

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.020174
ARTICLE
Adults with Congenital Heart Disease during the COVID-19 Era: One-Year Tertiary Center Experience
1Adult Cardiology Department, Madinah Cardiac Center, Madinah, Saudi Arabia
2Cardiology Department, Faculty of Medicine, Tanta University, Tanta, Egypt
*Corresponding Author: Fatma A. Taha. Email: fatmastaha@yahoo.com
Received: 10 November 2021; Accepted: 29 March 2022
Abstract: Background: Adult patients with congenital heart disease (ACHD) might be at high risk of Coronavirus disease-2019 (COVID-19). This study aimed to report on a one-year tertiary center experience regards COVID-19 infection in ACHD patients. Methods: This is a one-year (March-2020 to March-2021) tertiary-center retrospective study that enrolled all ACHD patients; COVID-19 positive patients’ medical records, and management were reported. Results: We recorded 542 patients, 205 (37.8%) COVID-19-positive, and 337 (62.2%) COVID-19-negative patients. Palliated single ventricle and Eisenmenger syndrome patients were more vulnerable to COVID-19 infection (P < 0.05*). Cardiovascular COVID-19 complications were arrhythmias in 47 (22.9%) patients, heart failure in 39 (19.0%) patients, cyanosis in 12 (5.9%) patients, stroke/TIA in 5 (2.4%) patients, hypertension and infective endocarditis in 2 (1.0%) patients for each, pulmonary hypertension and pulmonary embolism in 1 (0.5%) patient for each. 11 (5.4%) patients were managed with home isolation, 147 (71.7%) patients required antibiotics, 32 (15.6%) patients required intensive care unit (ICU), 8 (3.9%) patients required inotropes, 7 (3.4%) patients required mechanical ventilation, and 2 (1.0%) patients required extracorporeal membrane oxygenation (ECMO). Thromboprophylaxis was given to all 46 (22.4%) hospitalized patients. American College of Cardiology/American Heart Association classification revealed that complex lesions, and FC-C/D categories were more likely to develop severe/critical symptoms, that required mechanical ventilation and ECMO (P < 0.05*). Mortality was reported in 3 (0.6%) patients with no difference between groups (P = 0.872). 193 (35.6%) patients were vaccinated. Conclusions: COVID-19 infection in ACHD patients require individualized risk stratification and management. Eisenmenger syndrome, single ventricle palliation, complex lesions, and FC-C/D patients were more vulnerable to severe/critical symptoms that required ICU admission, mechanical ventilation, and ECMO. The vaccine was mostly tolerable.
Keywords: Adult congenital heart disease; COVID-19 era; COVID-19 pandemic; COVID-19 positive congenital heart disease patients; COVID-19 infected adults with congenital heart disease
Nomenclature
| ACE2 | Angiotensin-converting enzyme 2 |
| ACHA | Adult Congenital Heart Association |
| ACHD | Adult congenital heart disease |
| AP | Anatomical physiological |
| ARDS | Adult respiratory distress syndrome |
| ASA | Atrial septal aneurysm |
| ASD | Atrial septal defect |
| AV | Aortic valve |
| AVSD | Atrioventricular septal defect |
| BA | Bronchial asthma |
| BDG | Bidirectional Glenn |
| CAD | Coronary artery disease |
| CHD | Congenital heart disease |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CT | Computed tomography |
| CVD | Cardiovascular disease |
| CVS | Cardiovascular system |
| D-TGA | Dextro-transposition of the great arteries |
| ECMO | Extracorporeal membrane oxygenation |
| EPOCH | European Collaboration for Prospective Outcome Research in Congenital Heart Disease |
| FC | Functional class |
| HF | Heart failure |
| ICD | Implantable cardioverter defibrillator |
| ICU | Intensive care unit |
| NIV | Non-invasive ventilation |
| O2 | Oxygen |
| PAH | Pulmonary arterial hypertension |
| PAP | Pulmonary artery pressure |
| PDA | Patent ductus arteriosus |
| PFO | Patent foramen ovale |
| PPE | Personal protective equipment |
| PV | Pulmonary valve |
| PVR | Pulmonary vascular resistance |
| RT-PCR | Real-time polymerase chain reaction |
| RV | Right ventricle |
| SCD | Sudden cardiac death |
| SD | Standard deviation |
| TGA | Transposition of the great arteries |
| TIA | Transient ischemic attack |
| WHO | World Health Organization |
Coronavirus disease 2019 (COVID-19) is a disease caused by acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It was first reported in the city of Wuhan, China in 2019, before spreading and causing a pandemic [1]. Patients with COVID-19 can have a range of different presentations including fever, headache, dry cough, dyspnea, fatigue, and anosmia [2,3], and sometimes need intensive care and ventilation support [4–6].
Angiotensin-converting enzyme-2 (ACE2) has been confirmed as the entry point for SARS-CoV-2 into the host cells. It has been shown that SARS-CoV-2 has a higher affinity for the ACE2 receptor than SARS-CoV-1 [7,8]. This, in combination with the presence of ACE2 in the myocardial cells, may explain the mechanism of how COVID-19 affects the cardiovascular system (CVS). The importance of this interaction is supported by the studies that found that pre-existing cardiovascular disease (CVD) as coronary artery disease (CAD), heart failure (HF), and arrhythmias had a larger impact on mortality rate in COVID-19 patients than those who had chronic obstructive pulmonary disease (COPD) [4,9]. The exact mechanism of the cardiomyocytes damage is still unclear. It has been suggested that it can occur as a result of the COVID-19 cytokine storm or as a consequence of the oxidative stress resulting from the increased cardiomyocytes oxygen demand during adult respiratory distress syndrome (ARDS) [1]. For this reason, subjects that might be at high risk include patients with congenital heart disease (CHD) [1,10], and may include those with cyanosis, Eisenmenger syndrome, Fontan palliation, systemic right ventricles (RVs), and unrepaired complex CHD. This risk might be further multiplied if CHD is associated with additional comorbidities; ventricular dysfunction, arrhythmias, HF, and pulmonary arterial hypertension (PAH) [1,10–14]. While research is limited, some evidence are suggesting that CHD patients may have a worse prognosis if they are infected with COVID-19, than those without CHD [15,16]. This likely applies predominantly to adult CHD (ACHD) patients, as pediatric patients may not have had the time to develop these cardiovascular complications [16]. The latter has also been shown to have protective higher levels of ACE2 [17].
Since it is unknown whether ACHD patients are at high-risk for COVID-19 infection or not, we aimed in this study to report on a one-year tertiary center experience regards COVID-19 infection in ACHD patients and to consider the risk stratification, presentation, adverse outcomes, management, and prevention of the infected patients.
This is a retrospective cohort study that enrolled all ACHD patients who were followed in our center either as outpatients or inpatients, throughout the year from March-2020 to March-2021. COVID-19 infection reports were retrospectively collected. The studied ACHD patients were divided into COVID-19 positive and negative groups. COVID-19 was diagnosed according to the guidelines of the World Health Organization (WHO) [18]. Nasopharyngeal swabs with real-time polymerase chain reaction (RT-PCR) tests were applied if there were suspicion of symptoms and before admission for any elective procedures. When suspicion was proved by PCR, routine laboratory investigations, D-Dimer, and sometimes chest computed tomography (CT) was performed. COVID-19 positive patients’ presentation, outcome, and management were reported and related to the patient status; repaired versus unrepaired. Repaired patients defined as all patients who underwent any operative repair, percutaneous intervention, or both. Unrepaired patients defined as all patients who did not undergo any operative repair or percutaneous intervention.
This study complied with the Declaration of Helsinki ethical guidelines of 1975, as revised in 2013, and was reviewed and approved by the board committee of Madinah Cardiac Center, Madinah, Saudi Arabia, approval number MCC1722020. Informed written consent was obtained from all patients to participate in the study.
2.2 Data Collection and Data Quality
All the studied ACHD patients’ medical records were evaluated to report demographic data and clinical characteristics including baseline oxygen (O2) saturation, comorbid risk factors, cardiovascular comorbidities, cardiac diagnosis, functional class (FC), number and type of previous interventions, previously implanted devices/valves/pacemakers/implantable cardioverter defibrillator (ICD), previous medications as well as cardiac interventions and mortality during this year. Patients were classified according to the anatomical physiological (AP) classification depending on the American College of Cardiology/American Heart Association (ACC/AHA) 2018 ACHD classification into simple, moderate complexity, and complex lesions with a physiological stage of A, B, C, or D [19].
2.3 COVID-19 Positive Patients
In COVID-19 positive patients, the cardiovascular COVID-19 complications included arrhythmias, HF (systolic or diastolic), cyanosis, stroke/transient ischemic attack (TIA), hypertension, infective endocarditis, pulmonary arterial hypertension (mean pulmonary arterial pressure (PAP) > 25 mm Hg at rest or > 30 mm Hg during exercise or systolic pulmonary arterial pressure > 50% of systolic systemic blood pressure), pulmonary hemorrhage, pulmonary embolism, cardiogenic shock, and pericardial effusion were reported and analyzed. ARDS, and respiratory failure defined according to the Berlin Definition [20] were also reported. Patients with high serum D-Dimer > 0.5 ug/ml, high serum troponin > 0.04 ng/ml, and positive chest CT signs for COVID-19 infection were recorded.
Once an ACHD patient was diagnosed with COVID-19, the management was similar to the general population in addition to the management of the cardiovascular complications. Following the national protocol and guidelines [21], patients’ presentations were divided into asymptomatic, mild/moderate symptoms, severe and critical presentations and were managed as follows:
• Asymptomatic patients (Discovered accidentally): The patient underwent home isolation only.
• Patients with mild/moderate symptoms (No O2 requirements, no evidence of pneumonia, but with other COVID-19 symptoms, e.g., fever): The patients underwent home isolation, with symptom treatment (Antibiotics, analgesics, and vitamins). In case of new-onset cough or fever, inhaled budesonide was considered.
• Patients with severe symptoms (Clinical signs of pneumonia with one of the following: Respiratory rate ≥ 30/min, blood O2 saturation ≤ 90%, severe respiratory distress): The patients underwent intensive care unit (ICU) admission, antibiotics, and antifungals according to local antibiogram and institutional pneumonia management guidelines/pathways. Favipiravir or Remdesivir and systemic corticosteroids were considered.
• Patients with critical symptoms (ARDS, respiratory failure, sepsis, or septic shock): The patients underwent ICU admission, antibiotics, and antifungals according to local antibiogram and institutional pneumonia management guidelines/pathways. Remdesivir and systemic corticosteroids were considered. For patients with respiratory failure, mechanical ventilation and extracorporeal membrane oxygenation (ECMO) were considered.
• Thromboprophylaxis: Low molecular weight heparin (LMWH) was given to all patients who required hospital admission.
• Pregnancy and lactation: Management of infection with SARS-COV2 in pregnancy was mainly based on supportive care. Consideration of antiviral therapy was based on patient condition, and preference of the patient and the treating team.
2.5 Prevention and Approved Vaccines
Usual general protection measures were applied to the whole population including the ACHD patients [face mask, reduce non-essential contact, preference for homework and online school learning, with personal protective equipment (PPE) in the workplace].
Two approved vaccines were started to be given on 21/2/2021 to the whole population >18 years including ACHD patients: Pfizer-BioNTech (COVID-19 mRNA vaccine, COMIRNATY, USA) and Oxford-AstraZeneca (ChAdOx1-S [recombinant] COVID-19 vaccine, UK). Patients with acute PCR-confirmed COVID-19 did not vaccinate until after they have recovered from acute illness. Patients who were known to have an allergy against any of the vaccine component, or had a history of anaphylaxis following any vaccination, were not vaccinated until further evidence was available. All patients who received any type of the vaccines were reported.
This is a retrospective cohort study in which statistical analysis was performed using the SPSS statistical package (Version 25; SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to confirm the normality of the distribution. Quantitative data were stated as mean ± standard deviation (SD), and qualitative data were expressed using numbers and percentages. The comparisons between the continuous variables were performed using the paired sample t-test and the ANOVA test, and the comparison between the categorical variables was performed using the Chi-square test. A P-value of <0.05* was considered statistically significant at a confidence interval of 95%.
3.1 Demographic Data and Clinical Characteristics
Demographic data and clinical characteristics of the studied patients were summarized in Table 1.

In this study, we retrospectively reported 542 patients with ACHD; 205 (37.8%) patients were PCR-confirmed COVID-19 positive, and 337 (62.2%) patients were COVID-19 negative. No age or baseline O2 saturation difference between COVID-19 positive and negative patients, with significantly more males [116/205 (56.6%), P = 0.003*)], higher weight [65.5 ± 19.2 Kg, P = 0.013*], and with larger body surface area (BSA) [1.7 ± 0.3 m2, P = 0.008*] in COVID-19 positive patients. COVID-19 positive patients were more likely to have chronic respiratory disease [Bronchial asthma (BA)/Chronic obstructive pulmonary disease (COPD)], chronic anemia, and a family history of sudden cardiac death (SCD) (P < 0.05*). They displayed more impaired ventricular function, valvular regurgitation, valvular stenosis, pulmonary hypertension, and arrhythmias, and were more on previous cardiac medications, antiplatelets, and anticoagulants (P < 0.05*). COVID-19 negative patients were more likely to have previously implanted devices, valves, and conduits (P < 0.05*). Comorbid risk factors and cardiovascular comorbidities in COVID-19 positive and COVID-19 negative patients were displayed in Fig. 1.
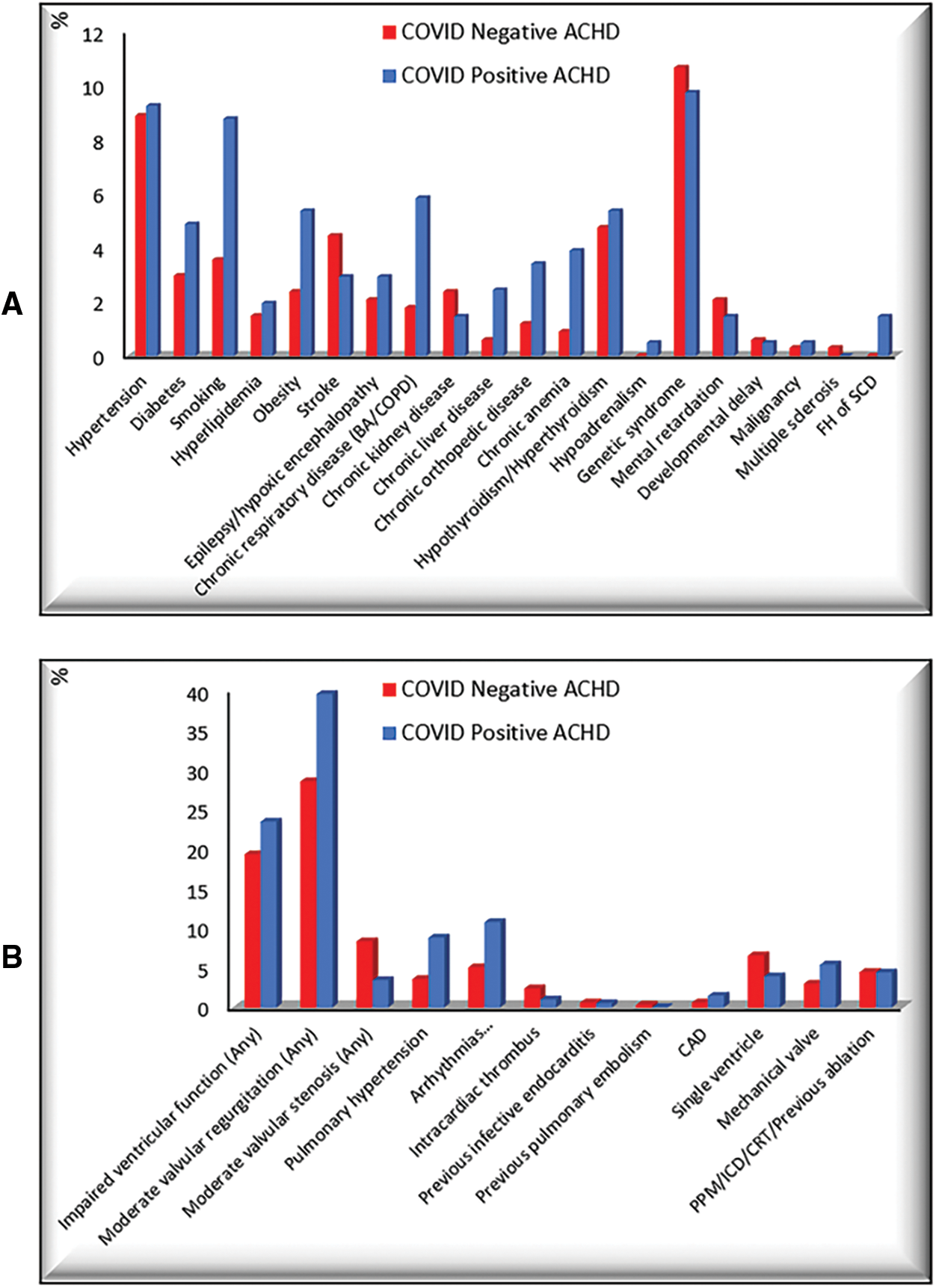
Figure 1: Comorbid risk factors and cardiovascular comorbidities in COVID-19 positive and COVID-19 negative patients. A: Comorbid risk factors, B: Cardiovascular comorbidities
CRT: Cardiac resynchronization therapy, ICD: Implantable cardioverter-defibrillator, PPM: Permanent pacemaker, SCD: Sudden cardiac death.
COVID-19 positive patients were more likely to have three or more previous interventions (Mostly combined percutaneous and surgical) (P = 0.002*), while COVID-19 negative patients had a single previous intervention (mostly percutaneous interventions) (P < 0.001*). COVID-19 positive patients were more subjected to multiple admissions and cardiac interventions during the period of the study (P < 0.05*). No difference in pregnancy and mortality rate between COVID-19 positive and negative patients during the period of the study (P < 0.05*).
3.2 Congenital Heart Disease Lesions and COVID-19 Infection
ACC/AHA anatomical physiological classification of the studied patients was displayed in Fig. 2.

Figure 2: ACC/AHA anatomical physiological classification of the studied patients. A1: COVID-19 positive patients, A2: COVID-19 negative patients
The anatomical classification denoted that simple CHD lesions were more reported among COVID-19 negative patients [118/337 (35.0%), P < 0.001*] and complex lesions were more recorded among COVID-19 positive patients [42/205 (20.5%), P < 0.001*], with no difference in moderate complexity lesions between both groups of patients. The physiological staging displayed that ACHD FC A patients were more presented in the COVID-19 negative group [112/337 (33.2%), P < 0.001*], while FC C and D patients were more presented in the COVID-19 positive group [45/205 (22%), P < 0.001*, and 32/205 (15.6%), P = 0.032*, respectively], with no difference as regard FC B category between both groups.
Three-hundred eighty-one (70.3%) patients were previously repaired vs. 161 (29.7%) patients were unrepaired. Various repaired lesions presented similarly in both COVID-19 positive and negative groups, except for the Bentall procedure and the palliated single ventricle (Bidirectional Glenn (BDG)/Fontan/Kawashima) patients who were more vulnerable to COVID-19 infection (P < 0.05*). Unrepaired lesions presented similarly in both COVID-19 positive and negative groups, except for the Eisenmenger patients who were more vulnerable to COVID-19 infection (P = 0.033*) [Supplementary Table].
3.3 COVID-19 Positive Patients
COVID-19 positive adult congenital heart disease patients’ presentation, outcome, and management were displayed in Table 2.

Among the 205 COVID-19 positive patients, 126 (61.5%) patients were repaired vs. 79 (38.5%) patients were unrepaired. Repaired patients presented mostly with mild/moderate symptoms and signs with higher O2 saturation at baseline and during infection (P < 0.001*), while unrepaired patients exhibited mostly critical presentation with lower O2 saturation (P < 0.05*).
The most common cardiovascular COVID-19 complications were arrhythmias in 47 (22.9%) patients [33 (26.2%) repaired vs. 14 (17.7%) unrepaired, P = 0.006*], then heart failure in 39 (19.0%) patients [21 (16.7%) repaired versus 18 (22.8%) patients, P = 0.631], followed by cyanosis in 12 (5.9%) patients [2 (1.6%) repaired vs. 10 (12.7%) unrepaired, P = 0.031*], stroke/TIA in 5 (2.4%) unrepaired patent foramen ovale (PFO)/atrial septal defect (ASD)/atrial septal aneurysm (ASA) patients, hypertension in 2 (1%) repaired patients, infective endocarditis in 2 (1%) unrepaired (patent ductus arteriosus) PDA patients, pulmonary hypertension and pulmonary hemorrhage in 1 (0.5%) post-Fontan patient, and pulmonary embolism in 1 (0.5%) unrepaired ASD patient. No patient developed cardiogenic shock or pericardial effusion/tamponade. 7 (3.4%) patients developed ARDS/Respiratory failure [2 (1.6%) repaired vs. 5 (6.3%) unrepaired, P = 0.035*].
Among the 47 (22.9%) patients with arrhythmias, 32 (15.6%) patients had new onset arrhythmias, and 15 (7.3%) patients had increased frequency of previous arrhythmias. 12 (5.9%) patients had atrial extra-systoles, 8 (3.9%) patients had supraventricular arrhythmias (SVT), 4 (2%) had atrial fibrillation (AF), and 23 (11.2) ventricular ectopics.
32 (15.6%) patients had high serum D-Dimer > 0.5 ug/ml [11 (8.7%) repaired vs. 21 (26.6%) unrepaired, P = 0.033*], 11 (5.4%) patients had high serum troponin > 0.04 ng/ml [6 (4.8%) repaired vs. 5 (6.3%) unrepaired, P = 0.611], and 59 (28.8%) patients had positive chest CT signs for COVID-19 infection [33 (26.2%) repaired vs. 26 (32.6%) unrepaired, P = 0.046*].
11 (5.4%) patients were managed with home isolation only, 147 (71.7%) patients required home isolation with antibiotic intake, 32 (15.6%) patients required ICU admission with non-invasive ventilation (NIV), 8 (3.9%) patients required inotropes, 7 (3.4%) patients required mechanical ventilation, and 2 (1%) patients from the unrepaired group required ECMO. Repaired patients were more likely to be managed with home isolation and antibiotics (P < 0.001*), and unrepaired patients were more likely to require mechanical ventilation and ECMO (P = 0.035, P = 0.049*, respectively). Thromboprophylaxis was given to all, 46 (22.4%), hospitalized patients [21 (16.7%) repaired vs. 25 (31.7%) unrepaired, P = 0.012*].
63/205 (30.7%) patients were firstly and accidentally discovered to have CHD lesions during their COVID-19 infection and 39/205 (19.0%) patients underwent cardiac interventions for these lesions either percutaneously or surgically after relieving of the acute stage of infection. Among these 63 patients, 31 (15.1%) patients had interatrial septal defect (ASD/PFO/ASA), 5 (2.4%) patients had small to moderate sized ventricular septal defect (VSD), 3 (1.5%) patients had VSD/Double chamber RV (DCRV), 4 (2.0%) patients had small PDA, 8 (3.9%) patients had bicuspid/unicuspid aortic valve (AV) with moderate to severe aortic stenosis, 3 (1.5%) patients had moderate to severe valvular pulmonary stenosis (PS), 2 (1.0%) patients had moderate form of Ebstein anomaly, 2 (1.0%) patient had subaortic membrane, 1 (0.5%) patient had congenitally corrected transposition of the great arteries (ccTGA), 2 (1.0%) patients had tetralogy of Fallot (TOF), 1 (0.5%) patient had double outlet right ventricle DORV/TOF/collaterals, and 1 (0.5%) patient had pulmonary atresia (PA)/VSD/collaterals.
6 (2.9%) of the COVID-19 positive patients had pregnancy during the period of the study, but only 2 (1.0%) showed infection during pregnancy, 1 (0.5%) patient revealed no complications, and the other 1 (0.5%) patient with a severe form of Ebstein anomaly experienced intrauterine fetal death. There were no maternal deaths and no cardiomyopathies. Furthermore, no fetal transmission was reported.
3.4 Risk Stratification of COVID-19 Positive Patients
Risk stratification of COVID-19 positive patients was summarized in Table 3.

5 (71.4%) of the critically ill presented patients had impaired ventricular function (P < 0.001*), 5 (71.4%) had pulmonary hypertension (P < 0.001*), and 1 (14.3%) had intracardiac thrombus (P = 0.002*). 9 (22.5%) of the severely presented patients had previous arrhythmias (P = 0.038*) and 3 (7.5%) had previous coronary artery disease (CAD) (P = 0.006*). Simple lesions were more to be asymptomatic (P < 0.001*), moderate complexity lesions were more to present with mild/moderate symptoms (P < 0.001*), while complex lesions were more to develop critical symptoms (P = 0043*). ACHD FC A and B category were more likely to be asymptomatic (P < 0.001*), FC C patients were more to present with severe symptoms (P = 0.027*), and FC D patients were more to develop critical symptoms (P = 0.231). Eisenmenger syndrome, ccTGA, single ventricle palliation (Fontan pathway and Kawashima), and complex lesions were more vulnerable to critical symptoms that required ICU admission, mechanical ventilation, or ECMO. COVID-19 positive patients’ presentation category concerning their anatomical diagnosis was displayed in Fig. 3.

Figure 3: COVID-19 positive patients’ presentation category concerning their anatomical diagnosis. A1: Repaired patients. A2: Unrepaired patients
ASA: Atrial septal aneurysm, ASD: Atrial septal defect, AV: Aortic valve, AVSD: Atrioventricular septal defect, BAV: Bicuspid aortic valve, cc-TGA: Congenitally corrected transposition of the great arteries, DCM: Dilated cardiomyopathy, D-TGA: Dextro-Transposition of the great arteries, HOCM: Hypertrophic obstructive cardiomyopathy, LVOT: Left ventricular outflow tract, MAPCAs: Multiple aortopulmonary collaterals, MV: Mitral valve, MVP: Mitral valve prolapse, PA: Pulmonary atresia, PDA: Patent ductus arteriosus, PFO: Patent foramen ovale, PS: Pulmonary stenosis, PV: Pulmonary valve, RV-PA conduit: Right ventricle to pulmonary artery conduit, SAM: subaortic membrane, TOF: Tetralogy of Fallot, TV: tricuspid valve, UAV: Unicuspid aortic valve, VSD: Ventricular septal defect.
3.5 Mortality among the Studied Patients
Three (0.6) patients passed away during the period of the study, with no difference between COVID-19 positive (one patient) and negative groups (2 patients) (P = 0.872). The 1 (0.5%) COVID-19 positive patient was a post-Fontan patient who had increased PAP with no arterio-venous or veno-venous collaterals, and with preserved renal and liver functions. During the patient COVID-19 infection, he developed sever uncontrollable pulmonary hemorrhage of about 2 Liters of blood, the patient was admitted in the ICU, and the hemorrhage was controlled with small dose coagulant, elective mechanical ventilation, with milrinone infusion, and other COVID-19 infection managements (antibiotics, and antivirals). Cardiac CT was done to exclude any new collateral development and denoted no new collaterals to intervene. On the 7th day, the patient released from mechanical ventilation, continued on anti-pulmonary hypertension medications, albumin compensation, and other supportive medications. On the 16th day, he developed another sever attack of pulmonary hemorrhage, impairment of renal function, again required mechanical ventilation with ultrafiltration then he developed multisystem failure and death. The 2 (0.6%) COVID-19 negative patients were one post-Fontan patient, and one with aortic interruption [Both patients showed end-stage heart failure and were not capable of heart transplantation].
In this cohort, a total of 193 (35.6%) ACHD patients were vaccinated with the first vaccine dose, all of them were >18 years; 70 (34.1%) patients from the COVID-19 positive group and 123 (36.5%) patients from the COVID-19 negative group (P =0.771). The vaccine was tolerable in most ACHD patients, only sometimes with low grade fever, rash, headache and body aches.
4.1 COVID-19 Infected Patients
In our experience, the number of COVID-19-infected ACHD patients in one year was 205/542 (37.8%). Some potential explanations for this average number of infected patients: Firstly, families tend to be very protective of ACHD patients, additionally, most stabilized ACHD patients were managed virtually during the active waves of the pandemic, and ACHD patients were relatively younger and less at risk for COVID-19 adverse outcomes. In our cohort, we did not observe the same critical respiratory outcomes and the higher mortality risk that have been previously described for CVDs [4–6] other than CHDs [11,22,23].
In this study, 116/205 (56.6%) COVID-19 positive patients were males (P = 0.003*), in line with the previous studies showing higher percentages of infection in males than in females [11,24], and against a recent study that recorded 51% females among the studied patients [25].
The most common cardiovascular COVID-19 complications were arrhythmias in 22.9% patients, followed by HF in 19.0% patients, then cyanosis in 5.9% patients, stroke/TIA in 2.4% patients, hypertension, and infective endocarditis in 1% patients for each, pulmonary hypertension/hemorrhage and pulmonary embolism in 0.5% patients for each. In the Italian study on seventy-six COVID-19-infected patients with CHD, HF was the most common cardiovascular complication (9%), followed by palpitations/arrhythmias (3%), stroke/TIA (3%), pulmonary hypertension (3%), and myocardial injury (1%) [26]. Guo et al. demonstrated that patients with underlying CVD were more prone to myocardial injury and HF during the course of COVID-19, and observed a high amount of myocardial involvement in CHD COVID-19-infected patients [22]. The explanation that arrhythmias and HF were the most common complications may be due to an element of myocarditis that might be diagnosed by the increased levels of serum troponin.
4.2 Risk Stratification Concerning Diagnosis and Comorbidities
ACHD is a cohort with a broad age range and a wide spectrum of clinical presentations ranging from completely asymptomatic individuals to various degrees of functional limitation and overt HF. Some patients also present with hypoxia, cyanosis, erythrocytosis, hyperuricemia, and other end-organ dysfunction. In this cohort, among the more vulnerable COVID-19 positive patients were the Eisenmenger and the palliated single ventricle patients. Eisenmenger patients suffer from delicate homeostatic adaptations that can be fatal when unbalanced. Additionally, Eisenmenger patients are vulnerable to ventricular dysfunction and may be vulnerable to thromboembolic phenomena that have been labeled with COVID-19 [27]. Theoretically, RV dysfunction has an unfavorable prognosis in COVID-19; RV that works against chronically increased pulmonary vascular resistance (PVR) may be susceptible to adverse changes from acute respiratory infection [28,29]. The Adult Congenital Heart Association (ACHA) and the International Society of ACHD gather data on the number of suspected and confirmed cases both in the United States and globally to better understand the outcomes in the ACHD population and stated that among the most vulnerable ACHD patients were those with Fontan physiology or various modifications of single ventricle palliation, who are dependent on low PVR for venous return. ARDS that typically increase the mean PAP ≥ 30 mmHg could be devastating to Fontan patients who are dependent on passive pulmonary blood flow. Additionally, Fontan patients are intolerant to positive pressure ventilation because the elevated intrathoracic pressure can adversely affect the venous return. Fontan patients are also prone to thromboembolic complications, which have been described with COVID-19 [27]. A survey of ACHD cardiologists at the European Collaboration for Prospective Outcome research in congenital heart disease (EPOCH) in over 20 European tertiary ACHD centers throughout nine countries indicated that patients with PAH, central cyanosis, and absence of a sub-pulmonary ventricle were considered at the highest risk of adverse COVID-19 outcomes [29].
In this study, patients with simple lesions were more likely to be asymptomatic, patients with moderate complexity lesions were more likely to present with mild/moderate symptoms, while patients with complex lesions were more vulnerable to develop critical symptoms. ACHD FC A and B categories were more likely to be asymptomatic, FC C patients were more to present with severe symptoms, and FC D patients were more to develop critical symptoms. In a single-center cohort of 53 CHD patients who were infected with COVID-19 (10 pediatric, 43 adults), advanced physiologic stage (C/D), but not increasing complexity of anatomy, was associated with a moderate or severe illness [30]. Also, in one study, based on the ACHD anatomy and physiological stage classification, any patient with complex CHD anatomy stage III or physiological stage B, C, or D symptoms could be considered at high risk for COVID-19 complications based on the decreased functional reserve [19]. Studies have tried to risk-stratify patients with viral pneumonia [31], and the MuLBSTA score may be a tool provider to see which patients are at high risk of morbidity and mortality [32]. A large-scale multicenter study based on collaboration between ACHA and the International Society for ACHD is currently underway. Preliminary findings reinforce the ACC recommendation to prioritize physiologic stage C/D patients. In the United Kingdom, the British Congenital Cardiac Association is conducting a nationwide multicenter survey to widely measure the impact of COVID-19 on CHD; this will permit a better understanding of groups at risk which will impact preventive strategies.
We suspect that the anatomy is insufficient alone to inform risk stratification and should be accompanied by the physiological aspects and patient status. Whereas patients with ACHD with repaired complex underlying anatomy might be at low risk than those with unrepaired simpler anatomical diagnoses who are symptomatic or have HF. Risk stratification should be individualized to each patient and should include patients’ physiological stage and lifestyle needs. Additional aspects of intrahospital triage and intensive care treatment of affected ACHD patients have been outlined [33,34].
In this study, 11 (5.4%) patients managed only with home isolation, 147 (71.7%) patients required home isolation with antibiotics intake, 32 (15.6%) patients required ICU admission with NIV, 8 (3.9%) patients required inotropes, 7 (3.4%) patients required mechanical ventilation, and 2 (1%) unrepaired patients required ECMO. No patient was ventilated for cardiac causes but respiratory failure. In agreement and with the respect to CHD, A total of 94 suspected or confirmed cases have been reported in 7 papers [26,35–40]. Amongst the 94 COVID-19 infected reported CHD cases, 79 patients (84%) were adults and 15 (16%) were children. Concerning critical care, of all 94 patients, 3 (3.2%) patients required ECMO, 6 (6.4%) received inotropes, 6 (6.4%) received positive pressure ventilation, and 3 (3.2%) were supported with NIV.
The addition of dexamethasone, remdesivir, and immunotherapies medications to the management of CHD patients necessitated careful dosing and thorough monitoring [41,42]. None of the medications that were often used in treating CHD manifestations and complications were shown to exacerbate the clinical course of COVID-19 in this population [43].
In this study, 2 (1.0%) patients showed COVID-19 infection during pregnancy, 1 (0.5%) patient revealed no complications, and the other 1 (0.5%) experienced intrauterine fetal death. There were no maternal deaths or cardiomyopathies. Furthermore, no fetal transmission was reported. The largest study on COVID-19 and pregnancy included 116 pregnant women in China. This Chinese cohort reported that 8 (6.9%) pregnant women experienced severe COVID-19 pneumonia similar to non-pregnant patients and stated that COVID-19 infection during pregnancy was not associated with an increased risk of spontaneous abortion or spontaneous preterm birth. There were also no cases of fetal deaths with only 1 (1%) neonatal death. Furthermore, there was no evidence of vertical transmission of COVID-19 infection from the mother to the baby and the breast milk samples tested negative for SARS-CoV-2 [44].
Despite these largely reassuring results, we advocate that when COVID-19 is diagnosed in pregnant ACHD patients, meticulous care should be provided by a multidisciplinary team including obstetricians, ACHD cardiologists, intensivists, obstetric anesthetists, infectious-disease specialists, and neonatologists.
The mortality of ACHD patients affected by COVID-19 seemed low. During the period of this study, only, 1 (0.5%) post-Fontan COVID-19 positive patient died by severe uncontrollable pulmonary hypertension and hemorrhage, vs. 2 (0.6%) COVID-19 negative patients; with no difference between the two groups. In agreement with the current results was the Italian cohort who stated that patients with CHD were less at risk for COVID-19 related mortality [26]. Also, Broberg et al. reported that COVID-19 mortality in ACHD was proportionate with the general population, and the most vulnerable patients were those with worse physiological stage, cyanosis and pulmonary hypertension, whereas anatomic complexity did not appear to predict infection severity [25].
Our study suggested that cardiovascular comorbidities; impaired ventricular function, pulmonary hypertension, cyanosis, and arrhythmias might play a role in determining COVID-19 mortality, rather than the anatomic cardiac disease itself. However, their studies displayed that pre-existing systolic dysfunction was not highlighted as a risk factor for mortality [4,29]. Further evidence is anticipated with larger populations to confirm these observations.
4.6 General Care, Psychological Considerations, and Vaccines Recommendations
In addition to the generally recommended measures of disease prevention, patients with ACHD required additional measures of infection prevention; avoidance of social or occupational activities, conversion of outpatient appointments to virtual clinics, postponement of non-urgent/elective procedures during the first acute wave of the pandemic, and maintaining a close link to vulnerable patients to ensure appropriate assessment and therapy. Although elective procedures were delayed, urgent surgical, catheter-based, or electrophysiological interventions did not postpone. After the first wave, all patients required essential interventions were achieved under full PPE.
The mental health of many patients was challenged by stressors including concerns about social isolation, employment, seeking cardiology care, vaccination, and mortality. We recommended empathizing with psychological considerations to COVID-19 and encouraging strategies that previously helped patients handle health anxiety.
Patients with ACHD should be vaccinated against influenza and pneumococcal pneumonia [45,46], and so, considered favorite candidates for the COVID-19 vaccine. In this cohort, a total of 193 (35.6%) patients were vaccinated. The ACC recommends that ACHD patients at a progressive, decompensated physiologic stage be considered among the highest risk cardiac patients and prioritized for vaccination [30].
Even though our cohort was dependent on COVID-19 infection reports and was conducted through a large number of ACHD patients in a tertiary center, the epidemic has been spreading rapidly, and the documentation of the asymptomatic infected ACHD patients who did not seek medical care was impossible. As by the end of the study, not all our ACHD patients were vaccinated, therefore, we will report on them in the post-vaccination era to present the influence of the vaccine on the spread, case rates, presentation, and outcomes of COVID-19 infection in ACHD patients in a separate study.
Adults with congenital heart disease (ACHD) represent a growing and complex population of patients who require individualized risk stratification, protection, and treatment of COVID-19 infection. Due to anatomical and pathophysiological heterogeneity, risk stratification should consider underlying anatomy and physiology. Despite, the management of the infection was more or less similar to the general population, Eisenmenger, single ventricle palliation, complex lesions, and FC C and D patients were more vulnerable to severe and critical symptoms that required ICU admission, mechanical ventilation, or ECMO. The vaccine was tolerable in most ACHD patients.
Acknowledgement: The authors are grateful to Madinah Cardiac Center, Madinah, Saudi Arabia, for the cooperation to undertake this study, and are grateful to all the participated patients in the study.
Authorship: Study conception and design: FT, RA, OA; data collection: FT; analysis and interpretation of results: FT, RA, FA; Draft manuscript preparation: FT. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Available upon request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Tan, W., Aboulhosn, J. (2020). The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. International Journal of Cardiology, 309, 70–77. DOI 10.1016/j.ijcard.2020.03.063. [Google Scholar] [CrossRef]
2. Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X. et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Journal of the American Medical Association, 325, 1061–1069. DOI 10.1001/jama.2020.1585. [Google Scholar] [CrossRef]
3. Shi, Y., Wang, G., Cai, X., Deng, J., Zheng, L. et al. (2020). An overview of COVID-19. Journal of Zhejiang University-Science B, 21(5), 343–360. DOI 10.1631/jzus.B2000083. [Google Scholar] [CrossRef]
4. Guzik, T. J., Mohiddin, S. A., Dimarco, A., Patel, V., Savvatis, K. et al. (2020). COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 116(10), 1666–1687. DOI 10.1093/cvr/cvaa106. [Google Scholar] [CrossRef]
5. Wang, C., Horby, P., Hayden, F., Gao, G. (2020). A novel coronavirus outbreak of global health concern. Lancet, 395, 470–473. DOI 10.1016/S0140-6736(20)30185-9. [Google Scholar] [CrossRef]
6. Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B. et al. (2020). A novel coronavirus from patients with pneumonia in China 2019. New England Journal of Medicine, 382(8), 727–733. DOI 10.1056/NEJMoa2001017. [Google Scholar] [CrossRef]
7. Cevik, M., Bamford, C., Ho, A. (2020). COVID-19 pandemic—A focused review for clinicians. Clinical Microbiology and Infection, 26(7), 842–847. DOI 10.1016/j.cmi.2020.04.023. [Google Scholar] [CrossRef]
8. Kang, Y., Chen, T., Mui, D., Ferrari, V., Jagasia, D. et al. (2020). Cardiovascular manifestations and treatment considerations in COVID-19. Heart, 106(15), 1132–1141. DOI 10.1136/heartjnl-2020-317056. [Google Scholar] [CrossRef]
9. Mehra, M. R., Desai, S. S., Kuy, S. R., Henry, T. D., Patel, A. N. (2020). Cardiovascular disease, drug therapy, and mortality in COVID-19. New England Journal of Medicine, 382(25), e102. DOI 10.1056/NEJMoa2007621. [Google Scholar] [CrossRef]
10. Morray, B. H., Gordon, B. M., Crystal, M. A., Goldstein, B. H., Qureshi, A. M. et al. (2020). Resource allocation and decision making for pediatric and congenital cardiac catheterization during the novel coronavirus SARS-CoV-2 (COVID-19) pandemic: A U.S. multi-institutional perspective. Journal of Invasive Cardiology, 32, 103–109. [Google Scholar]
11. Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q. et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. DOI 10.1056/NEJMoa2002032. [Google Scholar] [CrossRef]
12. Brida, M., Chessa, M., Gu, H., Gatzoulis, M. (2020). The globe on the spotlight: Coronavirus disease 2019 (COVID-19). International Journal of Cardiology, 310(20), 170–172. DOI 10.1016/j.ijcard.2020.04.006. [Google Scholar] [CrossRef]
13. Lu, X., Zhang, L., Du, H., Zhang, J., Li, Y. Y. et al. (2020). SARS-CoV-2 infection in children. New England Journal of Medicine, 382(17), 1663–1665. DOI 10.1056/NEJMc2005073. [Google Scholar] [CrossRef]
14. Dong, Y., Mo, X., Hu, Y., Qi, X., Jiang, F. et al. (2020). Epidemiology of COVID-19 among children in China. Pediatrics, 145(6), e20200702. DOI 10.1542/peds.2020-0702. [Google Scholar] [CrossRef]
15. Radke, R., Frenzel, T., Baumgartner, H., Diller, G. (2020). Adult congenital heart disease and the COVID-19 pandemic. Heart, 106(17), 1302–1309. DOI 10.1136/heartjnl-2020-317258. [Google Scholar] [CrossRef]
16. Gallego, P., Ruperti-Repilado, F., Schwerzmann, M. (2020). Adults with congenital heart disease during the coronavirus disease 2019 (COVID-19) pandemic: Are they at risk? Revista Española de Cardiología, 73(10), 795–798. DOI 10.1016/j.recesp.2020.06.026. [Google Scholar] [CrossRef]
17. Marchesi, C., Paradis, P., Schiffrin, E. (2008). Role of the renin-angiotensin system in vascular inflammation. Trends in Pharmacological Sciences, 29(7), 367–374. DOI 10.1016/j.tips.2008.05.003. [Google Scholar] [CrossRef]
18. World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
19. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation, 139, 698–800. DOI 10.1161/CIR.0000000000000603. [Google Scholar] [CrossRef]
20. Ranieri, V. M., Rubenfeld, G. D., Thompson, B. T., Ferguson, N. D., Caldwell, E. et al. (2012). Acute respiratory distress syndrome: The Berlin definition. Journal of the American Medical Association, 307, 2526–2533. DOI 10.1001/jama.2012.5669. [Google Scholar] [CrossRef]
21. Saudi Ministry of Health Protocol for Patients Suspected or Confirmed with COVID-19 supportive care and antiviral treatment of suspected or confirmed COVID-19 infection. https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19. [Google Scholar]
22. Guo, T., Fan, Y., Chen, M., Wu, X., Zhang, L. et al. (2020). Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). Journal of the American Medical Association Cardiology, 5(7), 811–818. DOI 10.1001/jamacardio.2020.1017. [Google Scholar] [CrossRef]
23. Li, B., Yang, J., Zhao, F., Zhi, L., Wang, X. et al. (2020). Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Research in Cardiology, 109(5), 531–538. DOI 10.1007/s00392-020-01626-9. [Google Scholar] [CrossRef]
24. Grasselli, G., Zangrillo, A., Zanella, A., Antonelli, M., Cabrini, L. et al. (2020). Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy Journal of the American Medical Association, 323(16), 1574–1581. DOI 10.1001/jama.2020.5394. [Google Scholar] [CrossRef]
25. Broberg, C. S., Kovacs, A. H., Sadeghi, S., Rosenbaum, M. S., Lewis, M. J. et al. (2021). COVID-19 in adults with congenital heart disease. Journal of the American College of Cardiology, 77(13), 1644–1655. DOI 10.1016/j.jacc.2021.02.023. [Google Scholar] [CrossRef]
26. Sabatino, J., Ferrero, P., Chessa, M., Bianco, F., Ciliberti, P. et al. (2020). COVID-19 and congenital heart disease: Results from a nationwide survey. Journal of Clinical Medicine, 9(6), 1774. DOI 10.3390/jcm9061774. [Google Scholar] [CrossRef]
27. Bikdeli, B., Madhavan, M. V., Jimenez, D., Chuich, T., Dreyfus, I. et al. (2020). COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. Journal of the American College of Cardiology, 75(23), 2950–2973. DOI 10.1016/j.jacc.2020.04.031. [Google Scholar] [CrossRef]
28. Argulian, E., Sud, K., Vogel, B., Bohra, C., Garg, V. P. et al. (2020). Right ventricular dilation in hospitalized patients with COVID-19 infection. Journal of the American College of Cardiology Cardiovascular Imaging, 13(11), 2459–2461. DOI 10.1016/j.jcmg.2020.05.010. [Google Scholar] [CrossRef]
29. Diller, G. P., Gatzoulis, A. M., Broberg, C. S., Aboulhosn, J., Brida, M. et al. (2020). Coronavirus disease 2019 in adults with congenital heart disease: A position paper from the ESC working group of adult congenital heart disease, and the International Society for Adult Congenital Heart Disease. European Heart Journal, 12, ehaa960. DOI 10.1093/eurheartj/ehaa960. [Google Scholar] [CrossRef]
30. COVID-19 Vaccination in Adults with Congenital Heart Disease.American College of Cardiology.https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines?. [Google Scholar]
31. Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F. et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395, 507–551. DOI 10.1016/S0140-6736(20)30211-7. [Google Scholar] [CrossRef]
32. Guo, L., Wei, D., Zhang, X., Wu, Y., Li, Q. et al. (2019). Clinical features predicting mortality risk in patients with viral pneumonia: The MuLBSTA score. Frontiers in Microbiology, 10, 2752. DOI 10.3389/fmicb.2019.02752. [Google Scholar] [CrossRef]
33. Lastinger, L., Daniels, C., Lee, M., Sabanayagam, A., Bradley, E. (2020). Triage and management of the ACHD patient with COVID-19: A single center approach. International Journal of Cardiology, 320, 178–182. DOI 10.1016/j.ijcard.2020.06.023. [Google Scholar] [CrossRef]
34. Radke, R., Frenzel, T., Baumgartner, H., Diller, G. (2020). Adult congenital heart disease and the COVID-19 pandemic. Heart, 106(17), 1302–1309. DOI 10.1136/heartjnl-2020-317258. [Google Scholar] [CrossRef]
35. Simpson, M., Collins, C., Nash, D., Panesar, L., Oster, M. (2020). Coronavirus disease 2019 infection in children with pre-existing heart disease. Journal of Pediatrics, 227, 302–307. DOI 10.1016/j.jpeds.2020.07.069. [Google Scholar] [CrossRef]
36. Shekerdemian, L. S., Mahmood, N. R., Wolfe, K. K., Riggs, B. J., Ross, C. E. et al. (2020). Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. Journal of the American Medical Association, 174, 868–873. DOI 10.1001/jamapediatrics.2020.1948. [Google Scholar] [CrossRef]
37. Ferrero, P., Piazza, I., Ciuffreda, M. (2020). COVID-19 in adult patients with CHD: A matter of anatomy or comorbidities? Cardiology in the Young, 30(8), 1196–1198. DOI 10.1017/S1047951120001638. [Google Scholar] [CrossRef]
38. Bezerra, R. F., Franchi, S. M., Khader, H., Castro, R. M., Liguori, G. R. et al. (2020). COVID-19 as a confounding factor in a child submitted to staged surgical palliation of hypoplastic left heart syndrome: one of the first reports of SARS-CoV-2 infection in patients with congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 161(2), 97–101. DOI 10.1016/j.jtcvs.2020.05.081. [Google Scholar] [CrossRef]
39. Salik, I., Mehta, B. (2020). Tetralogy of Fallot palliation in a COVID-19 positive neonate. Journal of Clinical Anesthesia, 66(11), 109914. DOI 10.1016/j.jclinane.2020.109914. [Google Scholar] [CrossRef]
40. Krishnan, U. S., Krishnan, S. S., Jain, S., Chavolla-Calderon, M. B., Lewis, M. et al. (2020). Severe acute respiratory syndrome associated with Coronavirus 2 infection in patients with down syndrome, congenital heart disease, and pulmonary hypertension: is down syndrome a risk factor? Journal of Pediatrics, 225, 246–248. DOI 10.1016/j.jpeds.2020.06.076. [Google Scholar] [CrossRef]
41. Iacobazzi, D., Baquedano, M., Madeddu, P., Caputo, M. (2020). COVID-19, State of the adult and pediatric heart: From myocardial injury to cardiac effect of potential therapeutic intervention. Frontiers in Cardiovascular Medicine, 14, 140. DOI 10.3389/fcvm.2020.00140. [Google Scholar] [CrossRef]
42. Giordano, R., Cantinotti, M. (2020). Congenital heart disease in the era of COVID-19 pandemic. General Thoracic and Cardiovascular Surgery, 1–3. DOI 10.1007/s11748-020-01417-z. [Google Scholar] [CrossRef]
43. Alsaied, T., Aboulhosn, J. A., Cotts, T. B., Daniels, C. J., Etheridge, S. P. et al. (2020). Coronavirus disease 2019 (COVID-19) pandemic implications in pediatric and adult congenital heart disease. Journal of the American Heart Association, 9(12), e017224. DOI 10.1161/JAHA.120.017224. [Google Scholar] [CrossRef]
44. Yan, J., Guo, J., Fan, C., Juan, J., Yu, X. et al. (2020). Coronavirus disease 2019 in pregnant women: A report based on 116 cases. American Journal of Obstetrics & Gynecology, 223(1), 1–4. DOI 10.1016/j.ajog.2020.04.014. [Google Scholar] [CrossRef]
45. Bare, I., Crawford, J., Pon, K., Farida, N., Dehghani, P. (2018). Frequency and consequences of influenza vaccination in adults with congenital heart disease. American Journal of Cardiology, 121(4), 491–494. DOI 10.1016/j.amjcard.2017.11.008. [Google Scholar] [CrossRef]
46. Lui, G. K., Saidi, A., Chair, V., Hatt, A. B., Burchill, L. J. et al. (2017). Diagnosis and management of noncardiac complications in adults with congenital heart disease: A scientific statement from the American Heart Association. Circulation, 136(20), 348–392. DOI 10.1161/CIR.0000000000000535. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |