 Open Access
Open Access
ARTICLE
Mid-Term Preliminary Results for Safety and Patency of the Occlutech Atrial Flow Regulator in an Animal Model
1 Division of Cardiology, Department of Pediatrics, Stead Family Children’s Hospital, University of Iowa Hospitals and Clinics, Iowa City, USA
2 Division of Cardiology, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, USA
3 Division of Cardiology, Heart Institute, Children’s Hospital of Colorado, University of Colorado at Denver, Aurora, USA
* Corresponding Author: Gareth Morgan. Email:
Congenital Heart Disease 2022, 17(3), 269-280. https://doi.org/10.32604/chd.2022.019973
Received 27 October 2021; Accepted 24 March 2022; Issue published 03 May 2022
Abstract
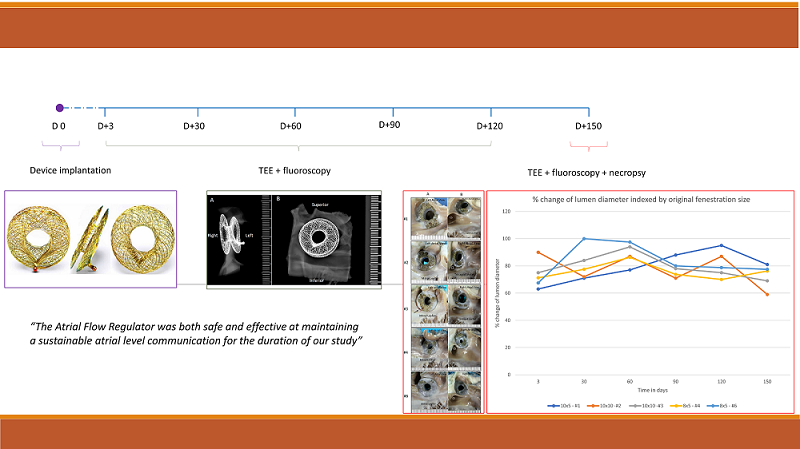
Objective: The Atrial Flow Regulator (AFR) is a double disc device made of self-expanding Nitinol wire mesh, structured around a central lumen. Once deployed via the transfemoral route, the device stents the atrial septum leaving a preselected fixed diameter atrial communication. We sought to evaluate the mid-term performance of the AFR by implanting the device in 5 healthy porcine hearts to assess safety and patency of the device fenestration over a period of 150 days. Method: Five AFR devices were implanted in 5 female Yucatan adult minipigs. The animals were survived to 150 days with periodic assessments at days +3, +30, +60, +90, +120, and +150. These assessments consisted of transesophageal echocardiography and fluoroscopic evaluation. The animals were sacrificed at day +150. Histological and pathological assessments were carried out to characterize neointimal tissue growth, inflammation, thrombus formation, endothelial coverage, endothelial maturity, and the presence of any luminal thrombus. Result: There were no unscheduled deaths. Patency was maintained in all 5 animals across the 150-day study. There was no statistically significant difference in the lumen diameter over the study duration. Neointimal growth was mild to moderate in all specimens and occurred mostly on the surfaces of the device in direct contact with the atrial septum. There was no evidence of any significant inflammatory response on routine blood work or by imaging or histological assessment. Scanning electron microscope (SEM) examination showed nearly complete surface coverage with endothelial tissue. The animals were in a healthy condition for the duration of the study with no attributable pathology and no adverse effects noted on distant organs in any of the 5 animals. Conclusion: As a continuation of our earlier work, this 150-day midterm animal study provides important safety and feasibility information. Our preliminary results show that the AFR is both safe and effective in maintaining a sustainable atrial level communication for the duration of the study.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools