

 | Congenital Heart Disease |  |
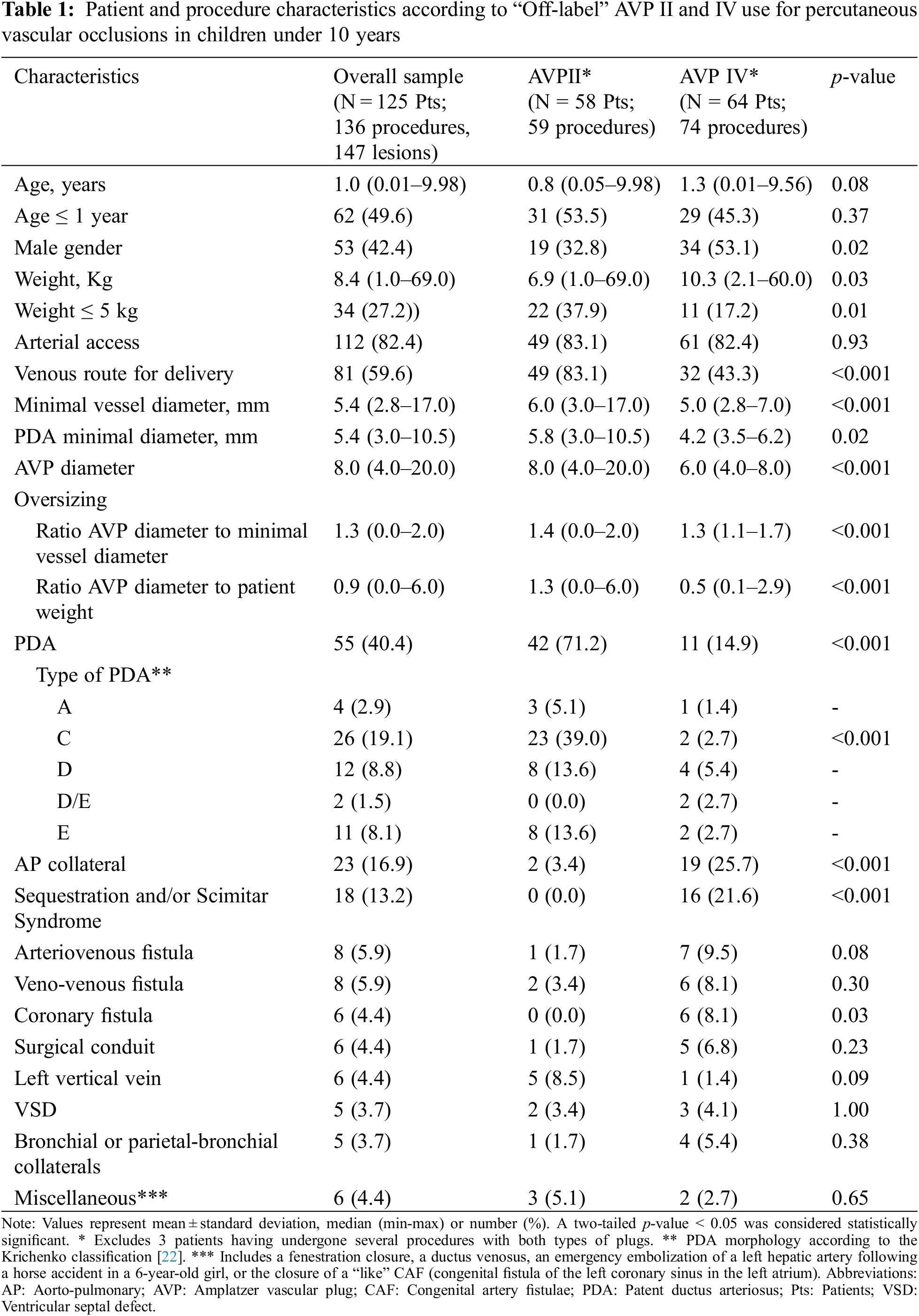
DOI: 10.32604/chd.2022.020835
ARTICLE
Efficacy, Safety and Characteristics of the Amplatzer Vascular Plug II and IV Utilization for Various Percutaneous Occlusions in Children under 10 Years
1Antilles-Guyane M3C Paediatric Cardiology Center, University Hospital of Martinique, Fort-de-France, France
2L’institut du Thorax, INSERM, CNRS, UNIV Nantes, CHU Nantes, Nantes, France
3Department of Paediatric Cardiology and Pediatric Cardiac Surgery, University Hospital of Nantes, Nantes, France
4Department of Paediatric and Congenital Heart Disease, University Hospital of Marseille, Marseille, France
5Department of Paediatric and Congenital Heart Disease, University Hospital of Lille-Nord de France, Lille, France
6Department of Paediatric and Congenital Heart Disease, Cardiothoracic Center of Monaco, Monaco
7Department of Paediatric and Congenital Heart Disease, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
8Clinical Research Department, CHU Martinique (University Hospital of Martinique), Fort-de-France, France
*Corresponding Author: Hugues Lucron. Email: hugues.lucron@chu-martinique.fr
Received: 15 January 2022; Accepted: 15 May 2022
Abstract: Objectives: We aim to describe the efficacy, safety, and characteristics of the Amplatzer Vascular Plug (AVP) II and IV “off-label” use for multiple cardiovascular occlusions in children under 10 years. Methods: Observational retrospective multicenter (2007–2020, 6 centers) review of paediatric procedures using AVP II or IV. Results: A total of 125 children (49.6% aged ≤ 1 year, 147 lesions) underwent 136 successive procedures (success rate: 98.5%) using 169 devices (109 AVP IV, 60 AVP II). The mean device diameter was 7.7 ± 3.2 mm (4–20 mm). The median AVP size to vessel diameter ratio was 1.3 (0–2). The median age and weight at implantation were 1.0 year (0.01–9.98) and 8.4 kg (1–69). Procedures were heterogeneous (55 patent ductus arteriosus (PDA), 28 collaterals, 18 sequestrations, 22 arteriovenous/veinovenous/coronary fistulas, 6 vertical veins, 6 conduits, 5 ventricular septal defects, 7 miscellaneous). Day 1 and 6-month occlusion rates were respectively 94.8% and 98.5%. Major adverse events (MAE) occurred in 5.2% of cases (no procedure-related deaths), and more frequently in weight ≤ 5 kg (p = 0.01), younger patients (p = 0.03) during PDA closure (p = 0.02) of tubular types (p = 0.02) using larger devices (p = 0.03) and AVP II (p = 0.003). Independent predictor of MAE risk was a higher AVP diameter to patient weight ratio (Odds-ratio: 2.33, 95% confidence interval 1.31–4.13, p = 0.004, optimal cut off: 1.45). Conclusions: Both AVPs are safe and effective for percutaneous occlusions in children under 10. Such devices represent an alternative “off label” use for well selected paediatric patients.
Keywords: Amplatzer vascular plug (AVP) II and IV; cardiovascular occlusions; off-label use; children under 10 years; efficacy; safety
Continuous development of new techniques and material has made catheter treatment of congenital vascular malformation or congenital heart disease (CHD) possible in children and infants [1–5]. Percutaneous treatment has become the “gold standard” for the majority of patent ductus arteriosus (PDA) closures in children, including premature or low weight infants [1–2,5]. Several devices have been developed to occlude multiple vascular structures in adults, but the more recent ones such as the Amplatzer vascular plug (AVP) II and IV (Abbott Vascular®. Santa Clara, California, 95054 USA) remain frequently used “off-label” in the paediatric population [6–13]. To the exception of PDA closures [13,14], published data on AVP II and IV use in children and infants remain scarce, particularly in regard to the heterogeneity of procedures and outcomes [15–20]. The aim of this study is to describe the efficacy, safety, and characteristics of AVP II and IV “off-label” paediatric utilization for various percutaneous vascular occlusions, including very large tubular PDAs, in children under 10 years.
In this observational multicenter study, a retrospective chart review of all children aged less than 10 years, having undergone a cardiac, vascular, or conduit occlusion procedure using AVP II or IV between January 2007 and March 2020, was conducted in 6 academic centers from the same European country. All cardiovascular abnormalities were potential candidates for AVP II or IV implantation. All patients were treated and followed-up through a dedicated nationwide network. All vascular abnormalities were potential candidates for AVP device implantation whenever another “on-label” device could not be selected, such as the one used during some PDA closures. All procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation, and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from each study participant’s parents or legal guardians, and the Institutional Review Board of one of the study centers approved all study procedures (IRB 2020/053).
2.2 Device and Delivery System
The tri-lobe AVP II (4 to 22 mm, 5 to 9 Fr delivery catheter) was first delivered in 2007. A few years later, the highly flexible AVP IV was developed for the adult peripheral vascular structure. The AVP IV (4 to 8 mm; length of 10 to 13.5 mm delivery catheter, 4 to 5 Fr) is adequate for highly tortuous vessels of less than 6 mm in diameter [16,20,21]. Both AVP II and IV are easily re-sheathable and repositionable after partial or complete deployment until best possible position is obtained for optimal release. The main AVP II and IV characteristics are summarized in Fig. 1.
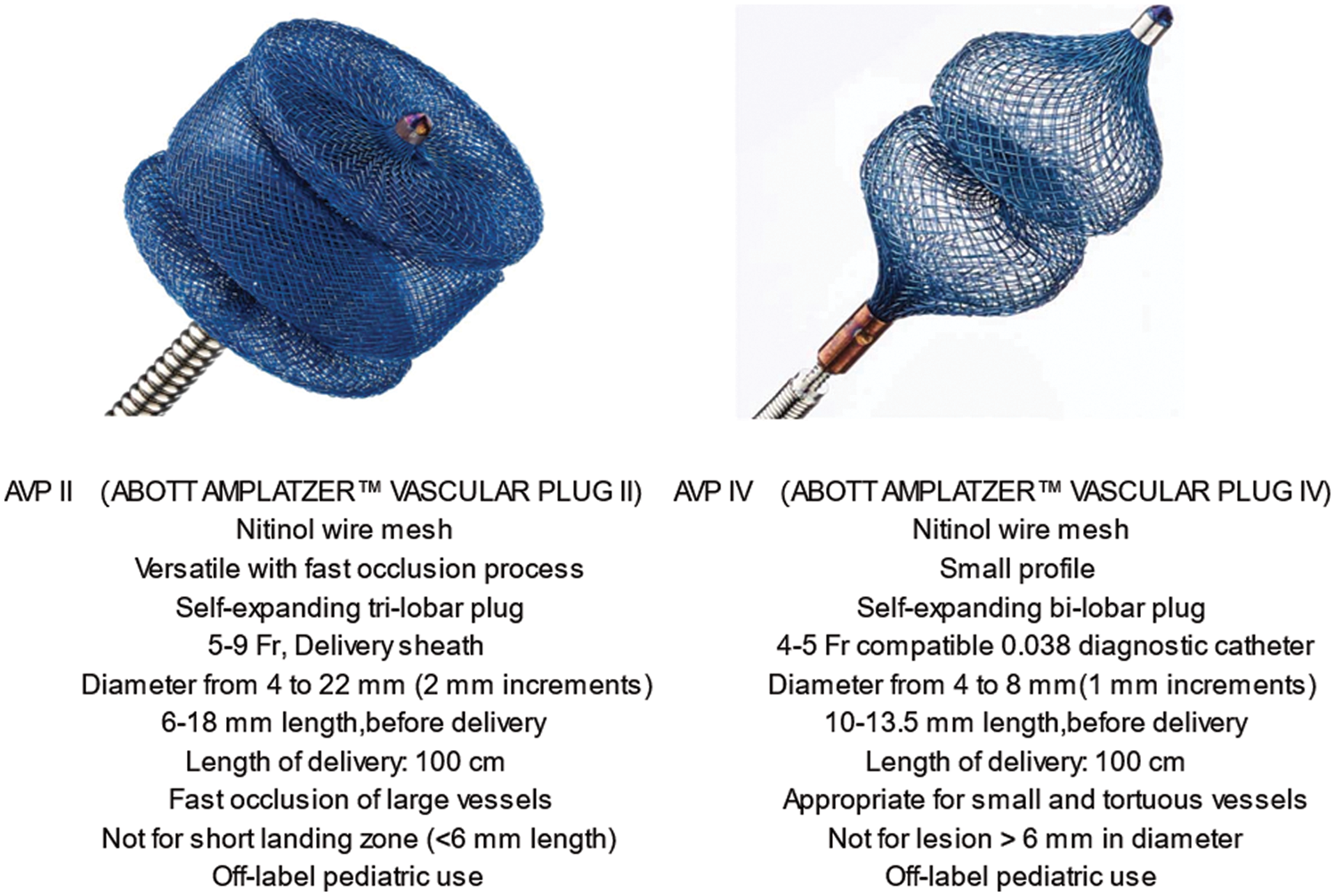
Figure 1: Main characteristics of AVP II and IV
2.3 Device Selection and Implantation Technique
Each device was chosen by the interventional paediatric cardiologists in order to obtain adequate occlusion without abnormal protrusion into nearby structures. Device selection was based on multiple parameters such as lesion anatomy (type, size, length), blood flow crossing the defect, vascular access, best technical approach, and finally, the availability of an appropriate landing zone. Due to its property of rapid occlusion and wide availability of sizes, AVP II was selected more frequently for the occlusion of large vessels (diameter > 6 mm), even in very young children. AVP IV was more often selected for closing smaller but longer and tortuous vessels, as well as small surgical conduits.
Overall, AVP was usually chosen 20% to 50% larger than the arterial native vessel size measured on initial angiography [12–14] considering the narrowest landing zone, as well as immediate upstream and downstream diameters. For vein and surgical conduits, AVP was more often upsized to 140%–150%. For non-tubular PDA, the chosen AVP size was between 120% and 150% of the minimum diameter, most often localized at the pulmonary end [14]. An over sizing ≥150% was always considered in case of severe pulmonary hypertension. Regarding large tubular PDAs, device size and selection was defined according to both maximal diameter and vessel length in lateral and right anterior oblique projections, with a device measuring 120%–160% of the minimal diameter and at least 1–2 mm more than the diameter of the largest PDA portion. Furthermore, our learning curve experience with AVP II in closure of very large tubular PDA included a cautious “pull through” with immediate device upsizing whenever needed [14]. It is to be noted that half of the participating centers always considered AVP II in infants for the occlusion of tubular PDAs > 6 mm, which would not have been possible with other commercially available devices, while the remaining centers referred these small patients for surgical PDA ligation [14].
Following parent/legal guardian consent, interventions were performed in a digital catheterization laboratory under conscious sedation or general anesthesia, according to age, procedure type, and team practice. All patients received IV heparin and standard antibiotic prophylaxis. Anterograde and retrograde route were used according either to the choice of the operators, the lesion to occlude, type and size of the selected device, findings during the procedures, or requested size of the delivery sheath. A few patients with either venous fistulae, partial cavo-pulmonary connection, single ventricle circulation, or interruption of the inferior vena cava, requested exclusive internal jugular vein access. All devices were deployed through a delivery sheath (AVP II) or a diagnostic catheter (AVP IV) using previously described techniques [6,10,12,21]. An additional echocardiographic guidance was performed whenever indicated, mainly in small infants [21]. For occlusion of large PDA using AVP II, the usual implantation technique was to contain 2 or 3 of the disks inside the body of the PDA whenever possible. Congenital artery fistulae (CAF) were closed with AVP IV only, predominantly in neonates and infants, using either a retrograde or antegrade approach, with or without arterio-venous loop. In all cases, once adequate position and occlusion without significant residual shunt was demonstrated, the AVP was released by unscrewing counter-clockwise. A final angiogram was usually performed to assess any residual shunt.
Demographic data and procedure characteristics such as vascular access, occlusion type, and implanted AVP types and number were recorded. PDA morphologies were defined according to Krinchenko’s classification [22]. To determine the real oversizing of the AVP plug, the ratio of plug diameter to either minimal vessel diameter or to patient weight was measured. In addition, considering the occlusion of very large PDAs in low weight patients, the ratio of plug size to minimal PDA diameter, as well as the ratio of minimal PDA diameter to patient weight, were calculated. Residual shunt presence was described. Additional interventions performed during the same procedure were listed.
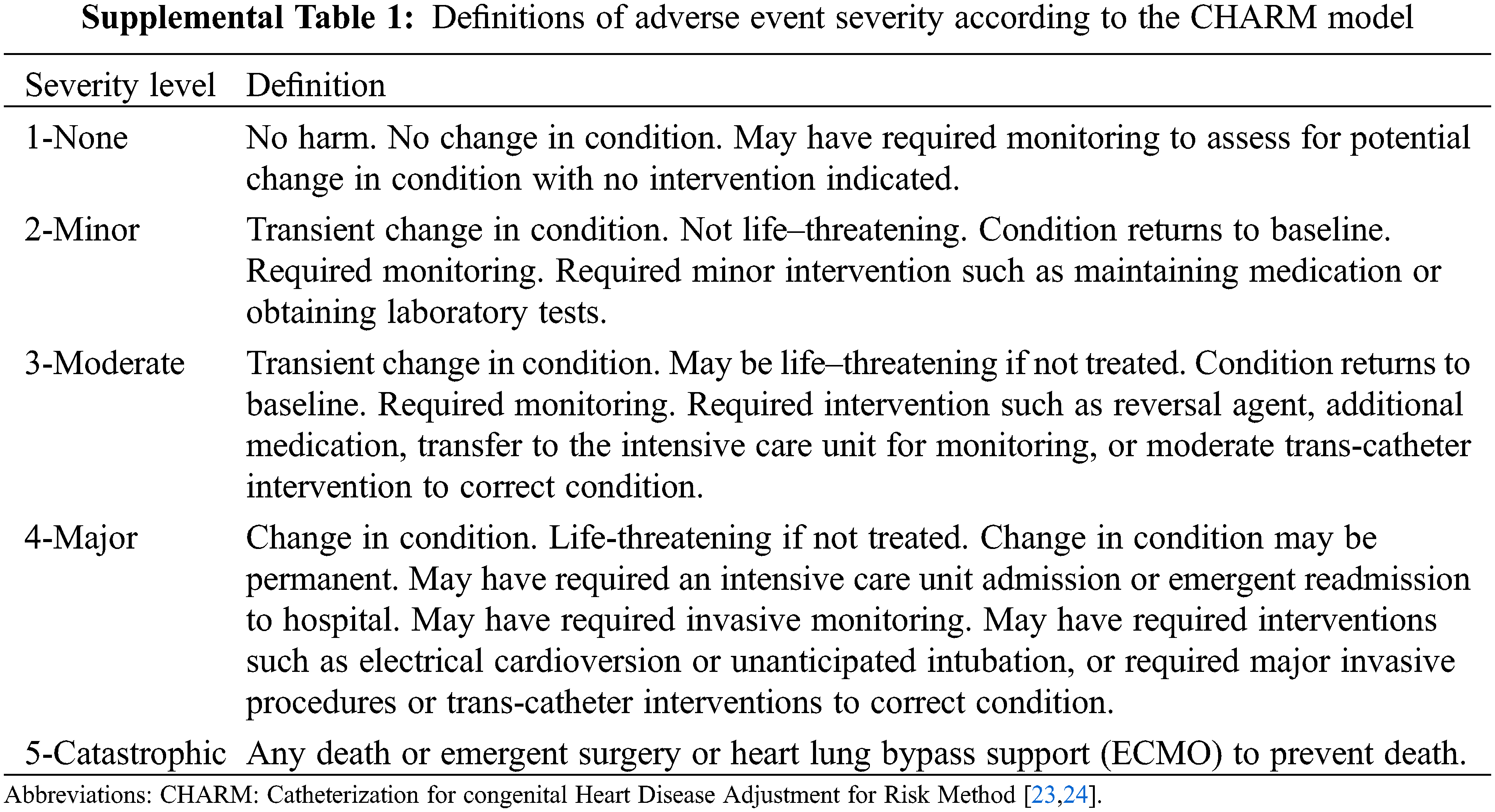
Immediate technical success was defined as successful plug delivery in an adequate position with no more than mild intra-prosthetic residual shunt and without residual para-prosthetic shunt around the device or compression of nearby structures. Occlusion rate was monitored immediately after the procedure, at day 1 and 6 months later using echocardiography or CT scan whenever indicated. Adverse events (severity level ≥3) were defined according to the previously published CHARM (Catheterization for congenital Heart Disease Adjustment for Risk Method) model (Supplemental Table 1) [23,24]. An adverse event of severity level 3 refers to transient changes in condition requiring treatment, with a condition returned to baseline after monitoring or moderate intervention (reversal agent, additional medication, and transfer to the intensive care unit for monitoring). Major Adverse Events (MAE, severity level ≥4) included any need for a new percutaneous procedure or surgical intervention. Cardiovascular event occurrence or death was monitored during at least 6-months post procedure.
Baseline patient and procedure characteristics were described and compared according to AVP type and MAE occurrence. Quantitative variables were described using mean ± standard deviation or median (minimum-maximum range). Categorical variables were presented as absolute values and percentages. Group comparisons were conducted with Student t-test, Wilcoxon-Mann-Whitney test, Chi-square test, or Fisher’s exact test. Univariate and multivariate logistic regression models were fitted to assess the independent effect of predictors on MAE risk. Receiver Operating Curve (ROC) analysis was used to establish optimal cut-off points for MAE prediction by the following ratios: (a) AVP plug diameter to patient weight; (b) AVP diameter to minimal vessel diameter; (c) AVP plug diameter to minimal PDA diameter; (d) minimal PDA diameter to patient weight. Variables with significant association in univariate analysis (p < 0.25) were retained for multivariate models. All statistical analyses were performed using SAS software (version 9.4, Cary NC, USA), with p-values < 0.05 considered statistically significant.
Over a 14 year-period, 125 children under 10 years (57.6% female, mean age: 2.8 ± 3.1 years, mean weight 13.9 ± 13.7 kg) underwent 136 procedures using either AVP II and/or AVP IV for the percutaneous treatment of 147 different lesions. Overall, 169 plugs (60 AVP II and 109 AVP IV) were successfully implanted out of the 171 initially engaged. As such, two procedures failed due to poor patient tolerance requiring withdrawal before release. The first procedure failure involved an infant who developed immediate left pulmonary artery (LPA) stenosis (Fig. 2) during closure of a large PDA (AVP II). The second failure concerned another patient in whom the obliteration (AVP IV) of a lobar sequestration collateral artery induced an immediate acute chest pain syndrome.
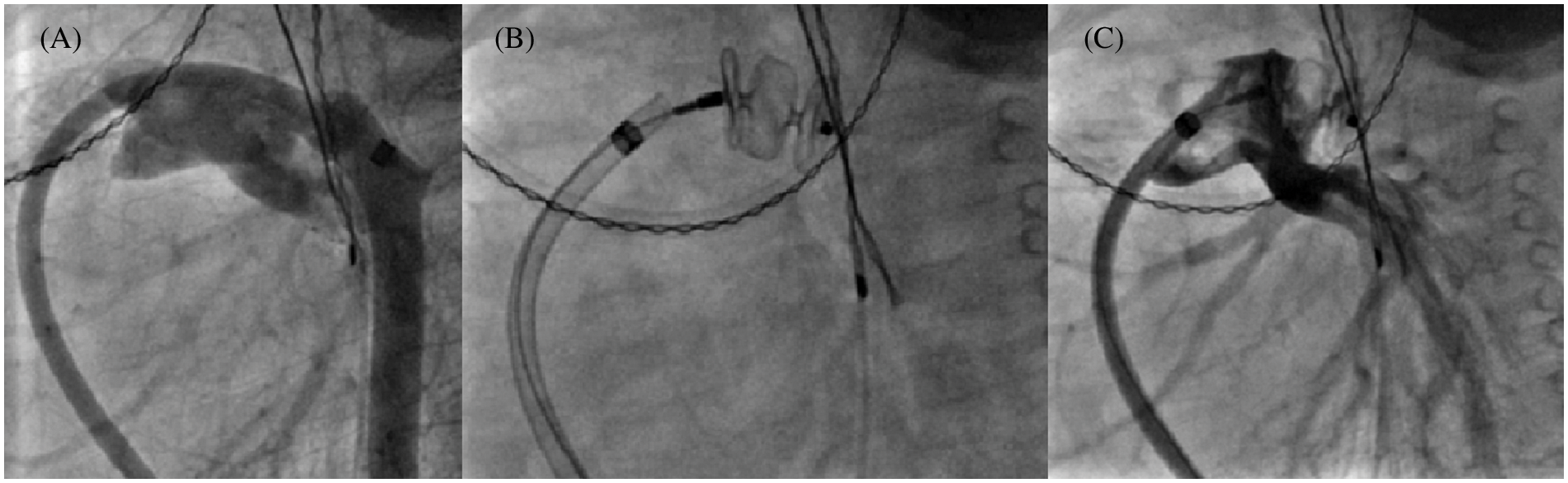
Figure 2: 10 mm AVP II implantation failure in a 6 kg infant. Multiple angiograms, lateral view. (A): 6.5 mm tubular PDA by venous approach with small aortic arch; (B): 10 mm AVP II positioning; (C): Left pulmonary artery stenosis which became rapidly severe
A wide variety of procedures were carried out throughout the study period (Fig. 3).
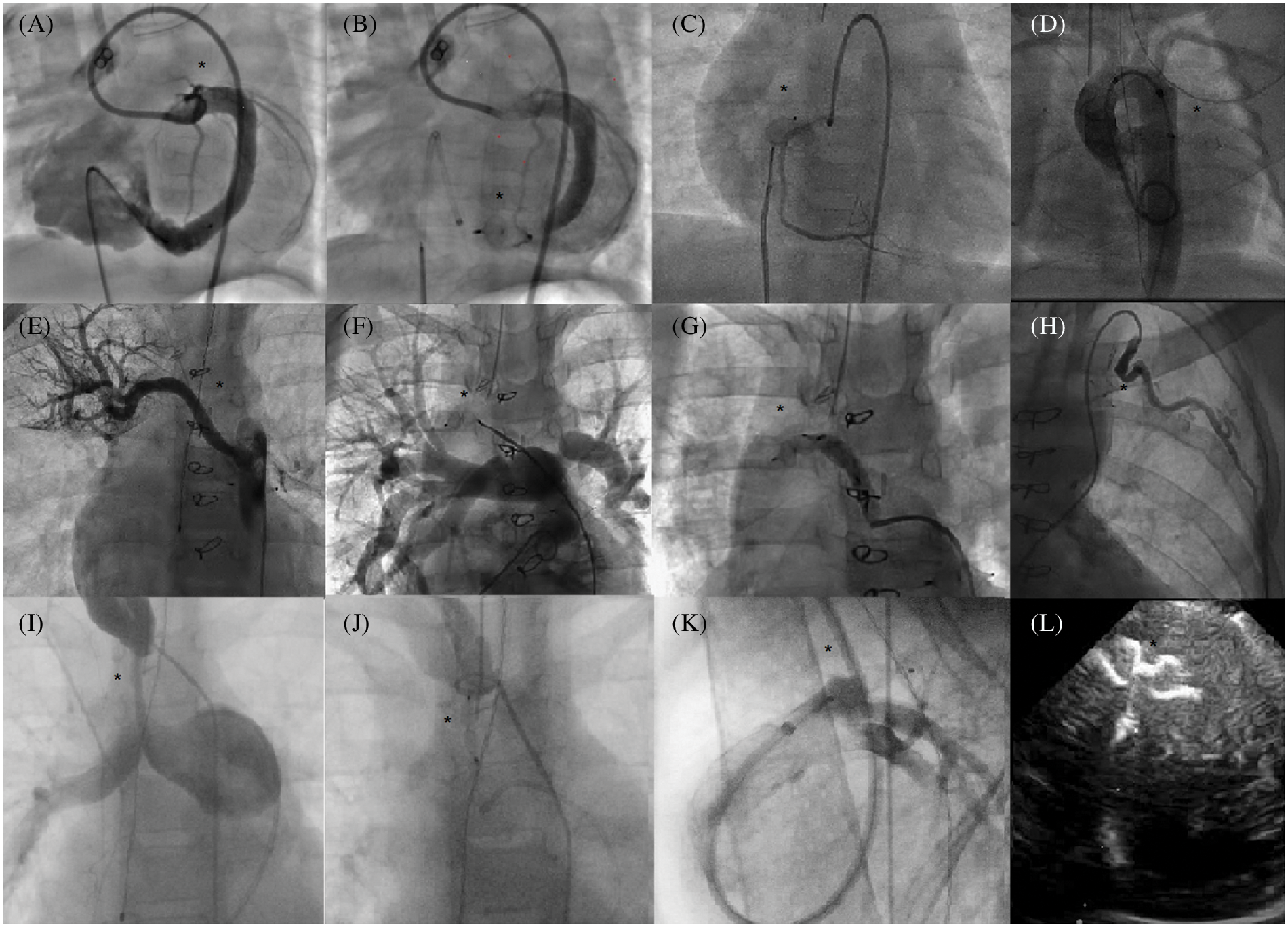
Figure 3: Use of AVP IV for multiple occlusions in children Angiograms (area of interest is designated by a black star). A, B: Retrograde CAF (congenital artery fistulae) closure (circumflex artery to right ventricle). C: Retrograde CAF closure (right coronary to right atrium). D: Rescue anterograde closure of left aortic sinus to left atrium fistulae. E, F, G: Selective embolization of a non-communicating aorto-pulmonary collateral. H: Selective embolization of an arterial collateral from the left internal mammary artery. I, J: Percutaneous occlusion of a right modified Blalock anastomosis. K: Venous closure of a patent ductus arteriosus via the jugular vein. Echocardiogram (Apical view) L: Muscular ventricular septal defect closure
Overall, 55 PDA occlusions were performed with a minimal diameter of 5.5 ± 1.6 mm and different morphologies [22]: 47.3% type C (tubular morphology, Fig. 4), 7.3% type A, 21.8% type D, 3.6% type D/E, and 20% type E.
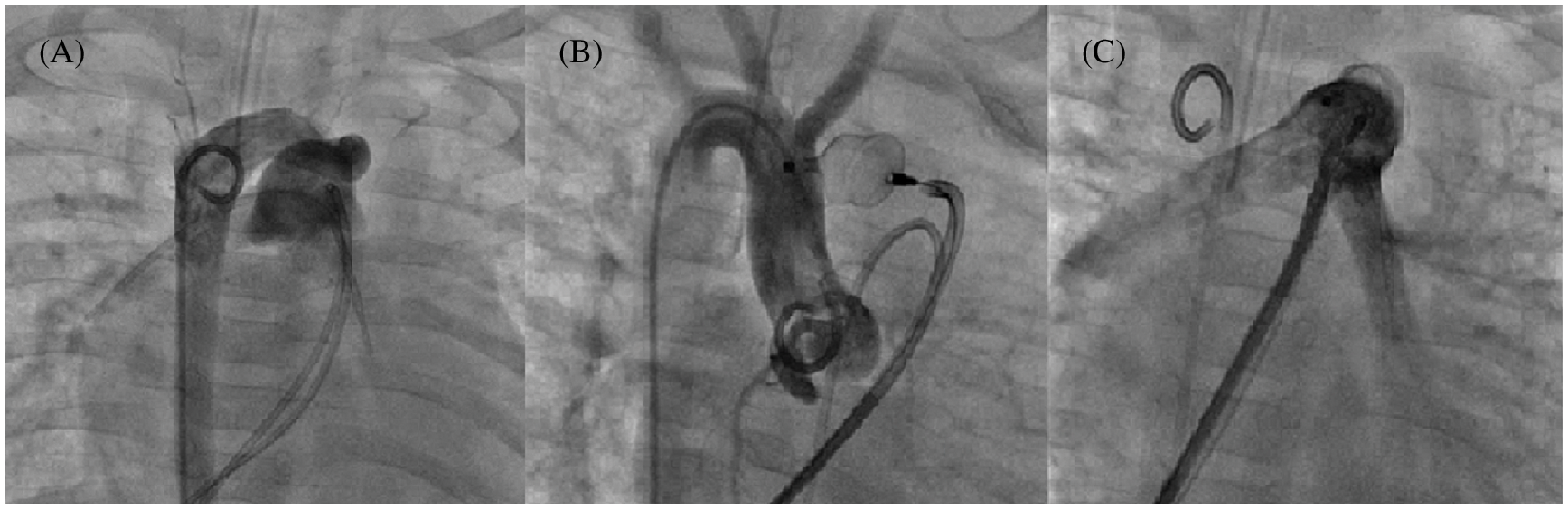
Figure 4: Tubular PDA closure using a 12 mm AVP II in a 6 kg infant. Multiple angiograms. (A): 7.6 mm tubular PDA with a right sided aortic arch; (B): AVP II deployment into the PDA without shunt; (C): Absence of pulmonary artery stenosis before device release
Furthermore, 80% of PDA closure procedures included both arterial and venous femoral access with arterio-venous loop, while 7 AVP II were implanted into PDA by venous route only, and 4 plugs (3 AVP II) by a pure arterial approach. Five AVP II, as well as 2 AVP IV, were implanted to replace “on-label” PDA devices. Patient and procedure characteristics according to AVP II and IV “Off-label” use in children are further summarized in Table 1.
Successful closures of anomalous or aberrant systemic arterial supply to a lung segment was achieved using 18 AVP IV and 2 AVP II. Six uncomplicated closures of single or multiple congenital artery fistulae (CAF) using 6 to 8 mm AVP IV in infants and neonates were also completed, for which delayed post-procedure angiograms demonstrated the absence of residual shunt or coronary thrombosis. The effective closures of 2 peri-membranous Ventricular Septal Defects (VSD) with aneurysm (AVP II) as well as 1 postoperative VSD (AVP IV) and 2 muscular VSDs (AVP IV, Fig. 3L) were also achieved. The miscellaneous group included single procedures such as closure of fenestration (Fontan circulation), closure of congenital fistula of the left aortic sinus to the left atrium (CAF “like”), ductus venosus closure, or left hepatic artery occlusion.
Overall, the median oversizing of devices was 130%, but was higher with AVP II than with AVP IV (1.4 vs. 1.3, p < 0.001), while the ratio of plug size to patient weight was also higher for AVP II implantations (1.3 vs. 0.5, p < 0.001). Moreover, in comparison to AVP IV, the use of AVP II was characterized by larger devices (mean diameter: 8.0 (4.0–20.0) mm vs. 6.0 (4.0–8.0) mm; p < 0.001) in younger children (53.5% of infants ≤1year vs. 45.3%, p = 0.37) often weighing less than 5 kg (37.9% vs. 17.2%, p = 0.01) and concerned more PDA closures (71.2% vs. 14.9%, p < 0.001) predominantly of type C (39.0% vs. 2.7%, p < 0.001) as well as the occlusion of larger vessels (6 mm vs. 5 mm, p < 0.001).
In contrast, AVP IV was more widely used for the occlusion of single or multiple AP collaterals (25.7% vs. 3.4%, p < 0.001) as well as for the occlusion of the arterial supply of pulmonary sequestration (16% vs. 0%, p < 0.001) or scimitar vein occlusion (1 case). AVP IV was also preferred for CAF closure (8.1% vs. 0%, p = 0.03). Overall, because of the number of lesions treated simultaneously during the same procedure, the number of AVP IV implanted per procedure was higher, compared to AVP II (min-max range: 1–5 for AVP IV vs. 0–1 for AVP II, p < 0.001). Finally, AVP II was more predominantly delivered using a venous route compared to AVP IV in this study (p < 0.001).
The positioning and release of at least one device was possible in 134 procedures, accounting for an immediate procedure success rate of 98.5%. Full occlusion rate observed at day 1 and 6-month post procedure were 94.8% and 98.5%, respectively. No difference was observed according to AVP type. Procedure efficacy and safety of AVP II and IV are detailed in Table 2.
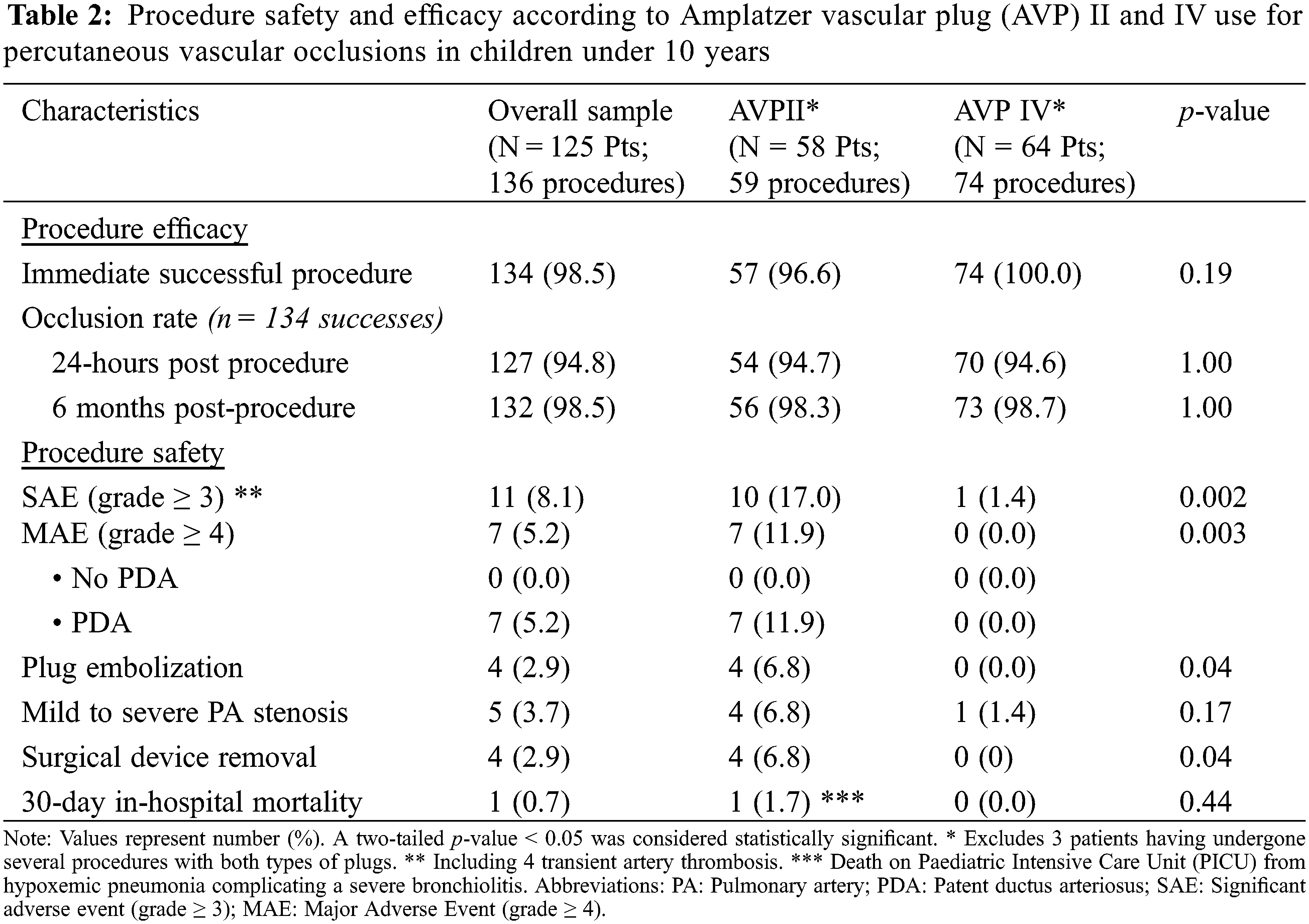
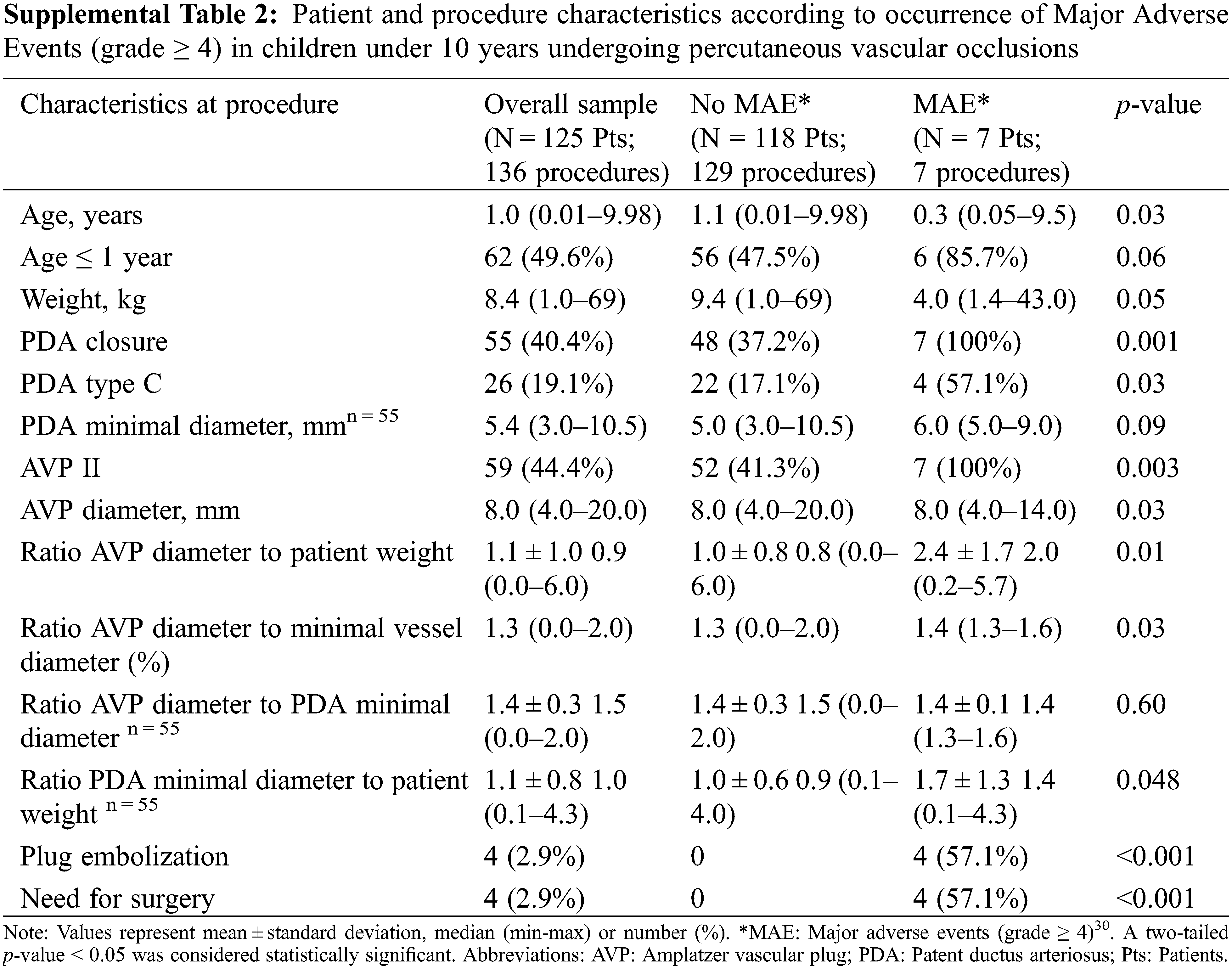
There was no procedure related death. Thirty-day in-hospital mortality occurred only in one patient who died due to hypoxemic pneumonia complicating a severe bronchiolitis, unrelated to procedure. Significant adverse events of severity level ≥3 (including 4-transient femoral artery thrombosis) occurred in a total of 8.1% of procedures. Patient and procedure characteristics according to occurrence of Major Adverse Events (MAE, grade ≥ 4) in children are summarized in Supplemental Table 2.
In total, 5.2% of patients underwent MAE, solely observed during catheter closure of large PDAs using AVP II. Observed MAE were predominantly device embolization or the need for urgent or delayed surgery. MAE occurred more frequently in younger patients (mean age 1.6 ± 3.5 years, vs. 2.8 ± 3.1 years in patients with no MAE, p = 0.03) with a lower mean weight (9.6 ± 14.8 kg vs. 14.2 ± 13.6 kg, p = 0.05). Furthermore, 5 (71.4%) patients with MAE weighed 5 kg or less. MAE prevalence was also higher with larger AVP plug size (mean diameter of 9.1 ± 2.3 mm vs. 7.7 ± 3.3 mm in patients with no MAE, p = 0.03) and a higher ratio of the AVP to vessel diameter (1.4 vs. 1.3, p = 0.03).
In all, there were a total of 4 device embolization, always observed within the first 24 h (Fig. 5), 2 of which were recovered by snaring technique in the catheter laboratory, and 2 by surgical removal.
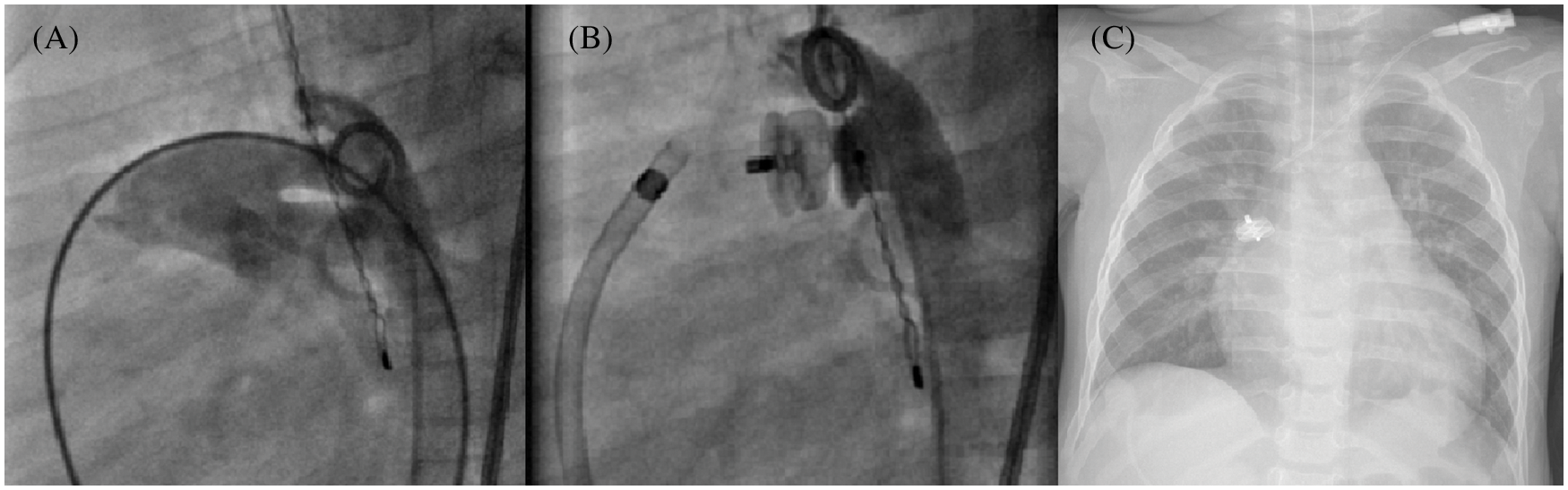
Figure 5: Early embolization of a 10 mm AVP II after PDA closure. Multiple angiograms, lateral view, and chest X-ray. (A): Aorta with a 7 mm tubular PDA; (B): Post device release final result; (C): Device embolization into the right pulmonary artery
Additional surgical procedures were performed in 2 patients presenting delayed severe LPA stenosis. The latter were documented 2 weeks after PDA closure in a 3.2 kg baby (10 mm AVP II) and 8 weeks after the procedure in another infant weighing 4.9 kg (10 mm PDA, 14 mm AVP II). This patient presented with a device “attraction” of the descending aorta and severe plication of the LPA. The last MAE involved a 3.6 kg infant already on inotropic support at the entrance to the catheterization room who presented a cardiac arrest during the first aortography. He was successfully resuscitated. There was no EKG modification, with unremarkable immediate coronary angiogram. PDA closure was successfully achieved using a 6 mm AVP II. Three other mild LPA stenosis did not require treatment, with uneventful follow-ups. There was no haemolysis, severe bleeding, cardiac arrhythmia, or late embolization.
Using univariate logistic regression analysis (Table 3), MAE was significantly associated with a weight ≤5 kg (Odds ratio, OR: 7.9, 95% Confidence Interval, CI: 1.5–42.8; p = 0.02), type C PDA (OR: 6.5, 95% CI: 1.36–31.04, p = 0.02), greater device oversizing illustrated by the ratio of AVP diameter to minimum vessel diameter ≥1.38 (OR: 5.56, 95% CI: 1.04–29.90, p = 0.046). We also observed a potential association between MAE and higher values of either AVP diameter to patient weight ratio (OR: 2.3, 95% CI: 1.3–4.0, p = 0.003; optimal cut-off: 1.45) or PDA diameter to patient weight ratio (OR: 2.4, 95% CI: (1.0–5.8, p = 0.048; optimal cut-off: 1.25).
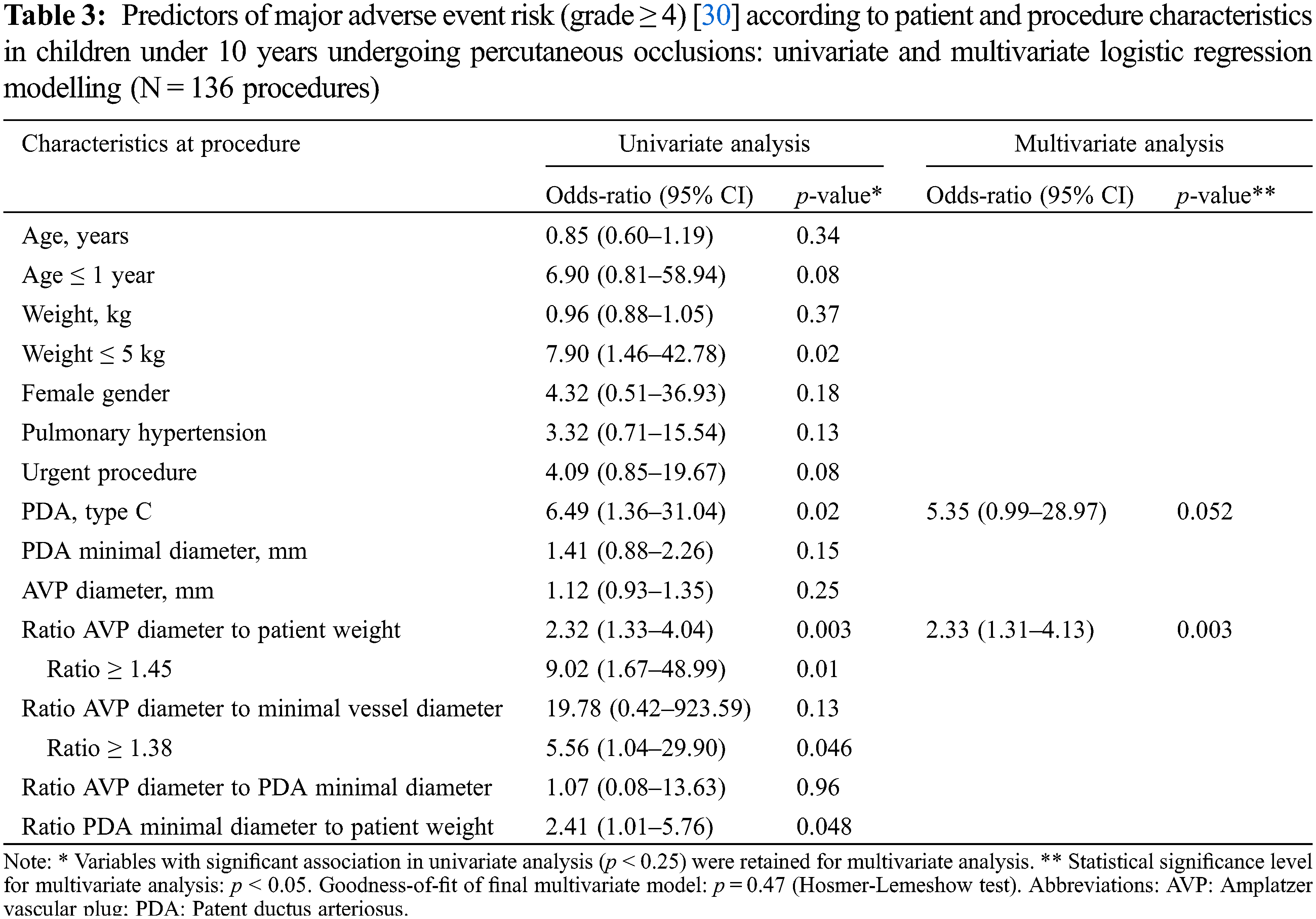
Multivariate analysis (Table 3) retained a high ratio of AVP diameter to patient weight as an independent predictor (OR: 2.33%, 95% CI: 1.3–4.1, p = 0.004), with an optimal cut-off value set at 1.45. As such, a ratio ≥1.45 predicted a significant 9-fold increase of MAE risk (OR: 9.02, 95% CI: 1.67–49.00, p = 0.01). Furthermore, an association between MAE and PDA of tubular morphology was also supposed, with an OR of 5.35 (95% CI: 0.99–28.97, p = 0.052).
The cohort reported in this paper is the largest paediatric series describing multiple indications of AVP II and IV use, with a total of 169 implanted devices (55.6% of AVP IV). In this multicenter experience, the off-label utilization of such devices appears effective in children and infants (median age: 1 year). The overall rate of successful AVP implantation (98.5%) constitutes a major indicator of efficacy which compares favourably with previous reports on use of AVPs and other devices formally developed for PDA or vascular occlusions [1–2,4–14,21,25–29].
The instruction manual of the manufacturer recommends an AVP II or IV oversizing of 30%–50%, which is underlined by our study results (median oversizing percentage 130%). We report several occlusions of large vessels (median > 5 mm) which is remarkable and rarely reported in infants till now. Off-label use of AVPs with even greater oversizing (150%–160%) could sometimes be indicated in cases of high blood flow (CAF, large arterial collaterals, VSD, large PDAs), severe pulmonary hypertension, surgical conduits, as well as very large, short or even slightly elastic lesions in infants and young children [14,25–28].
In our study, the higher number of AVP IV implanted per procedure compared to AVP II reflects the concomitant treatment of several lesions, such as multiple collaterals or multiple feeding vessels. Thus, using an oversizing of 120%–160%, and with the exception of a few complex arterio-venous malformations that cannot be treated exclusively with plugs, the achievement of selective or supra-selective occlusions does not require any additional occluders or coils implantations [12–14]. In addition, the rare replacement of a plug by another one of larger size is primarily motivated by potential risk of secondary mobilization, and rarely indicated to obtain immediate complete occlusion.
This compares very favourably with the use of more recent devices such as the microvascular plug (MVP, Medtronic™, Minneapolis, MN, USA), for which implantation may require significant oversizing (30% to 60% for venous occlusions, 40% to 70% for arterial occlusions) and the complementary use (20%) of coils or other occluders at the same site to obtain a similar occlusion rate [4]. Overall, this illustrates the very good occlusive capacity of AVP II and IV which is highlighted in the present study, by a low rate (5.2%) of trivial to mild residual intra-prosthetic shunt at Day 1 post-procedure, predominantly described after VSD and CAF closure [25,26]. The 6-month occlusion rate is also excellent (98.5%), suggesting adequate mid-term device thrombosis with subsequent endothelialisation.
Moreover, to the best of our knowledge, we report the largest patient series in which AVP II and IV are used to occlude several anatomical shunt varieties including large malformations [8,12,14,16,18,21,25–28]. In our experience, AVP II and IV offer excellent paediatric “off-label” alternatives for closure of surgical conduits, aorto-pulmonary collaterals, arterio-venous fistulae, acquired veno-venous communications, sequestration closure, and other miscellaneous lesions [5,8,11,12,15,19]. We also underline that AVP IV seems to be a safe alternative for large CAF occlusions in low-weight infants, even if CAF closure in adults and children offer variable results [6–7,20,28,29]. Finally, despite some limited experience with various VSD types, we observe that both AVP devices could be effective in percutaneous VSD closure [25,26].
Furthermore, although recent plugs such as the smallest MVP types [4] can be deployed efficiently using micro catheters to access distal lesions that are not easily treatable in children, we describe that AVP IV can be safely implanted in infants, even of low weight. In addition to an interesting almost immediate thrombotic capacity, this device is also of great flexibility. AVP IV does not require, contrary to MVPs [4], a straight deployment zone or major oversizing to increase its thrombogenic potential. Additionally, even though AVP IV increases in length upon deployment, it remains usable in the majority of paediatric vascular embolization of vessels ≤6 mm with a sufficient landing zone [10,15,17]. Like MVPs [4], AVP IV can also be directly deployed via the arterial route in very large coronary fistulas in small infants, as sustained by our study results. When considering lesions of larger diameter, the profile advantage of MVPs is perhaps less obvious, especially since the larger sized MVPs also require the use of 4 or 5 Fr catheters for delivery, in addition to longer lengths for implantation [4]. Nevertheless, given its design, AVP IV might not be the most suitable device compared to some of the smaller MVPs or coils indicated for very distal embolization within small vessels (<3 mm) [4]. Finally, the major limitation to the use of AVP IV in children remains its size, as well as the length of the lesion to be treated. Indeed, the length of AVP IV combined with a maximum diameter of 8 mm avoids its deployment for closure of large (≥6 mm) tubular PDAs in infants. This particular point is in line with the current lack of devices suitable for the treatment of large lesions in infants on the market.
AVP II could prove to be a potential alternative, as underlined by our study results. AVP II is also a relatively “low-cost device” which requires most often 5 to 6 Fr standard delivery catheters [6,12,14,18]. This profile might limit an arterial use in low weight infants with some risk of complications related to vascular access, such as transient femoral artery thrombosis. On the other hand, larger devices up to 14–16 mm can be easily deployed using 6 Fr delivery catheter, which remains accessible in young children [6]. While AVP II allows a fast occlusion, it might currently be under used in children and infants. This might explain the low rate of AVP II implantation in our multicentre study, where it was mainly used for closure of large PDAs [27] and lesions, such as venous ones, that were often too large to fill with AVP IV [12].
Pertaining to the safety of “off-label” AVP use, we did not experience any major complications using AVP IV, as reported by several authors [1,17,21]. Interestingly, all MAE in our study solely involved PDA closure with AVP II in symptomatic infants ≤5 kg, well identified as being at higher risk during interventional cardiac catheterization [27,30]. On the other hand, this study also brings to light that AVP II remains a suitable alternative for the closure of very large PDAs in symptomatic infants weighing 2 to 6 kg, in whom the deployment of labelled devices might be impossible or at high risk of complications. AVP II also remains well adapted for some elongated PDAs, such as those without significant aortic ampoule, where it could prevent aortic obstruction during PDA closure [12–13,27]. Moreover, the occurrence of embolization or severe LPA stenosis in our patients underlines that AVP II might not yet have the optimal design for closing all PDAs in infants [27,31]. Nevertheless, AVP II proves to be effective and perfectly safe in all other occlusions and adjusts to variable landing zones. It is to be noted that at the time of implantation, further oversizing might be required with AVP II to achieve better device stability, as we observed while treating a few lesions with high blood flow such as tubular PDAs and arterial collaterals. Even though this oversizing increases the chances of reliable implantation, it might also be a source of significant complications. Therefore, based on our experience, we modestly recommend the respect of an optimal ratio of AVP II diameter to patient weight <1.45 in low-weight infants, as well as a minimal PDA diameter to patient weight ratio <1.25. Based on this principle, the use of an AVP II > 8 mm for closure of PDA > 6 mm might not be recommended for children <5 kg.
Finally, an overall AVP II or IV oversizing of more than 140% compared to vessel diameter is probably not recommended in low-weight children, except in certain particular cases. This is consistent with general manufacturer recommendations for the use of these two devices in adults.
Our retrospective study design might have introduced potential interpretation bias. Firstly, we did not take into account center heterogeneity among the 6 participating institutions, in terms of patient volume, procedure type, and operator-biased device selection. Secondly, large tubular PDAs in infants of low weight were not considered for AVP catheter occlusion in half of the participating centers, which preferred surgical procedures for this high-risk patient group [14]. Finally, even in skilled hands, procedure safety, efficacy, and feasibility remain intricately linked to operator experience.
This multi-institutional study reports the largest and youngest group of catheter-treated children (50% infants <1 year), with AVP II or IV “off-label” use for the occlusion of heterogeneous congenital cardiovascular malformations. For a wide range of indications, we demonstrate that both devices are perfectly safe and effective in children under 10 years. Despite some MAEs that were solely related to PDA closure in infants, AVP II might still represent an alternative for the closure of well-selected atypical or large tubular PDAs. New plugs with a small profile and an adapted design are required to further improve the management of very large vessels in small infants. In the meantime, effective paediatric labelling of AVP II and IV seems essential and will contribute to the optimization of future catheter techniques in children.
Acknowledgement: The authors would like to acknowledge Ms. Mélanne Ghahraman and Mrs. Maria Minassian, for their assistance with manuscript proofreading.
Authorship Contribution: H.L: Methodology conception, data curation, formal analysis, writing–original draft, writing–review & editing, supervision. A.E.B, C.O: interpretation of data, writing-review & editing. A.H, P.G, F.B, C.D, S.K, M.T: investigation, data curation, interpretation of data and formal analysis. M.B: formal analysis, writing-review & editing. R.B: formal analysis, statistics, writing review & editing, final approval. F.G: conception, investigation, supervision, writing-review & editing, final approval.
Availability of Data and Material: Data supporting the findings of this study are encrypted and stored in an institutional repository. Data can be made available upon request to the corresponding author.
Funding Statement: The authors received no financial support for the conduct, authorship and publication of the present research.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. VanLoozen, D., Sandoval, J. P., Delaney, J. W., Pedra, C., Calamita, P. et al. (2018). Use of Amplatzer vascular plugs and Amplatzer duct occluder II additional sizes for occlusion of patent ductus arteriosus: A multi-institutional study. Catheterization and Cardiovascular Interventions, 92, 1323–1328. DOI 10.1002/ccd.27824. [Google Scholar] [CrossRef]
2. Godart, F., Houeijeh, A., Domanski, O., Guillaume, M. P., Brard, M. et al. (2018). Is the new occlutech duct occluder an appropriate device for transcatheter closure of patent ductus arteriosus? International Journal of Cardiology, 261, 54–57. DOI 10.1016/j.ijcard.2018.03.059. [Google Scholar] [CrossRef]
3. Kitano, M., Fujimoto, K., Kato, A., Kurosaki, K., Shiraishi, I. (2021). Efficacy and safety of the atrial septal defect closure for patients with absent or malaligned aortic rim using a figulla flex II device flared and straddling behind the aorta. Congenital Heart Disease, 16(3), 269–283. DOI 10.32604/CHD.2021.015308. [Google Scholar] [CrossRef]
4. Haddad, R. N., Bonnet, D., Malekzadeh-Milani, S. (2022). Embolization of vascular abnormalities in children with congenital heart diseases using medtronic micro vascular plugs. Heart Vessels, 37(7), 1271–1282, DOI 10.1007/s00380-021-02007-6. [Google Scholar] [CrossRef]
5. Sathanandam, S. K., Gutfinger, D., O’Brien, L., Forbes, T. J., Gillespie, M. J. et al. (2020). Amplatzer piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheterization and Cardiovascular Interventions, 96, 1266–1276. DOI 10.1002/ccd.28973. [Google Scholar] [CrossRef]
6. Ramakrishnan, S. (2015). Vascular plugs–A key companion to interventionists–“Just plug it”. Indian Heart Journal, 67(4), 399–405. DOI 10.1016/j.ihj.2015.07.001. [Google Scholar] [CrossRef]
7. Mottin, B., Baruteau, A. E., Boudjemline, Y., Piechaud, J. F., Godart, F. et al. (2016). Transcatheter closure of coronary artery fistulas in infants and children: A French multicenter study. Catheterization and Cardiovascular Interventions, 87, 411–418. DOI 10.1002/ccd.26320. [Google Scholar] [CrossRef]
8. Kubicki, R., Stiller, B., Hummel, J., Höhn, R., Fleck, T. et al. (2019). Collateral closure in congenital heart defects with Amplatzer vascular plugs: Single-center experience and a simplified delivery technique for exceptional cases. Heart and Vessels, 34, 134–140. DOI 10.1007/s00380-018-1232-3. [Google Scholar] [CrossRef]
9. Barwad, P., Ramakrishnan, S., Kothari, S. S., Saxena, A., Gupta, S. K. et al. (2013). Amplatzer vascular plugs in congenital cardiovascular malformations. Annals of Pediatric Cardiology, 6, 132–140. DOI 10.4103/0974-2069.115255. [Google Scholar] [CrossRef]
10. Pech, M., Mohnike, K., Wieners, G., Seidensticker, R., Seidensticker, M. et al. (2011). Advantages and disadvantages of the Amplatzer vascular plug IV in visceral embolization: Report of 50 placements. Cardiovascular and Interventional Radiology, 34, 1069–1073. DOI 10.1007/s00270-011-0150-x. [Google Scholar] [CrossRef]
11. Hundt, W., Kalinowski, M., Kiessling, A., Heverhagen, J. T., Eivazi, B. et al. (2012). Novel approach to complex pulmonary arteriovenous malformation embolization using detachable coils and Amplatzer vascular plugs. European Journal of Radiology, 81, e732–e738. DOI 10.1016/j.ejrad.2012.01.030. [Google Scholar] [CrossRef]
12. Schwartz, M., Glatz, A. C., Rome, J. J., Gillespie, M. J. (2010). The Amplatzer vascular plug and Amplatzer vascular plug II for vascular occlusion procedures in 50 patients with congenital cardiovascular disease. Catheterization and Cardiovascular Interventions, 76, 411–417. DOI 10.1002/ccd.22370. [Google Scholar] [CrossRef]
13. Garay, F. J., Aguirre, D., Cárdenas, L., Springmuller, D., Heusser, F. (2015). Use of the Amplatzer vascular plug II device to occlude different types of patent ductus arteriosus in paediatric patients. Journal of Interventional Cardiology, 28, 198–204. DOI 10.1111/joic.12188. [Google Scholar] [CrossRef]
14. Delaney, J. W., Fletcher, S. E. (2013). Patent ductus arteriosus closure using the Amplatzer® vascular plug II for all anatomic variants. Catheterization and Cardiovascular Interventions, 81, 820–824. DOI 10.1002/ccd.24707. [Google Scholar] [CrossRef]
15. Wiegand, G., Sieverding, L., Bocksch, W., Hofbeck, M. (2013). Transcatheter closure of abnormal vessels and arteriovenous fistulas with the Amplatzer vascular plug 4 in patients with congenital heart disease. Pediatric Cardiology, 34, 1668–1673. DOI 10.1007/s00246-013-0701-9. [Google Scholar] [CrossRef]
16. Webb, M. K., Hunter, L. E., Kremer, T. R., Huddleston, C. B., Fiore, A. C. et al. (2020). Extracardiac fontan fenestration device closure with Amplatzer vascular plug II and septal occluder: Procedure results and medium-term follow-up. Pediatric Cardiology, 41, 703–708. DOI 10.1007/s00246-019-02283-0. [Google Scholar] [CrossRef]
17. Adelmann, R., Windfuhr, A., Bennink, G., Emmel, M., Sreeram, N. (2011). Extended applications of the Amplatzer vascular plug IV in infants. Cardiology in the Young, 21, 178–181. DOI 10.1017/S104795111000171X. [Google Scholar] [CrossRef]
18. Tabori, N. E., Love, B. A. (2008). Transcatheter occlusion of pulmonary arteriovenous malformations using the Amplatzer vascular plug II. Catheterization and Cardiovascular Interventions, 71, 940–943. DOI 10.1002/ccd.21474. [Google Scholar] [CrossRef]
19. Miyabayashi, K., Furukawa, T., Ohtsuki, M., Kishiro, M., Shimizu, T. (2017). Lobar occlusion of pulmonary arteriovenous malformations with Amplatzer vascular plug. Pediatrics International, 59, 837–838. DOI 10.1111/ped.13282. [Google Scholar] [CrossRef]
20. Tang, L., Wang, Z. J., Tang, J. J., Fang, Z. F., Hu, X. Q. et al. (2020). Transcatheter closure of large coronary-cameral fistulas using the patent ductus arteriosus occluder or Amplatzer vascular plugs. International Heart Journal, 61, 1220–1228. DOI 10.1536/ihj.20-169. [Google Scholar] [CrossRef]
21. Baruteau, A. E., Lambert, V., Riou, J. Y., Angel, C. Y., Belli, E. et al. (2015). Closure of tubular patent ductus arteriosus with the Amplatzer vascular plug IV: Feasibility and safety. World Journal for Pediatric and Congenital Heart Surgery, 6, 39–45. DOI 10.1177/2150135114558070. [Google Scholar] [CrossRef]
22. Krichenko, A., Benson, L. N., Burrows, P., Möes, C. A., McLaughlin, P. et al. (1989). Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. American Journal of Cardiology, 63, 877–880. DOI 10.1016/0002-9149(89)90064-7. [Google Scholar] [CrossRef]
23. Bergersen, L., Gauvreau, K., Jenkins, K. J., Lock, J. E. (2008). Adverse event rates in congenital cardiac catheterization: A new understanding of risks. Congenital Heart Disease, 3, 90–105. DOI 10.1111/j.1747-0803.2008.00176.x. [Google Scholar] [CrossRef]
24. Bergersen, L., Gauvreau, K., Foerster, S. R., Marshall, A. C., McElhinney, D. B. et al. (2011). Catheterization for congenital heart disease adjustment for risk method (CHARM). Journal of American College of Cardiology: Cardiovascular Interventions, 4, 1037–1046. DOI 10.1016/j.jcin.2011.05.021. [Google Scholar] [CrossRef]
25. Hua, N., Aquino, P., Owada, C. Y. (2016). Transcatheter closure of perimembranous ventricular septal defects with the Amplatzer vascular plug-II. Cardiology in the Young, 26, 1194–1201. DOI 10.1017/S1047951115002206. [Google Scholar] [CrossRef]
26. Mijangos-Vázquez, R., El-Sisi, A., Sandoval Jones, J. P., García-Montes, J. A., Hernández-Reyes, R. et al. (2020). Transcatheter closure of perimembranous ventricular septal defects using different generations of Amplatzer devices: Multicenter experience. Journal of Interventional Cardiology, 2020, 8948249. DOI 10.1155/2020/8948249. [Google Scholar] [CrossRef]
27. Jain, S. M., Pradhan, P. M., Sen, S., Dalvi, B. V. (2020). Transcatheter closure of elongated and pulmonary hypertensive patent arterial duct in infants using Amplatzer vascular plug II. Cardiology in the Young, 30, 243–248. DOI 10.1017/S1047951120000104. [Google Scholar] [CrossRef]
28. Aggarwal, V., Mulukutla, V., Qureshi, A. M., Justino, H. (2018). Congenital coronary artery fistula: Presentation in the neonatal period and transcatheter closure. Congenital Heart Disease, 13, 782–787. DOI 10.1111/chd.12653. [Google Scholar] [CrossRef]
29. El-Sabawi, B., Al-Hijji, M. A., Eleid, M. F., Cabalka, A. K., Ammash, N. M. et al. (2020). Transcatheter closure of coronary artery fistula: A 21-year experience. Catheterization and Cardiovascular Interventions, 96, 311–319. DOI 10.1002/ccd.28721. [Google Scholar] [CrossRef]
30. Backes, C. H., Cua, C., Kreutzer, J., Armsby, L., El-Said, H. et al. (2013). Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheterization and Cardiovascular Interventions, 82, 786–794. DOI 10.1002/ccd.24726. [Google Scholar] [CrossRef]
31. Chien, Y. H., Wang, H. H., Lin, M. T., Lin, H. C., Lu, C. W. et al. (2020). Device deformation and left pulmonary artery obstruction after transcatheter patent ductus arteriosus closure in preterm infants. International Journal of Cardiology, 312, 50–55. DOI 10.1016/j.ijcard.2020.02.065. [Google Scholar] [CrossRef]
Supplemental Tables
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |