

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019480
ARTICLE
Combined Echocardiography and Lung Ultrasound for Extubation Outcome Prediction in Children after Cardiac Surgery
1Department of Echocardiography, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
2Department of Pediatric Cardiac Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
3Pediatric Intensive Care Unit, Department of Pediatric Cardiac Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
4Department of Echocardiography, Fuwai Yunnan Cardiovascular Hospital, Kunming, China
*Corresponding Authors: Hao Wang. Email: hal61112@126.com; Hong Meng. Email: drfwmh@126.com
Received: 28 November 2021; Accepted: 20 January 2022
Abstract: Background: Children are at risk of extubation failure after congenital heart disease surgery. Such cases should be identified to avoid possible adverse consequences of failed extubation. This study aimed to identify ultrasound predictors of successful extubation in children who underwent cardiac surgery. Methods: Children aged 3 months to 6 years who underwent cardiac surgery (if they were intubated for >6 h and underwent a spontaneous breathing trial) were included in this study. Results: We included 83 children who underwent surgery for congenital heart disease. Transthoracic echocardiography and lung ultrasound were performed immediately before spontaneous breathing trials. Upon spontaneous breathing trial completion, respiratory parameters, including arterial blood gas analysis and frequency-to-tidal volume ratio, were similarly recorded. For outcome assessment, all children were followed up for 48 h after extubation. We successfully extubated 57 children (68.7%). These children were significantly older and weighed more but had shorter aortic cross-clamp and cardiopulmonary bypass times. Children who could not be weaned or extubated had prolonged total mechanical ventilation and pediatric intensive care unit stay. In the multivariate regression analysis, a lung ultrasound score ≥12 and ejection fraction ≥40% immediately before spontaneous breathing trials were the only independent predictors of successful extubation. When combined, the lung ultrasound score and an ejection fraction ≥40% showed a better diagnostic performance than every other isolated variable (lung ultrasound, N-terminal-pro-B-type natriuretic peptide, and frequency-to-tidal volume ratio). Conclusions: The combination of lung ultrasound and transthoracic echocardiography immediately before the spontaneous breathing trial effectively predicts extubation outcomes in children after cardiac surgery.
Keywords: Lung; echocardiography; intensive care units; pediatric; airway extubation; ventilator weaning; pro-brain natriuretic peptide
Despite advances in the approach for immediate extubation after congenital heart surgery (CHS) [1], a significant number of children who undergo surgery for congenital heart disease (CHD) still require mechanical ventilation (MV). Unfortunately, extubation failure occurs in 10%–19% of these patients [2]. Failed extubation may lead to severe cardiorespiratory decompensation and is associated with prolonged MV, prolonged lengths of intensive care and hospital stays, with higher hospital costs and mortality rates [3,4]. Spontaneous breathing trials (SBTs) predict extubation success in pediatric patients following CHS; however, approximately 17% of the patients who are extubated after passing an SBT require reintubation [5]. Hence, to avoid adverse consequences, children at risk of weaning and extubation failure should be identified.
In children who have undergone CHS, the most common extubation failure etiologies are cardiac dysfunction, unresolved lung disease, airway edema, and decreased respiratory drive [2,6]. However, traditional weaning indices, such as respiratory frequency, maximal inspiratory pressure, and frequency-to-tidal volume ratio (f/VT), are poor predictors of extubation failure in children who have passed an SBT [7]. Their limitations may be related to an emphasis on respiratory failure and poor diagnostic value in heart failure, which equally plays an important role in extubation failure. Therefore, an accurate extubation outcome prediction tool should be able to assess both respiratory function and cardiac performance.
Ultrasonography is a useful bedside tool for the evaluation of cardiopulmonary failure in intensive care units (ICUs) [8,9]. Transthoracic echocardiography (TTE) can measure left ventricular (LV) diastolic and systolic performance and can be used to identify high-risk patients prior to SBT [10,11]. Following pediatric cardiac surgery, lung ultrasound (LUS) has high diagnostic accuracy for pulmonary complications, including pleural effusion, pneumothorax, pulmonary edema, and lung consolidation [12]. Moreover, ultrasound is a reliable tool for the detection of abnormal diaphragmatic motion (ADM) [13,14]. Thus, comprehensive ultrasound techniques, including LUS and TTE, may help identify both unresolved lung disease and cardiac and diaphragmatic dysfunctions [8].
Ultrasonography is a fast, non-invasive bedside technique that can predict weaning success and post-extubation failure in adults [8,15]. However, the systematic use of multiorgan ultrasound in determining extubation readiness in children undergoing cardiac surgery has not been studied extensively. Therefore, we conducted a prospective observational study to evaluate the usefulness of combined TTE and LUS in predicting extubation success in children after CHS.
2.1 Patients and Study Protocol
This prospective observational study was approved by the Institutional Review Board of the Chinese Clinical Trial Registry (No. ChiECRCT20200377) and registered with the Fundamental Research Funds for the Central Universities (No. 3332020018). Written informed consent was obtained from the guardians/parents of all children that participated in this study. Children who were on MV for >6 h and eligible for their first SBT after cardiac surgery between January and July 2020 were screened for inclusion. The inclusion criteria were children aged 3 months to 6 years and those scheduled for primary complete corrective surgery and cardiopulmonary bypass (CPB) repair under general anesthesia.
Children were eligible for an SBT if they met all criteria [15], and the intervention was approved by the physician in charge. SBT was performed with a pressure support ventilation of 10 cm H2O for 60 min. Weaning failure was defined by the failure of SBT. Weaning success was defined as the patient passed SBT and removed the endotracheal tube. Extubation success was defined as the patient not requiring MV support (invasive or non-invasive) in the following 48 h [5].
Ultrasound examinations were conducted immediately before SBT with pressure support of a positive end-expiratory pressure of 5 cm H2O and fraction of inspired oxygen ≤40%. For the LUS, four patterns corresponding to different degrees of aeration loss were defined as follows: 0) normal aeration, A-lines and 1 or 2 B-lines; 1) moderate loss of lung aeration, multiple well-defined B-lines; 2) severe loss of lung aeration, multiple coalescent B-lines; and 3) complete loss of lung aeration resulting in lung consolidation [16]. The LUS score was calculated as the sum of the 12 regions (between 0 and 36). The diaphragmatic motion was assessed by either B-mode or M-mode and classified into four patterns [17]. Left ventricular ejection fraction (LVEF) was assessed using the monoplane Simpson’s method by manually tracing the LV endocardial border in the apical 4-chamber and 2-chamber views, requiring a clear delineation of the blood-endocardium interface and excluding the papillary muscles. Two-dimensional fractional area change (FAC) was used to estimate right ventricular (RV) function quantitatively. Percentage FAC = 100 × end-diastolic area (area ED)–end-systolic area/area ED. The endocardial border was traced in the apical four-chamber views from the tricuspid annulus along the free wall to the apex and back to the annulus. Pulmonary hypertension (PH) and severe PH were defined as systolic pulmonary artery pressures exceeding 50% and 75% of systemic systolic pressure, respectively [18].
Demographic variables, including age, sex, CHD type, risk adjustment for CHS-1 (RACHS-1) score, MV duration before SBT, total MV time, and the ICU stay duration, were recorded. Laboratory data, including the N-terminal-pro-B-type natriuretic peptide (NT-proBNP) levels, were similarly recorded immediately before SBT. Clinical variables were monitored by the ICU nurse. Upon SBT completion, respiratory parameters, including arterial blood gas analysis and f/VT ratio, were recorded.
Continuous variables were described as mean ± standard deviation or median (interquartile range) and were compared using Student’s t-test or Mann–Whitney U test, as appropriate. Categorical variables were expressed as numbers and percentages. Proportion comparisons were performed using the chi-square test. The discriminatory power of predictors was quantified by measuring the area under the receiver operating characteristic curve (AUC ROC). Logistic regression analysis was used to construct a prediction model for the binary outcome of successful extubation.
Additional information on the protocols and methods is presented in Supplemental Digital Content 1.
We screened 195 children with CHD, 91 of whom met the inclusion criteria and underwent an SBT trial. Of the 83 enrolled patients, 57 (68.7%) were successfully extubated (Fig. 1). Table 1 presents the clinical characteristics. Children who underwent successful extubation were significantly older and weighed more than those who were not successfully extubated (both p = 0.002); however, they had shorter aortic cross-clamp and CPB times (p = 0.002 and 0.007, respectively). We found no significant differences in gender, preoperational LVEF, RACHS-1 score, and MV duration before SBT (p = 0.107, 0.085, 0.976, and 0.535, respectively) between the groups. Children who underwent successful extubation had significantly lower NT-proBNP levels and heart rates (p = 0.001 and 0.023, respectively) but higher systolic blood pressures before SBT and lower f/VT ratios after SBT (p = 0.013 and 0.034, respectively). Children who failed weaning or extubation had prolonged total MV and PICU stay durations (both p < 0.001). The causes of weaning or extubation failure were cardiac dysfunction (n = 10), lung disease (n = 9), airway edema (n = 6), and diaphragmatic paralysis (n = 1).
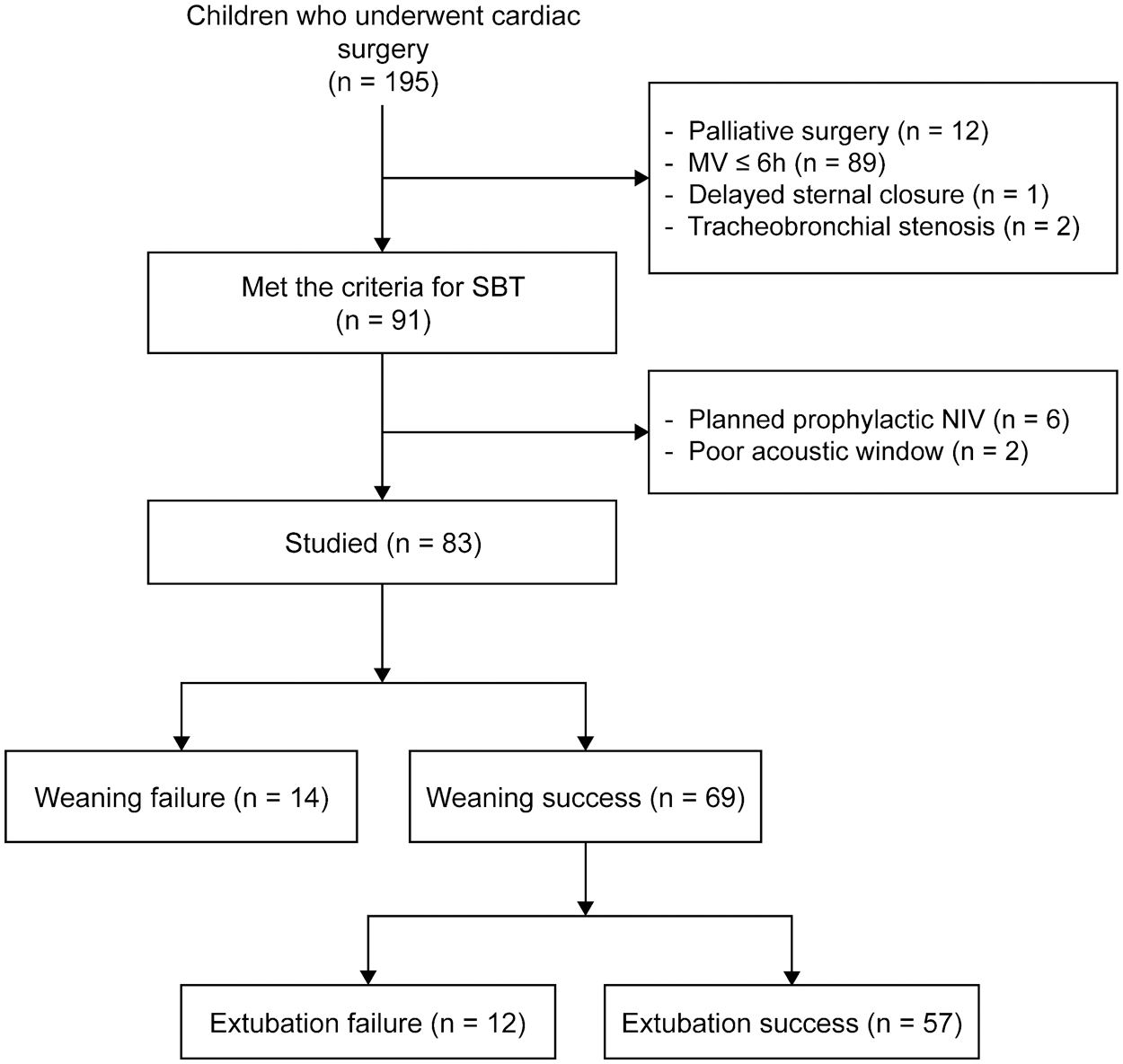
Figure 1: Study flowchart. MV: mechanical ventilation; SBT: spontaneous breathing trial; NIV: non-invasive mechanical ventilation
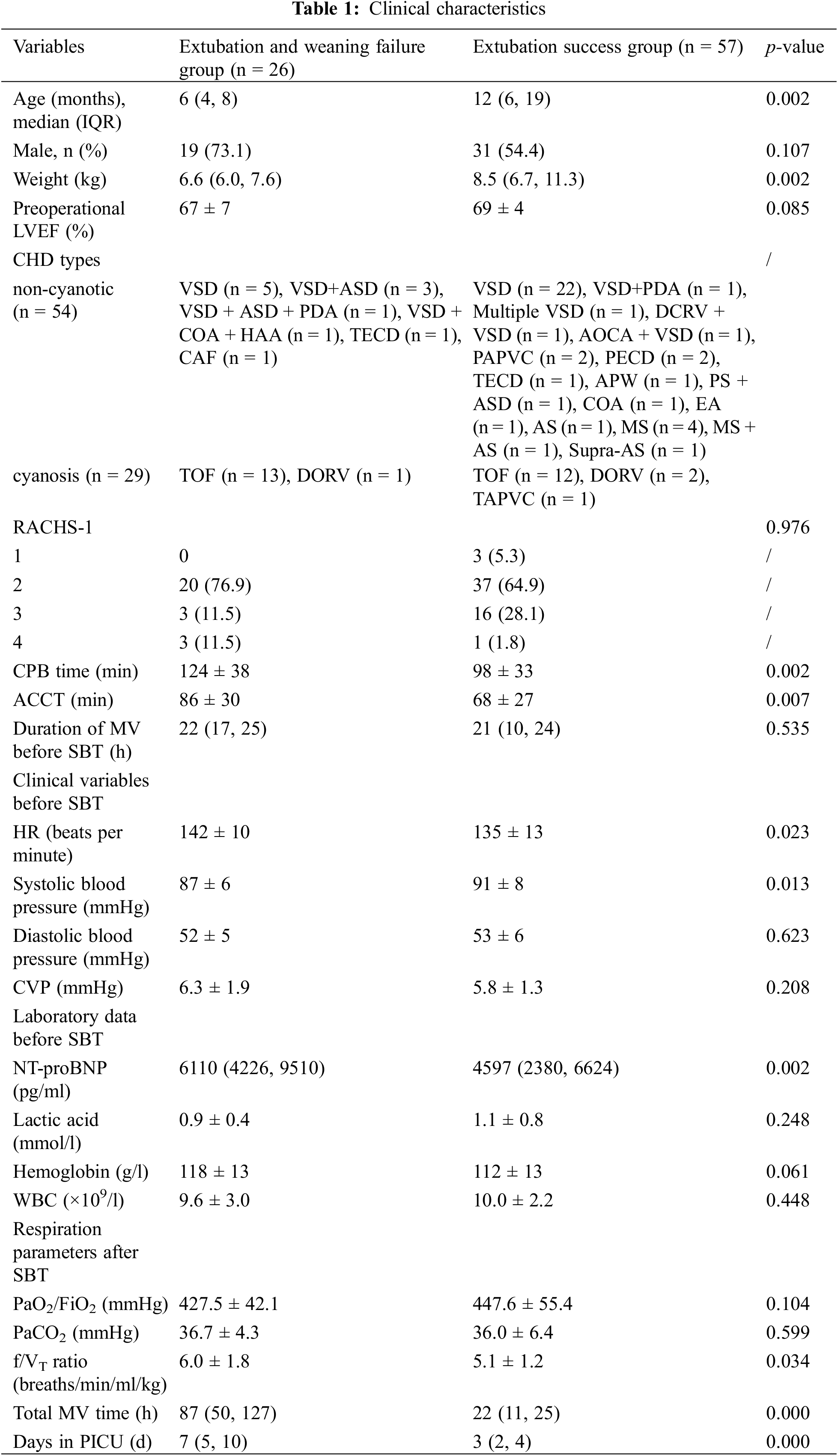
LVEF: left ventricular ejection fraction, CHD: congenital heart disease, VSD: ventricular septal defect, ASD: articular septal defect, PDA: patent ductus arteriosus, COA: coarctation of aorta, HAA: aortic arch hypoplasia, TOF: tetralogy of fallot, DORV: double outlet right ventricle, TECD/PECD: complete/partial type endocardial cushion defect, CAF: coronary artery fistula, DCRV: double chambered right ventricle, AOCA: anomalous origin of coronary artery, TAPVC/PAPVC: total/partial anomalous pulmonary venous connection, EA: Ebstein’s anomaly, AS: aortic stenosis, MS: mitral stenosis, APW: aortopulmonary window, PS: pulmonary artery stenosis, RACHS-1: risk adjustment for congenital heart surgery 1, CPB: cardiopulmonary bypass, ACCT: aortic cross-clamp time, MV: mechanical ventilation, SBT: spontaneous breathing trial, FiO2: fraction of inspired oxygen, RR: respiratory rate, RSBI: rapid shallow breathing index, NT-proBNP: N-terminal-pro-B-type natriuretic peptide, PaO2: arterial oxygen tension, PaCO2: arterial carbon dioxide tension, WBC: white blood cell count, IQR: interquartile range, f/VT: frequency-to-tidal volume.
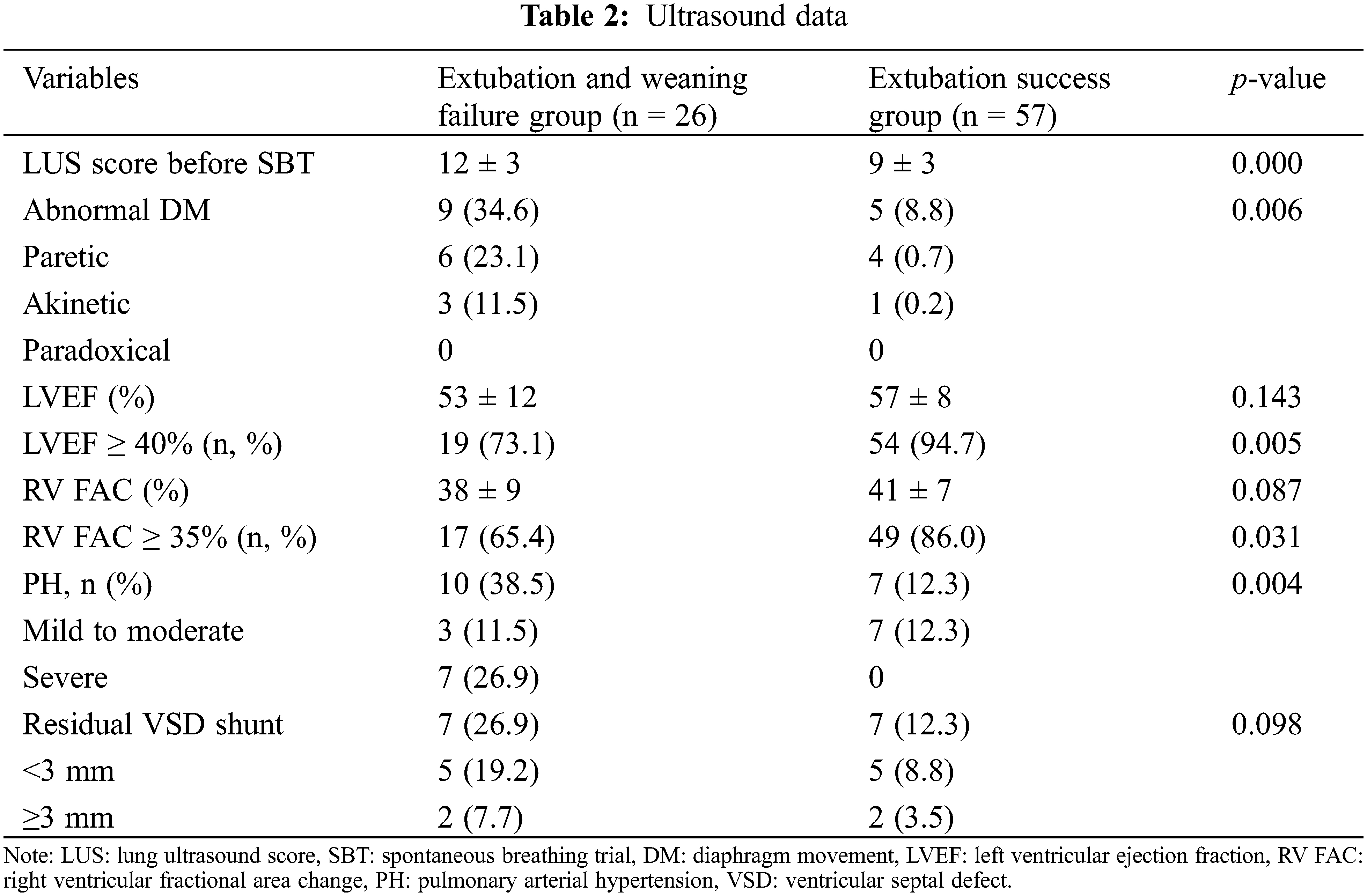
Table 2 shows the ultrasound data for these children. We found a significant difference in the LUS scores between the groups (p < 0.001). The number of children with PH and ADM was higher in the weaning or extubation failure group than the extubation success group (p = 0.004 and 0.006, respectively). There were no statistically significant differences in the LVEF and RV FAC between the groups (p = 0.143 and 0.087, respectively); however, the number of children who had an LVEF ≥ 40% or RV FAC ≥ 35% were significantly higher in the extubation success group than in the weaning or extubation failure group (p = 0.005 and 0.031, respectively). There were no significant differences in the number of children with residual ventricular septal defects between the groups (p = 0.098).
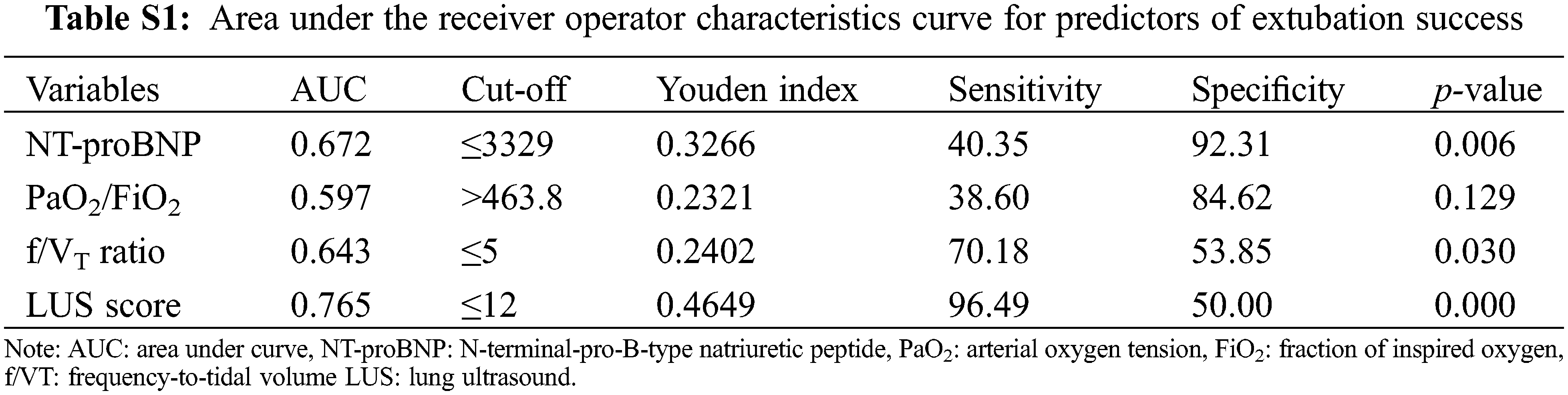
Supplemental Digital Content 2 (Table S1) shows the AUC ROC for the extubation success predictors, as well as the best cut-off points based on the combination of higher sensitivity and specificity for each one.
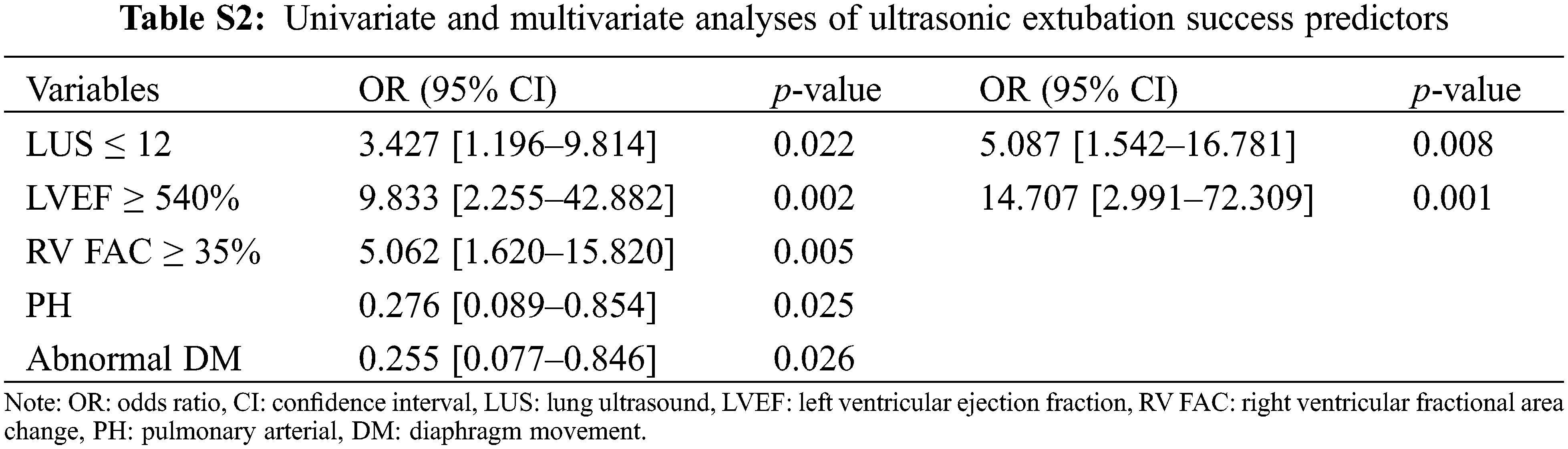
Supplemental Digital Content 3 (Table S2) summarizes the univariate and multivariate logistic regression analysis results. In the multivariate regression analysis, LUS scores ≤ 12 and EF ≥ 40% before SBT were the only independent extubation success predictors. When these parameters were combined, they yielded an AUC of 0.838 (0.560–0.771) with a sensitivity and specificity of 94.74% and 73.08%, respectively (p < 0.000), which showed better diagnostic performance than every other isolated variable (vs. LUS, NT-proBNP, and f/VT ratio; p = 0.0426, 0.0495, and 0.0240, respectively; Fig. 2).
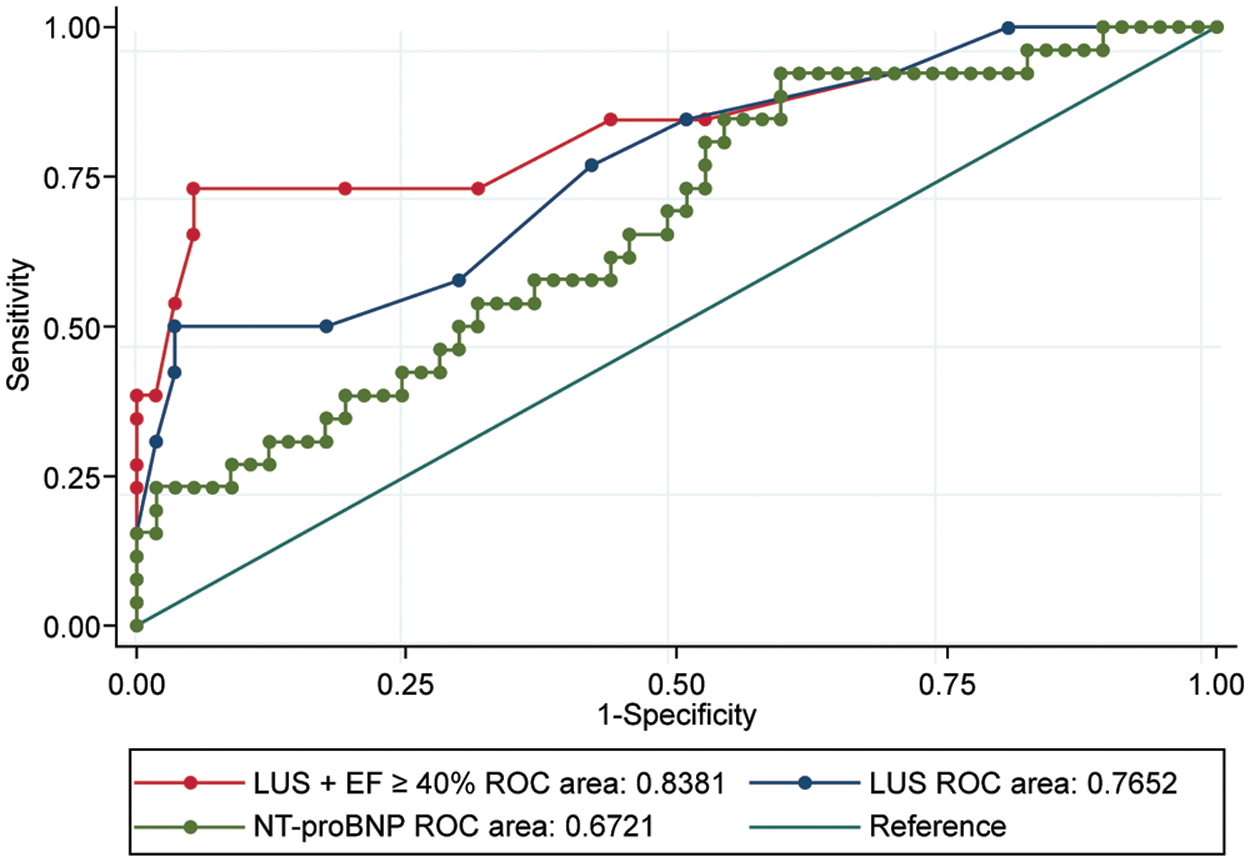
Figure 2: Predictive values. Ability of LVEF ≥40% combined with LUS, isolated LUS, and NT-proBNP immediately before SBT to predict extubation success. LVEF: left ventricular ejection fraction, LUS: lung ultrasound score, NT-proBNP: N-terminal-pro-B-type natriuretic peptide, SBT: spontaneous breathing trial, ROC: receiver operating characteristic
The quadratic weighted kappa for concordance between the ultrasound operators was 0.816 (standard error = 0.058), whereas the observed and expected random concordance were 83.33% and 9.67%, respectively.
We investigated the usefulness of multiorgan ultrasound examinations for predicting extubation outcomes in pediatric patients after CHS. Our findings suggest that combined TTE and LUS effectively predict extubation outcomes in pediatric patients. The independent extubation success predictors were an LVEF ≥ 40% and an LUS score ≥ 12.
In our study, 31% (26/83) of the children failed their initial SBT or extubation, a higher rate than in previous studies [2,4]. In this study, we excluded low-risk patients with early extubation. Besides, fewer children with CHD visited the hospital during the COVID-19 outbreak, with a higher proportion of patients than usual with severe illness. The most common etiologies of weaning or extubation failure were cardiac dysfunction and lung disease in our study. Postoperative pulmonary complications are common after heart surgery and may result in extubation failure, causing high morbidity and increased costs, especially in neonates and children [19]. CPB can induce a systemic inflammatory response and lead to pulmonary edema [20]. Additionally, ventilation cessation during CPB results in alveolar collapse and atelectasis [20]. LUS is a reliable tool in ICUs during the weaning process since it can measure the reduction in pulmonary aeration resulting from either pulmonary edema or derecruitment [8]. In a recent study, Cantinotti et al. demonstrated the prognostic value of LUS score as an independent predictor of ICU length of stay and extubation time after pediatric cardiac surgery, supporting its routine use in this setting [21].
This finding is consistent with the findings of Rahman et al. [15,22]. However, these authors reported specificities and AUCs for predicting successful extubation that were higher than those observed in our study. We attribute this to the study population. Contrary to the subjects included in the studies by Rahman et al. [15,22], who were unselected pediatric and adult ICU patients, respectively, we included infants and children with CHD who may have had higher extubation failure rates due to heart failure. This may similarly explain the poor respiratory parameter performance (arterial oxygen tension/fraction of inspired oxygen and f/VT) in predicting extubation success in our study, which was consistent with the results of previous research [7]. Therefore, extubation success prediction using LUS scores has a high sensitivity but low specificity. Consequently, a single parameter is inadequate for accurate extubation outcome prediction.
Echocardiography is essential in congenital cardiology because of its clear cardiac anatomy definition and cardiac chamber’s size and function measurement [23]. Moreover, TTE has been proven useful in identifying heart failure during SBT in adults [8,24,25]. Of the 10 patients who failed to extubate due to cardiac dysfunction, seven had an LVEF < 40%. In the case of volume overload and LV systolic insufficiency, SBT may induce cardiogenic pulmonary edema, leading to failed extubation. Here, an LVEF ≥ 40% immediately before SBT was an independent predictor of extubation success. Notably, the Simpson method was used to measure the LV systolic function in our study, which has been validated in children of various sizes [26]. However, for children with abnormally shaped left ventricles, the modified Simpson algorithm, which uses a combination of short-axis and long-axis views, may be considered [27]. A combined TTE and LUS approach may offer a more comprehensive understanding of the patient’s status at a lower cost and shorter duration since it allows integrated evaluation of both the heart and lungs in a single examination [9,28]. A recent retrospective study with a large sample size also showed that LUS performed as a completion of routine echocardiography with minimal cost and significantly reduced the amount of chest radiographic examinations [29].
After CHD surgery, perioperative NT-proBNP and BNP levels in children serve as sensitive predictors of prolonged MV and ICU stay duration [30,31]. In our study, the NT-proBNP levels immediately before SBT were not good predictors of extubation success. Considering that we exclusively enrolled pediatric patients who underwent their first SBT within the first 10–48 h postoperatively, the NT-proBNP test timing, which was affected by the CPB time, differed, and the NT-proBNP levels changed with time postoperatively.
Ultrasound is a highly sensitive tool for diagnosing ADM [17], a common complication in pediatric cardiac surgery associated with prolonged mechanical ventilatory support. In our patients, the lack of statistical significance for ADM as a predictor of extubation outcome in the multivariate analysis could be due to the underrepresentation of extreme cases in our cohort. In most cases, the diaphragmatic motion was recovered [32]. Similarly, although PH and a residual ventricular septal defect have been associated with higher mortality post-cardiac surgery, neither of these findings have been upheld consistently, nor were these factors independent predictors of extubation outcome in our cohort.
Here, the finding of prolonged MV and ICU stay durations in patients who failed weaning or extubation was consistent with previously published results [4]. Studies have assessed LUS usefulness in extubation readiness estimation in children [21,33]. However, none have reviewed the systematic use of multiorgan ultrasound in children undergoing cardiac surgery. Comprehensive ultrasound allows for the detection of cardiac dysfunction and lung disease, which were the most common reasons for extubation or weaning failure in our study. Therefore, since combined ultrasound does not only help intensivists in identifying high-risk children but also in recognizing the main causes of weaning or extubation failure, it should be considered when extubating pediatric patients from MV after cardiac surgery to improve the likelihood of successful extubation.
The limitations of our study are as follows: (a) This was a single-center study with a relatively small number of patients and no validation cohort. (b) LV diastolic performance was not evaluated since the study population included children with CHD, some having mitral regurgitation or stenosis, as these indices have not been validated for diastolic function measurement in these cases. (c) We did not use the diaphragmatic thickening fraction, an extubation outcome predictor, to diagnose ADM. As the diaphragm is extremely thin in children, small measurement errors may result in thickening fraction overestimation or underestimation. Therefore, we used a combined ultrasound technique for both the B- and M-modes, as reported by Gil-Juanmiquel et al. [17], as a reliable ADM diagnosis method. (d) Patients extubated in the operating room were excluded. A recent study proved that perioperative LUS-guided recruitment maneuvers were practical and beneficial in pediatric patients undergoing cardiac surgery [34]. Therefore, we believe that LUS would play a more important role in these immediate extubation cases. (e) We excluded children undergoing palliative surgery. Because pulmonary artery blood flow in these children is excessive, infrequent, or unevenly distributed, pulmonary conditions may not be consistent with pulmonary ultrasound scores. Nevertheless, further study is warranted to determine whether combined ultrasound technology used in the extubation readiness assessment could lead to a reduction in MV duration.
In summary, combined LUS and TTE immediately before SBT can effectively predict extubation outcomes in children post-cardiac surgery. This new approach provides additional information for pediatric cardiac extubation postoperatively. Further randomized and blinded, large-scale studies are needed to validate whether this method improves the extubation success rate.
Acknowledgement: The members of the writing group gratefully acknowledge Editage for assistance in English editing.
Author Contributions: M.L.: conceptualized and designed the study, carried out the study, and drafted the manuscript. H.M. and H.W.: participated in the design of the study, performed the ultrasound, and interpreted the data. L.Z.: participated in the design of the study, data collection, statistical analysis, and follow-up of the patients. Y.Z.: collected data. C.L. and Z.L.: performed the ultrasound. H.W.: Conceptualized the research, supervised the research, and revised the manuscript.
Data Availability Statement: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This study was supported by the Fundamental Research Funds for the Central Universities (No. 3332020018) and Yunnan Provincial Cardiovascular Disease Clinical Medical Center Project (No. FZX2019-06-01). Dr. Li received funding from the Fundamental Research Funds for the Central Universities.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Varghese, J., Kutty, S., Abdullah, I., Hall, S., Shostrom, V. et al. (2016). Preoperative and intraoperative predictive factors of immediate extubation after neonatal cardiac surgery. Annals of Thoracic Surgery, 102(5), 1588–1595. DOI 10.1016/j.athoracsur.2016.04.030. [Google Scholar] [CrossRef]
2. Harrison, A. M., Cox, A. C., Davis, S., Piedmonte, M., Drummond-Webb, J. J. et al. (2002). Failed extubation after cardiac surgery in young children: Prevalence, pathogenesis, and risk factors. Pediatric Critical Care Medicine, 3(2), 148–152. DOI 10.1097/00130478-200204000-00011. [Google Scholar] [CrossRef]
3. Abu-Sultaneh, S., Hole, A. J., Tori, A. J., Benneyworth, B. D., Lutfi, R. et al. (2017). An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatric Critical Care Medicine, 18(10), e463–e471. DOI 10.1097/PCC.0000000000001285. [Google Scholar] [CrossRef]
4. Mastropietro, C. W., Cashen, K., Grimaldi, L. M., Gowda, K. M. N., Piggott, K. D. et al. (2017). Extubation failure after neonatal cardiac surgery: A multicenter analysis. Journal of Pediatrics, 182(suppl 1), 190–196.e4. DOI 10.1016/j.jpeds.2016.12.028. [Google Scholar] [CrossRef]
5. Ferreira, F. V., Sugo, E. K., Aragon, D. C., Carmona, F., Carlotti, A. P. (2019). Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: A randomized controlled trial. Pediatric Critical Care Medicine, 20(10), 940–946. DOI 10.1097/PCC.0000000000002006. [Google Scholar] [CrossRef]
6. Foster, C. B., Spaeder, M. C., McCarter, R. J., Cheng, Y. I., Berger, J. T. (2013). The use of near-infrared spectroscopy during an extubation readiness trial as a predictor of extubation outcome. Pediatric Critical Care Medicine, 14(6), 587–592. DOI 10.1097/PCC.0b013e31828a8964. [Google Scholar] [CrossRef]
7. Farias, J., Alía, I., Retta, A., Olazarri, F., Fernández, A. et al. (2002). An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Medicine, 28(6), 752–757. DOI 10.1007/s00134-002-1306-6. [Google Scholar] [CrossRef]
8. Bouhemad, B., Mojoli, F., Nowobilski, N., Hussain, A., Rouquette, I. et al. (2020). Use of combined cardiac and lung ultrasound to predict weaning failure in elderly, high-risk cardiac patients: A pilot study. Intensive Care Medicine, 46(3), 475–484. DOI 10.1007/s00134-019-05902-9. [Google Scholar] [CrossRef]
9. Alsaddique, A., Royse, A. G., Royse, C. F., Mobeirek, A., El Shaer, F. et al. (2016). Repeated monitoring with transthoracic echocardiography and lung ultrasound after cardiac surgery: Feasibility and impact on diagnosis. Journal of Cardiothoracic and Vascular Anesthesia, 30(2), 406–412. DOI 10.1053/j.jvca.2015.08.033. [Google Scholar] [CrossRef]
10. Moschietto, S., Doyen, D., Grech, L., Dellamonica, J., Hyvernat, H. et al. (2012). Transthoracic echocardiography with Doppler tissue imaging predicts weaning failure from mechanical ventilation: Evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Critical Care, 16(3), R81. DOI 10.1186/cc11339. [Google Scholar] [CrossRef]
11. Caille, V., Amiel, J. B., Charron, C., Belliard, G., Vieillard-Baron, A. et al. (2010). Echocardiography: A help in the weaning process. Critical Care, 14(3), 1–7. DOI 10.1186/cc9076. [Google Scholar] [CrossRef]
12. Cantinotti, M., Ait Ali, L., Scalese, M., Giordano, R., Melo, M. et al. (2018). Lung ultrasound reclassification of chest X-ray data after pediatric cardiac surgery. Pediatric Anesthesia, 28(5), 421–427. DOI 10.1111/pan.13360. [Google Scholar] [CrossRef]
13. Palkar, A., Narasimhan, M., Greenberg, H., Singh, K., Koenig, S. et al. (2018). Diaphragm excursion-time index: A new parameter using ultrasonography to predict extubation outcome. Chest, 153(5), 1213–1220. DOI 10.1016/j.chest.2018.01.007. [Google Scholar] [CrossRef]
14. Llamas-Alvarez, A. M., Tenza-Lozano, E. M., Latour-Pérez, J. (2017). Diaphragm and lung ultrasound to predict weaning outcome: Systematic review and meta-analysis. Chest, 152(6), 1140–1150. DOI 10.1016/j.chest.2017.08.028. [Google Scholar] [CrossRef]
15. Soummer, A., Perbet, S., Brisson, H., Arbelot, C., Constantin, J. M. et al. (2012). Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Critical Care Medicine, 40(7), 2064–2072. DOI 10.1097/CCM.0b013e31824e68ae. [Google Scholar] [CrossRef]
16. Caltabeloti, F. P., Monsel, A., Arbelot, C., Brisson, H., Lu, Q. et al. (2014). Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: A lung ultrasound observational study. Critical Care, 18(3), 1–11. DOI 10.1186/cc13859. [Google Scholar] [CrossRef]
17. Gil-Juanmiquel, L., Gratacós, M., Castilla-Fernández, Y., Piqueras, J., Baust, T. et al. (2017). Bedside ultrasound for the diagnosis of abnormal diaphragmatic motion in children after heart surgery. Pediatric Critical Care Medicine, 18(2), 159–164. DOI 10.1097/PCC.0000000000001015. [Google Scholar] [CrossRef]
18. Hansmann, G. (2017). Pulmonary hypertension in infants, children, and young adults. Journal of the American College of Cardiology, 69(20), 2551–2569. DOI 10.1016/j.jacc.2017.03.575. [Google Scholar] [CrossRef]
19. Ng, C. S., Wan, S., Yim, A. P., Arifi, A. A. (2002). Pulmonary dysfunction after cardiac surgery. Chest, 121(4), 1269–1277. DOI 10.1378/chest.121.4.1269. [Google Scholar] [CrossRef]
20. Wynne, R., Botti, M. (2004). Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: Clinical significance and implications for practice. American Journal of Critical Care, 13(5), 384–393. DOI 10.4037/ajcc2004.13.5.384. [Google Scholar] [CrossRef]
21. Cantinotti, M., Giordano, R., Scalese, M., Marchese, P., Franchi, E. et al. (2020). Prognostic value of a new lung ultrasound score to predict intensive care unit stay in pediatric cardiac surgery. Annals of Thoracic Surgery, 109(1), 178–184. DOI 10.1016/j.athoracsur.2019.06.057. [Google Scholar] [CrossRef]
22. Rahman, D. A. A., Saber, S., El-Maghraby, A. (2020). Diaphragm and lung ultrasound indices in prediction of outcome of weaning from mechanical ventilation in pediatric intensive care unit. Indian Journal of Pediatrics, 87(6), 413–420. DOI 10.1007/s12098-019-03177-y. [Google Scholar] [CrossRef]
23. Beghetti, M. (2017). Echocardiographic evaluation of pulmonary pressures and right ventricular function after pediatric cardiac surgery: A simple approach for the intensivist. Frontiers in Pediatrics, 5, 184. DOI 10.3389/fped.2017.00184. [Google Scholar] [CrossRef]
24. Ferré, A., Guillot, M., Lichtenstein, D., Mezière, G., Richard, C. et al. (2019). Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Medicine, 45(5), 601–608. DOI 10.1007/s00134-019-05573-6. [Google Scholar] [CrossRef]
25. Sharma, V., Rao, V., Manlhiot, C., Fremes, S., Wąsowicz, M. (2017). A derived and validated score to predict prolonged mechanical ventilation in patients undergoing cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 153(1), 108–115. DOI 10.1016/j.jtcvs.2016.08.020. [Google Scholar] [CrossRef]
26. Silverman, N. H., Schiller, N. B. (1984). Cross sectional echocardiographic assessment of cardiac chamber size and ejection fraction in children. Ultrasound in Medicine & Biology, 10(6), 757–769. DOI 10.1016/0301-5629(84)90236-9. [Google Scholar] [CrossRef]
27. Mercier, J. C., DiSessa, T. G., Jarmakani, J. M., Nakanishi, T., Hiraishi, S. et al. (1982). Two-dimensional echocardiographic assessment of left ventricular volumes and ejection fraction in children. Circulation, 65(5), 962–969. DOI 10.1161/01.CIR.65.5.962. [Google Scholar] [CrossRef]
28. Cantinotti, M., Giordano, R., Volpicelli, G., Kutty, S., Murzi, B. et al. (2016). Lung ultrasound in adult and paediatric cardiac surgery: Is it time for routine use? Interactive Cardiovascular and Thoracic Surgery, 22(2), 208–215. DOI 10.1093/icvts/ivv315. [Google Scholar] [CrossRef]
29. Cantinotti, M., Giordano, R., Gargani, L., Marchese, P., Franchi, E. et al. (2020). Could judicious use of lung ultrasound reduce radiographic examinations in pediatric cardiac surgery patients. Journal of Clinical Anesthesia, 61, 109638. DOI 10.1016/j.jclinane.2019.109638. [Google Scholar] [CrossRef]
30. Qu, J., Liang, H., Zhou, N., Li, L., Wang, Y. et al. (2017). Perioperative NT-proBNP level: Potential prognostic markers in children undergoing congenital heart disease surgery. Journal of Thoracic and Cardiovascular Surgery, 154(2), 631–640. DOI 10.1016/j.jtcvs.2016.12.056. [Google Scholar] [CrossRef]
31. Cantinotti, M., Giordano, R., Scalese, M., Molinaro, S., Della Pina, F. et al. (2015). Prognostic role of BNP in children undergoing surgery for congenital heart disease: Analysis of prediction models incorporating standard risk factors. Clinical Chemistry and Laboratory Medicine, 53(11), 1839–1846. DOI 10.1515/cclm-2014-1084. [Google Scholar] [CrossRef]
32. Smith, B. M., Ezeokoli, N. J., Kipps, A. K., Azakie, A., Meadows, J. J. (2013). Course, predictors of diaphragm recovery after phrenic nerve injury during pediatric cardiac surgery. Annals of Thoracic Surgery, 96(3), 938–942. DOI 10.1016/j.athoracsur.2013.05.057. [Google Scholar] [CrossRef]
33. El Amrousy, D., Elgendy, M., Eltomey, M., Elmashad, A. E. (2020). Value of lung ultrasonography to predict weaning success in ventilated neonates. Pediatric Pulmonology, 55(9), 2452–2456. DOI 10.1002/ppul.24934. [Google Scholar] [CrossRef]
34. Song, I. K., Kim, E. H., Lee, J. H., Kang, P., Kim, H. S. et al. (2018). Utility of perioperative lung ultrasound in pediatric cardiac surgery: A randomized controlled trial. Anesthesiology, 128(4), 718–727. DOI 10.1097/ALN.0000000000002069. [Google Scholar] [CrossRef]
Supplemental Digital Content 1
Materials and Methods
This prospective observational study, which followed the STrengthening the Reporting of OBservational studies in Epidemiology guidelines, was approved by the Institutional Review Board of the Chinese Clinical Trial Registry (No. ChiECRCT20200377) and registered with the Fundamental Research Funds for the Central Universities (No. 3332020018). It was conducted in a 40-bed pediatric ICU at a tertiary care hospital, China’s National Center for Cardiovascular Diseases.
Study Population
Children on mechanical ventilation (MV) for >6 h and who were eligible for their first spontaneous breathing trial (SBT) after cardiac surgery between January and July 2020 were screened for inclusion. The inclusion criteria were: 1) an age of 3 months to 6 years; and 2) primary congenital heart disease repair and cardiopulmonary bypass (CPB) under general anesthesia. The exclusion criteria were: 1) presence of preoperative pneumonia, pneumothorax, pleural effusion, or tracheobronchial stenosis; 2) MV before surgery; 3) left ventricular ejection fraction <40% before surgery; 4) previous spontaneous breathing trial (SBT) failure; 5) palliative or staging cardiac surgery; 6) delayed sternal closure; 7) extracorporeal membrane oxygenation support before or after surgery; and 8) planned prophylactic non-invasive MV.
Study Protocol
All children were transferred from the operating room to the pediatric intensive care unit (PICU) after cardiac surgery. In the PICU, the children received synchronized intermittent mandatory ventilation with a tidal volume of 10–20 mL/kg bodyweight and positive end-expiratory pressure (PEEP) of 4–8 cmH2O. Children were considered eligible for an SBT if they met all the criteria (1) and if the intervention was approved by the physician in charge. The SBT criteria were as follows: fully awake with a stable condition; adequate gas exchange as indicated by oxygen saturation >90%, fraction of inspired oxygen (FiO2) ≤50%, PEEP ≤5 cmH2O, and peak inspiratory pressure ≤20 cmH2O; adequate respiratory drive and appropriate level of consciousness with intact cough and gag reflexes; hemodynamic stability (dopamine or epinephrine doses <10 µg/kg/min and <0.1 µg/kg/min, respectively); pH >7.30 on arterial blood gas analysis; absence of bleeding; and absence of electrolyte disturbances. When a patient met all the criteria and was enrolled in the study, the ultrasound was performed.
After the measurements were performed, the child underwent an SBT. SBT was performed using continuous positive airway pressure with a PEEP of 5 cmH2O, pressure support of 10 cmH2O, and FiO2 ≤40%. The SBT duration was 60 min. For children who showed poor SBT tolerance, full ventilatory support was immediately recommenced. SBT failure was defined when a patient presented with any of the following criteria: increased respiratory work (i.e., accessory respiratory muscle use, retractions, and paradoxical breathing), tachypnea, tachycardia, hypotension, diaphoresis, oxygen saturation <90%, arterial carbon dioxide tension >55 mmHg or an increase of >10 mmHg, or pH <7.30 on arterial blood gas analysis [1]. Children who successfully passed the SBT were extubated and received supplemental oxygen via a facemask. Extubation success was defined as no need for MV support (invasive or non-invasive) in the following 48 h [1].
Lung Ultrasound
Experienced operators (C.L. and L.Z.Z.) performed the ultrasonography immediately before SBT using a CX-50 (Philips, Amsterdam, Netherlands) or Vivid-i (GE Healthcare, Chicago, IL, USA) device with a wideband 9–12 MHz linear transducer and phased array probe. Comprehensive thoracic ultrasound comprised lung ultrasound (LUS) and diaphragm ultrasound assessment.
For the LUS, all intercostal spaces of the upper and lower parts of the anterior, lateral, and posterior regions of the left and right chest (12 regions) were examined [2]. Each region of interest was identified using anatomical landmarks: from the sternum to the anterior axillary line for anterior lung regions, from the anterior to the posterior axillary lines for lateral lung regions, and from the posterior axillary line to the spine for posterior lung regions. The upper and lower parts of the anterior, lateral, and posterior lung regions were determined using the horizontal mamillary line. Four ultrasound patterns that corresponded to the different degree of aeration loss were identified in each intercostal space: 1) normal aeration, characterized by the presence of lung sliding with horizontal A-lines and, occasionally, 1 or 2 isolated vertical B-lines; 2) moderate loss of lung aeration, characterized either by multiple well-defined, regularly spaced “B-lines” that were 7-mm apart arising at the pleural line and corresponding with interstitial edema; 3) severe loss of lung aeration, characterized by multiple coalescent vertical B-lines beginning at the pleural line and corresponding with alveolar edema; and 4) complete loss of lung aeration resulting in lung consolidation, characterized by the presence of a tissue pattern that contained either hyperechoic punctiform or tubular images that were representative of static and dynamic air bronchograms, respectively. The worst ultrasound pattern observed in 1 or several intercostal spaces was considered the region of interest. A value (0, 1, 2, or 3) was attributed to each region examined, and the LUS score was calculated as the sum of the 12 regions. The sum of these gave an LUS score between 0 and 36.
The diaphragmatic motion was assessed either by B-mode or M-mode [3]. In B-mode, the probe was positioned on the posterior axillary line on one side with the transducer notch pointing at 12 o’clock toward the axilla. In M-mode, the probe was placed in the subcostal region parallel to the intercostal space using the M-mode with the cursor crossing the diaphragm. Then, the highest and lowest peak points were assessed as markers for the range of diaphragmatic movement. Diaphragmatic motion was classified as follows: 1) “normal,” if the diaphragm moved toward the transducer during inspiration with >4 mm excursion and there was a <50% difference between the movement of the two hemidiaphragms; 2) “paretic,” if the amplitude was <4 mm and the difference between the two hemidiaphragms was >50%; 3) “akinetic,” if there was no movement and a straight line was observed in M-mode; and 4) “paradoxical,” when the diaphragm moved away from the transducer during inspiration (3).
Echocardiography
The transthoracic echocardiographic examination was performed at the same time as thoracic ultrasound, immediately before SBT, by the same operator. Left ventricular ejection fraction was assessed using the monoplane Simpson method [4]. Two-dimensional fractional area change (FAC) was used to quantitatively estimate right ventricular (RV) function, with a lower reference value of 35% for normal RV systolic function. Percentage FAC = 100 × end-diastolic area (area ED)–end-systolic area/area ED. The endocardial border was traced in the apical four-chamber views from the tricuspid annulus along the free wall to the apex, then back to the annulus, and along the interventricular septum at end-diastole and end-systole [5].
Doppler echocardiography was used to determine the systolic pulmonary artery pressure (SPAP). SPAP was calculated as the sum of the estimated right atrial pressure and the peak pressure gradient between the peak right ventricle and right atrium, as estimated by application of the modified Bernoulli equation to the peak velocity, as represented by the tricuspid regurgitation Doppler signal [5]. Pulmonary hypertension (PH) and severe PH were defined as pulmonary artery systolic pressures that exceeded 50% and 75% of systemic systolic pressures, respectively [6].
Statistics
Continuous variables were described as means ± standard deviation (SD) or medians (interquartile range) and were compared using Student’s t-test or Mann–Whitney U test, as appropriate. Categorical variables were expressed as numbers and percentages. Proportion comparisons were performed using the chi-square test. The discriminatory power of predictors was quantified by measuring the area under the receiver-operating characteristic curve (AUC ROC). The results are expressed as the AUC and 95% confidence interval (CI) for this area. Logistic regression analysis was used to construct a prediction model for the binary outcome of successful extubation. Odds ratios were given with 95% CIs. Concordance between different ultrasound operators was calculated using quadratic weighted kappa. A p-value < 0.05 was considered statistically significant.
References
1. Ferreira, F. V., Sugo, E. K., Aragon, D. C., Carmona, F., Carlotti, A. P. (2019). Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: A randomized controlled trial. Pediatric Critical Care Medicine, 20(10), 940–946. DOI 10.1097/PCC.0000000000002006.
2. Caltabeloti, F. P., Monsel, A., Arbelot, C., Brisson, H., Lu, Q. et al. (2014). Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: A lung ultrasound observational study. Critical Care, 18(3), 1–11. DOI 10.1186/cc13859.
3. Gil-Juanmiquel, L., Gratacós, M., Castilla-Fernández, Y., Piqueras, J., Baust, T., et al. (2017). Bedside ultrasound for the diagnosis of abnormal diaphragmatic motion in children after heart surgery. Pediatric Critical Care Medicine, 18(2), 159–164. DOI 10.1097/PCC.0000000000001015.
4. Lai, W. W., Geva, T., Shirali, G. S., Frommelt, P. C., Humes, R. A. et al. (2006). Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Journal of the American Society of Echocardiography, 19(12), 1413–1430. DOI 10.1016/j.echo.2006.09.001.
5. Rudski, L. G., Lai, W. W., Afilalo, J., Hua, L., Handschumacher, M. D. et al. (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography, 23(7), 685–713; 786–788. DOI 10.1016/j.echo.2010.05.010.
6. Hansmann, G. (2017). Pulmonary hypertension in infants, children, and young adults. Journal of the American College of Cardiology, 69(20), 2551–2569. DOI 10.1016/j.jacc.2017.03.575.
Supplemental Digital Content 2
Supplemental Digital Content 3
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |