

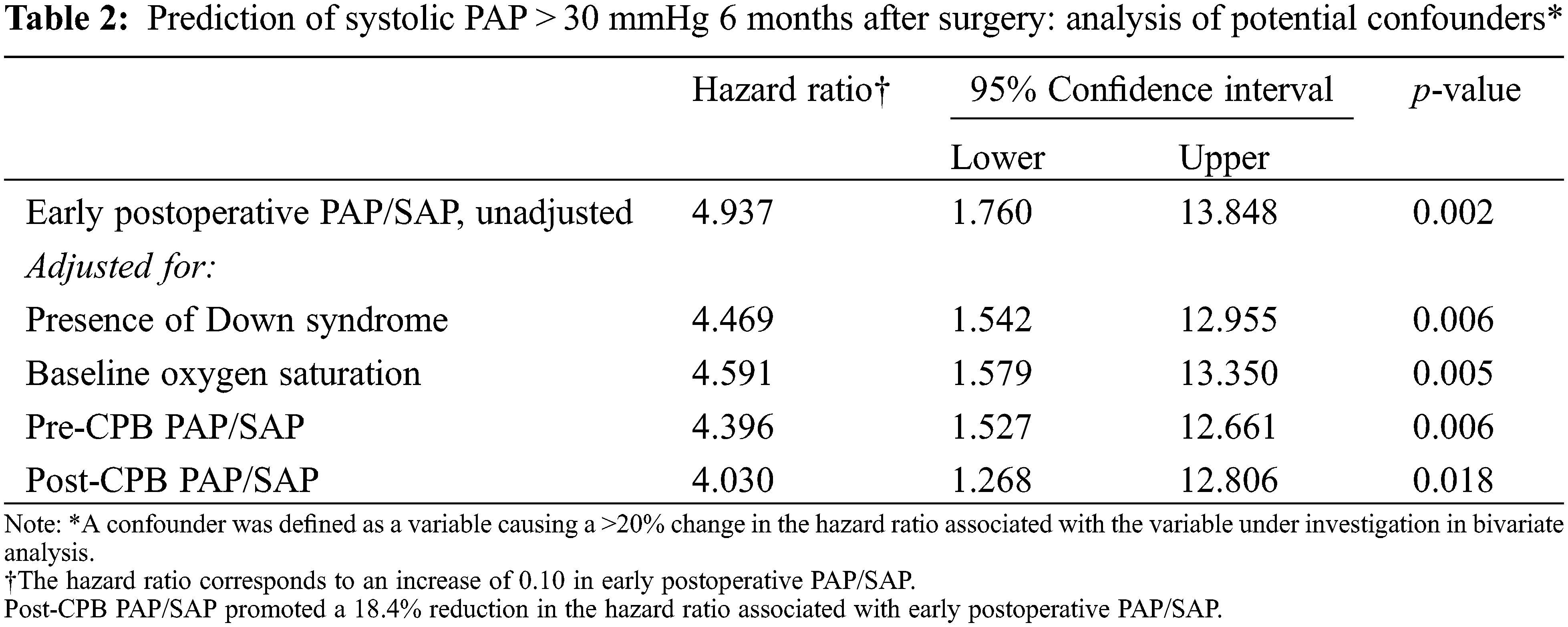
 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019382
ARTICLE
Prediction of Pulmonary Arterial Pressure Level after Repair of Congenital Cardiac Communications and Discharge from the Hospital: Role of Down Syndrome and Early Postoperative Hemodynamics
Heart Institute (InCor), University of São Paulo School of Medicine, São Paulo, Brazil
*Corresponding Author: Antonio Augusto Lopes. Email: aablopes@usp.br
#Part of a doctoral thesis by Eloisa S. Carvalho to the Program in Cardiology, University of São Paulo School of Medicine, São Paulo, Brazil
Received: 21 October 2021; Accepted: 11 January 2022
Abstract: Background: Postoperative pulmonary hypertension limits the success of surgical treatment in some patients with unrestrictive congenital cardiac communications. Identifying patients at risk of developing postoperative pulmonary hypertension is important to individualize follow-up strategies. Methods: We analyzed a prospective cohort of 52 pediatric patients (age 3 to 35 months) looking for perioperative predictors of mildly elevated pulmonary arterial pressure 6 months after surgery, defined as a systolic pressure greater than 30 mmHg by transthoracic echocardiography. This corresponds to a mean pulmonary arterial pressure of >20 mmHg. Clinical, echocardiographic and hemodynamic parameters were investigated. Perioperative hemodynamics was assessed by directly measuring pulmonary and systemic arterial pressures using indwelling catheters. Early postoperative pulmonary hemodynamics was defined as the mean pulmonary/systemic mean arterial pressure ratio (PAP/SAP) obtained per patient during the first 6 h of postoperative care. Results: Among the factors that were investigated as possible predictors, perioperative hemodynamics and the presence of Down syndrome were initially selected using univariate analysis (p < 0.030). Early postoperative PAP/SAP was correlated with PAP/SAP obtained in the operating room just after cardiopulmonary bypass (r = 0.70, p < 0.001), and it was higher in subjects with Down syndrome than in nonsyndromic individuals (p = 0.003). Early postoperative PAP/SAP was the only predictor selected using multivariate analysis. It was characterized as an independent predictor after adjustments for possible confounders. An early postoperative PAP/SAP of >0.35 was 76% sensitive and 74% specific at predicting a systolic pulmonary arterial pressure of >30 mmHg 6 months after surgery (hazard ratio with 95% CI 8.972 [2.428–33.158], p = 0.002). Conclusion: The hypertensive early postoperative behavior of the pulmonary circulation was strongly but not exclusively associated with Down syndrome, and it was characterized as an independent predictor of altered pulmonary arterial pressure after discharge from the hospital.
Keywords: Pulmonary hypertension; congenital heart disease; Down syndrome; pediatric cardiac surgery
Pulmonary vascular abnormalities are present in almost all patients with unrestrictive congenital cardiac communications and may either constitute a problem while assigning them to surgical repair of cardiac lesions or limit the success of treatment if surgery is not offered in a timely fashion (early in life) [1–4]. In addition to the age at operation, the presence of extracardiac syndromes, especially Down syndrome (trisomy 21), and the complexity of the cardiac anomaly have been considered classical risk factors for perioperative complications and progression of pulmonary vascular disease postoperatively [5,6]. The potential for reversion of pulmonary vascular abnormalities following surgery has been estimated based on pulmonary vascular responsiveness to vasodilator agents, which is generally assessed during preoperative cardiac catheterization. It can also be predicted based on the structure of pulmonary vessels, biological behavior of pulmonary vascular cells, and circulating and tissue biomarkers [7–10]. However, there have been relatively few follow-up studies to confirm predictions in the pediatric population.
Cardiac catheterization with pulmonary vasodilator testing has been considered the gold standard procedure to evaluate cardiopulmonary hemodynamics and decide about surgery in patients with congenital heart disease and pulmonary hypertension [4]. Nevertheless, cardiac catheterization is not performed in all patients, and it is widely known that hemodynamic parameter estimation is difficult in subjects for whom pulmonary blood flow and systemic blood flow need to be calculated separately [11]. On the other hand, theoretically, preoperative hemodynamic evaluation should be considered to be limited in providing adequate prognostic information in the sense that parameters are obtained in the presence of cardiac lesions and therefore altered blood flow conditions. Postoperatively, patients can be evaluated free of cardiac shunts, but except for instances where they are seen for research purposes, invasive procedures are performed late after surgery to confirm the diagnosis of postoperative pulmonary hypertension, which is associated with poor prognosis [12].
To start dealing with this “too-early-or-too-late” dilemma, we decided to assess pulmonary hemodynamics just after surgical repair of cardiac communications in pediatric subjects and verify whether hemodynamic parameters could predict mild elevations of pulmonary arterial pressure after discharge from the hospital. Several studies have focused on pulmonary vascular tone instability following cardiac surgery, and much has been learned about patient management in the setting of postoperative intensive care [13]. Less is known about the behavior of the pulmonary circulation after hospital discharge. In a recent study, we looked for possible predictors of persistent pulmonary hypertension in patients with moderate to severe pulmonary vasculopathy who were given oral sildenafil pre-and postoperatively [14]. The present study involved patients with less severe pulmonary hypertension who were discharged from the hospital under no specific medications. We analyzed potential predictors of pulmonary artery pressure level 6 months after surgery and examined the particular role of Down syndrome.
The study was based on a prospective cohort of pediatric patients who were referred to the Heart Institute (InCor), University of São Paulo School of Medicine, São Paulo, Brazil for surgical repair of congenital cardiac anomalies. Patients were consecutively enrolled from November 2016 to July 2020 on the basis of the following criteria: age 1 month to 3 years; hearts with biventricular physiology; presence of unrestrictive post tricuspid communications; clinical and echocardiographic features suggestive of at least moderately elevated pulmonary arterial pressure: a loud second heart sound in the pulmonary area, absence of intense systolic murmurs over the precordium and absence of significant interventricular pressure gradients thus suggesting elevation of right ventricular systolic pressure; absence of features suggestive of advanced pulmonary vasculopathy with significant right-to-left shunting across the septal defects and peripheral oxygen saturation <85%; absence of extra cardiac syndromes except for Down syndrome. Having met the inclusion criteria, patients entered the study if their parents or relatives agreed to participate and signed an informed consent form.
The initial evaluation consisted of a detailed clinical history, physical examination, chest radiographs, and ECG. The diagnosis of Down syndrome was confirmed by genetic testing prior to referral to the Heart Institute. Transthoracic echocardiography was used for assessing cardiovascular anatomy and hemodynamic parameters. Patients were assigned to surgery on the basis of noninvasive diagnostic evaluation. Clinical and radiographic signs of pulmonary over circulation were generally associated with a pulmonary/systemic blood flow ratio greater than 2.50 by transthoracic echocardiography and normal (>93%) peripheral oxygen saturation. Patients with a pulmonary/systemic blood flow ratio <2.00 and peripheral oxygen saturation <93% with no signs of congestive heart failure or failure to thrive were presumed to have higher levels of pulmonary vascular resistance, thus requiring special attention perioperatively. Systolic and mean pulmonary arterial pressure were estimated by transthoracic echocardiography based on parameters derived from the tricuspid regurgitant jet and pulmonary regurgitant jet, respectively. The pulmonary/systemic blood flow ratio was calculated based on systolic flow parameters in the right and left ventricular outflow tracts. Pulmonary venous flow was estimated by measuring the velocity-time integral of blood flow in pulmonary veins. Values <20.0 cm are generally associated with heightened pulmonary vascular resistance in pediatric patients with congenital cardiac communications [15,16]. All patients were deemed operable and assigned to total repair of the cardiac anomalies. None of them presented with features that generally preclude surgery in this population: pulmonary/systemic blood flow ratio <1.50 with predominant right-to-left flow across the septal defect and peripheral oxygen saturation persistently below 85%.
2.3 Perioperative Monitoring and Care
All patients underwent open cardiac surgery for repair of cardiac lesions. Intraoperatively, levels of pulmonary arterial pressure and systemic arterial pressure were recorded before and after cardiopulmonary bypass (CPB). Pulmonary and systemic arterial lines were placed for postoperative hemodynamic monitoring. In our institution, a pulmonary catheter is routinely inserted by the surgeon intraoperatively in patients with unrestrictive cardiac communications with moderate to severe pulmonary hypertension. Inhaled nitric oxide was started after CPB termination (20 ppm) and maintained in the postoperative intensive care unit. We routinely use inhaled nitric oxide postoperatively in these patients as an attempt to prevent life-threatening hemodynamic disturbances associated with right and left heart failure. Postoperatively, patients received fentanyl, midazolam, and ketamine singly or in combination for analgesia/sedation and milrinone, epinephrine and norepinephrine as inotropic/vasoactive agents. The amount of drugs used was computed using the vasoactive-inotropic score [17]. Mild cardiopulmonary instabilities characterized by a rise in pulmonary arterial pressure with a decrease in peripheral oxygen saturation were generally reversed by manual ventilation and chest physiotherapy. Severe and sustained instabilities associated with systemic hypotension were managed by means of deep sedation, systemic alkalinization, changes in mechanical ventilation parameters and doses of inotropic/vasoactive drugs, and use of pulmonary vasodilators (milrinone, sildenafil, and prostaglandin E1 in addition to inhaled nitric oxide).
2.4 Postoperative Pulmonary Arterial Pressure
Pulmonary and systemic arterial pressure levels were subsequently recorded in the postoperative intensive care unit with readings taken at 2-h intervals (12 times a day) for 2.5 days. This was the minimum time of invasive monitoring required for study purposes. Longer periods of hemodynamic monitoring were necessary in patients with unstable clinical curse. Curves were constructed for mean pulmonary arterial pressure and pulmonary/systemic mean arterial pressure ratio (PAP/SAP) and were comparatively analyzed between patients with and without Down syndrome. We also analyzed pulmonary and systemic arterial pressures specifically during the first 6 h of intensive care unit stay. In that period, patients were still deeply sedated, stable on mechanical ventilation receiving 20 ppm inhaled nitric oxide, and free of major post-CPB instabilities. We computed the PAP/SAP ratio and used the mean of the first four values to define early postoperative hemodynamics (early postoperative PAP/SAP). This parameter was compared to the PAP/SAP ratio registered in the operating room (post-CPB PAP/SAP) in terms of the ability to predict the outcome.
Patients were followed up for 6 months after hospital discharge and then re-evaluated noninvasively. We assessed pulmonary hemodynamics by measuring systolic pulmonary arterial pressure using transthoracic echocardiography. In particular, we looked for possible perioperative predictors of a systolic pulmonary arterial pressure of >30 mmHg. We chose this level because it corresponds to a mean pulmonary arterial pressure of >20 mmHg (mean PAP∼0.61 × systolic PAP + 2 mmHg), i.e., the lower limit of mild pulmonary hypertension according to current literature [18,19]. Echocardiographic evaluation 6 months after surgery was performed by a single professional (CRPC, coauthor) in a blinded fashion. Post-CPB PAP/SAP, early postoperative PAP/SAP, and the presence of Down syndrome were among the potential predictors we analyzed.
Unless otherwise specified, results of numeric variables are presented as medians with interquartile ranges. Categorical variables are reported as the number of cases and proportions or percentages. All numeric variables had their distributions tested for closeness to the normal (Gaussian) distribution. Whenever necessary, dependent variables were subjected to Box-Cox transformation before applying statistical tests. Differences within subjects were analyzed using the t test for paired data. Differences between groups were tested using the general linear model. The relationship between variables was tested by calculating Pearson’s coefficient of correlation. Postoperative pulmonary arterial pressure curves were analyzed using the general linear model for repeated measures. Logistic regression analysis was used to identify possible predictors of hemodynamic outcome. The corresponding hazard ratios with 95% confidence intervals (CIs) are provided. All variables with a p-value of <0.10 in univariate analysis were included in multivariate analysis. Once a predictor was identified, bivariate analysis was performed to look for possible confounders. A confounder was defined as a variable causing a >20% change in the hazard ratio associated with the predictor. Receiver operating characteristic curves were constructed, and cutoff values for predictors are provided with respective sensitivity and specificity levels. In all assessments, 0.05 was assumed to be the level of significance. All tests were performed using the IBM SPSS Statistical software (version 26, Armonk, NY, USA). The sample size was calculated based on the following: approximately one-third of patients were expected to have abnormal pulmonary arterial pressure 6 months after surgery, and 5 to 10 events (abnormal pulmonary pressure) would be necessary to analyze each of the main predictors under investigation, namely, early postoperative hemodynamics and Down syndrome (∼15 events required). Thus, the study was planned to include ∼45 patients.
A flow diagram showing how patients were selected is depicted in Fig. 1. Fifty-two patients were enrolled with an age range of 3 to 35 months (11(7–15) months, median with interquartile range) and male to female ratio of 17:35. The weight and height were 6.42(5.45–7.80) Kg and 66(62–74) cm, respectively. Phenotypic characteristics of Down syndrome were present in 34 of them (65%). All patients had unrestrictive post tricuspid cardiac communications (ventricular septal defect [n = 32] or atrioventricular septal defect [n = 20]) either isolated or associated with atrial septal defect (secundum type) or patent ductus arteriosus. Baseline pulmonary/systemic blood flow ratio (2.15[1.73–2.90]), velocity-time integral of blood flow in pulmonary veins (21.2[19.9–24.9] cm) and peripheral oxygen saturation (96%[93%–98%]) suggested that pulmonary vascular resistance was elevated in some individuals. Preoperative systolic pulmonary artery pressure and mean pulmonary artery pressure were 75(64–80) mmHg and 45(33–55) mmHg, respectively.
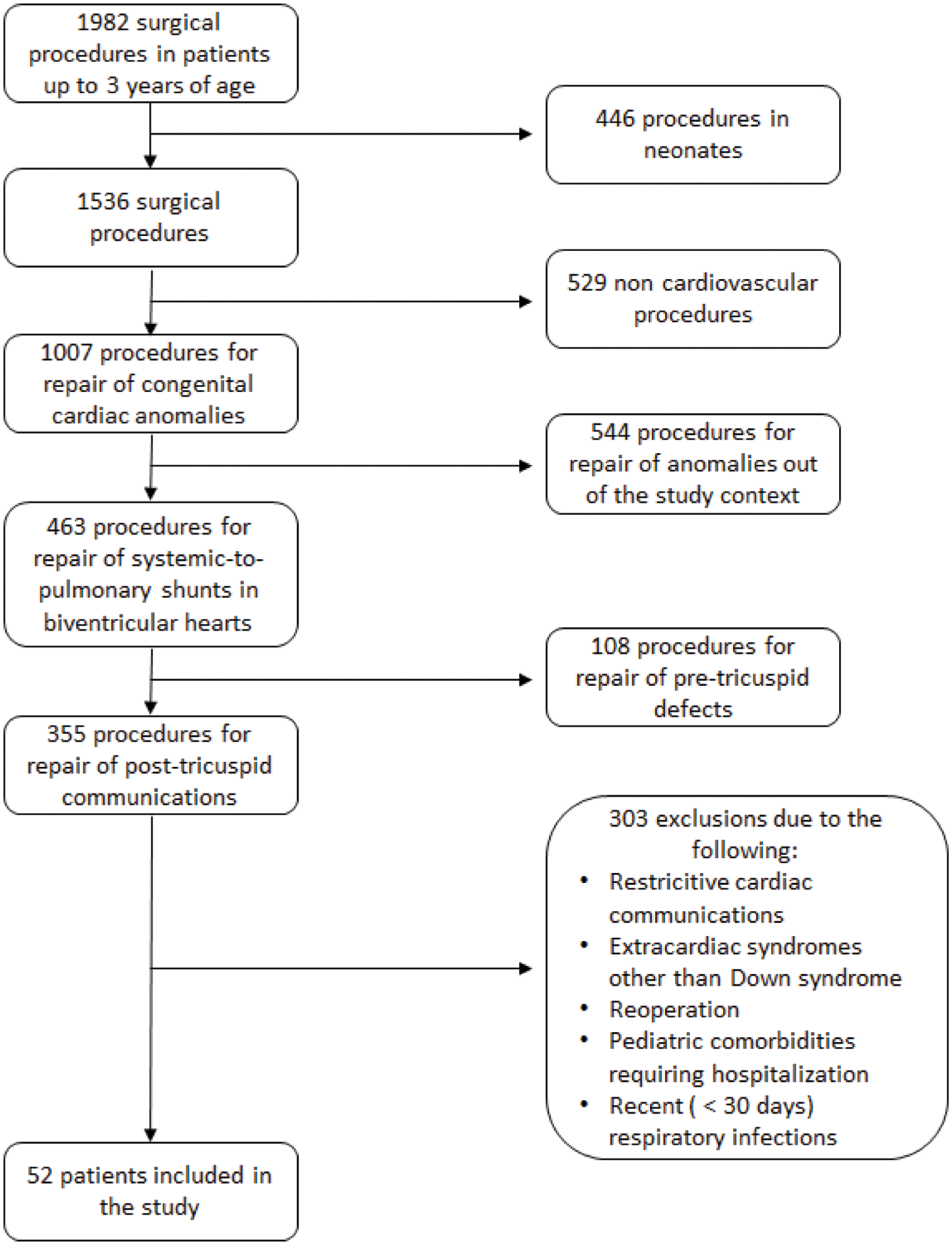
Figure 1: Flow diagram showing included and excluded patients in the period of the study
All patients underwent surgical repair of cardiac lesions under CPB. Postoperative hemodynamic measurements were performed under inhaled nitric oxide. The mean pulmonary arterial pressure decreased, and the mean systemic arterial pressure increased after CPB in comparison with pre-CPB levels (32(26–38) mmHg to 21(18–25) mmHg, p < 0.001 and 46(39–53) mmHg to 53(49–59) mmHg, p < 0.001, respectively). As a result, PAP/SAP ratio decreased from 0.74(0.61–0.87) to 0.39(0.33–0.47) (p < 0.001). Despite the continuous use of inhaled nitric oxide postoperatively, several patients had heightened pulmonary arterial pressure with mean pressure levels approaching or exceeding 30 mmHg in many instances. Pressure curves for subjects with and without Down syndrome are shown in Fig. 2. Patients with Down syndrome had higher pulmonary arterial pressure during the first 20 h of postoperative care than nonsyndromic individuals. Early postoperative PAP/SAP (0.37[0.32–0.46], range 0.19 to 0.74) was significantly correlated with PAP/SAP registered in the operating room immediately after CPB (r = 0.70, p < 0.001), and it was higher in patients with Down syndrome than in nonsyndromic patients (0.40[0.33–0.48] and 0.34[0.29–0.39], p = 0.003, respectively). When the general linear model was used to investigate multiple factors that might have influenced early postoperative PAP/SAP, post-CPB PAP/SAP and Down syndrome but not CPB time or the vasoactive-inotropic score in the early postoperative period remained in the model (respective p-values, <0.001, 0.024, 0.354 and 0.877). In addition, there was a weak but significant correlation between early postoperative PAP/SAP and the duration of mechanical ventilation (r = 0.32, p = 0.021).
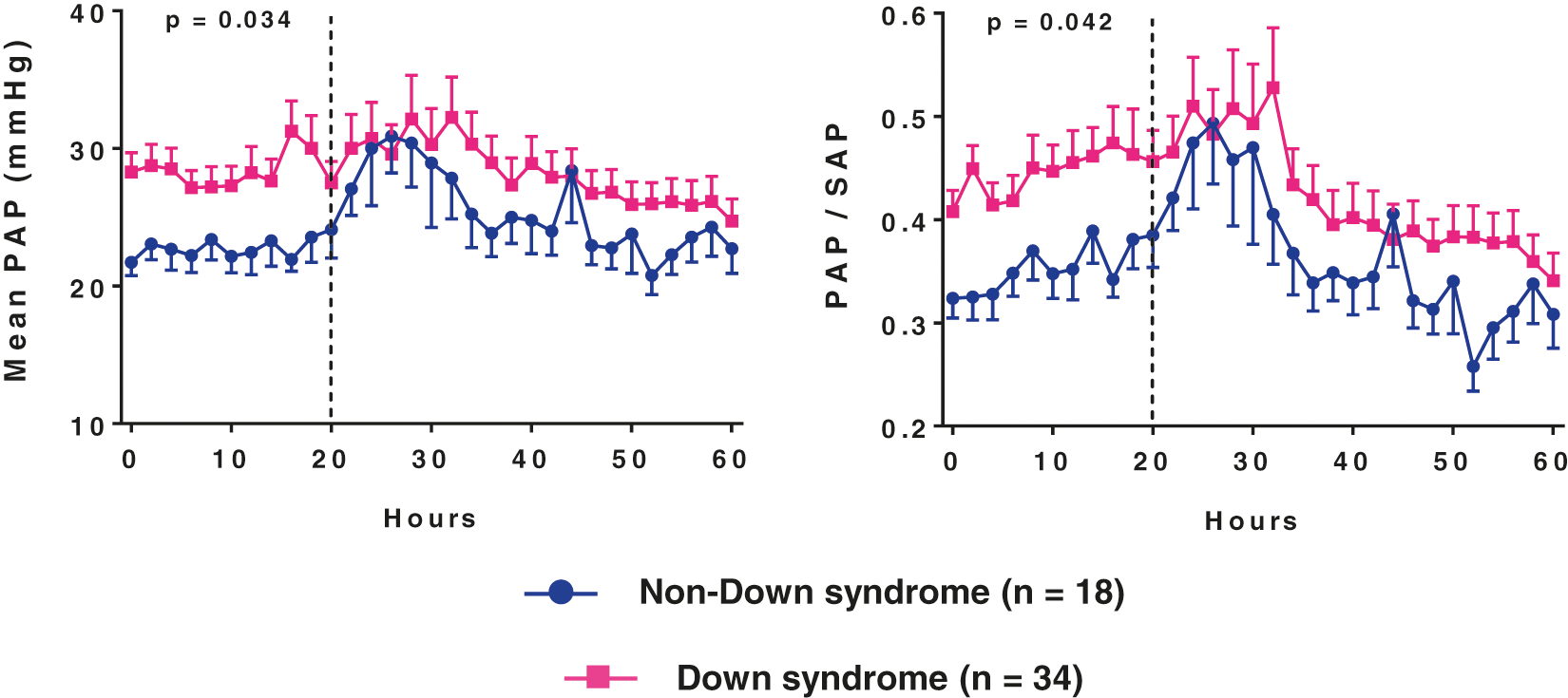
Figure 2: Mean pulmonary arterial pressure (PAP) and pulmonary/systemic mean arterial pressure ratio (PAP/SAP) during the first 2.5 days of postoperative care (readings at 2-h intervals)
Data are presented as means with SE. The p-values correspond to the analysis of the first 20 h. They were obtained using the general linear model for repeated measures after Box-Cox transformation of the dependent variables
3.3 Relevant Postoperative Events
Postoperatively, 17 patients had elevations of pulmonary arterial pressure above the systemic arterial pressure level. Some episodes were isolated and promptly reversed by manual ventilation and sedation. Others were associated with a >20% decrease in systemic arterial pressure and/or a decrease in peripheral oxygen saturation to levels <90%. Eventually, instabilities were not severe enough to require major changes in therapeutic strategies but lasted long or were clustered. There were no deaths associated with these events, although some influenced the duration of mechanical ventilation. One patient died of septicemia. Another patient died of severe and sustained systemic hypotension followed by bradycardia unresponsive to vasoactive drugs, including vasopressin. The fatal outcome was unrelated to pulmonary hypertension, infection, or relevant residual cardiac lesions.
Patients were mechanically ventilated for 7(6–10) days. The duration of mechanical ventilation was influenced by early postoperative hemodynamics (early postoperative PAP/SAP) but not Down syndrome (p = 0.027 and p = 0.775, respectively, in bivariate analysis). Factors associated with long times of mechanical ventilation (e.g., >10 days) other than pulmonary hypertension were delayed sternal closure, respiratory disturbances, infection, arrhythmias, seizures, and other neurological events. Inhaled nitric oxide was maintained during the entire period of mechanical ventilation, except in patients in whom ventilation was prolonged for reasons other than pulmonary hypertension.
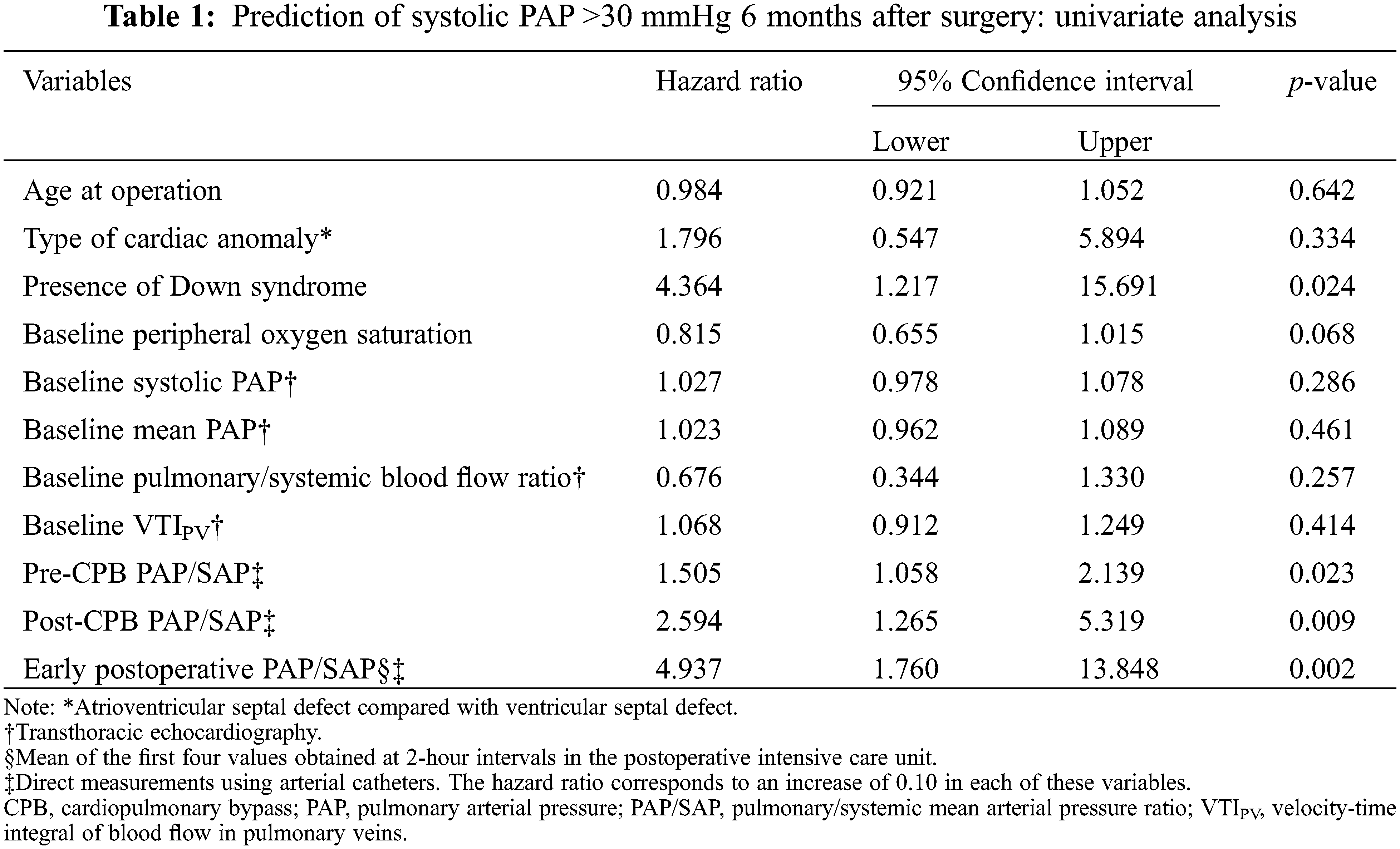
Fifty patients were discharged from the hospital and subjected to echocardiographic evaluation 6 months after surgery as an attempt to identify perioperative predictors of a postoperative systolic pulmonary arterial pressure >30 mmHg. Twenty-five patients were above this level. By univariate analysis, five potential predictors were identified with a p-value < 0.10 (Table 1). However, using the forward LR procedure for variable selection in multivariate analysis, only early postoperative PAP/SAP remained in the model as a predictor. Subsequently, we performed bivariate analysis to investigate whether variables not included in the model (i.e., the presence of Down syndrome, baseline peripheral oxygen saturation and pre-and post-CPB PAP/SAP) could act as confounders. Table 2 shows that the hazard ratio associated with early postoperative PAP/SAP was not significantly changed by any of the aforementioned variables. Receiver operating characteristic curves further confirmed that early postoperative PAP/SAP was even better than post-CPB PAP/SAP in predicting postoperative systolic pulmonary arterial pressure levels greater than 30 mmHg. The cutoff level of 0.35 was associated with the best levels of sensitivity and specificity at predicting postoperative pulmonary arterial pressure (Fig. 3). The hazard ratio associated with an early postoperative PAP/SAP of >0.35 vs. values ≤0.35 was 8.97 (95% CI 2.43–33.16, p = 0.001). Early postoperative PAP/SAP was also tested for prediction of postoperative systolic pulmonary artery pressure > 40 mmHg. Because only five patients were above this level, the prediction was less robust. The hazard ratio associated with an early postoperative PAP/SAP of >0.43 (best cutoff value) was 11.64 (95% CI 1.17–115.59, p = 0.048).
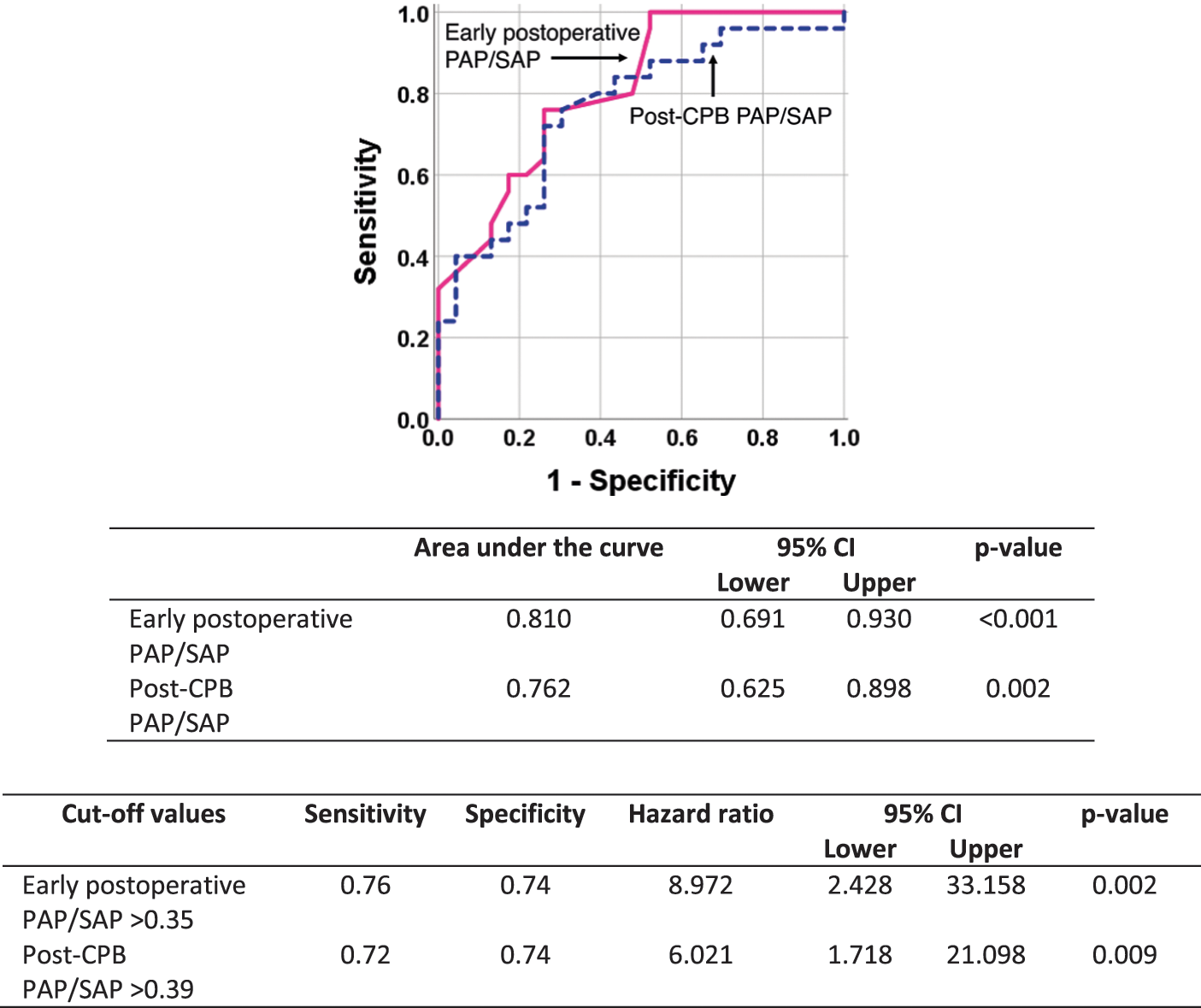
Figure 3: Receiver operating characteristic curves showing the role of post-cardiopulmonary bypass (CPB) pulmonary/systemic mean arterial pressure ratio (PAP/SAP) and early postoperative PAP/SAP in predicting a systolic pulmonary arterial pressure >30 mmHg 6 months after cardiac surgery
The interaction that we observed between early postoperative PAP/SAP and Down syndrome, i.e., higher values of the former in syndromic individuals, made it difficult to interpret prediction when both factors were included in the statistical model. To solve this problem, we performed subgroup analysis working with the outcome as a numeric variable. The results are shown in Fig. 4. For de entire cohort, the 6-month postoperative systolic pulmonary arterial pressure was 32(23–35) mmHg (range 19 to 54 mmHg). In patients with early postoperative PAP/SAP ≤ 0.35, those with Down syndrome had higher 6-month postoperative pulmonary arterial pressure levels than the nonsyndromic patients, although the mean values were below 30 mmHg in both subgroups. In patients with early postoperative PAP/SAP > 0.35, 6-month postoperative pulmonary arterial pressure was elevated (>30 mmHg), with similar levels in the subgroups. The ratio of syndromic to nonsyndromic individuals in this group was 2.7:1. Thus, early postoperative PAP/SAP, which was higher but not exclusively elevated in syndromic subjects, seemed to be the link between Down syndrome and abnormal pulmonary artery pressure 6 months after surgery. Of the 32 Down syndrome patients who were evaluated after hospital discharge, 12 had normal systolic pulmonary arterial pressure. In this subgroup, the age at operation was 4 to 35 months (11(6–24) months); eight patients had complete atrioventricular septal defect; eight had early postoperative PAP/SAP ≤ 0.35; and only one had major postoperative cardiopulmonary instabilities as described.
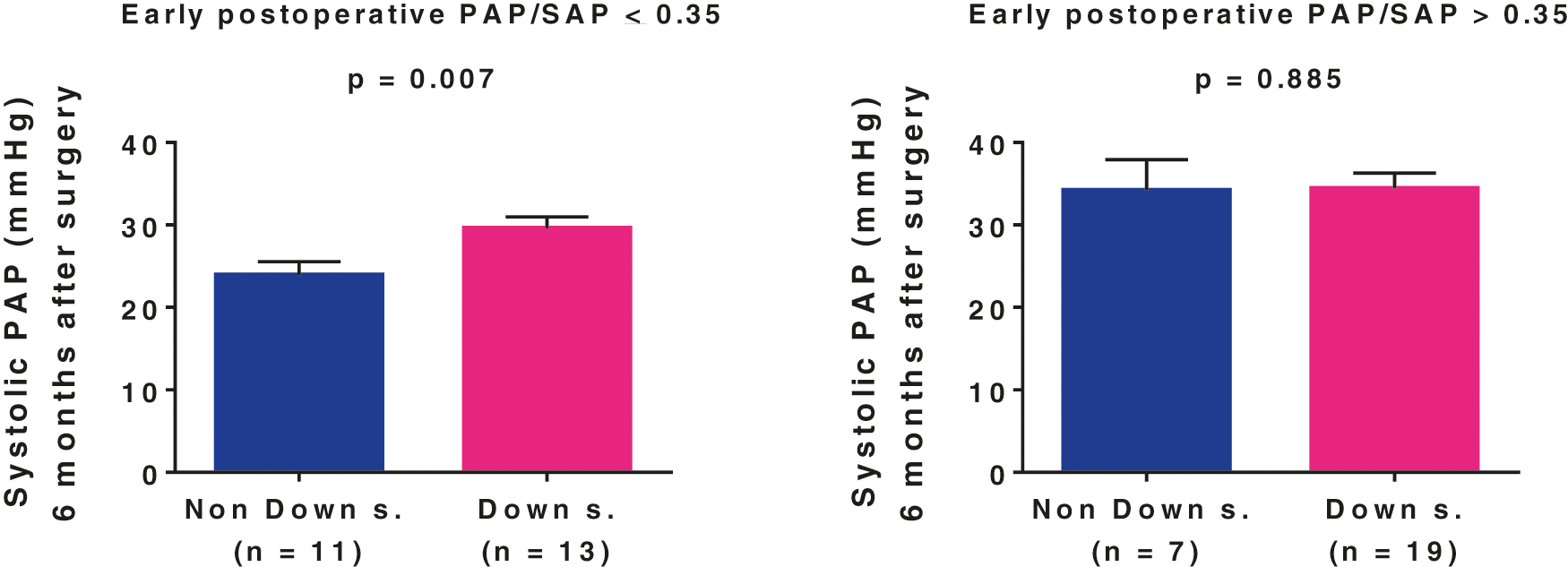
Figure 4: Role of early postoperative pulmonary/systemic mean arterial pressure ratio (PAP/SAP) and Down syndrome in determining pulmonary arterial systolic pressure 6 months after surgical repair of unrestrictive congenital cardiac communications
Data are presented as means with SE. Comparisons between subgroups was carried out using the general linear model after Box-Cox transformation of the dependent variable
In this study, we looked for possible predictors of abnormal pulmonary arterial pressure 6 months after surgical repair of congenital cardiac communications. In some sense, our results contrast with the common belief that young patients not presenting with signs of moderate to severe pulmonary hypertension and therefore not requiring preoperative cardiac catheterization or pulmonary vasodilator therapy generally have complete normalization of pulmonary hemodynamics postoperatively. In the present cohort, 25% of patients had a 6-month postoperative pulmonary arterial pressure greater than 35 mmHg. Five of them were above 40 mmHg with levels up to 54 mmHg. Identifying patients with mild elevation of pulmonary pressure after discharge from the hospital sounds like a good strategy for early diagnosis of postoperative pulmonary hypertension. In a previous series (unpublished observations), a young patient had systolic pulmonary arterial pressure levels of 37, 37, 38, 46 and 52 mmHg at 6, 12, 42, 72 and 84 months after surgery, respectively, thus requiring postoperative cardiac catheterization and specific therapeutic measures. In the present study, early postoperative PAP/SAP, as defined, was identified as an independent predictor of 6-month postoperative systolic pulmonary arterial pressure by univariate, bivariate and multivariate analyses. Down syndrome per se and other parameters that were assessed perioperatively did not remain in the predictive model using multivariate analysis.
To establish the study outcome, we assumed that a relationship does exist between systolic pulmonary artery pressure and mean pulmonary artery pressure. In fact, this relationship has been consistently demonstrated in most forms of pulmonary hypertension [20]. Using transthoracic echocardiography to look for a possible association between systolic and mean pulmonary artery pressure in pediatric subjects with congenital cardiac shunts (unpublished data), the regression equation we obtained (mPAP = 0.62 sPAP-3 mmHg, R2= 0.79, p < 0.001) was very similar to that reported for adults with pulmonary hypertension (mPAP = 0.61 sPAP + 2 mmHg) [21]. Postoperatively, we were able to estimate systolic pulmonary arterial pressure in all patients based on reliable flow curves of tricuspid valve regurgitation. Although the limits of the echocardiographic estimation of systolic pulmonary arterial pressure are widely known, we think that we had a favorable scenario for obtaining accurate data. First, no patients had pulmonary stenosis pre-or postoperatively. Second, residual right atrioventricular valve regurgitation is almost always present after repair of atrioventricular septal defects. Third, tricuspid valve leaflets are frequently desinserted and reinserted during the repair of ventricular septal defects, leading to residual tricuspid regurgitation in most patients.
By measuring post-CPB PAP/SAP and early postoperative PAP/SAP, which were significantly correlated, we were able to examine the behavior of the pulmonary circulation just after elimination of the cardiac shunt. Of course, parameters were still assessed in the presence of factors that could potentially affect pulmonary vascular tone, such as the presence of vasoconstrictor substances in circulation, post-CPB systemic inflammatory reaction, pre-and postoperative endothelial cell dysfunction and transient changes in pH and alveolar oxygen tension [22]. However, both post-CPB PAP/SAP and early postoperative PAP/SAP were obtained during nitric oxide administration, which was expected to reduce the effects of pulmonary vasoconstrictors [13,23]. Postoperative pulmonary hemodynamics is also influenced by preoperative factors, of which the severity of pulmonary vascular remodeling plays a pivotal role [24]. Furthermore, there have been studies supporting a role for genetic factors. Reports by Summar et al. [25] and Canter et al. [26] showed a relationship between postoperative elevation of pulmonary arterial pressure and genetic variations of the mitochondrial enzyme carbamyl-phosphate synthetase I. An amino acid change from threonine to asparagine at residue 1405 (within the binding site of enzyme cofactor N-acetylglutamate) is associated with changes in the bioavailability of arginine (hepatic urea cycle), which is the precursor for nitric oxide synthesis [27]. In another line of investigation, Loukanov and coworkers demonstrated an association between post-CPB elevation of pulmonary arterial pressure and the endothelial nitric oxide synthetase gene polymorphism Glu298Asp in children with congenital heart disease [28]. Based on then findings, we speculate that postoperative elevation of pulmonary arterial pressure, as observed in some of our patients, is not merely a result of the global response to surgery with the release of pulmonary vasoconstrictors but rather a phenotypic manifestation modulated by genes that need to be further studied and characterized. The association we observed between early postoperative, and 6-month postoperative pulmonary arterial pressure is alignment with this possibility.
There is general agreement that Down syndrome is associated with early development of pulmonary hypertension. Several disturbances have been pointed out as causes of pulmonary vascular abnormalities in subjects with Down syndrome, including abnormal lung development and pulmonary hypoplasia, airway obstruction and acquired lung disease, increased hemodynamic stress secondary to left-to-right intracardiac shunting, and endothelial dysfunction either related or unrelated to hemodynamic changes [6]. Genetic and proteomic components have also been identified, including increased expression of antiangiogenic factors encoded on chromosome 21 [29] and heightened circulating levels of specific proinflammatory cytokines, suggesting increased interferon signaling [30]. Although congenital heart disease is considered a major risk factor for developing pulmonary hypertension in this population, it is known that some patients do surprisingly well perioperatively and postoperatively. In the present study, 12 of 32 Down syndrome patients who were evaluated after hospital discharge had normal pulmonary arterial pressure according to the established criterion. It seems that patients with Down syndrome and congenital heart disease constitute a heterogeneous population in terms of pulmonary vasoreactivity and postoperative pulmonary hypertension. Further studies are required for a better characterization of patient subpopulations based on new methodologies for structural quantification of the pulmonary arterial tree, new blood biomarkers, genetic testing, and transcriptomic analysis of circulating cells [31]. Until then, preoperative hemodynamic evaluation and postoperative hemodynamic monitoring will continue providing data of potential prognostic value in terms of identifying patients at risk for pulmonary hypertension late after surgery.
In summary, we observed that even patients with moderate to severe pulmonary hypertension, i.e., those not requiring preoperative invasive diagnostic evaluation or pulmonary vasodilator therapy, may have abnormal levels of pulmonary arterial pressure after repair of cardiac communications and discharge from the hospital. Unsuspected systolic pressure levels greater than 40 mmHg may be found in some of them. In our cohort, the hypertensive behavior of the pulmonary circulation in the early postoperative period was tightly associated with Down syndrome. However, because it was not observed exclusively in syndromic individuals, it became a better predictor of elevated pulmonary arterial pressure after hospital discharge than Down syndrome itself. Importantly, postoperative hemodynamics was assessed invasively and with the use of inhaled nitric oxide. Therefore, our conclusions cannot be generalized. Down syndrome patients not presenting with significant changes in pulmonary hemodynamics early after surgery did not see to be at risk of developing pulmonary hypertension thereafter, although longer follow-up observations are necessary to confirm this hypothesis. Further studies are required for a better characterization of factors that determine the behavior of the pulmonary circulation pre-and postoperatively in this population.
Ethical Approval and Consent: The study protocol was approved by the Institutional Scientific and Ethics Committee–Heart Institute-InCor–University of São Paulo School of Medicine, São Paulo, Brazil, Approval No. 2.068.696.
Author Contribution: Eloisa Sassá Carvalho: Conceptualization, Methodology, Investigation, Data curation, Writing–original draft. Maria Francilene S. Souza: Conceptualization, Methodology, Investigation, Data curation, Writing–original draft. Kelly Cristina O. Abud, Claudia R. P. Castro and Juliano G. Penha: Methodology, Investigation, Data curation, Writing–original draft. Ana Maria Thomaz and Vanessa A. Guimarães: Investigation, Data curation, Writing–original draft. Antonio Augusto Lopes: Conceptualization, Funding acquisition, Methodology, Investigation, Project administration, Supervision, Formal analysis, Writing–review & editing.
Acknowledgement: We acknowledge the collaborative work of Pediatric Cardiologists and Cardiac Surgeons and all colleagues of the multiprofessional team involved in assisting patients at the Heart Institute (InCor), São Paulo, Brazil. We also express our gratitude to Mrs. Roseli Polo for her technical assistance in all phases of the study.
Funding Statement: This work was supported by FAPESP-Foundation for Research Support of the State of São Paulo, São Paulo, Brazil [Grant # 2015/21587-5].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Lindberg, L., Olsson, A. K., Jögi, P., Jonmarker, C. (2002). How common is severe pulmonary hypertension after pediatric cardiac surgery? Journal of Thoracic and Cardiovascular Surgery, 123(6), 1155–1163. DOI 10.1067/mtc.2002.121497. [Google Scholar] [CrossRef]
2. Giglia, T. M., Humpl, T. (2010). Preoperative pulmonary hemodynamics and assessment of operability: Is there a pulmonary vascular resistance that precludes cardiac operation? Pediatric Critical Care Medicine, 11, S57–S69. DOI 10.1097/PCC.0b013e3181d10cce. [Google Scholar] [CrossRef]
3. Myers, P. O., Tissot, C., Beghetti, M. (2014). Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circulation Journal, 78(1), 4–11. DOI 10.1253/circj.cj-13-1263. [Google Scholar] [CrossRef]
4. Kozlik-Feldmann, R., Hansmann, G., Bonnet, D., Schranz, D., Apitz, C. et al. (2016). Pulmonary hypertension in children with congenital heart disease (PAH-CHD, PPHVD-CHD). Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European paediatric pulmonary vascular disease network, endorsed by ISHLT and DGPK. Heart, 102(Suppl 2), ii42–ii48. DOI 10.1136/heartjnl-2015-308378. [Google Scholar] [CrossRef]
5. D’Alto, M., Mahadevan, V. S. (2012). Pulmonary arterial hypertension associated with congenital heart disease. European Respiratory Review, 21(126), 328–337. DOI 10.1183/09059180.00004712. [Google Scholar] [CrossRef]
6. Bush, D., Galambos, C., Dunbar Ivy, D. (2021). Pulmonary hypertension in children with Down syndrome. Pediatric Pulmonology, 56(3), 621–629. DOI 10.1002/ppul.24687. [Google Scholar] [CrossRef]
7. Rabinovitch, M. (2000). Pathobiology of pulmonary hypertension: Impact on clinical management. Seminars in Thoracic and Cardiovascular Surgery Pediatric Cardiac Surgery Annual, 3, 63–81. DOI 10.1053/tc.2000.6507. [Google Scholar] [CrossRef]
8. Lévy, M., Maurey, C., Celermajer, D. S., Vouhé, P. R., Danel, C. et al. (2007). Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. Journal of American College of Cardiology, 49(7), 803–810. DOI 10.1016/j.jacc.2006.09.049. [Google Scholar] [CrossRef]
9. Smadja, D. M., Gaussem, P., Mauge, L., Israël-Biet, D., Dignat-George, F. et al. (2009). Circulating endothelial cells: A new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation, 119(3), 374–381. DOI 10.1161/CIRCULATIONAHA.108.808246. [Google Scholar] [CrossRef]
10. van der Feen, D. E., Bossers, G. P. L., Hagdorn, Q. A. J., Moonen, J. R., Kurakula, K. et al. (2020). Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Science Translational Medicine, 12(554), 1–28. DOI 10.1126/scitranslmed.aaw4974. [Google Scholar] [CrossRef]
11. Wilkinson, J. L. (2001). Haemodynamic calculations in the catheter laboratory. Heart, 85(1), 113–120. DOI 10.1136/heart.85.1.113. [Google Scholar] [CrossRef]
12. Haworth, S. G., Hislop, A. A. (2009). Treatment and survival in children with pulmonary arterial hypertension: The UK pulmonary hypertension service for children 2001–2006. Heart, 95(4), 312–317. DOI 10.1136/hrt.2008.150086. [Google Scholar] [CrossRef]
13. Kaestner, M., Schranz, D., Warnecke, G., Apitz, C., Hansmann, G. et al. (2016). Pulmonary hypertension in the intensive care unit. expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. the european paediatric pulmonary vascular disease network, endorsed by ISHLT and DGPK. Heart, 102(Suppl 2), ii57–ii66. DOI 10.1136/heartjnl-2015-307774. [Google Scholar] [CrossRef]
14. Thomaz, A. M., Kajita, L. J., Aiello, V. D., Zorzanelli, L., Galas, F. R. et al. (2019). Parameters associated with outcome in pediatric patients with congenital heart disease and pulmonary hypertension subjected to combined vasodilator and surgical treatments. Pulmonary Circulation, 9(3), 1–13. DOI 10.1177/2045894019837885. [Google Scholar] [CrossRef]
15. Ribeiro, Z. V., Tsutsui, J. M., Miranda, R., Mohry, S., Mathias, W. et al. (2010). Ecocardiografia-Doppler e parâmetros hemodinâmicos em cardiopatias congênitas com hiperfluxo pulmonar [Doppler echocardiography and hemodynamic parameters in congenital heart disease with increased pulmonary flow]. Arquivos Brasileiros de Cardiologia, 94(5), 592–600. DOI 10.1590/S0066-782X2010005000042. [Google Scholar] [CrossRef]
16. R Rivera, I. R., Mendonça, M. A., Andrade, J. L., Moises, V., Campos, O. et al. (2013). Pulmonary venous flow index as a predictor of pulmonary vascular resistance variability in congenital heart disease with increased pulmonary flow: A comparative study before and after oxygen inhalation. Echocardiography, 30(8), 952–960. DOI 10.1111/echo.12163. [Google Scholar] [CrossRef]
17. Gaies, M. G., Jeffries, H. E., Niebler, R. A., Pasquali, S. K., Donohue, J. E. et al. (2014). Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: An analysis from the pediatric cardiac critical care consortium and virtual PICU system registries. Pediatric Critical Care Medicine, 15(6), 529–537. DOI 10.1097/PCC.0000000000000153. [Google Scholar] [CrossRef]
18. Kolte, D., Lakshmanan, S., Jankowich, M. D., Brittain, E. L., Maron, B. A. et al. (2018). Mild pulmonary hypertension is associated with increased mortality: A systematic review and meta-analysis. Journal of the American Heart Association, 7(18), e009729. DOI 10.1161/JAHA.118.009729. [Google Scholar] [CrossRef]
19. Rosenzweig, E. B., Abman, S. H., Adatia, I., Beghetti, M., Bonnet, D. et al. (2019). Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. The European Respiratory Journal, 53(1), 1801916. DOI 10.1183/13993003.01916-2018. [Google Scholar] [CrossRef]
20. Chemla, D., Humbert, M., Sitbon, O., Montani, D., Hervé, P. (2015). Systolic and mean pulmonary artery pressures: Are they interchangeable in patients with pulmonary hypertension? Chest, 147(4), 943–950. DOI 10.1378/chest.14-1755. [Google Scholar] [CrossRef]
21. Chemla, D., Castelain, V., Humbert, M., Hébert, J. L., Simonneau, G. et al. (2004). New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest, 126(4), 1313–1317. DOI 10.1378/chest.126.4.1313. [Google Scholar] [CrossRef]
22. Adatia, I. (2011). Pulmonary hypertension and postoperative congenital heart disease. In: Beghetti, M. et al. (editorsPediatric Pulmonary Hypertension, pp. 209–232. Munich: Elsevier Urban & Fischer. [Google Scholar]
23. Miller, O. I., Tang, S. F., Keech, A., Pigott, N. B., Beller, E. et al. (2000). Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: A randomised double-blind study. Lancet, 356(9240), 1464–1469. DOI 10.1016/S0140-6736(00)02869-5. [Google Scholar] [CrossRef]
24. Rabinovitch, M. (1999). Pulmonary hypertension: Pathophysiology as a basis for clinical decision making. The Journal of Heart and Lung Transplantation, 18(11), 1041–1053. DOI 10.1016/S1053-2498(99)00015-7. [Google Scholar] [CrossRef]
25. Summar, M. L., Hall, L., Christman, B., Barr, F., Smith, H. et al. (2004). Environmentally determined genetic expression: Clinical correlates with molecular variants of carbamyl phosphate synthetase I. Molecular Genetics and Metabolism, 81(Suppl 1), S12–S19. DOI 10.1016/j.ymgme.2003.11.014. [Google Scholar] [CrossRef]
26. Canter, J. A., Summar, M. L., Smith, H. B., Rice, G. D., Hall, L. D. et al. (2007). Genetic variation in the mitochondrial enzyme carbamyl-phosphate synthetase I predisposes children to increased pulmonary artery pressure following surgical repair of congenital heart defects: A validated genetic association study. Mitochondrion, 7(3), 204–210. DOI 10.1016/j.mito.2006.11.001. [Google Scholar] [CrossRef]
27. Pearson, D. L., Dawling, S., Walsh, W. F., Haines, J. L., Christman, B. W. et al. (2001). Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. The New England Journal of Medicine, 344(24), 1832–1838. DOI 10.1056/NEJM200106143442404. [Google Scholar] [CrossRef]
28. Loukanov, T., Hoss, K., Tonchev, P., Klimpel, H., Arnold, R. et al. (2011). Endothelial nitric oxide synthase gene polymorphism (glu298asp) and acute pulmonary hypertension post cardiopulmonary bypass in children with congenital cardiac diseases. Cardiology in the Young, 21(2), 161–169. DOI 10.1017/S1047951110001630. [Google Scholar] [CrossRef]
29. Galambos, C., Minic, A. D., Bush, D., Nguyen, D., Dodson, B. et al. (2016). Increased lung expression of anti-angiogenic factors in down syndrome: Potential role in abnormal lung vascular growth and the risk for pulmonary hypertension. PLoS One, 11(8), e0159005. DOI 10.1371/journal.pone.0159005. [Google Scholar] [CrossRef]
30. Sullivan, K. D., Evans, D., Pandey, A., Hraha, T. H., Smith, K. P. et al. (2017). Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Scientific Reports, 7(1), 14818. DOI 10.1038/s41598-017-13858-3. [Google Scholar] [CrossRef]
31. van der Feen, D. E., Bartelds, B., de Boer, R. A., sBerger, R. (2019). Assessment of reversibility in pulmonary arterial hypertension and congenital heart disease. Heart, 105(4), 276–282. DOI 10.1136/heartjnl-2018-314025. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |