

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019065
ARTICLE
Coronary Artery Complications after Right Ventricular Outflow Tract Reconstruction Surgery
1Department of Pediatrics, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, South Korea
2Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, South Korea
3Department of Radiology, Seoul National University Hospital, Seoul, South Korea
*Corresponding Author: Eun Jung Bae. Email: eunjbaek@snu.ac.kr
Received: 01 September 2021; Accepted: 20 December 2021
Abstract: Background: Mechanisms and clinical manifestations of coronary artery complications after right ventricular outflow tract reconstruction surgery are not well known. Methods: Patients who had coronary artery complications after pulmonary valve replacement or the Rastelli procedure at a single tertiary centre were retrospectively analysed. Results: Coronary artery complications were identified in 20 patients who underwent right ventricular outflow tract reconstruction surgery. The median age at diagnosis of coronary artery complication was 21 years (interquartile range: 13–25 years). Mechanisms of coronary artery complications were compression by adjacent materials in 12 patients, dynamic compression of intramural course of coronary artery in two patients, and intraoperative injury in six patients. Congenital coronary artery anomalies were identified in 50% (10/20) of patients. Four patients presented with early postoperative haemodynamic instability. Fourteen patients showed late onset symptoms or signs of coronary insufficiency, including chest pain, ventricular dysfunction, or ventricular arrhythmias. Coronary artery stenosis was incidentally found on cardiac computed tomography angiography in two asymptomatic patients. Four patients underwent surgical interventions, and one patient underwent percutaneous coronary intervention for coronary stenosis. One patient with recurrent ventricular tachycardia required an implantable cardioverter-defibrillator. There were two deaths in patients with intraoperative coronary injury. Conclusion: Preoperative coronary evaluation and long-term follow-up for the development of coronary artery complications are required in patients undergoing right ventricular outflow tract reconstruction surgery to prevent ventricular dysfunction, arrhythmias, and death, especially among those with congenital coronary anomalies.
Keywords: Congenital heart disease; right ventricular outflow tract reconstruction surgery; coronary artery disease
After repair of tetralogy of Fallot, patients with significant pulmonary regurgitation often require pulmonary valve replacement (PVR) [1]. In other conotruncal anomalies, such as pulmonary atresia with ventricular septal defect (VSD), truncus arteriosus, double outlet right ventricle and transposition of great arteries with pulmonary stenosis, the Rastelli procedure, which includes a VSD closure and extracardiac conduit interposition between the right ventricle (RV) and pulmonary artery (PA), is performed [2,3]. In patients undergoing such right ventricular outflow tract (RVOT) reconstruction surgery, mortality has been reported to range from 1.2% to 8.5%, and reoperation due to a prosthetic valve or conduit failure has been reported in 7.1%–51% [1–4]. It is also known that an increase in the number of sternotomies is associated with an increase in operative mortality [5,6]. Therefore, strategies should be developed to reduce the mortality rates in patients undergoing multiple RVOT reconstruction surgeries.
Coronary artery complications (CAC) after RVOT reconstruction surgery, which may be one of the causes of postoperative mortality, have been overlooked and there have been only two case reports [7,8]. Therefore, this study was conducted to raise awareness of early diagnosis of CAC and to improve outcomes by investigating the mechanism and clinical manifestations of CAC after RVOT reconstruction surgery in 20 patients. An initial presentation of this study was made on 19 patients [9]; however, another patient with CAC was later identified.
2.1 Study Design and Participants
This retrospective analysis complies with the ethical guidelines of the 1975 Declaration of Helsinki, and it was approved by the Institutional Review Board of Seoul National University Hospital (date of approval: July 4th, 2019; Number: H-1905-078-1033). The requirement for informed consent was waived due to the retrospective study design and the absence of any clinical intervention performed.
We identified patients with congenital heart disease (CHD) who underwent PVR or the Rastelli procedure from March 1987 to February 2019 at Seoul National University Children’s Hospital. Among these patients, those with more than 50% of coronary artery stenosis or coronary occlusion on cardiac computed tomography angiography (CTA) and/or invasive coronary angiography (ICA) were finally enrolled for analysis. Because coronary artery stenosis after coronary artery transfer surgery is a well-known complication [10–12], we excluded patients who previously underwent arterial switch operation or Ross operation.
We collected patients’ demographic data and medical history, including diagnosis of CHD, number of previous open cardiac surgeries, and type and size of prosthetic valve or conduit. Patients’ symptoms, such as chest pain, dyspnoea on exertion, palpitation, or cardiac arrest were evaluated. Resting, exercise, and ambulatory electrocardiograms (ECG) were analysed to identify ventricular tachycardia (VT), newly developed ST segment changes, or pathological Q-waves [13]. We reviewed echocardiography to identify newly developed systolic ventricular failure, regional wall motion abnormalities (RWMA), and increased myocardial echogenicity in the territory of the affected coronary artery [14]. In patients who underwent cardiac magnetic resonance imaging (CMR), we assessed global and regional ventricular function and late gadolinium enhancement of myocardium [13]. Persistent or reversible myocardial perfusion defects were identified in patients who underwent myocardial single-photon emission computed tomography (SPECT) [15].
Categorical variables were reported in frequency and percentage. Continuous variables were presented as median and interquartile range (IQR).
Of the 544 patients who underwent PVR or the Rastelli procedure from March 1987 to February 2019 (32 years), CAC was diagnosed in 20 patients (3.7%) following RVOT reconstruction surgery. All patients’ demographic data, diagnoses and treatment for the underlying CHDs are described in Table 1. The median age of the patients at the time of the study was 25 years (IQR, 18-27 years), except two patients died. The median number of previous open cardiac surgeries and RVOT reconstruction surgeries before diagnosis of CAC were 3 (IQR, 2–4) and 2 (IQR, 1–3), respectively.
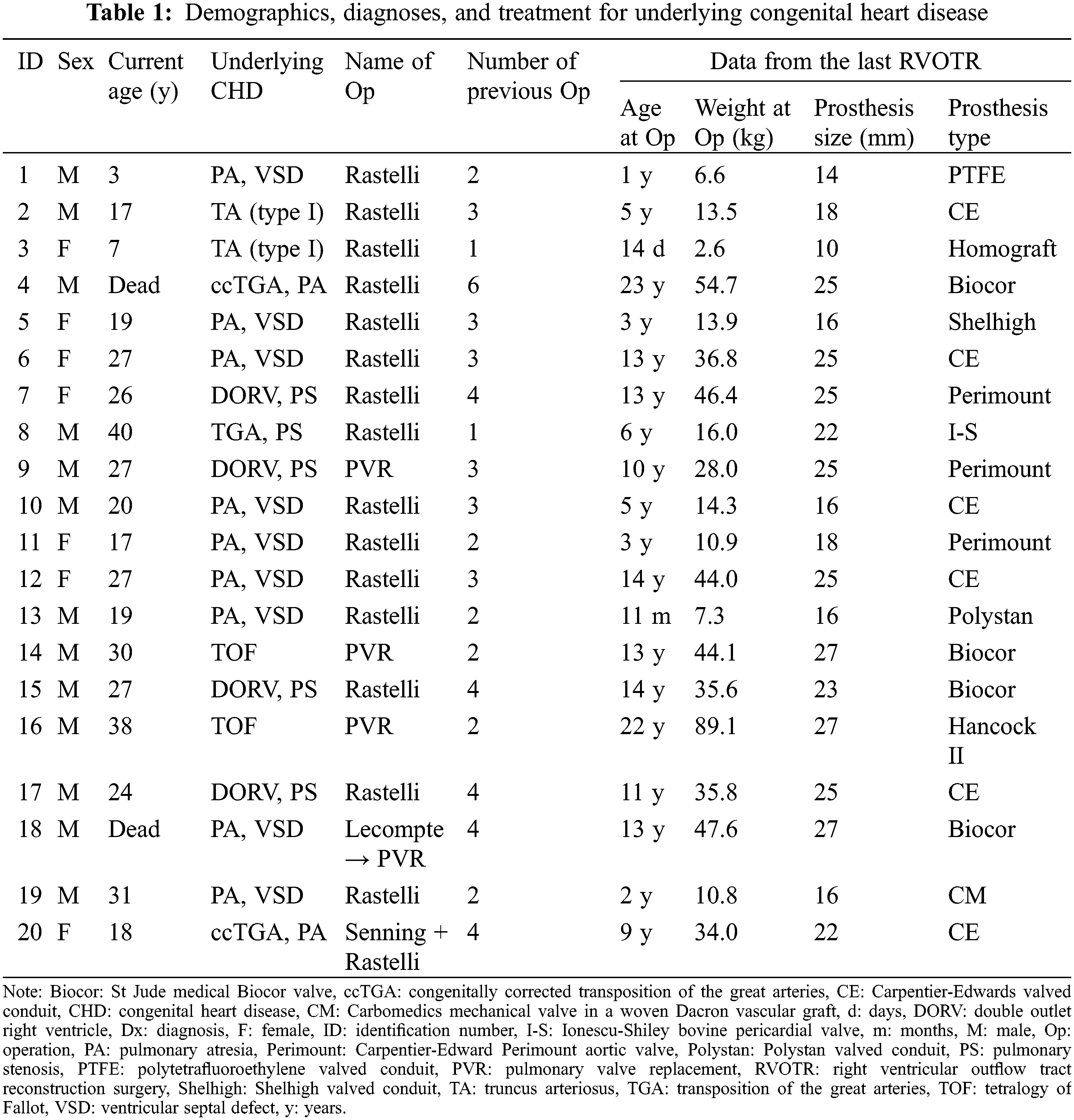
Mechanisms of CAC were compression by an adjacent RVOT patch, conduit, or prosthetic valve in 12 patients; dynamic compression of intramural course of the coronary artery in two patients; and intraoperative injury in six patients. The right coronary artery (RCA) was involved in ten patients; left anterior descending coronary artery (LAD) in eight patients; and left main coronary artery (LCA) in two patients. The types of CAC were classified according to the mechanism of CAC, involved coronary artery, and presence of coronary artery anomaly (Table 2 and Fig. 1).
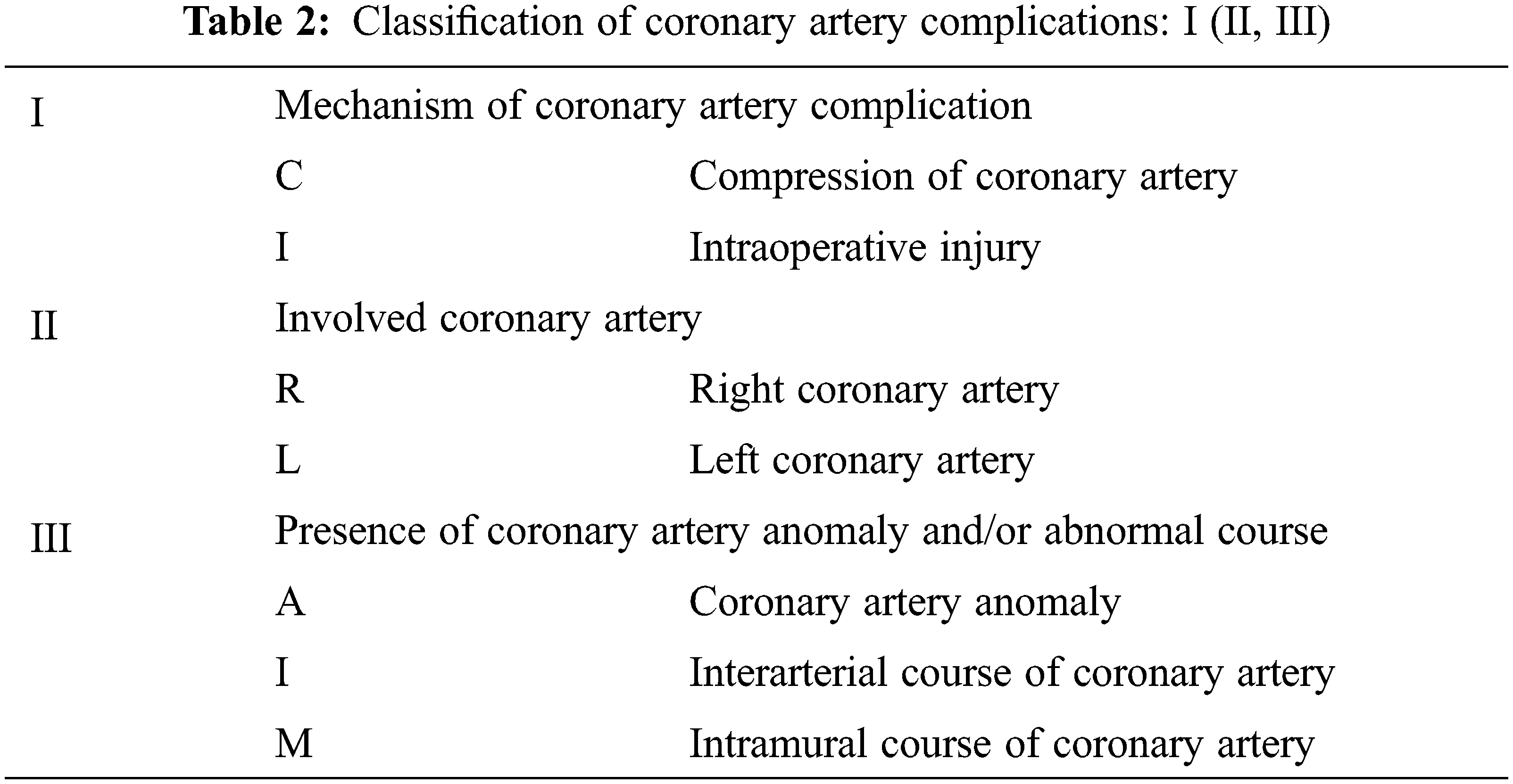
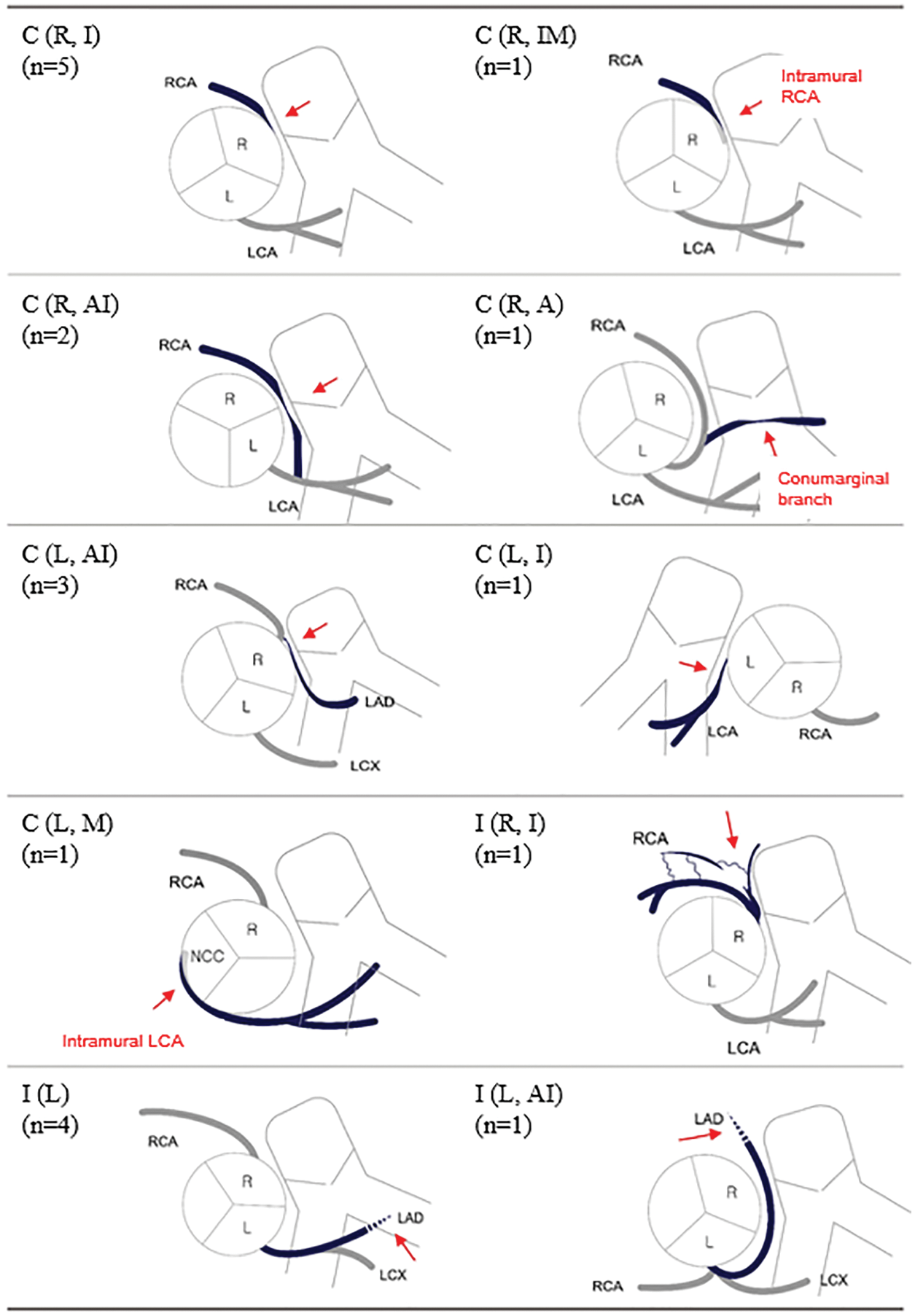
Figure 1: Schematic diagrams of coronary artery complications after right ventricular outflow reconstruction surgery. Red arrows indicate areas of compressed or damaged coronary arteries
Note: L: left coronary cusp, LAD: left anterior descending coronary artery, LCA: left main coronary artery, LCX: left circumflex coronary artery, n: number of patients, NCC: non-coronary cusp, R: right coronary cusp, RCA: right coronary artery. *The types of coronary artery complications were classified according to the rules described in Table 2.
3.3 Clinical Manifestations of CAC
The clinical features of patients were divided into the following three categories according to the presence of symptoms or signs of coronary insufficiency and the timing of their onset (Table 3). The median age at diagnosis of CAC was 21 years (IQR, 13–25 years). The median time to diagnosis of CAC from the last RVOT reconstruction surgery was 9 years (IQR, 3–12 years). Congenital coronary anomaly was identified in half of patients with CAC.
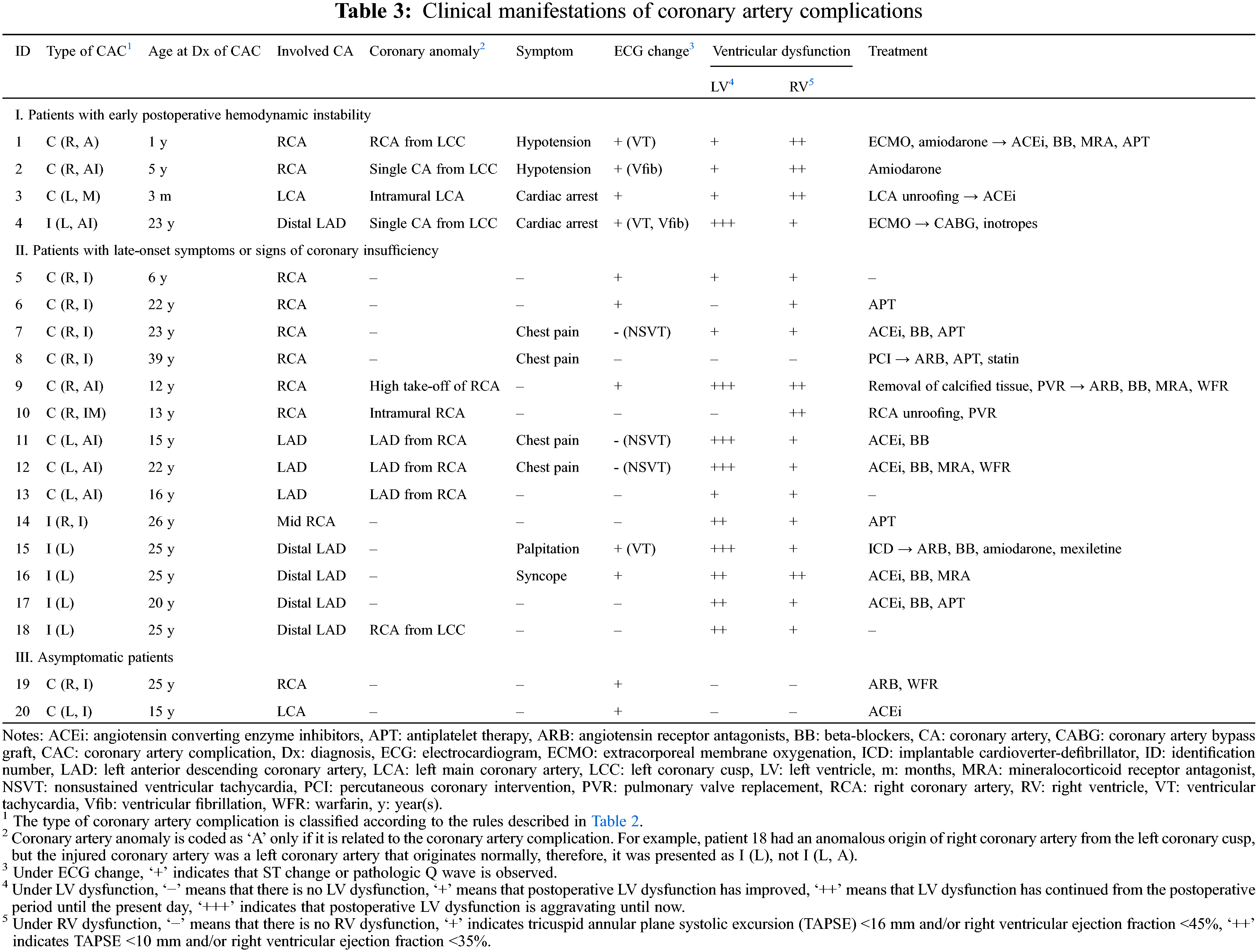
3.3.1 Patients with Early Postoperative Haemodynamic Instability
Three patients (patients 1, 2, and 4) experienced ventricular arrhythmias within postoperative day 3, and two of them needed extracorporeal membrane oxygenation support. In patients 1 and 2, the RCA was temporarily compressed by RV-to-PA conduit immediately after the operation; however, the tissue oedema around the conduit improved over time, prompting resolution of RCA compression without surgical intervention. Patient 4 was a 23-year-old man, who experienced intraoperative cardiac arrest during the 3rd RV-PA conduit change surgery. Postoperative echocardiography showed left ventricular (LV) dilatation, wall thinning, and severe dysfunction (ejection fraction [EF]: 15%). On the 3rd postoperative day, ICA confirmed LAD injury, and on-pump beating coronary artery bypass graft surgery was performed. However, he had recurrent VT and ventricular fibrillation, and eventually died due to brain injury.
Another infant (patient 3), who underwent Rastelli operation for truncus arteriosus at the age of 14 days, experienced cardiac arrest two months after the surgery. An ECG before the arrest showed ST depressions on lead V1–6 and ST elevations in lead III and aVF (Fig. 2A). Echocardiography showed progressing LV outflow tract obstruction (peak pressure gradient; 85 mmHg), LV dysfunction (EF 40%), and increased myocardial echogenicity of the interventricular septum (Fig. 2B). She underwent LV outflow tract obstruction relief surgery and unroofing of intramural course of the LAD, which was discovered in the operating room. After the surgery, ST changes normalised and LV dysfunction improved (Figs. 2C and 2D).
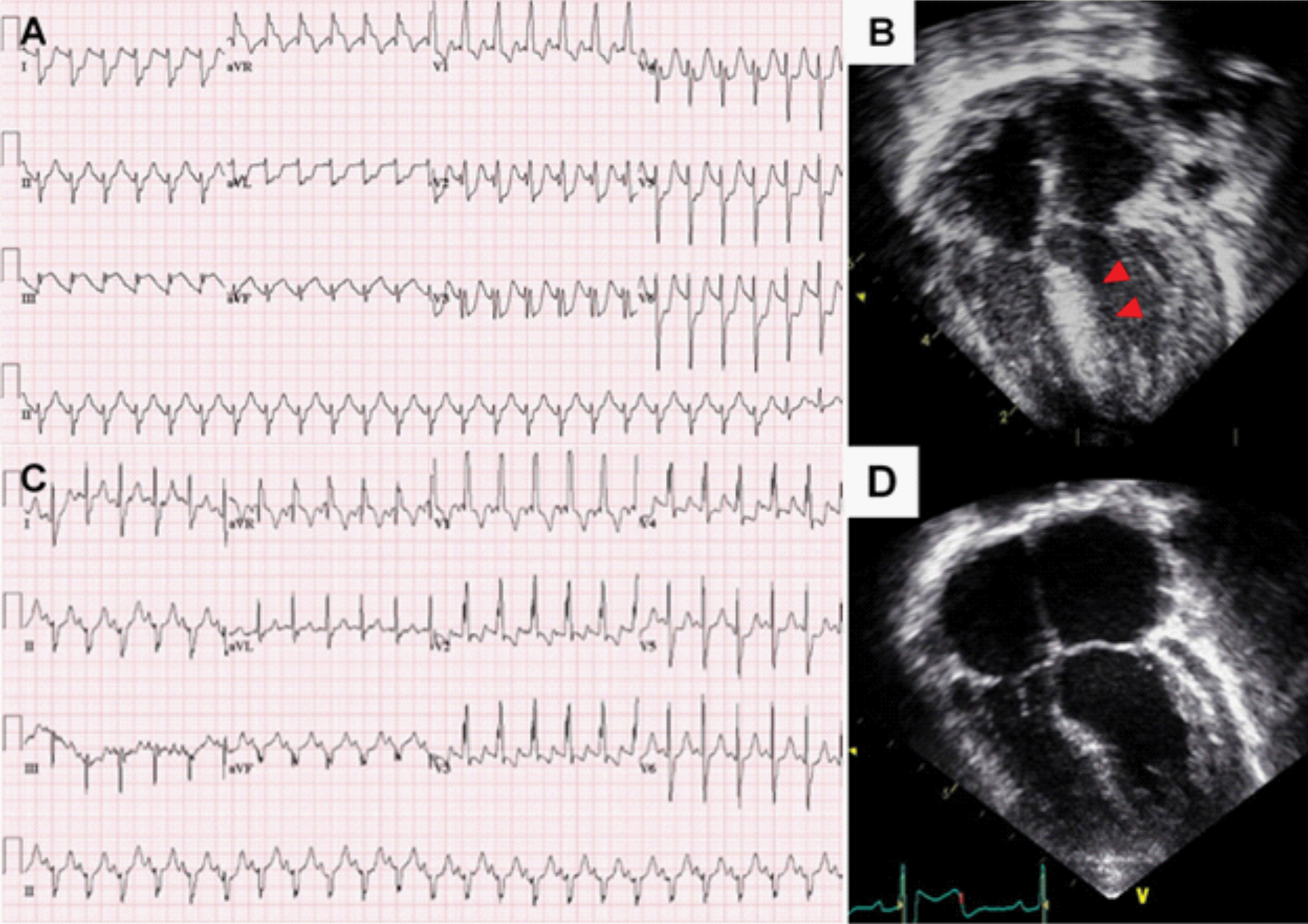
Figure 2: Acute coronary event in patient 3 who underwent Rastelli procedure for truncus arteriosus
Note: (A) Electrocardiogram before cardiac arrest showed ST depressions on lead V1–6 and ST elevations in lead III and aVF. (B) Echocardiography after cardiac arrest showed increased septal echogenicity due to myocardial ischemia. (C) After unroofing of intramural course of left coronary artery, ST changes were normalised. (D) Before discharge, echocardiography showed normalized interventricular septal wall echogenicity.
3.3.2 Patients with Late-Onset Symptoms or Signs of Coronary Insufficiency
Thirteen patients showed ventricular dysfunction on echocardiography or CMR at median 3.5 years after the RVOT reconstruction surgery; however, the median time to diagnosis of CAC was 10 years after the RVOT reconstruction surgery. Among the patients with ventricular dysfunction, one patient (patient 18) who had distal LAD injury and interarterial course of RCA experienced sudden death one year after diagnosis of CAC. The last follow-up echocardiography showed mild LV dysfunction (EF 45%) with decreased apical wall motion and CMR showed delayed enhancement of LV anteroinferior wall. However, he had been observed for CAC without any medical or surgical intervention since he was in a good functional class. Unfortunately, the patient died at the age of 26 years at another hospital and the cause of death could not be found. Another patient (patient 15) was admitted due to sustained VT, 11 years after the second PVR. Echocardiography showed severe LV dysfunction (EF 20%) with increased echogenicity of the interventricular septum (Fig. 3A). CMR and myocardial SPECT suggested myocardial infarction in the LAD territory (Figs. 3B and 3C). Although coronary CTA showed normal proximal coronary arteries, ICA revealed significant mid-LAD hypoplasia and scarce septal branches. He required an implantable cardioverter-defibrillator (ICD) due to recurrent VT and persistent LV dysfunction.
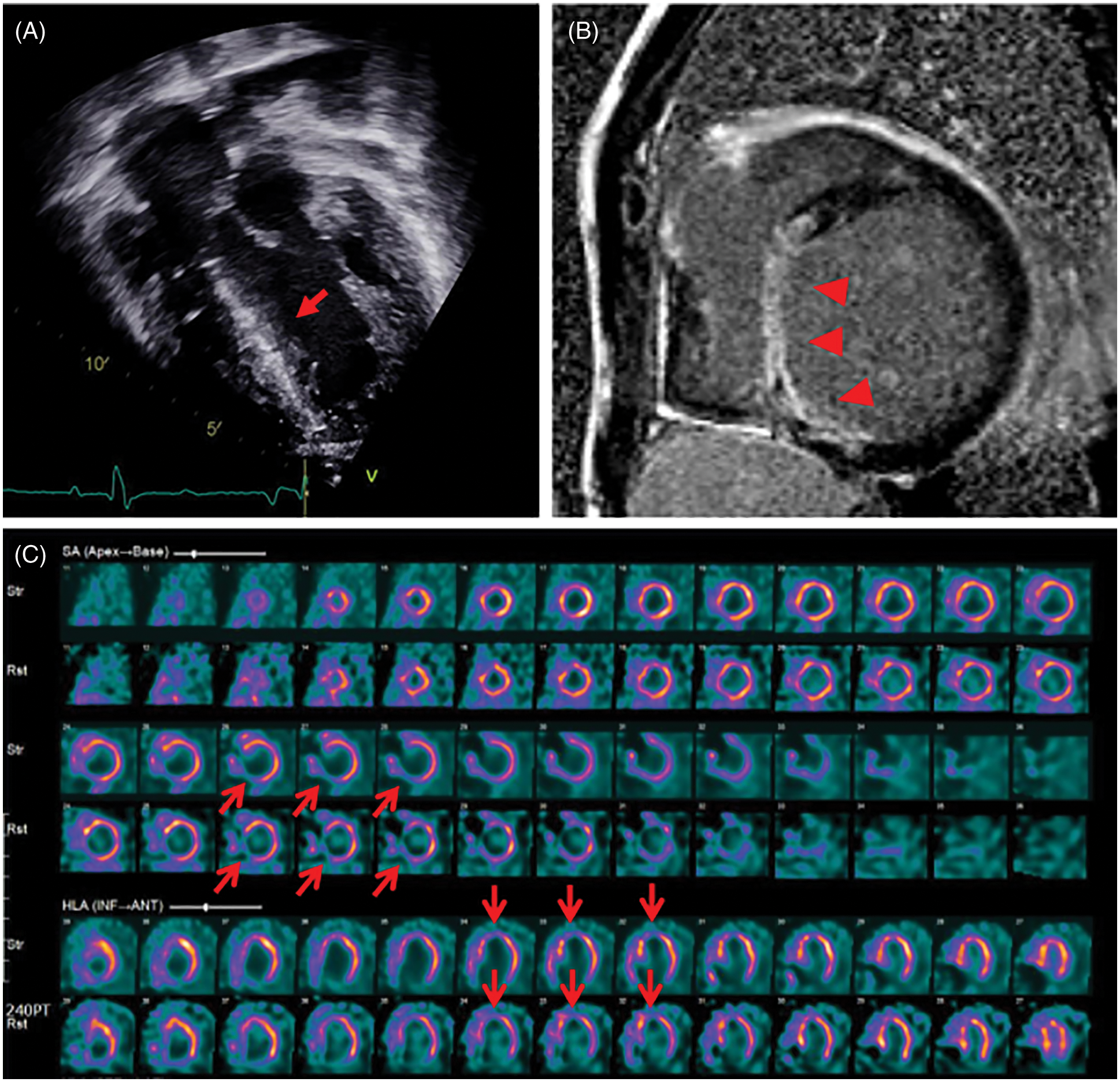
Figure 3: Lately detected myocardial perfusion defect in patient 15 who underwent Rastelli procedure for double outlet right ventricle with pulmonary stenosis
Note: (A) Echocardiography showing increased echogenicity of the interventricular septum (red arrow). (B) Cardiac magnetic resonance imaging shows diffuse transmural delayed enhancement (red arrow heads) in the basal to apical inferoseptal and inferior wall. (C) Myocardial single-photon emission computed tomography shows a persistent perfusion defect on stress and rest in the mid-basal inferoseptal and basal anteroseptal wall (red open arrows).
Four patients complained of atypical chest pain, of whom three had ventricular dysfunction and non-sustained VT on Holter monitoring. The other one patient was a 39-year-old man (patient 8) who visited the emergency department with atypical chest pain at 33 years after the RVOT reconstruction surgery. Even though ECG showed no ST-T change, echocardiography revealed normal ventricular function, and cardiac enzymes were within normal ranges; ICA showed severe RCA ostial stenosis adjacent to the conduit and aneurysmal dilatation of proximal RCA (Fig. 4A). Percutaneous coronary balloon angioplasty and drug-eluting stent insertion was performed with Resolute Onyx™ (Medtronic Inc., Santa Rosa, California, USA) 2.75 × 12 mm, and the stenosis was improved (Fig. 4B).
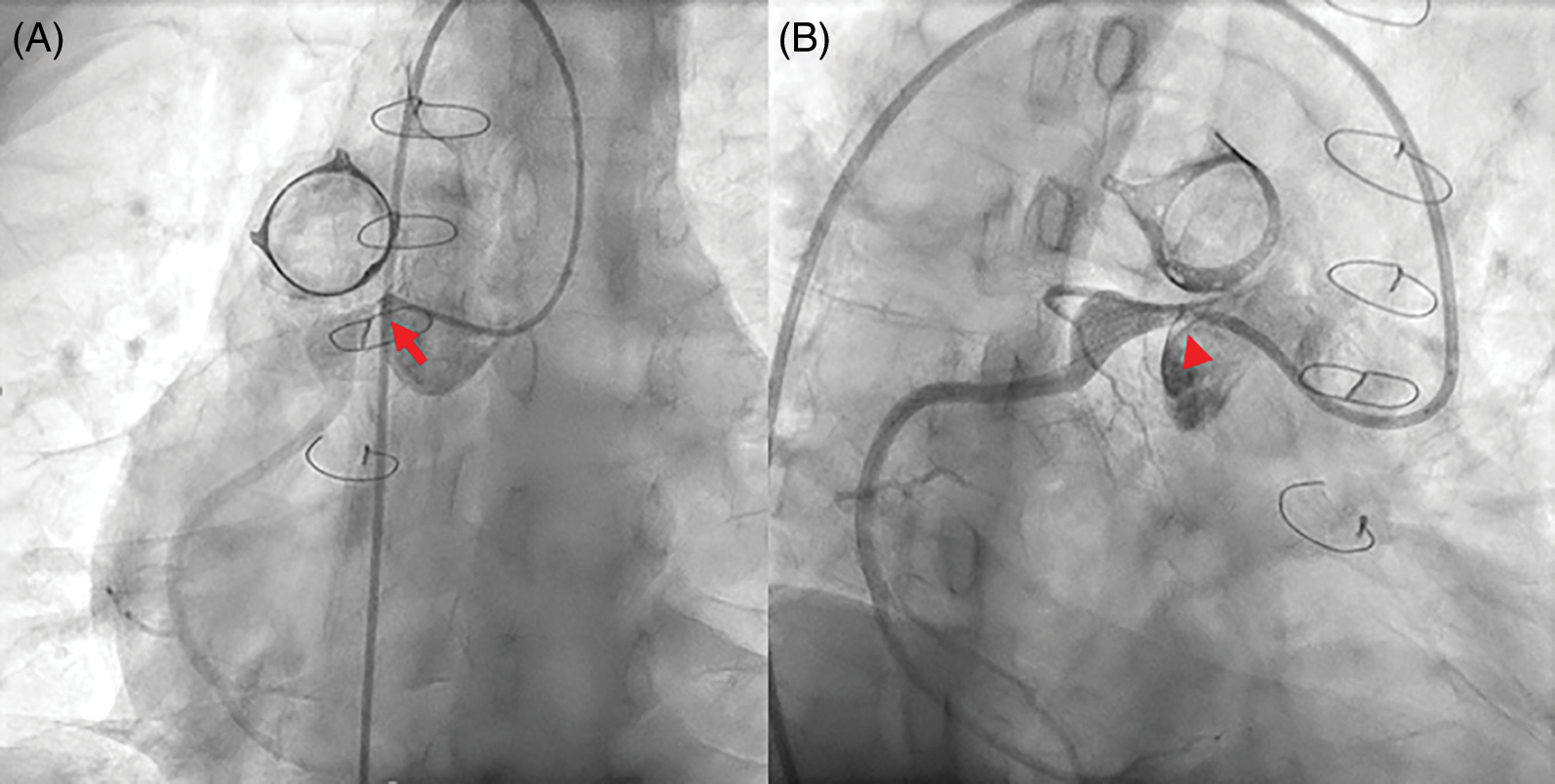
Figure 4: Percutaneous coronary intervention in patient 8 who performed Rastelli procedure for transposition great arteries and pulmonary stenosis.
Note: (A) Selective right coronary angiography showing severe right coronary artery ostial stenosis (red arrow) adjacent to the conduit and aneurysmal dilatation of the proximal portion. (B) After percutaneous coronary intervention, the stenosis is improved (red arrowhead).
In two patients (patients 19 and 20), coronary artery stenosis was incidentally detected in cardiac CTA. They did not have any symptoms, ventricular dysfunction, or decrease in perfusion upon myocardial SPECT. However, retrospective ECG analysis revealed ST depressions in inferolateral leads on exercise ECG in patient 19, and transient ST depressions in lateral precordial leads on postoperative day 1 ECG in patient 20.
Four patients underwent surgical treatment for the CAC. In addition to the previously mentioned two patients (patients 3 and 4), patient 9 underwent surgical removal of calcified tissue during the fourth PVR and patient 10 underwent unroofing of intramural course of the RCA during the third PVR. In patient 9, a postoperative cardiac CTA showed improved RCA stenosis (Figs. 5A and 5B); however, CMR performed at 4 years postoperatively showed no improvement of RV dysfunction (RV EF: 29.4%) and RWMA in the RV inferior and anterior wall.
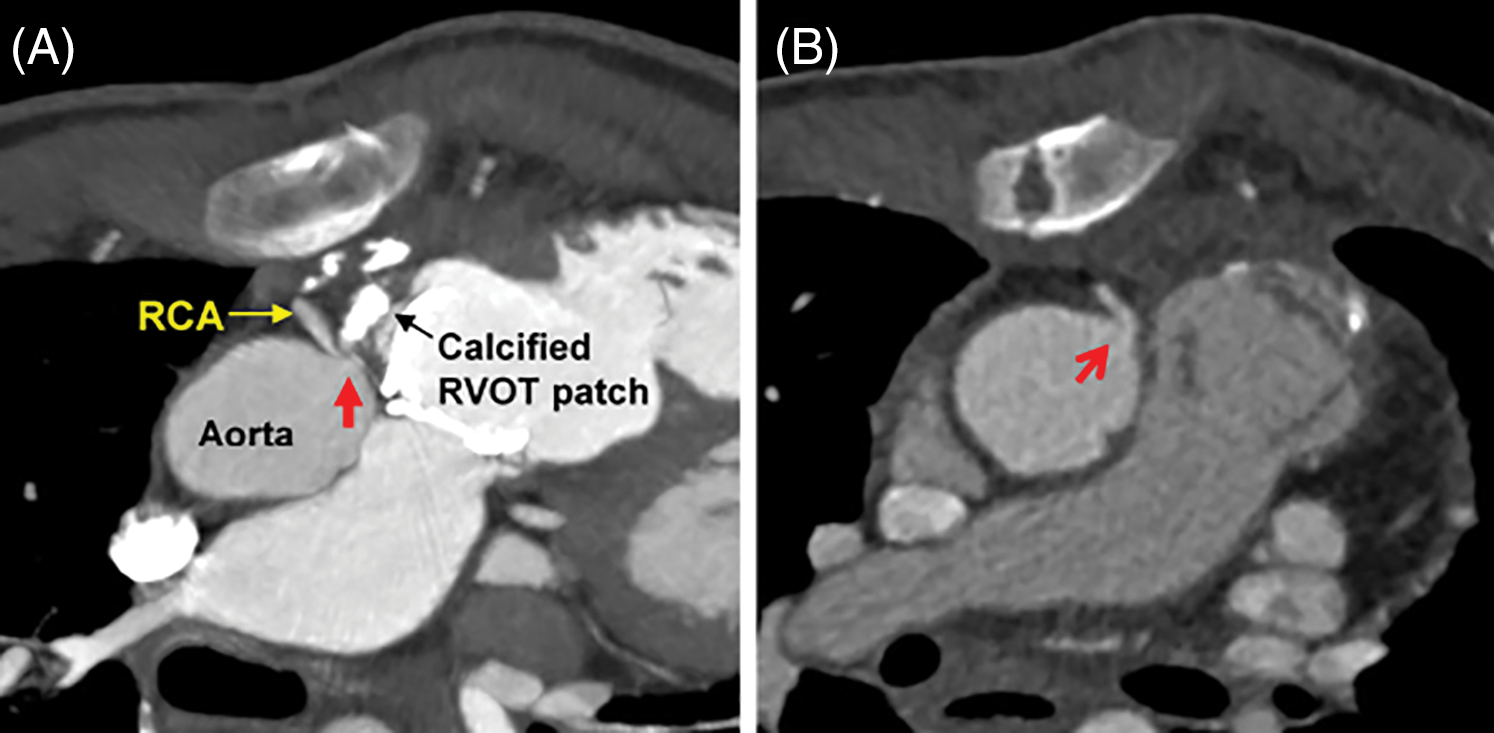
Figure 5: Surgical intervention in patient 9, who had undergone pulmonary valve replacement for double outlet right ventricle and pulmonary stenosis
Note: (A) Cardiac computed tomography angiography showing the ostium of the right coronary artery compressed by the adjacent calcified patch (red arrow). (B) After removal of calcified tissue and replacement with a new prosthetic valve, the coronary compression was relieved (red open arrow). RCA: right coronary artery, RVOT: right ventricular outflow tract.
Including two patients who underwent interventional and surgical treatment for CAC (patients 8 and 9), and the patient with ICD implantation (patient 15), a total twelve patients had been taking medication for heart failure. Six patients were prescribed anti-platelet drugs, and three patients were taking warfarin (Table 3). The clinical courses of all patients are summarized in Fig. 6.
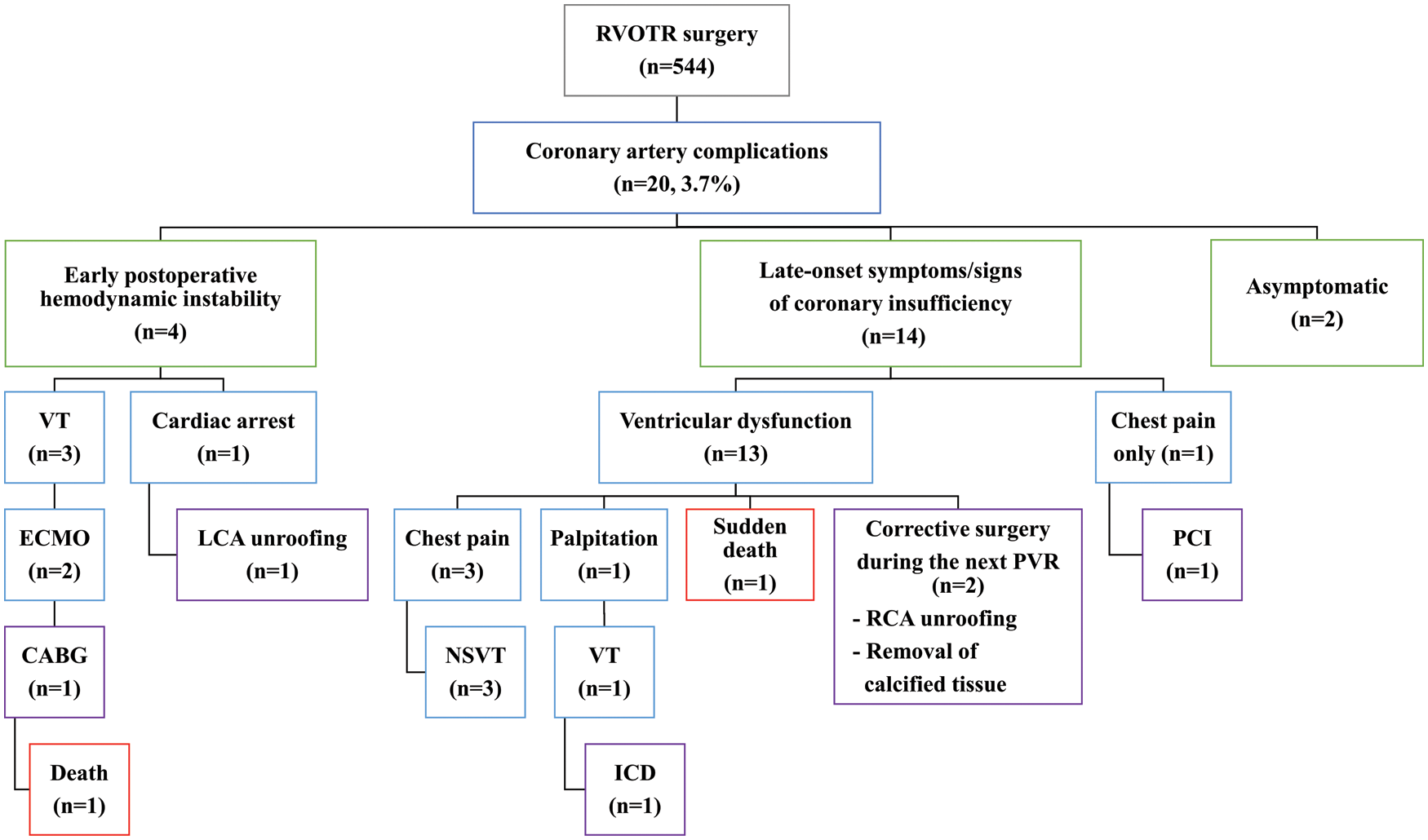
Figure 6: A summary of clinical courses of all patients with coronary artery complications after right ventricular outflow tract reconstruction surgery
Note: CABG: coronary artery bypass graft, ECMO: extracorporeal membrane oxygenation. LCA: left main coronary artery, ICD: implantable cardioverter defibrillator, NSVT: nonsustained ventricular tachycardia, PCI: percutaneous coronary intervention, PVR: pulmonary valve replacement, RCA: right coronary artery, RVOTR: right ventricular outflow tract reconstruction surgery, VT: ventricular tachycardia.
Following RVOT reconstruction surgery, CAC had developed at a younger age than typical coronary artery disease (CAD). CAC after RVOT reconstruction surgery have different mechanisms compared with typical CAD, which is caused by the accumulation of atherosclerotic plaque in the coronary arteries. A calcified conduit or prosthetic valve and anatomically narrow space between the aorta and RVOT can cause coronary artery stenosis, kinking, or occlusion after RVOT reconstruction surgery. The risk of the CAC can be increased in patients with coronary artery anomaly. Moreover, multiple previous open cardiac surgeries can pose a risk of intraoperative coronary injury during the RVOT reconstruction surgery. However, current clinical practice guidelines of adult CHD do not cover this problem, and indications of imaging and functional evaluation are not established in the follow-up of patients after RVOT reconstruction surgery [16].
Coronary artery malformations are frequently associated with conotruncal anomalies [17,18]. Further, the position of the coronary orifices on the aortic/truncal circumference varies according to the degree of rotation of the outflow tract [19]. Therefore, in all patients undergoing RVOT reconstruction surgery for conotruncal anomalies, it is necessary to confirm the presence of coronary anomaly and the course of the coronary artery before the surgery. If a coronary anomaly is suspected in echocardiography, coronary CTA should be performed before the initial RVOT reconstruction surgery to prevent CAC. Particularly, an anomalous aortic origin of the coronary artery (AAOCA) with an inter-arterial course is associated with myocardial ischaemia, arrhythmia, syncope, and sudden death, as it is prone to dynamic compression during physical exercise [20–22]. AAOCA with intramural course, where the proximal part of the anomalous vessel embedded in the aortic tunica media, is the most threatening feature [22]. If these malignant features are diagnosed by preoperative imaging or confirmed in the operating room, concomitant surgical correction could be performed upon RVOT reconstruction. In case of an intramural course of the coronary artery, coronary unroofing corrects the mechanism of dynamic, lateral phasic, systolic compression of the arterial wall [22]. In patients with AAOCA without intramural course or a single coronary artery, changing the position of the PA can prevent postoperative coronary compression [22].
After RVOT reconstruction surgery, patients with chest pain, ventricular dysfunction, or ventricular arrhythmias require exclusion of coronary problems. However, only four patients complained of atypical chest pain at the time of diagnosis of CAD in this study. Based on our institutional experience, even in asymptomatic patients, yearly screening ECG and echocardiography are recommended to identify de novo ST changes or pathologic Q-waves and to find ventricular dysfunction or RWMA. If there are abnormal findings in these screening tests, coronary CTA may be considered as the initial test to diagnose CAC [13]. If CAC is suspected in the coronary CTA, functional studies such as CMR or SPECT, should be performed [13]. CMR offers additional information, including regional ventricular function and myocardial viability and myocardial SPECT may unmask ischaemia in asymptomatic patients [23]. Gadolinium enhancement CMR can reveal a typical pattern of scarred myocardium in patients who have already experienced myocardial infarction [24]. In patients with uncertain diagnoses with the above-mentioned non-invasive tests, ICA with invasive functional evaluation, such as fractional flow reserve or instantaneous wave-free ratio, should be considered for confirmation of CAC and risk-stratification [13,25,26]. Intravascular ultrasonography is also a preferred method to evaluate the mechanisms responsible for ischaemia and to guide proper management of AAOCA and other significant coronary anomalies [27].
If a patient is planning to undergo redo surgery for pulmonary valve or conduit change, cardiac CTA should be performed preoperatively to find coronary anomaly or coronary compression by adjacent materials and to prevent intraoperative coronary injury. In patients suspected of coronary problems on preoperative CTA, ICA and functional evaluations should be considered before the redo surgery. Although there are multiple preoperative imaging studies on the location of coronary arteries, if a surgeon is still not certain of the exact location of the coronary arteries of a patient, intraoperative fluorescence coronary imaging may be considered to avoid coronary artery injury during the redo RVOT reconstruction surgery [28,29]. If a significant coronary stenosis is confirmed, concomitant correction should be performed during the reoperation for prosthetic valve failure. Relief of compression of the coronary artery, unroofing of intramural course of the coronary artery, or coronary artery bypass graft surgery could be performed, according to the mechanism and degree of CAC.
It is difficult to decide when and how to treat of CAC after RVOT reconstruction surgery, especially in those without symptoms or further surgical plans. In patients with severe ventricular dysfunction or fatal ventricular arrhythmia immediately after surgery and suspected extensive coronary artery injury, surgical correction should be considered immediately. However, if a CAC is diagnosed late by imaging and functional studies in asymptomatic patients, a comprehensive assessment of the need for surgery is required depending on the risks and benefits thereof. This is because surgical correction of a CAC may not improve ventricular dysfunction and arrhythmia in patients who already suffered irreversible damage to the myocardium. In the haemodynamically stable patient, PCI or medical treatment could be considered besides surgical treatment. Patients with symptomatic heart failure due to coronary stenosis should be managed with beta-blockers, angiotensin converting enzyme inhibitors, and mineralocorticoid receptor antagonists [30]. ICD is recommended for patients with documented VT causing haemodynamic instability and LV EF <35%, to reduce the risk of sudden death and all-cause mortality [13,30]. A summary of our institution’s evaluation and follow-up protocol for patients who undergo RVOT reconstruction surgery is shown in Fig. 7.
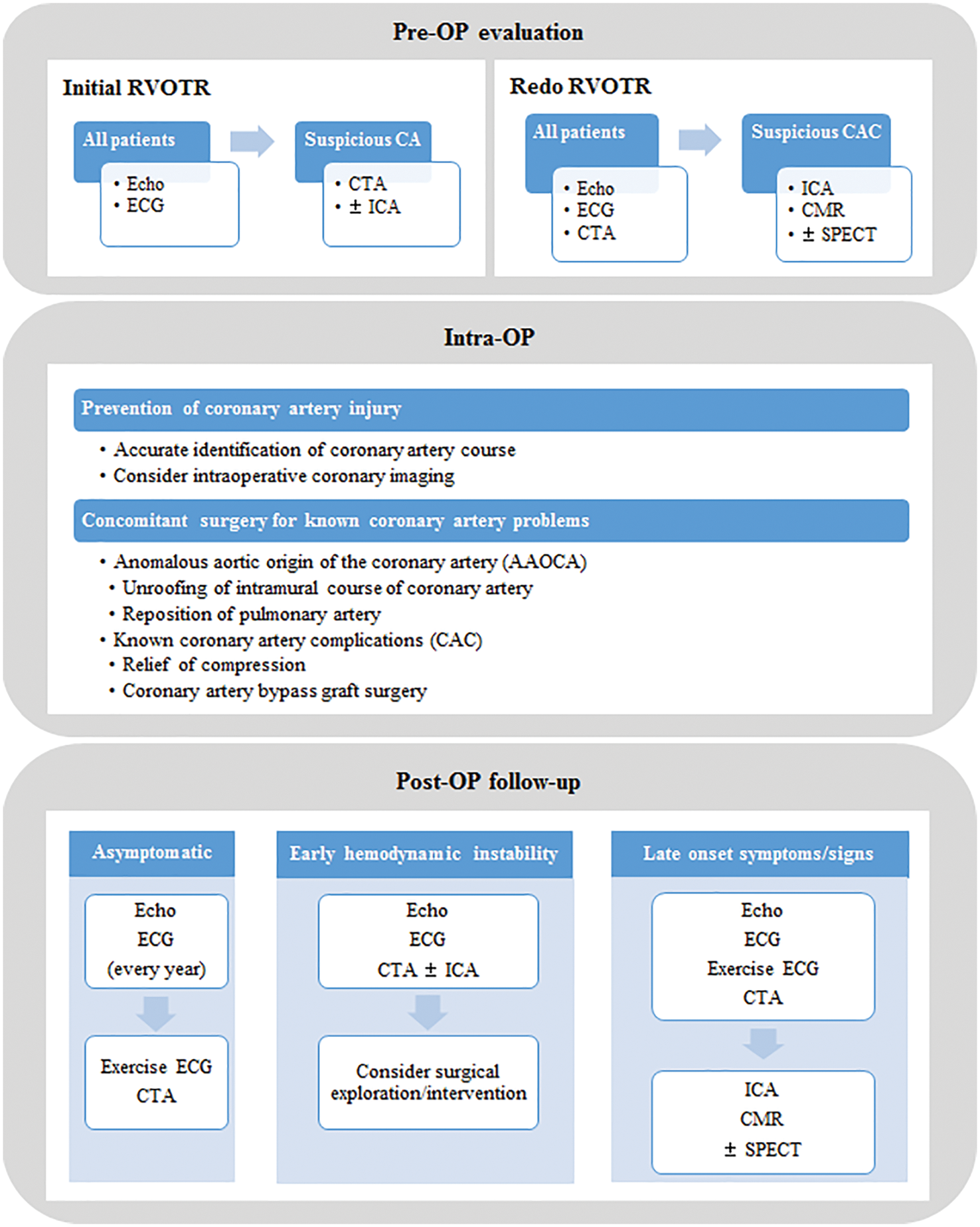
Figure 7: A summary of preoperative evaluation and follow-up protocol
Note: CA: coronary anomaly, CAC: coronary artery complication, CMR: cardiac magnetic resonance imaging, CTA: cardiac computed tomography angiography, ECG: electrocardiogram, Echo: echocardiography, ICA: invasive coronary angiography, OP: operation, RVOTR: right ventricular outflow tract reconstruction surgery, SPECT: single-photon emission computed tomography.
There are some limitations of this study. First, due to the retrospective study design, the evaluation and treatment of CAC were performed inconsistently according to patients’ clinical statuses and physicians’ preferences. In particular, CTA and ICA were not performed in all patients who underwent RVOT reconstruction surgery. A retrospective study was conducted only on patients with CAC found in clinical practice. Therefore, it is possible that more patients actually developed CAC asymptomatically, thereby evading subsequent detection. More systemic diagnostic criteria of CAC after RVOT reconstruction surgery and treatment guidelines must be established in the future. Second, the results of stress echocardiography and invasive functional studies were not available in this study. If such functional studies could be performed, the clinical importance of CAC and the policy of surgical or interventional treatment may be determined. Finally, evaluation and management of each type of CAC have not been separately discussed since the risk factor analysis by type has not been done yet. A further study is necessary to investigate the risk factors for CAC in patients who have undergone RVOT reconstruction surgery and to identify measures to prevent development of CAC.
Preoperative and postoperative long-term coronary assessment should be performed in all patients undergoing RVOT reconstruction surgery, especially those with congenital coronary artery anomaly. Guidelines for the prevention, diagnosis, and management of CAC should be prepared to prevent ventricular dysfunction, arrhythmias, and death after RVOT reconstruction surgery.
Author Contribution: Hye Won Kwon-Data curation, Formal analysis, Investigation, Writing–original draft. Mi Kyoung Song-Data curation, Data acquisition. Sang Yun Lee-Data curation, Methodology. Gi Beom Kim-Data curation, Resources. Jae Gun Kwak–Conceptualisation, Supervision. Sungkyu Cho-Data curation, Validation. Woong-Han Kim–Conceptualisation, Supervision. Whal Lee–Validation, Visualisation. Eun Jung Bae-Project administration, Conceptualisation, Supervision, Writing–review & editing.
Data Sharing: Data sharing of anonymized data may be possible upon request to the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jain, A., Oster, M., Kilgo, P., Grudziak, J., Jokhadar, M. et al. (2012). Risk factors associated with morbidity and mortality after pulmonary valve replacement in adult patients with previously corrected tetralogy of Fallot. Pediatric Cardiology, 33(4), 601–606. DOI 10.1007/s00246-012-0185-z. [Google Scholar] [CrossRef]
2. Huang, E. S., Herrmann, J. L., Rodefeld, M. D., Turrentine, M. W., Brown, J. W. (2019). Rastelli operation for D-transposition of the great arteries, ventricular septal defect, and pulmonary stenosis. World Journal of Pediatric and Congenital Heart Surgery, 10(2), 157–163. DOI 10.1177/2150135118817765. [Google Scholar] [CrossRef]
3. Backer, C. L., Mavroudis, C. (2003). The Rastelli operation. In: Operative techniques in thoracic and cardiovascular surgery, pp. 121–130. Netherlands: Elsevier. [Google Scholar]
4. Lee, C., Kim, Y. M., Lee, C. H., Kwak, J. G., Park, C. S. et al. (2012). Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: Implications for optimal timing of pulmonary valve replacement. Journal of the American College of Cardiology, 60(11), 1005–1014. DOI 10.1016/j.jacc.2012.03.077. [Google Scholar] [CrossRef]
5. Jacobs, J. P., Mavroudis, C., Quintessenza, J. A., Chai, P. J., Pasquali, S. K. et al. (2014). Reoperations for pediatric and congenital heart disease: An analysis of the Society of Thoracic Surgeons (STS) congenital heart surgery database. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 17(1), 2–8. DOI 10.1053/j.pcsu.2014.01.006. [Google Scholar] [CrossRef]
6. Lee, C., Lee, C. H., Kwak, J. G. (2016). Outcomes of redo pulmonary valve replacement for bioprosthetic pulmonary valve failure in 61 patients with congenital heart disease. European Journal of Cardiothoracic Surgery, 50(3), 470–475. DOI 10.1093/ejcts/ezw037. [Google Scholar] [CrossRef]
7. Said, S. M., Burkhart, H. M., Phillips, S. D., Dearani, J. A. (2011). Left main coronary artery compression by right ventricle-to-pulmonary artery conduit relieved by anterior translocation of the right pulmonary artery. World Journal of Pediatric Congenital Heart Surgery, 2(3), 502–504. DOI 10.1177/2150135111403780. [Google Scholar] [CrossRef]
8. Daskalopoulos, D. A., Edwards, W. D., Driscoll, D. J., Danielson, G. K., Puga, F. J. (1983). Coronary artery compression with fatal myocardial ischemia. A rare complication of valved extracardiac conduits in children with congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 85(4), 546–551. DOI 10.1016/S0022-5223(19)37539-7. [Google Scholar] [CrossRef]
9. Kwon, H., Bae, E. J., Kim, G. B., Lee, S. Y., Song, M. K. et al. (2020). Coronary artery diseases after right ventricular outflow tract reconstruction surgery. European Heart Journal, 41(Suppl. 2), 3207. DOI 10.1093/ehjci/ehaa946.3207. [Google Scholar] [CrossRef]
10. Khairy, P., Clair, M., Fernandes, S. M., Blume, E. D., Powell, A. J. et al. (2013). Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation, 127(3), 331–339. DOI 10.1161/CIRCULATIONAHA.112.135046. [Google Scholar] [CrossRef]
11. Ou, P., Khraiche, D., Celermajer, D. S., Agnoletti, G., Le Quan Sang, K. H. et al. (2013). Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. Journal of Thoracic and Cardiovascular Surgery, 145(5), 1263–1269. DOI 10.1016/j.jtcvs.2012.06.009. [Google Scholar] [CrossRef]
12. da Costa, F. D., Takkenberg, J. J., Fornazari, D., Balbi Filho, E. M., Colatusso, C. et al. (2014). Long-term results of the Ross operation: An 18-year single institutional experience. European Journal of Cardiothoracic Surgery, 46(3), 415–422. DOI 10.1093/ejcts/ezu013. [Google Scholar] [CrossRef]
13. Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E. et al. (2020). ESC guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal, 41(3), 407–477. DOI 10.1093/eurheartj/ehz425. [Google Scholar] [CrossRef]
14. Wolk, M. J., Bailey, S. R., Doherty, J. U., Douglas, P. S., Hendel, R. C. et al. (2014). ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: A report of the American college of cardiology foundation appropriate use criteria task force, American heart association, American society of echocardiography, American society of nuclear cardiology, Heart failure society of america, Heart rhythm society, Society for cardiovascular angiography and interventions, Society of cardiovascular computed tomography, Society for cardiovascular magnetic resonance, and Society of thoracic surgeons. Journal of the American College of Cardiology, 63, 380–406. DOI 10.1016/j.jacc.2013.11.009. [Google Scholar] [CrossRef]
15. Dvorak, R. A., Brown, R. K., Corbett, J. R. (2011). Interpretation of SPECT/CT myocardial perfusion images: Common artifacts and quality control techniques. RadioGraphics, 31(7), 2041–2057. DOI 10.1148/rg.317115090. [Google Scholar] [CrossRef]
16. Baumgartner, H., Bonhoeffer, P., de Groot, N. M., de Haan, F., Deanfield, J. E. et al. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249. [Google Scholar] [CrossRef]
17. Ratajska, A., Zlotorowicz, R., Blazejczyk, M., Wasiutynski, A. (2005). Coronary artery embryogenesis in cardiac defects induced by retinoic acid in mice. Birth Defects Research Part A: Clinical and Molecular Teratology, 73, 966–979. DOI 10.1002/(ISSN)1542-0760. [Google Scholar] [CrossRef]
18. Tomanek, R. J., Yu, Q., Lo, C. W. (2015). Coronary anomalies in mice with congenital heart defects. Anatomical Record, 298(2), 408–417. DOI 10.1002/ar.23056. [Google Scholar] [CrossRef]
19. Houyel, L., Bajolle, F., Capderou, A., Laux, D., Parisot, P. et al. (2013). The pattern of the coronary arterial orifices in hearts with congenital malformations of the outflow tracts: A marker of rotation of the outflow tract during cardiac development? Journal of Anatomy, 222(3), 349–357. DOI 10.1111/joa.12023. [Google Scholar] [CrossRef]
20. Basso, C., Maron, B. J., Corrado, D., Thiene, G. (2000). Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. Journal of the American College Cardiololgy, 35(6), 1493–1501. DOI 10.1016/S0735-1097(00)00566-0. [Google Scholar] [CrossRef]
21. Frommelt, P. C. (2009). Congenital coronary artery abnormalities predisposing to sudden cardiac death. Pacing and Clinical Electrophysiology, 32(Suppl. 2), S63–S66. DOI 10.1111/j.1540-8159.2009.02387.x. [Google Scholar] [CrossRef]
22. Grani, C., Kaufmann, P. A., Windecker, S., Buechel, R. R. (2014). Diagnosis and management of anomalous coronary arteries with a malignant course. Interventional Cardiology, 14, 83–88. DOI 10.15420/icr.2019.1.1. [Google Scholar] [CrossRef]
23. Grani, C., Buechel, R. R., Kaufmann, P. A., Kwong, R. Y. (2017). Multimodality imaging in individuals with anomalous coronary arteries. JACC: Cardiovascular Imaging, 10(4), 471–481. DOI 10.1016/j.jcmg.2017.02.004. [Google Scholar] [CrossRef]
24. Kim, R. J., Wu, E., Rafael, A., Chen, E. L., Parker, M. A. et al. (2000). The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. New England Journal of Medicine, 343(20), 1445–1453. DOI 10.1056/NEJM200011163432003. [Google Scholar] [CrossRef]
25. de Bruyne, B., Pijls, N. H., Kalesan, B., Barbato, E., Tonino, P. A. et al. (2012). Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. New England Journal of Medicine, 367(11), 991–1001. DOI 10.1056/NEJMoa1205361. [Google Scholar] [CrossRef]
26. Tonino, P. A., de Bruyne, B., Pijls, N. H., Siebert, U., Ikeno, F. et al. (2009). Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New England Journal of Medicine, 360(3), 213–224. DOI 10.1056/NEJMoa0807611. [Google Scholar] [CrossRef]
27. Angelini, P. (2007). Coronary artery anomalies: An entity in search of an identity. Circulation, 115(10), 1296–1305. DOI 10.1161/CIRCULATIONAHA.106.618082. [Google Scholar] [CrossRef]
28. Pourmoghadam, K. K., Bunnell, A. P., O’Brien, M. C., DeCampli, W. M. (2014). Avoiding coronary injury in congenital heart surgery by laser-assisted indocyanine green dye imaging. World Journal for Pediatric and Congenital Heart Surgery, 5, 326–329. DOI 10.1177/2150135113514459. [Google Scholar] [CrossRef]
29. Feins, E. N., Si, M. S., Baird, C. W., Emani, S. M. (2020). Intraoperative coronary artery imaging for planning. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 23, 11–16. DOI 10.1053/j.pcsu.2020.02.001. [Google Scholar] [CrossRef]
30. Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F. et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal, 18(8), 891–975. DOI 10.1002/ejhf.592. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |