 Open Access
Open Access
ARTICLE
Efficacy, Safety and Characteristics of the Amplatzer Vascular Plug II and IV Utilization for Various Percutaneous Occlusions in Children under 10 Years
1 Antilles-Guyane M3C Paediatric Cardiology Center, University Hospital of Martinique, Fort-de-France, France
2 L’institut du Thorax, INSERM, CNRS, UNIV Nantes, CHU Nantes, Nantes, France
3 Department of Paediatric Cardiology and Pediatric Cardiac Surgery, University Hospital of Nantes, Nantes, France
4 Department of Paediatric and Congenital Heart Disease, University Hospital of Marseille, Marseille, France
5 Department of Paediatric and Congenital Heart Disease, University Hospital of Lille-Nord de France, Lille, France
6 Department of Paediatric and Congenital Heart Disease, Cardiothoracic Center of Monaco, Monaco
7 Department of Paediatric and Congenital Heart Disease, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France
8 Clinical Research Department, CHU Martinique (University Hospital of Martinique), Fort-de-France, France
* Corresponding Author: Hugues Lucron. Email:
Congenital Heart Disease 2022, 17(4), 421-436. https://doi.org/10.32604/chd.2022.020835
Received 15 January 2022; Accepted 15 May 2022; Issue published 04 July 2022
Abstract
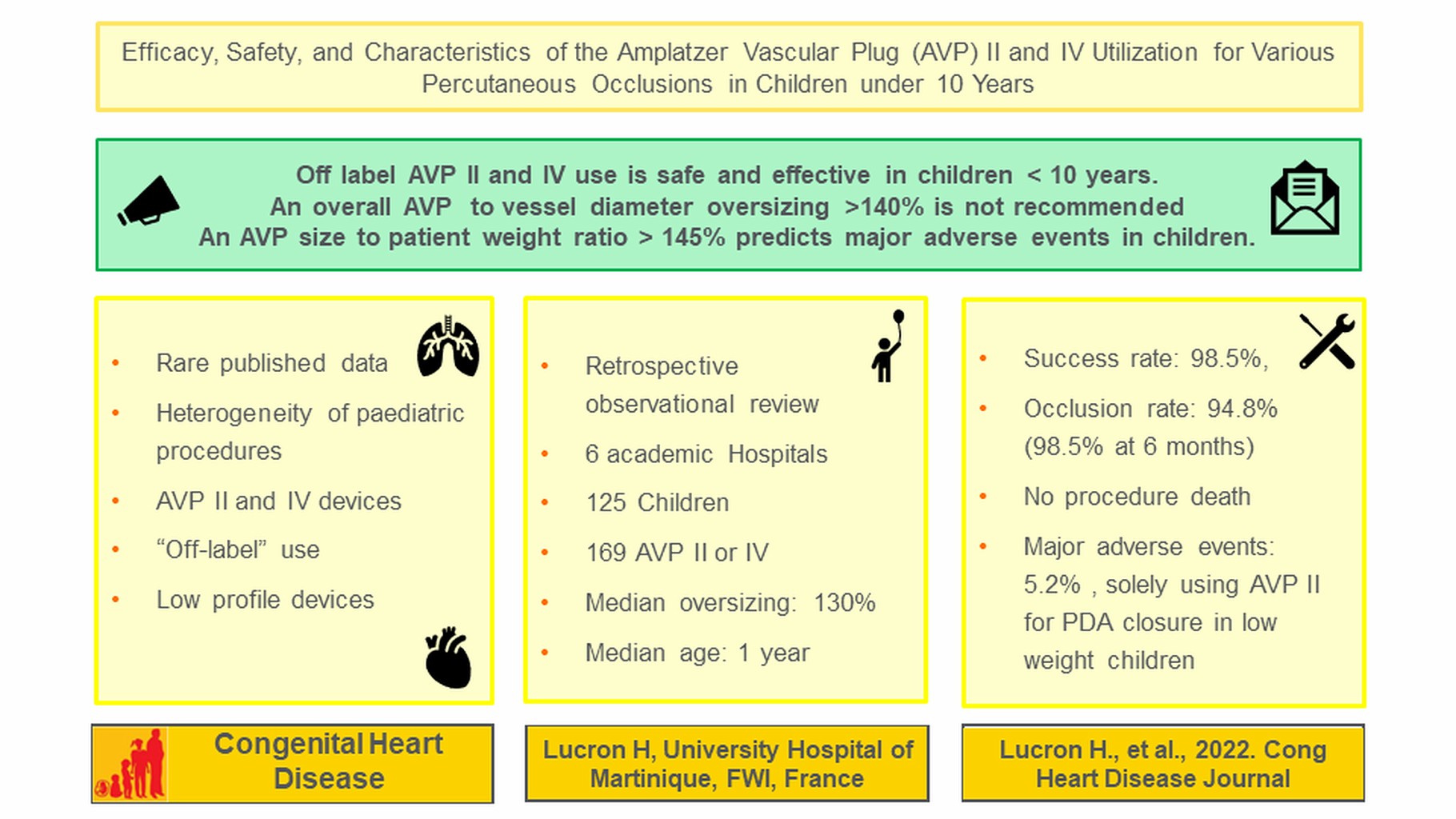
Objectives: We aim to describe the efficacy, safety, and characteristics of the Amplatzer Vascular Plug (AVP) II and IV “off-label” use for multiple cardiovascular occlusions in children under 10 years. Methods: Observational retrospective multicenter (2007–2020, 6 centers) review of paediatric procedures using AVP II or IV. Results: A total of 125 children (49.6% aged ≤ 1 year, 147 lesions) underwent 136 successive procedures (success rate: 98.5%) using 169 devices (109 AVP IV, 60 AVP II). The mean device diameter was 7.7 ± 3.2 mm (4–20 mm). The median AVP size to vessel diameter ratio was 1.3 (0–2). The median age and weight at implantation were 1.0 year (0.01–9.98) and 8.4 kg (1–69). Procedures were heterogeneous (55 patent ductus arteriosus (PDA), 28 collaterals, 18 sequestrations, 22 arteriovenous/veinovenous/coronary fistulas, 6 vertical veins, 6 conduits, 5 ventricular septal defects, 7 miscellaneous). Day 1 and 6-month occlusion rates were respectively 94.8% and 98.5%. Major adverse events (MAE) occurred in 5.2% of cases (no procedure-related deaths), and more frequently in weight ≤ 5 kg (p = 0.01), younger patients (p = 0.03) during PDA closure (p = 0.02) of tubular types (p = 0.02) using larger devices (p = 0.03) and AVP II (p = 0.003). Independent predictor of MAE risk was a higher AVP diameter to patient weight ratio (Odds-ratio: 2.33, 95% confidence interval 1.31–4.13, p = 0.004, optimal cut off: 1.45). Conclusions: Both AVPs are safe and effective for percutaneous occlusions in children under 10. Such devices represent an alternative “off label” use for well selected paediatric patients.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools