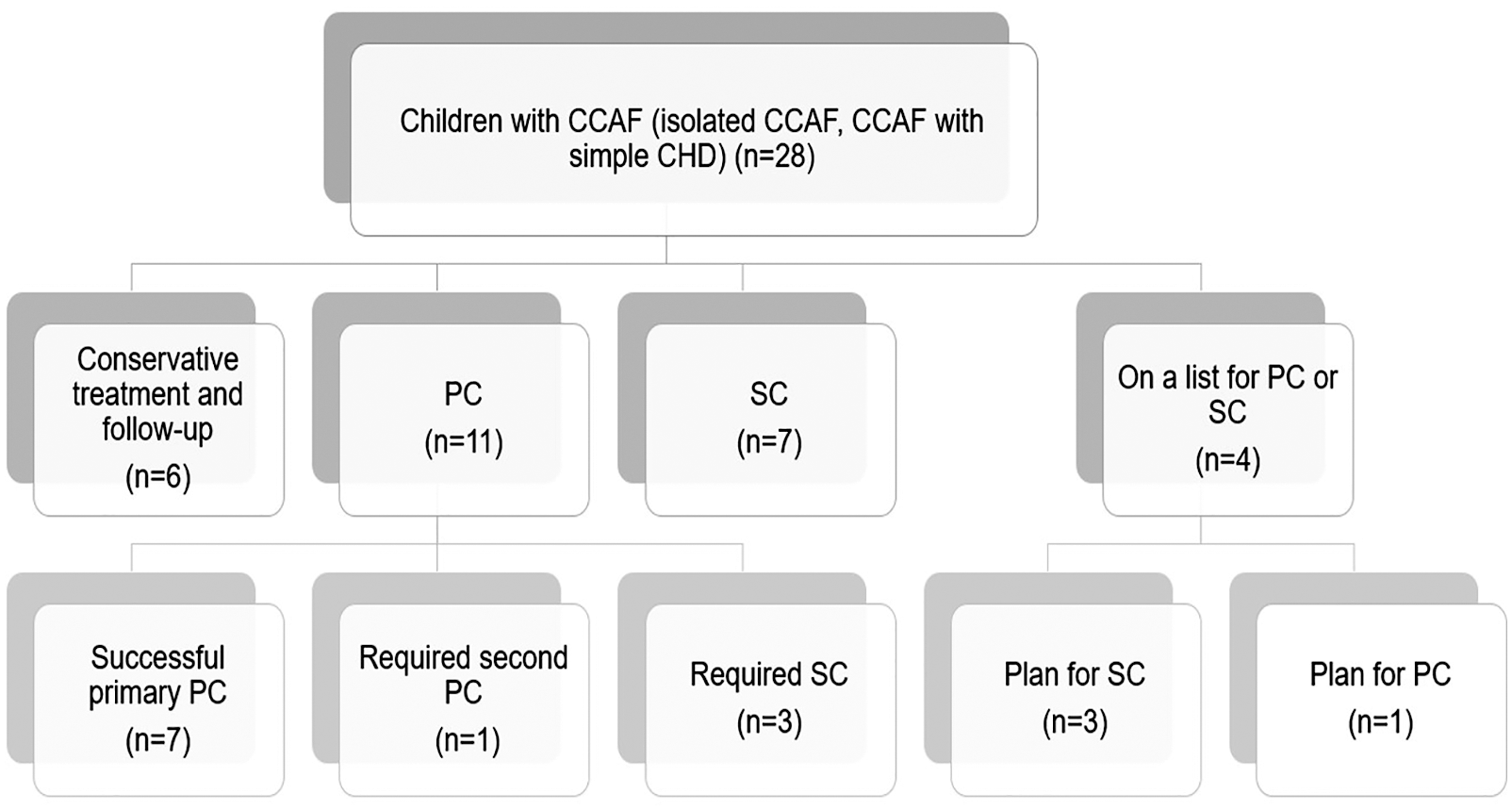
Figure 1: Treatment flow chart for all patients in the study (n = 28) (CCAF, congenital coronary fistula; PC, percutaneous closure; SC, surgical closure)
4 MACE, Clinical and Imaging Outcomes
4.1 Conservative Treatment and Watchful Follow-Up
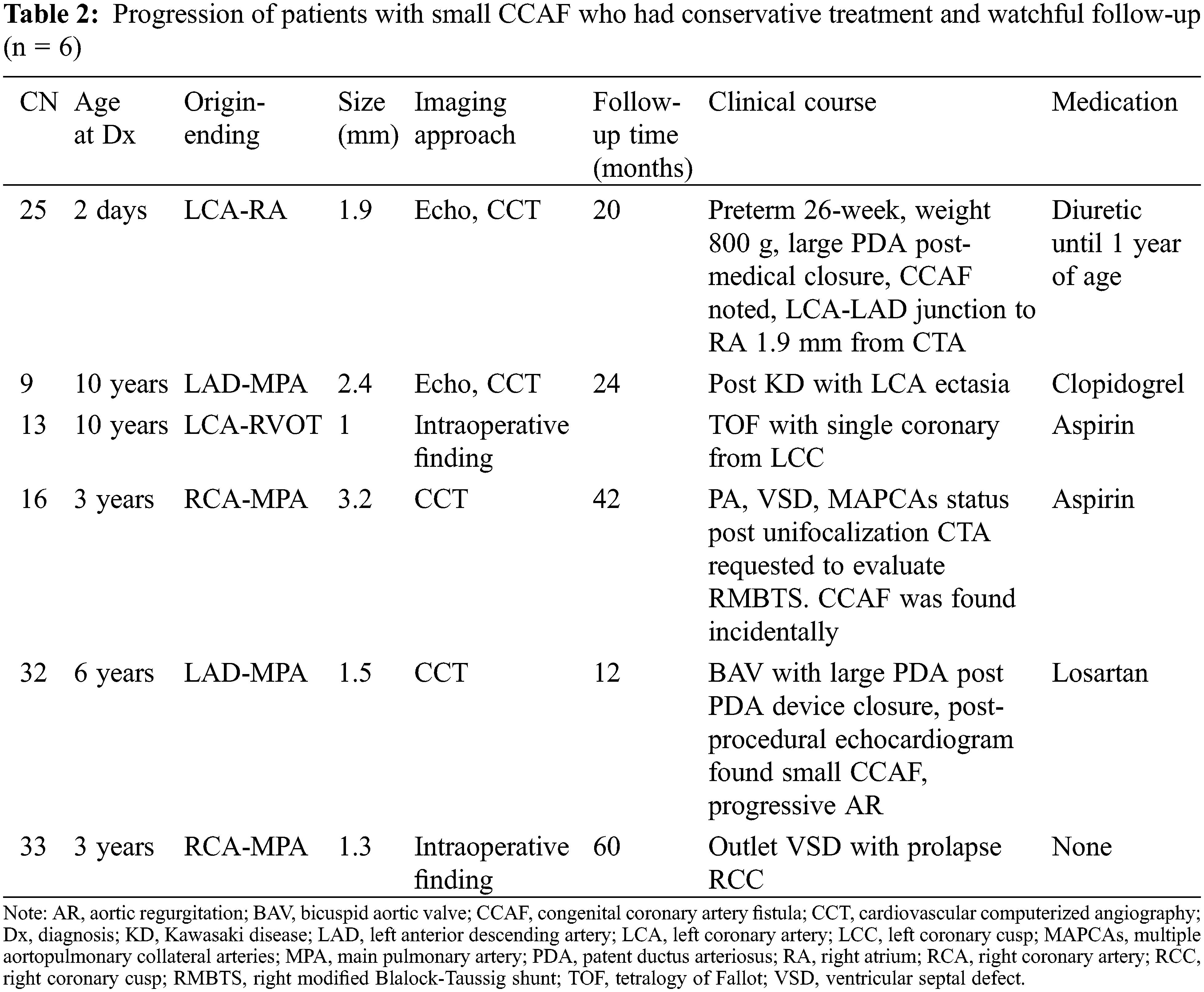
Six patients were treated with watchful follow-ups (Table 2). All patients had single opening fistula (diameter from 1.3 to 3.2 mm). The youngest age at diagnosis was 2 days in a female neonate who was born at the gestational age of 26 weeks with a birthweight of 800 g. With respiratory distress syndrome, large PDA with a CCAF size of 1.7 mm. She had CHF mainly from a large PDA. Ventilator support and medical closure for PDA were required. Her clinical condition greatly improved, and therefore, her CCAF was left untreated, and she was given a watchful follow up. She was discharged home with low dose diuretic. During a follow-up, she was thriving. The CCT at 1.5 years of age showed a small sized 1.9 mm CCAF from the LCA-LAD junction to the RA. Diuretic was discontinued. This patient was thriving and has been followed-up regularly. Of the six patients, three were diagnosed with small CCAF by incidental findings from CCT, which was performed due to their primary cardiac condition (CN9, CN16, and CN 32). The other two patients (CN13, CN33) were found intraoperatively during surgical repair for their congenital heart disease. One patient had PA/VSD post-unifocalization with a medium-sized CCAF diameter of 3.2 mm. This patient had an inadequate pulmonary vascular bed, and could not undergo further surgery, and was classified in this group. During a follow-up period (range 1–5 years), all patients were doing well, without signs of CHF or ischemic cardiomyopathy. No MACE was reported.
Eleven patients underwent PC. The median age at diagnosis and treatment was 3 years (range 2 months–14 years) and 6 years old (range 1.5–15 years), respectively. All patients had single fistulous opening (diameter 2.7–10.9 mm). TTE was used as a diagnostic tool and then the therapeutic diagnosis was confirmed by traditional CAG. Hemodynamic data from cardiac catheterization showed significant left to right shunt with a median Qp:Qs of 2. Overall, seven patients had successful primary PC without significant residual shunt. One patient had a post-procedural residual shunt requiring a re-PC. Of the 11 patients, three required subsequent SC due to 1 device migration and 2 unsuccessful PCs (Fig. 1) (Table S1).
In 7 patients with successful PC [median age 6 years (range 2.5–15 years)], an AmPlatzer Vascuar Plug II (AVP II) was commonly used to close the fistulous tract. No procedure had related complications such as wound infection, bacterial arteritis, device migration, fistula dissection, or hemolysis in this group. The average hospital length of stay was 3 days (including the pre-operative period). Medical treatment for heart failure could be discontinued after 6 months following the intervention. Residual shunt with spontaneous resolution was noted in a 4-year-old patient (CN24), who had a 6 mm diameter CCAF from RCA to RA and who underwent PC using a 6 mm AVP II. The tiny residual shunt was noted on the postoperative echocardiogram at one month. After dual antiplatelet therapy for 6 months, the subsequent echocardiogram showed no residual lesion, and the CMR 3 years later confirmed good cardiac function without residual lesion or thrombosis. During follow-ups from 2–14 years, all patients were relatively asymptomatic and were classified as NYHA FC I. Five patients had surveillance follow-up with CCT or CMR. Importantly, one patient with a large LCA-RVOT CCAF (10.9 mm diameter at the terminal site) had transient thrombus formation after PC using 14 AVP II, but was without ischemic heart symptoms at the subsequent CCT 6-months post-procedure. Warfarin and aspirin were prescribed. The CMR at one-year follow-up showed normal wall motion with LVEF of 56.2% and resolved thrombus formation. Warfarin was then discontinued.
The patient who required re-PC was a 12-year-old female with Down syndrome status post total repair of PAVC. The CCAF from RCA to RA with a terminal diameter of 6.8 mm was found during a postoperative TTE. Coil embolization was performed using a 038–8–8 Gianturco coil but the procedure was not successfully deployed. Re-intervention was performed successfully two months later using a 10 mm AVP II. Warfarin and aspirin were prescribed. At a recent follow-up at 6 months, the patient was classified as NYHA FC I, asymptomatic, and no residual shunt presented.
Following a primary PC, three cases required SC according to different indications. The first case was of a 1.5-year-old girl who had CCAF from LAD to RV apex with a terminal diameter of 3.1 mm. She underwent the procedure for 6 mm AVP II implantation and had ST elevation intraoperatively after the device was deployed. Emergency surgical ligation was performed, and total occlusion of the mid-LAD was noted due to the device migration. Her total length of stay in hospital was 8 days. Heart failure was controlled. All medications were discontinued at the 1-year follow-up. At the 5-year follow-up, post-procedure, she was asymptomatic and placed on Aspirin. The TTE showed no residual CCAF with normal LV function. Her recent CCT showed no thrombus formation or coronary dilation. The second patient was a 6-year-old boy who had muscular VSD in addition to CCAF from proximal RCA to RV (terminal diameter of 3 mm) with Qp:Qs of 2.56. PC was attempted with a 10 mm, and then followed with a 12 mm AVP II. Nevertheless, a significant residual shunt was present, and the procedure was then abandoned. Consultation was made with the surgery team. Surgical ligation of CCAF together with VSD closure was performed a year later. At this time, he has been followed-up for 9 years with a good functional class and LV function. During the CMR surveillance at 7-year post-procedure, a scar at the mid-postero-inferior LV was noted with a LVEF of 76%. The third patient was a 5-year-old boy who had CCAF from RCA to RA with a terminal site diameter of 8 mm. PC was performed unsuccessfully due to a failed loop creation. CCT showed a proximal type CCAF with a severely dilated proximal RCA of 12.6 mm. Elective SC was performed two months later. At the 6-month follow-up, he was asymptomatic with no residual shunt.
Primary treatment with SC was chosen for 7 patients [median age at diagnosis of 1 month (range 4 days–7 years)], all of whom had isolated CCAF with symptoms of heart failure. All of the preoperative TTEs showed good LV function. The median age at the time of the repair was 2 years (range 1 month–9 years). A complex fistula (i.e., 2 openings) was noted in one patient. All patients had dilated fistulous tracts and one had the drainage site to the coronary sinus (Fig. 2). The surgical procedures included surgical ligation/direct closure (n = 5), pericardial patch closure (n = 1), and fistulous ligation with aneurysmorraphy (n = 1). In addition, three patients underwent surgical ligation subsequent to the attempted PC, as previously described. Ten patients who underwent SC had no operative mortality. The median cardiopulmonary bypass and cross clamp time was 34 min (range 15–86 min) and 15 min (range 10–54 min), respectively. Postpericardiotomy syndrome and transient asymptomatic ST depression was noted in one patient. Average hospital length of stay was eight days (including a pre-operative day). At the median time of follow-up [3.3 years (range 0.1–8 years)], the ten patients survived without ischemic heart or heart failure symptoms. Four patients had postoperative CCT and CMR surveillance. Two patients with post-operative thrombus formation in the dilated fistulous tract had a stenosis of the distal coronary artery without perfusion deficit and they had preserved LV function. Characteristics of the original CCAF were complex: 2 large openings in 1 patient and a large-diameter drainage site with a dilated distal coronary artery in one patient (Figs. 3 and 4) (Table S1).
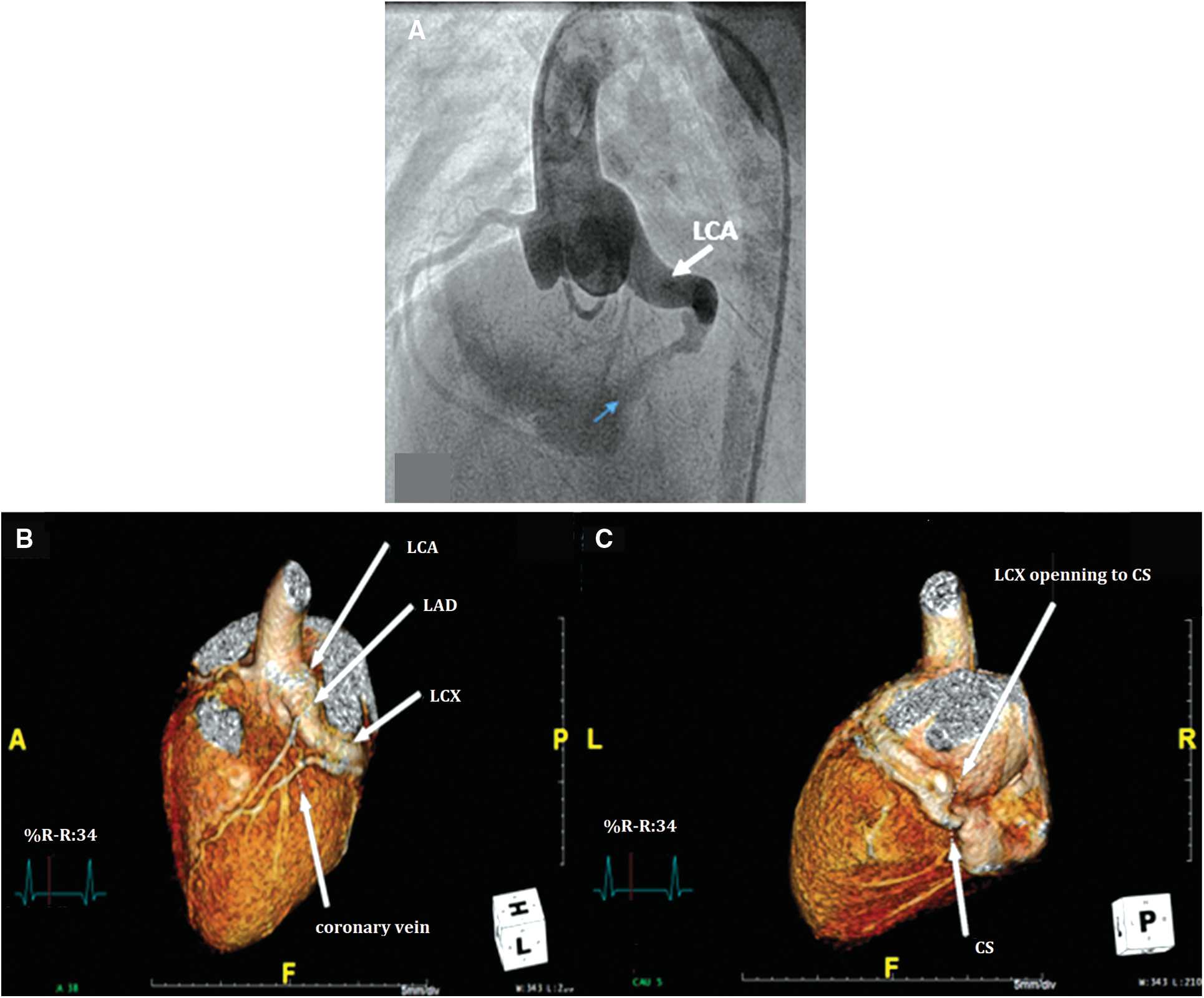
Figure 2: Pre-operative images of congenital coronary artery fistula (CCAF) from left circumflex artery (LCx) to coronary sinus (CS) in a 6-year-old boy. A. Aortic root angiogram demonstrating the dilated left coronary artery (LCA) with tortuous CCAF to the CS opening (blue arrow); B, C. Reconstructed cardiovascular computerized tomography showing CCAF from LCx to CS with tubular ectasia of LCA (LAD, left anterior descending artery)
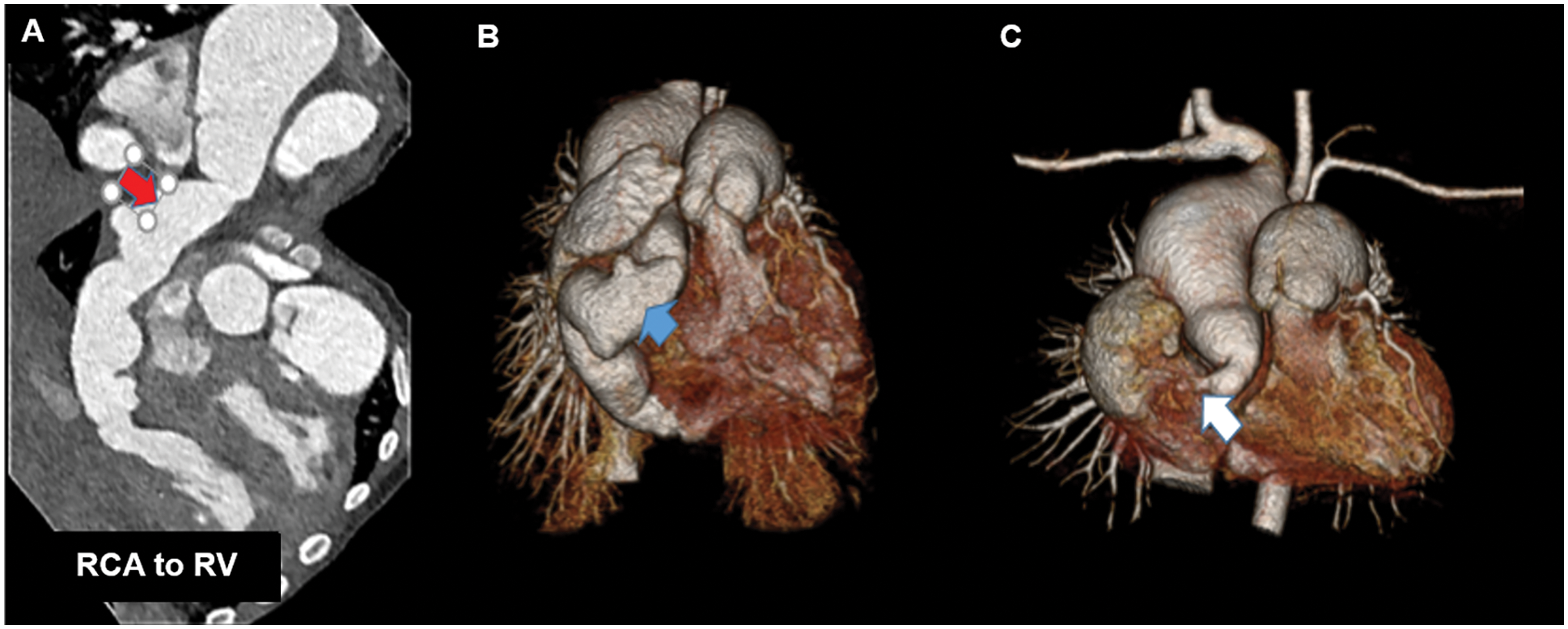
Figure 3: Pre- and post-operative cardiovascular computerized tomography (CCT) in a one-year-old girl with a large congenital coronary artery fistula (CCAF) from the right coronary artery (RCA) to the right ventricle (RV); A, B. Pre-operative CCT demonstrating the large tortuous RCA and the CCAF from RCA to RV (red arrow); C. Post-operative CCT showing total RCA occlusion (white arrow) compared to pre-operation (blue arrow) in B
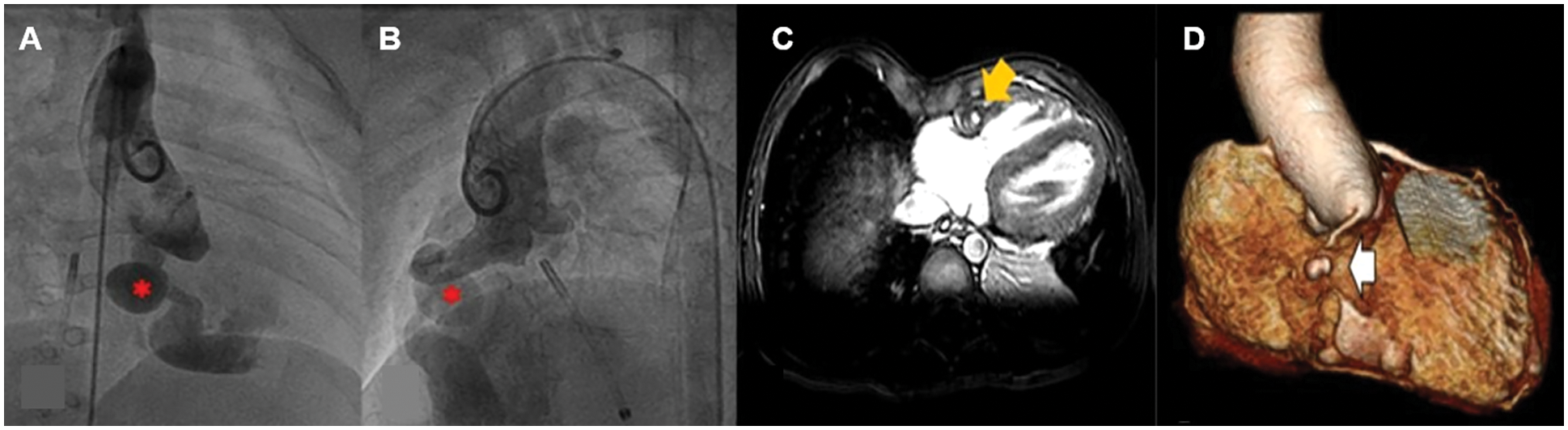
Figure 4: Pre-operative aortic root angiography in a patient at one year of age and cardiac image at 6-year post-operation with large congenital coronary artery fistula (CCAF) from the right coronary artery (RCA) to the right ventricle (RV); A, B. Aortic root angiography showing aneurysmal dilation of RCA and CCAF from proximal RCA to RV (*); C, D. Post-operative CMR showing filling defect (yellow arrow) at the mid-RCA due to intraluminal thrombus. The 3-D reconstruction shows a small, mid-RCA aneurysm (white arrow). No RCA flow was observed distal to the aneurysm
Four children (age at diagnosis 3 days–18 years) were waiting for closure intervention. Three patients were to undergo SC and among these, two were to undergo a repair of their underlying heart defects (redo pulmonary valve replacement and a primary repair of TOF). One had isolated CCAF (LCA to RA) with two drainage openings. One patient with RCA to an RV fistula was on the waiting list for PC.
This cohort study describes the long-term clinical and imaging outcomes of 28 children with CCAF at a single medical center in Thailand. Closure of moderate to large defects was performed by either PC or SC in usual ways for 18 patients. No operative mortality was reported, though acute coronary syndrome following PC occurred in one patient, requiring emergency surgical removal and fistula ligation. Of 10 patients who had imaging surveillance post-intervention, thrombotic occlusion of fistula without evidence of MI was noted in three of the patients (1 PC, 2 SC). The presence of a small area of myocardial scar was observed in one patient who had SC following a failed PC without ischemic or heart failure symptoms. In addition, watchful follow-up was used to reveal hemodynamically insignificant fistula without complication in the series. The result from this series indicates that thromboembolic risk does not end with the repair, even though it might be asymptomatic.
CCAF is a rare anomaly, and the pathophysiology depends on the pressure resistance between the fistula connection, the site, and the opening size at the termination. Coronary cameral fistulae were the most common fistulous connections in our series, which is similar to previous reports, illustrating that the shunt commonly involves the right coronary system and mostly drains into the right atrium or right ventricle [1,13]. Most of the children presented with an audible murmur (82%) or heart failure due to the volume load (35%). No ECG abnormalities were reported in our series. Practitioners are recommended to not rely solely on an uneventful resting ECG, which can occur in CCAF. The modality of treatment at the medical center is based on size and clinical symptoms. In contrast to the adult guidelines [5,6,10], ventricular dysfunction or ischemic cardiomyopathy in children is uncommon and none of the patients in our series reported chest pain or ischemic heart symptoms even though most of them had medium-to large-sized CCAF (82%). A small CCAF can be followed with watchful follow-up since spontaneous closure has been reported at a rate of 1%–2% [6,9,16]. In any case, neither spontaneous closure nor MACE was noted in the 5-year follow-up of five patients in our series who had small CCAF with conservative treatment.
Closure intervention has been recommended for medium and large CAF since fistula-related complications can arise with age, even when asymptomatic and especially in adult patients [5,6,10]. PC is indicated for CAF with favorable anatomy, including a single, narrow drainage site, proximal fistula origin, absence of multiple fistulae or large branching vessels adjacent to the optimal plugging position, and when the catheter and device can be implemented to safely close the fistula [5,8,9,12]. Different incidences of clinical outcomes and complications have been reported in prior publications, including post-coronary dilatation, residual shunt, thrombosis with or without MI or coronary stenosis, and death [9,11,12]. Long-term ischemic events have been found in association with a drainage site into the coronary sinus and with fistulae that originate from distal segments of ectatic coronary vessels, especially for sizes that are greater than 10 mm [5,12]. In the present study, moderate to large fistulae can be closed percutaneously despite some tortuosity. The proximal type with a single opening was noted. Among the 11 patients that underwent PC, 2 had residual shunt (18%) and 1 had device migration (9%), resulting in mid-RCA occlusion. Device mismatching was a plausible reason for these complications. PC with coil embolization is suitable for a fistula opening less than 5 mm, while a vascular plug is recommended for patients with a fistula opening that is larger by choosing the device number to be 1.5–2 times larger than the lesion [1,5,15]. SC is the mainstay therapy for medium to large CCAF and it has excellent immediate outcomes. Nevertheless, a risk of cardiopulmonary bypass and median sternotomy exists, and surgical complications can include bleeding, postpericardiectomy syndrome, arrhythmia, myocardial infarction, cerebrovascular accident, and mediastinitis, which can occur early post-operatively [8,13]. Similar to PC, residual shunt/recanalization and coronary thrombosis has been reported in long-term follow-up (3.6%–9.6% and 9.6%–34.2%, respectively) [8,11,14,17]. In this study, three children (10.7%; 1 PC and 2 SC) were noted to have thrombotic occlusion of the fistula without evidence of MI, which raises a concern that propagated coronary thrombosis is not uncommon following CCAF closure.
To our knowledge, no specific postprocedural thromboprophylaxis following percutaneous closure (PC) or surgical closure (SC) of congenital coronary artery fistula (CCAF) closure is typically recommended by the standard guidelines [18,19]. Furthermore, treatment strategies for thrombotic occlusion of the fistula in children are debated, unlike Kawasaki disease, which has been well- described for the management of coronary aneurysm with thrombus. The recent retrospective review of CCAF in neonate and infants [20] showed the usage of thromboprohylaxis; antiplatelets and anticoagulation in 90% of patients post closure. Suboptimal remodeling was also reported in some patients. Therefore, thromboprophylaxis post-closure of CCAF and treatment of coronary thrombosis is variable, depending on the surgeon’s preference. In our center, a mainstay thromboprophylaxis following closure of CCAF is antiplatelet (aspirin; ASA). If a large fistula segment or aneurysmal dilation occurs, anticoagulant; warfarin is often added. In the three patients who experienced propagated coronary thrombosis, two patients were prescribed ASA and warfarin post-closure due to a previous large fistula segment. The third patient had only ASA postoperatively. The coronary thrombus was subsequently shown in her 3-month post-operative echocardiography, and clopidogrel was added. All patients were relatively asymptomatic.
Currently, CCT is widely accepted as the gold standard imaging modality to evaluate coronary anomalies and CMR plus coronary magnetic resonance angiography is a standard tool used to evaluate myocardial function, myocardial viability, and the delineation of the coronary anatomy. In our center, the traditional protocol for the follow-up for patients with CCAF used interval echocardiography postoperatively. Life-long clinical assessment and echocardiography is routinely used in the follow-up. Presently, both CCT and CMR and an anesthetic team are available for pediatric patients in the center. We recommend a follow-up cardiac imaging for surveillance after defect closure (ideally 1–5 years post closure), or at follow-up intervals for patients with symptoms to evaluate possible recanalization, thrombus, or ischemia, as described in the adult guidelines [5]. Regarding a conservative treatment group, we routinely schedule patients for a follow-up, to assess clinical signs and symptom with serial transthoracic echocardiogram, which includes a functional and flow study. No ischemic symptoms or left ventricular dysfunction were reported. The patients had very small lesions, which would not likely lead to heart failure or the steal phenomenon and consequent ischemic heart disease. Therefore, no patients in this group were scheduled for strain echocardiography, stress echocardiography, or adenosine stress CMR since clinical signs and symptoms were not present. In any case, it would be of interest to include a tissue Doppler and strain echocardiography in the protocol for these patients in the future.
SC or PC is indicated to close CCAF with significant shunt. Most of the patients in our study were in a good functional class after the defect closure. Nevertheless, thrombus formation can occur after the procedure (up to 10.7%, even in asymptomatic patients). A scar at the LV myocardium is rare but it has been reported. Post-intervention surveillance by cardiovascular imaging is recommended once after defect closure (ideally 1–5 years post closure), or at an interval follow-up in patients with symptoms to evaluate recanalization, thrombus, or ischemia. Life-long clinical assessment and echocardiographic follow-up is warranted. Watchful follow-up is acceptable for hemodynamically insignificant fistulae without complication.
Acknowledgement: The authors acknowledge the faculty staff of Cardiovascular Thoracic Surgery, Faculty of Medicine Siriaj Hospital for their support and involvement with the care of patients. The authors also thank Dr. Glen Wheeler for his proofreading and editing.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: CV, PP; data collection: PP; analysis and interpretation of results: CV, PP; draft manuscript preparation: CV, PP. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Diab, K. A., Boujemline, Y., Hijazi, Z. M. (2021). Update on shunt closure in neonates and infants. Expert Review of Cardiovascular Therapy, 19(6), 475–492. DOI 10.1080/14779072.2021.1922079. [Google Scholar] [CrossRef]
2. Ouchi, K., Sakuma, T., Ojiri, H. (2020). Coronary artery fistula in adults: Incidence and appearance on cardiac computed tomography and comparison of detectability and hemodynamic effects with those on transthoracic echocardiography. Journal of Cardiology, 76(6), 593–600. DOI 10.1016/j.jjcc.2020.06.005. [Google Scholar] [CrossRef]
3. Yamanaka, O., Hobbs, R. E. (1990). Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheterization and Cardiovascular Diagnosis, 21, 28–40. DOI 10.1002/(ISSN)1097-0304. [Google Scholar] [CrossRef]
4. Karazisi, C., Eriksson, P., Dellborg, M. (2017). Coronary artery fistulas: Case series and literature review. Cardiology, 136(2), 93–101. DOI 10.1159/000447445. [Google Scholar] [CrossRef]
5. Al-Hijji, M., El Sabbagh, A., El Hajj, S., AlKhouli, M., El Sabawi, B. et al. (2021). Coronary artery fistulas: Indications, techniques, outcomes, and complications of transcatheter fistula closure. JACC Cardiovascular Interventions, 14(13), 1393–1406. DOI 10.1016/j.jcin.2021.02.044. [Google Scholar] [CrossRef]
6. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2021). 2020 ESC guidelines for the management of adult congenital heart disease. European Heart Journal, 42(6), 563–645. DOI 10.1093/eurheartj/ehaa554. [Google Scholar] [CrossRef]
7. Armsby, L. R., Keane, J. F., Sherwood, M. C., Forbess, J. M., Perry, S. B. et al. (2002). Management of coronary artery fistulae: Patient selection and results of transcatheter closure. Journal of the American College of Cardiology, 39(6), 1026–1032. DOI 10.1016/S0735-1097(02)01742-4. [Google Scholar] [CrossRef]
8. Wang, X., Pang, C., Liu, X., Wang, S., Zhang, Z. et al. (2020). Congenital coronary artery fistula in pediatric patients: Transcatheter versus surgical closure. BMC Cardiovascular Disorder, 20(1), 484–492. DOI 10.1186/s12872-020-01769-7. [Google Scholar] [CrossRef]
9. Buccheri, D., Chirco, P. R., Geraci, S., Caramanno, G., Cortese, B. (2018). Coronary artery fistulae: Anatomy, diagnosis and management strategies. Heart, Lung & Circulation, 27(8), 940–951. DOI 10.1016/j.hlc.2017.07.014. [Google Scholar] [CrossRef]
10. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation, 139(14), e637–e697. DOI 10.1161/cir.0000000000000602. [Google Scholar] [CrossRef]
11. Li, X., Song, L., Xu, M., Zhang, G., Jin, J. (2020). Long-term follow-up of pediatric patients after congenital coronary artery fistula closure. Pediatric Cardiology, 41(7), 1346–1353. DOI 10.1007/s00246-020-02379-y. [Google Scholar] [CrossRef]
12. El-Sabawi, B., Al-Hijji, M. A., Eleid, M. F., Cabalka, A. K., Ammash, N. M. et al. (2020). Transcatheter closure of coronary artery fistula: A 21-year experience. Catheterization and Cardiovascular Interventions, 96(2), 311–319. DOI 10.1002/ccd.28721. [Google Scholar] [CrossRef]
13. Kalisz, K., Sanders, A. E., Avery, R., Allen, B. D. (2020). Coronary artery fistulas: A review of the current and future roles of imaging. Journal of Thoracic Imaging, 36(6), 333–344. DOI 10.1097/rti.0000000000000557. [Google Scholar] [CrossRef]
14. Wang, Y., Yang, Y., Xia, L., Ding, W., Ji, Q. et al. (2021). Surgical correction of coronary artery ectasia combining congenital coronary artery fistula. Congenital Heart Disease, 16(1), 95–106. DOI 10.32604/CHD.2021.014276. [Google Scholar] [CrossRef]
15. Firouzi, A., Hosseini, Z., Khajali, Z., Saedi, S., Alemzadeh Ansari, M. J. (2020). Transcatheter closure of coronary artery fistulae: A literature review. Congenital Heart Disease, 15(1), 21–31. DOI 10.32604/CHD.2020.011515. [Google Scholar] [CrossRef]
16. Schleich, J. M., Rey, C., Gewillig, M., Bozio, A. (2001). Spontaneous closure of congenital coronary artery fistulas. Heart, 85(4), DOI 10.1136/heart.85.4.e6. [Google Scholar] [CrossRef]
17. Cheung, D. L., Au, W. K., Cheung, H. H., Chiu, C. S., Lee, W. T. (2001). Coronary artery fistulas: Long-term results of surgical correction. Annals of Thoracic Surgery, 71(1), 190–195. DOI 10.1016/S0003-4975(00)01862-2. [Google Scholar] [CrossRef]
18. Giglia, T. M., Massicotte, M. P., Tweddell, J. S., Barst, R. J., Bauman, M. et al. (2013). Prevention and treatment of thrombosis in pediatric and congenital heart disease: A scientific statement from the American Heart Association. Circulation, 128(24), 2622–2703. DOI 10.1161/01.cir.0000436140.77832.7a. [Google Scholar] [CrossRef]
19. Nair, A. G., Oladunjoye, O. O., Trenor III, C. C., LaRonde, M., van den Bosch, S. J. et al. (2018). An anticoagulation protocol for use after congenital cardiac surgery. Journal of Thoracic Cardiovascular Surgery, 156(1), 343–352.e4. DOI 10.1016/j.jtcvs.2018.02.106. [Google Scholar] [CrossRef]
20. Gowda, S. T., Latson, L., Sivakumar, K., Hiremath, G., Crystal, M. et al. (2021). Anatomical classification and posttreatment remodeling characteristics to guide management and follow-up of neonates and infants with coronary artery fistula: A multicenter study from the coronary artery fistula registry. Circulation Cardiovascular Interventions, 14(12), e009750. DOI 10.1161/CIRCINTERVENTIONS.120.009750. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |