

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.018479
ARTICLE
Carotid Artery Cut-Down in Pediatric Cardiac Catheterization: When and How?
1Diyarbakir Gazi Yasargil Training and Research Hospital, Pediatric Cardiovascular Surgery, Diyarbakir, Turkiye
2Firat Universitesi, Pediatric Cardiology, Elazig, Turkiye
3Diyarbakir Gazi Yasargil Training and Research Hospital, Pediatric Cardiology, Diyarbakir, Turkiye
*Corresponding Author: Onur Doyurgan. Email: onurdoyurgan@gmail.com
Received: 27 July 2021; Accepted: 27 October 2021
Abstract: Background: Vascular access used for pediatric cardiac catheterization is one of the most important factors that affects the success of the procedure. We aimed to compare the effect, success, and complications of cardiac catheterizations performed by carotid cut-down or femoral puncture in newborns or young infants. Methods: We included who underwent catheterization in our department between 28 January 2017 and 15 April 2021. These patients underwent balloon aortic valvuloplasty, balloon coarctation angioplasty, ductal stenting, diagnostic procedures for aortic arch pathologies, and modified Blalock-Taussig in-shunt intervention. Patients were divided into two groups: femoral puncture (group = 1) and carotid cut-down (CC, group = 2). Results: Seventy-two catheterization procedures were performed in 64 patients; 32 (44.4%) were performed via the femoral approach and 40 (55.6%) were performed via the carotid approach. Sixteen (22.2%) procedures were diagnostic and 56 (77.8%) procedures were interventional. CC was performed in 13 (32.5%) patients with failed femoral intervention. Patients in the CC group had shorter durations of procedure, vascular access, and anesthesia, compared with the femoral access group (80.9 and 116.2 min, p = 0.001; 12.9 and 22.5 min, p = 0.001; 140.9 and 166.6 min, p = 0.001, respectively). Patients who underwent CC had fewer complications than did patients in the femoral access group (2.5% and 21.8%, respectively; p = 0.01); larger sheats were used in CC patients (p = 0.028). Conclusion: The carotid artery can be successfully used as a primary catheterization route, particularly in patients with small body weight and patients who require rapid vascular access, or stenting of the vertical duct.
Keywords: Cardiac catheterization; carotid artery; cut-down; pediatric cardiology; surgery
Cannulation of the femoral artery for cardiac catheterization is usually easy in adults. However, it is occasionally difficult and slow in newborns and infants. Loss of peripheral pulse, lower extremity circulatory disturbance, subcutaneous hematoma, bleeding, local femoral artery damage-related occlusion, permanent stenosis, arteriovenous fistula, pseudoaneurysm, dissection, and femoral nerve damage are among the complications that can occur in patients after femoral puncture [1,2]. Femoral artery occlusion can also lead to serious complications such as claudication, poor leg growth, and (rarely) extremity loss [3]. Previously, Glatz et al. [4] reported an incidence of 20%–30% for acute arterial occlusion after femoral artery catheterization in pediatric patients weighing <4 kg. These patients had clinically indistinct findings; the risk of acute arterial occlusion was directly correlated with sheath size and inversely correlated with patient weight. Common carotid artery cannulation for cardiac catheterization is advantageous in young children. Because the carotid artery is larger than the femoral artery, it can support a larger sheath and provide easier access when a vertical angled intervention is required for left heart lesions (e.g., aortic stenosis, aortic coarctation, intermittent aortic arch anomaly, modified Blalock-Taussig [mBT] shunt, and vertical patent ductus arteriosus) [3,5–7]. Surgical cut-down of the common carotid artery for infants undergoing cardiac catheterization was first described by Azzolina et al. [8] in 1973 as an alternative to the femoral route. Cardiac catheterization may lead to several complications because it is an invasive procedure. Vascular damage and cerebrovascular events are important potential complications of carotid artery interventions. However, previous studies showed good arterial patency after carotid artery interventions [9,10]. Cardiac catheterization interventions through the carotid artery have a lower risk of vascular complications, particularly in newborns and infants with low body weight [4,11].
In this study, we achieved vascular access by carotid cut-down (CC) to reduce vascular complications and reduce the procedure time in newborns and young infants with either vertical left-sided cardiac lesions or inadequate/unsuccessful femoral puncture approach. We aimed to compare the effects of CC and femoral puncture during pediatric cardiac catheterization procedures on the procedure duration and the complication and success rates.
Between 28 January and 15 April 2021, 986 patiens underwent cardiac catheterization in our department. Of these patients, 64 fulfilled the inclusion criteria and were included in the study. In total, 72 cardiac catheterization procedures were performed in 64 patients. Cardiac catheterization procedures included balloon aortic valvuloplasty, balloon coarctation angioplasty, diagnostic angiography for aortic arch pathologies, and ductal stenting for ductus-dependent pulmonary circulation or in-shunt intervention after mBT shunt procedure. Patients were divided into femoral puncture (group 1) and CC (group 2) groups, according to the selected vascular access site. Demographic and perioperative data were retrospectively analyzed using the hospital database. Written informed consent was obtained from patients their family members. The study protocol was approved by the Health Sciences University, Gazi Yaşargil Training and Research Hospital Ethics Committee, Turkey (approval date/number: 29.5.2021/763). The study was conducted in accordance with the Declaration of Helsinki.
All catheterization procedures were performed in a fully equipped catheterization laboratory with patients intubated and under general anesthesia. Heart rate, respiratory rate, oxygen saturation, and blood pressure were monitored throughout the procedure. Arterial blood gases were measured before and after the procedure. The patients underwent a detailed physical examination and preoperative echocardiography to evaluate the cardiac lesions. Body weight, duct structure, presence of near-atretic aortic valve or near-atretic aortic coarctation, infection in the femoral region, and occlusion of the femoral artery were recorded. The algorithm (study flow chart) that clearly defines which patients the carotid cutdown method is preferred as the primary method of intervention is shown in Fig. 1.
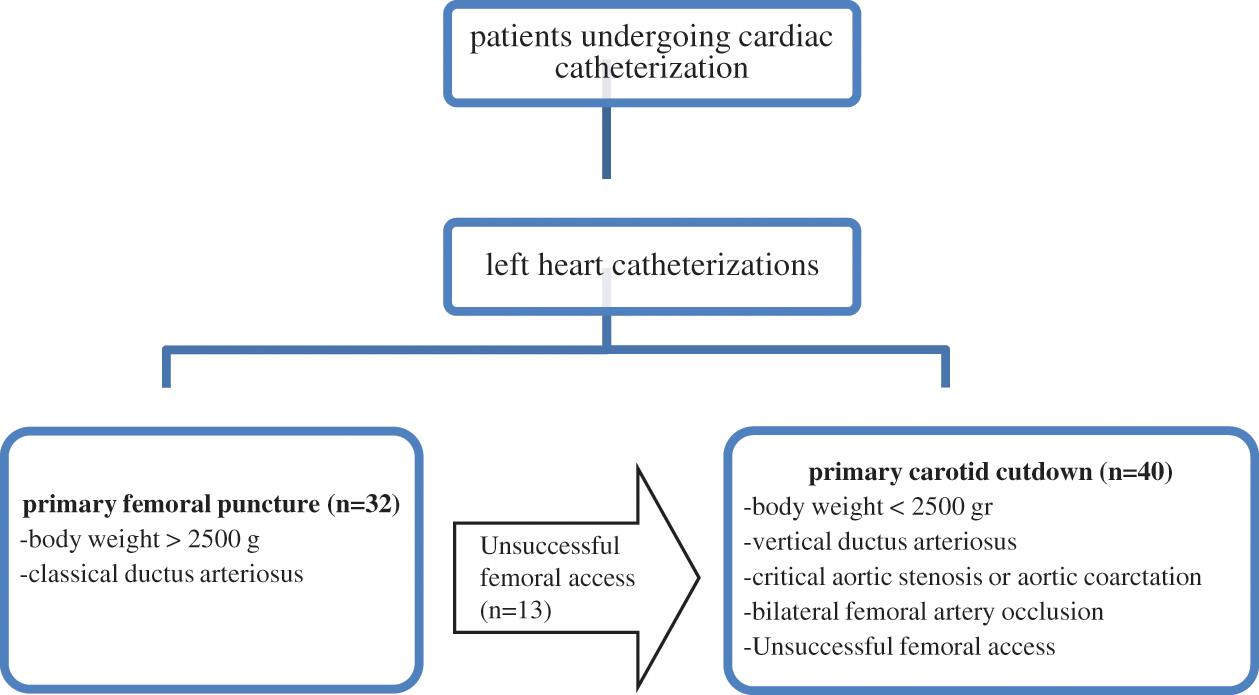
Figure 1: Study flow chart
Catheterization through the carotid artery was performed in patient with any of the following characteristics: failed two femoral artery attempts, body weight of <2,500 grams, risk factors for femoral catheterization, intervention difficulty (e.g., skeletal deformities, contractures, or puncture site infection), and/or diseases that required a vertical approach to reach the cardiac lesions. The CC side was selected on the basis of preoperative echocardiography findings.
Immediately after sheath placement, 100 U/kg of heparin sodium was administered; the dose was repeated if the activated clotting time was <200 s. Protamine was not administered to reverse the effect of heparin.
All patients were administered prophylactic antibiotics (intravenous cefazolin, 25 mg/kg) at 30 min before the procedure and two times after the procedure. Closed wound dressing with povidone-iodine was applied to patients in the CC group. After the procedure, patients were transferred to the intensive care unit for monitoring via echocardiography. Patients in the CC group were followed up by the radiology department within 24 h and at 6 months postoperatively; they underwent carotid color Doppler ultrasonography and neurological examination during outpatient monitoring. Further investigations were performed as indicated on the basis of the neurological findings. However, no examination or imaging findings related to the CC procedure were found. Patient demographic data, intra- and postoperative variables, technical success, and follow-up outcomes were compared between groups.
2.2.1 Direct Percutaneous Puncture of Common Femoral Artery
In the femoral puncture group, after premedication and induction of general anesthesia, femoral artery puncture was performed using a Seldinger needle; an appropriately sized sheath was placed into the artery with the help of a 0.018 inch wire. The sheath was removed after the procedure and compression was applied for at least 10 min to control the bleeding. Then, the patient was transferred to the intensive care unit for monitoring of bleeding and circulation. Repeat echocardiography was performed at 1 day postoperatively.
CC was performed in the catheterization room by the pediatric cardiovascular surgery team. All CC were performed by single pediatric cardiovascular surgeon (O.D.) with using loops. The patient was placed on the table in the supine position. The neck was extended by placing a pillow under the shoulders and the head was turned 45° to the opposite side. This maneuver, which is frequently used to achieve vascular access from the neck, allowed easy access to the carotid artery (Fig. 2A). After the skin had been wiped with 10% povidone-iodine solution (Kim-Pa, Povidone, 10% Povidone-iodine), a longitudinal skin incision was made along the medial edge of the sternocleidomastoid muscle. The common carotid artery was then liberated and returned with a silk suture.
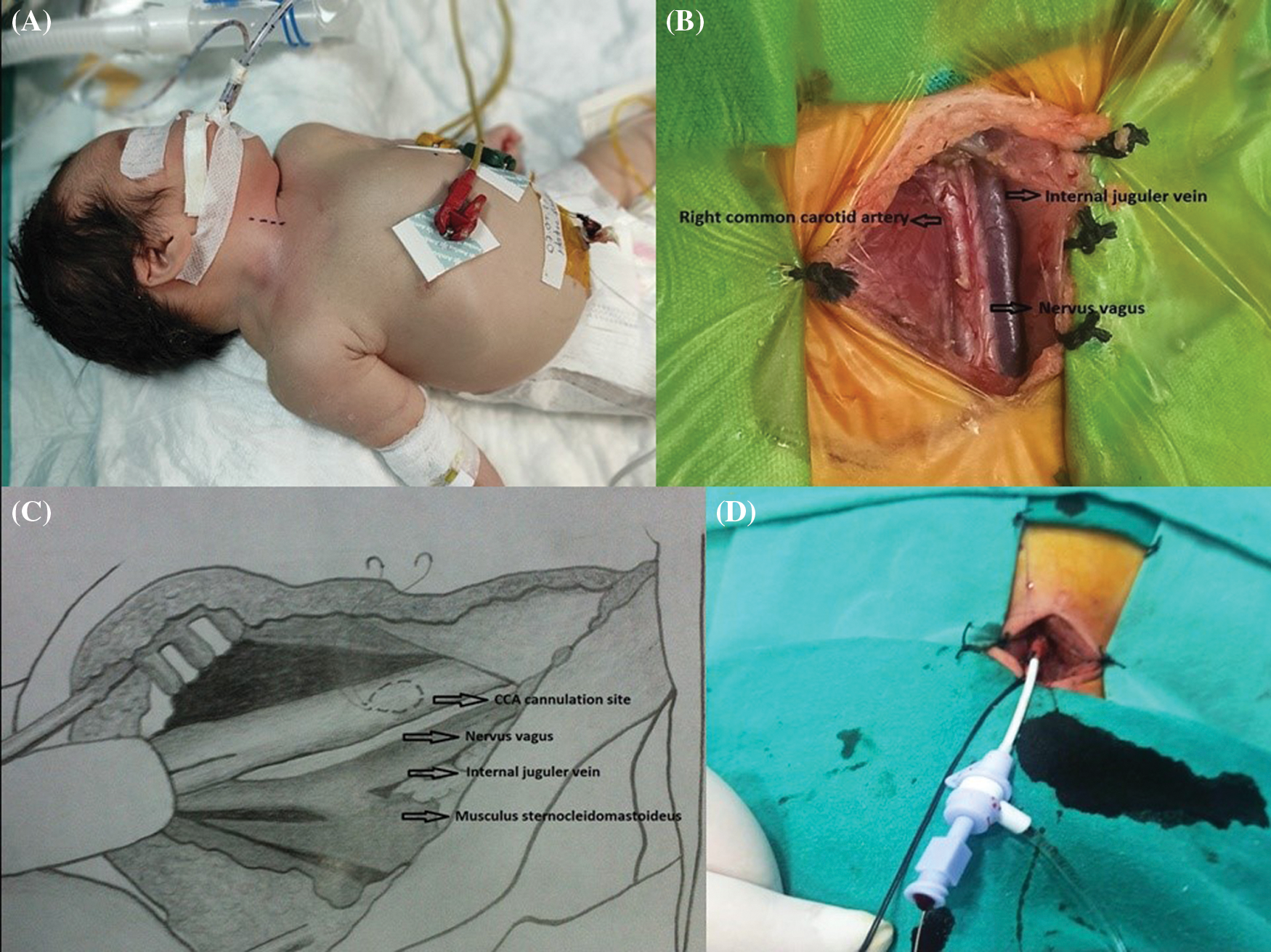
Figure 2: (A) Patient position for surgical cut-down approach to the carotid artery. Dashed line indicates surgical incision line at the medial edge of the sternocleidomastoid muscle. (B) The common carotid artery (CCA) was 3 mm in diameter in a newborn weighing 2,250 g. (C) CCA cannulation site and purse-string suture. (D) The artery was suspended with a silk suture and a needle was inserted through the middle of the purse-string suture. The wire and dilator were advanced. The sheath was advanced into the artery up to the mark. The purse-string suture was tightened and the sheath was fixed to the snare with a silk suture
2.2.3 Artery Cannulation of the Exposed Common Carotid Artery
After surgical cut-down, a purse-string suture (Prolene 6.0, Ethicon Inc., Somerville, NJ, USA) was applied to the puncture in the adventitia of the carotid artery and a tourniquet was placed. A needle was inserted into the artery through the middle of the purse-string suture. A soft-tipped guide wire was inserted through the needle into the ascending aorta. The sheath was advanced to the level (2 cm) previously marked with the silk suture, then inserted into the artery (Figs. 2B–2D). After the wire had been removed, the tourniquet was tightened; the sheath was fixed to the tourniquet with a silk suture. After the catheterization procedure, the sheath was removed and the purse-string suture was tied. Some patients required 1–2 extra sutures with 7.0 Prolene (Ethicon Inc., Somerville, NJ, USA) to control the bleeding. Patients in the CC group were monitored in the intensive care unit for possible neck swelling or respiratory problems.
SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Parametric variables are presented as means ± standard deviations; non-parametric variables are presented as medians (ranges). Categorical variables are presented as numbers and percentages. The chi-squared test and Fisher’s exact test were used to evaluate categorical variables. Student’s t-test was used to compare normally distributed continuous variables; the Mann-Whitney U test was used to compare non-normally distributed continuous variables. p < 0.05 was considered statistically significant.
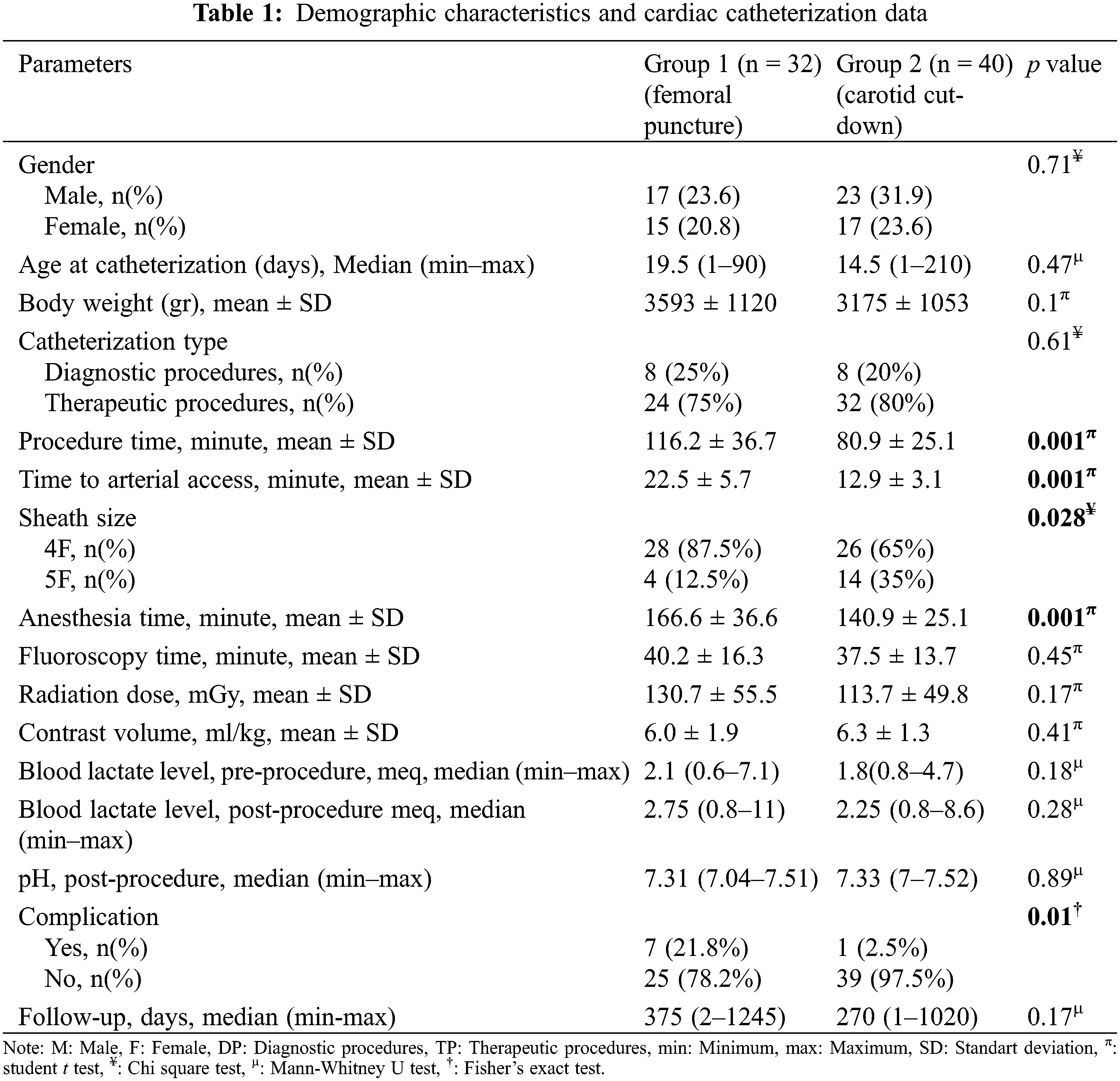
Seventy-two catheterization procedures were performed in 64 patients; 32 (44.4%) procedures were performed via the femoral route and 40 (55.6%) procedures were performed via the carotid route. Sixteen (22.2%) procedures were diagnostic and 56 (77.8%) procedures were interventional. The median patient age at catheterization was 27.4 days (1 day to 7 months) and the mean body weight was 3.36 ± 1.09 kg. In 13 (32.5%) of the 40 patients in group 2 (6 ductal stent, 3 balloon coarctation angioplasty, 2 balloon aortic valvuloplasty and 2 diagnostic heart catheterization procedures), the femoral route was attempted first. CC was performed in patients who failed femoral intervention. Patients in group 2 had shorter durations of operation, vascular access, and anesthesia, as well as fewer complications and larger sheaths, compared with patients in group 1 (p = 0.001, p = 0.001, p = 0.001, p = 0.01 and p = 0.028, respectively). The demographic characteristics and cardiac catheterization data of the patients are shown in Table 1.
The patients were diagnosed with ventricular septal defect + pulmonary atresia (26.3%), pulmonary atresia with intact ventricular septum (25%), aortic coarctation (20.8%), aortic stenosis (15.2%), stent/mBT shunt stenosis (8.3%), and aortic interruption (4.1%). A comparison of diagnoses between groups is presented in Fig. 3.
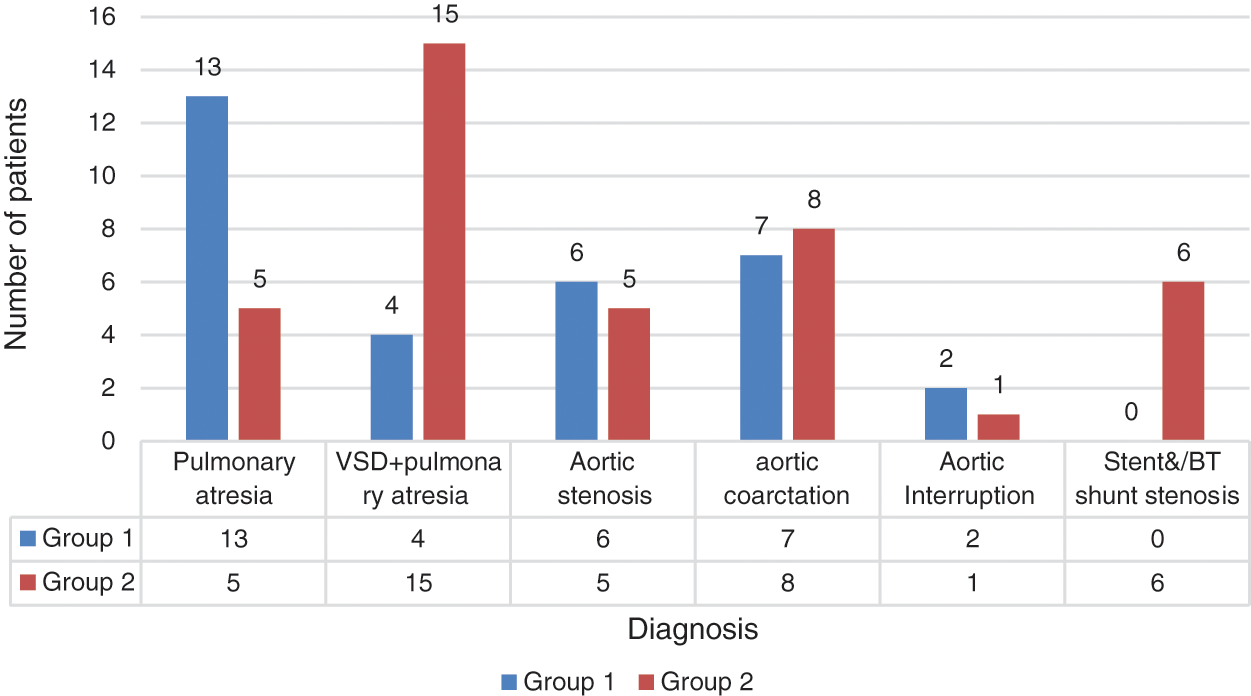
Figure 3: Distribution of patient diagnoses between groups
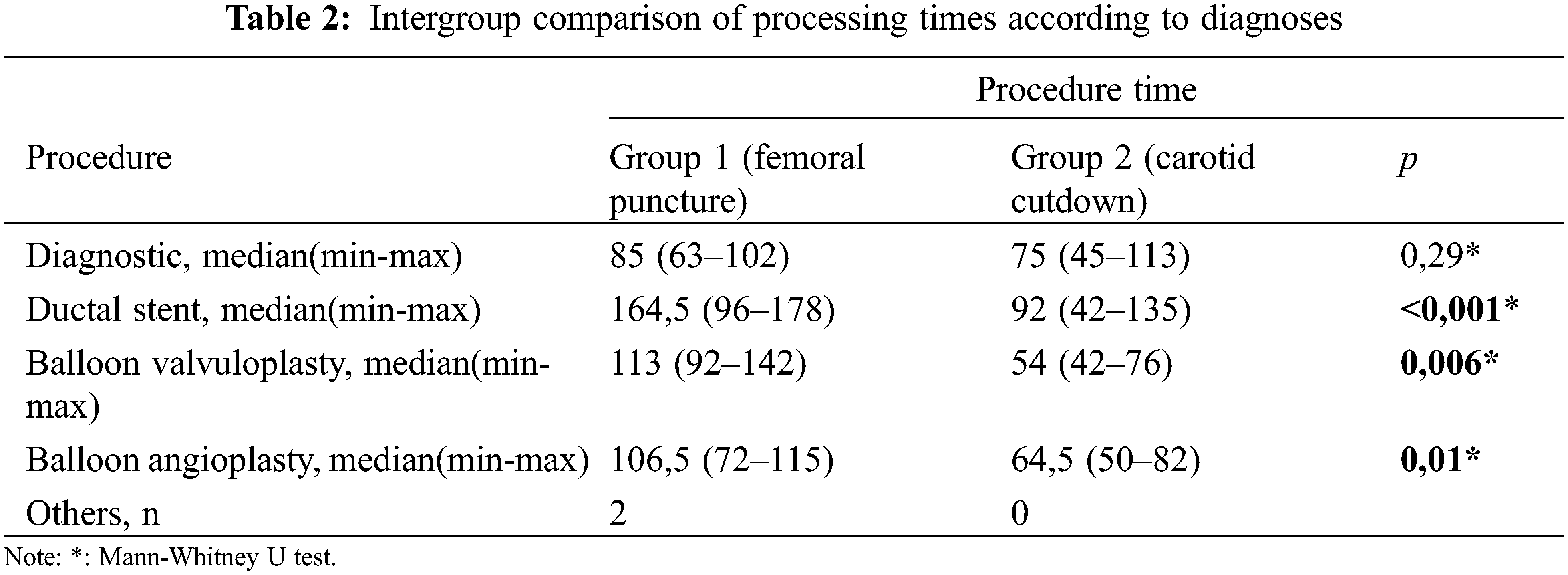
Although CC was performed more frequently in patients with ventricular septal defect + pulmonary atresia and stent/mBT shunt stenosis, femoral puncture was used more frequently in patients with intact ventricular septum + pulmonary atresia (p = 0.008). Angiographic images of a patient with intact ventricular septum + pulmonary atresia who underwent ductal stenting via CC are shown in Fig. 4. In the comparison made according to the diagnostic groups, there was no difference in the duration of the procedure between the femoral and CC groups in patients who underwent the diagnostic procedure. In patients diagnosed with ductal stent, balloon aortic valvuloplasty and balloon coarctation angioplasty, the procedure times were found to be significantly lower in the CC group compared to the femoral group (Table 2).
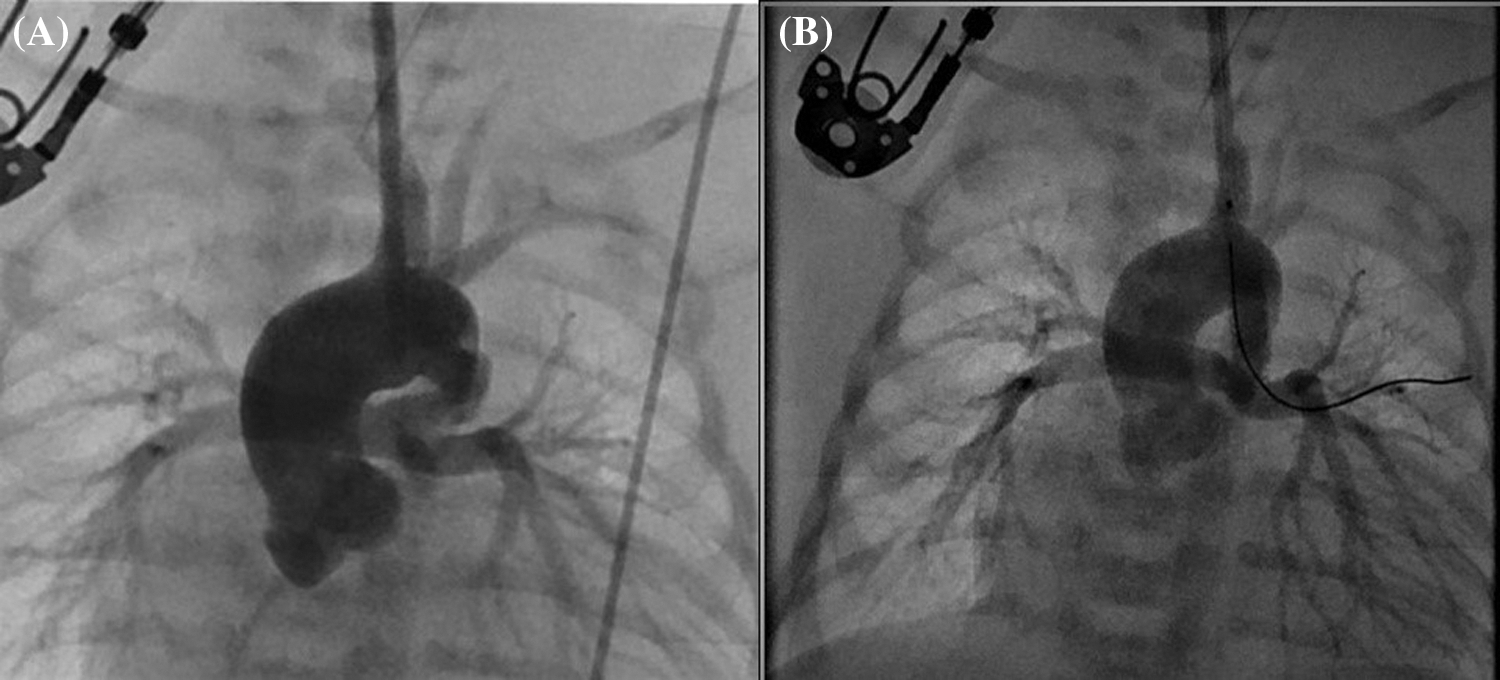
Figure 4: (A) Angiographic image obtained before the placement of a ductal stent for a vertical and tortuous ductus arteriosus, with contrast administered through the sheath placed with carotid cut-down, in a patient with pulmonary atresia and intact ventricular septum. (B) Angiographic image of the same patient after ductal stent placement
The most common indications for the procedures were diagnostic catheterization and ductal stent placement in both groups. The indications for the catheterization procedures are presented in Fig. 5.
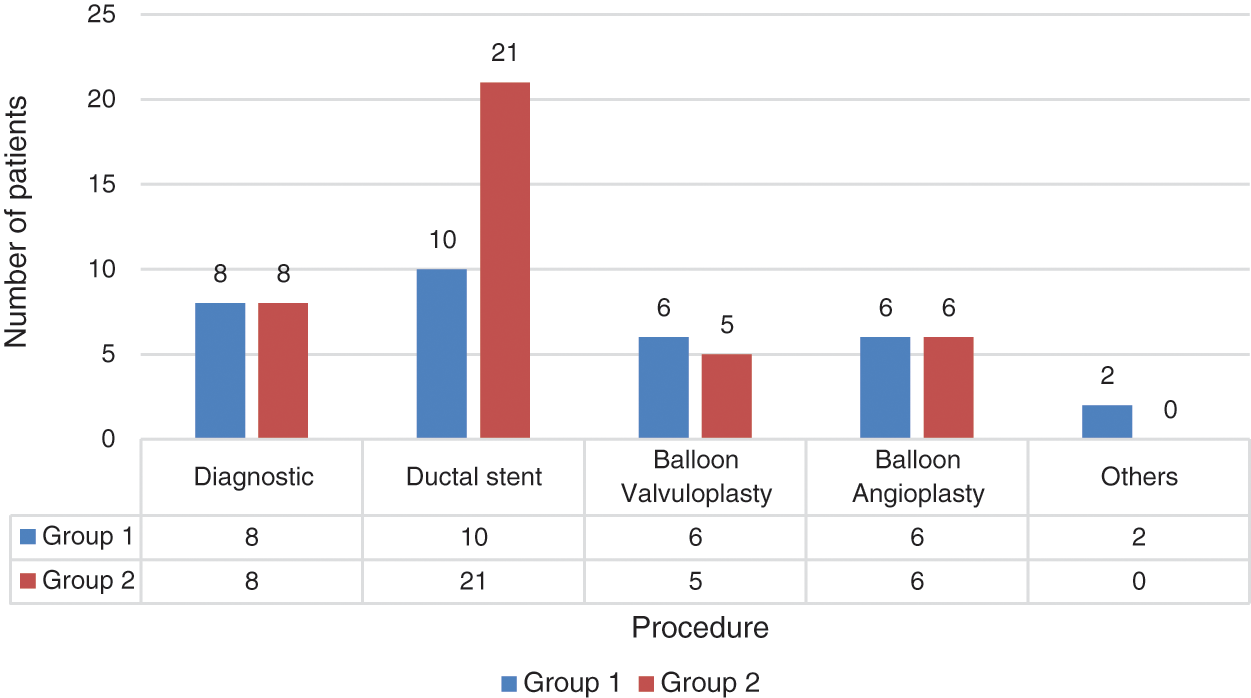
Figure 5: Distribution of catheterization procedures performed between groups
The complication rate was 21.8% (n = 7) in the femoral puncture group. The complications included circulatory disorders in the lower extremities because of vascular obstruction (n = 3, 9.4%), local hematoma (n = 2, 6.2%), arterial thrombus (n = 1, 3.1%), and stent migration to the pulmonary artery (n = 1, 3.1%). Patients with lower extremity circulatory disorders were treated with low molecular weight heparin. In the CC group, no complications were observed, except for wound infection in 1 patient (2.5%) who was treated with the appropriate antibiotics. Carotid Doppler USG was performed at the 6th month after procedure for all patients. Carotid artery stenosis was not observed in any patient in the carotid doppler USG. None of the study patients died during follow-up.
Pediatric cardiac catheterization procedures are used for anatomical and hemodynamic diagnosis and treatment in patients with congenital heart disease [12]. The increasing use of interventional procedures has led to growing interest in the associated complication rates. The success of the procedure depends on many factors, such as procedure complexity; operator experience; materials used; vascular route used; and patient diagnosis, age, and weight. In this study, we compared the catheterization procedures performed using femoral puncture or CC; we found that the CC group had shorter durations of procedure, vascular access, and anesthesia, as well as fewer complications, compared with the femoral puncture group.
In many centers, the femoral artery is the preferred route for left ventricular catheterization. Because of its straight course, the femoral artery is easy to manipulate and allows repeated attempts. However, this procedure is performed blindly by palpation; the inability to visualize the artery makes the procedure risky in patients with anatomical variations. Patients with a younger age or low body weight have an increased risk of arterial thrombosis during femoral artery catheterization [13]. Varan et al. [14] reported that the rate of vascular injury was 54.5% for infants who weighed <2,000 g and underwent femoral catheterization. Femoral arterial thrombosis prolongs the hospital stay, increases the cost, and contributes to increased rates of early and late morbidity and mortality [15]. In infants with severe aortic stenosis, near-atretic aortic coarctation, or a vertical curved duct, it may be technically difficult to advance the catheter in a retrograde manner through the small-diameter femoral artery [16]. The common carotid artery approach is the preferred alternative to the femoral approach [3]. The CC approach may be technically and anatomically superior because it avoids femoral arterial complications during procedures such as balloon aortic valvuloplasty, ductal stent placement, and systemic-to-pulmonary shunt interventions in newborns and infants [5,17]. Previous studies reported that, because of its vertical angle, the ductus can be safely selected as the intervention site of the carotid tract in cases that involve an unsuccessful femoral approach, stenting of a vertical patent ductus arteriosus, systemic-to-pulmonary artery shunt intervention, vertical patent ductus arteriosus stent redilatation, infants with a body weight <2,500 g, and/or a high risk of femoral vascular injury [18,19]. In our study, 13 (32.5%) patients underwent CC because femoral puncture had been unsuccessful. Of these 13 patients, 6 were undergoing ductal stent placement, 3 were undergoing balloon coarctation angioplasty, 2 were undergoing balloon aortic valvuloplasty, and 2 were undergoing diagnostic heart catheterization.
There are no studies concerning the temporary interruption of cerebral blood flow in pediatric patients. However, the long-term outcomes of patients after extracorporeal membrane oxygenation are good, although the carotid artery is ligated [16,20]. This is because the brain arterial system has a good compensatory blood circulation mechanism because of the Willis polygon and carotid system (external and internal).
Many recent studies have reported catheterization by percutaneous carotid puncture. Justino and Petit reported that vascular complications related to the intervention site occurred in 12.7% of 47 percutaneous carotid catheterization procedures performed in 42 patients. These complications included thrombotic occlusions (n = 2), hematomas (n = 2), non-occlusive thrombus (n = 1), and pseudoaneurysm (n = 1) [5]. Polat [19] reported that vascular complications associated with the carotid artery entry occurred in 19% of patients, despite the use of percutaneous interventions under ultrasound guidance. Therefore, despite the use of ultrasound guidance, vascular complications related to the entry site remain possible. The common carotid artery lies adjacent to the internal jugular vein laterally and the vagus nerve posteriorly. Therefore, if the common carotid artery is punctured without seeing the vessel, the vagus nerve or the internal jugular vein may be damaged.
Hematoma, bleeding, intravascular thrombus, and pseudoaneurysm formation can occur at the puncture site after percutaneous carotid catheterization. Choudhry et al. reported that post-procedural carotid artery pseudoaneurysm occurred in 10% of 20 infants younger than 3 months of age who underwent catheterization via the carotid artery [17]. The rates of vascular complications related to the catheter insertion site in patients who underwent percutaneous carotid catheterization were higher in previous studies than in our study; notably, our study included patients who underwent CC. These data suggest that CC may avoid possible complications of the percutaneous method, particularly among premature patients with a birth weight of <2,500 g.
Catheterization via CC is more invasive than the percutaneous technique, although it has fewer complications. In our study, none of the typical complications (e.g., hematoma, intravascular thrombus, bleeding, or pseudoaneurysm) were observed with the CC approach. Only one (2.5%) CC patient experienced a complication (wound infection) related to the intervention site. The low complication rate among our CC patients was presumably related to direct visualization of the carotid artery. We did not use the percutaneous carotid method because of the high risk of complications reported by previous studies, as well as our experience with cut-down procedures. Therefore, we avoided the possible complications of percutaneous intervention and reduced the operative duration for our patients.
Vascular complications reportedly increase with increasing size of the sheath placed in the femoral artery. Because the carotid artery is larger than the femoral artery, access through the carotid artery is preferable in patients with femoral artery/vein occlusion; this access helps to avoid femoral artery/vein complications, particularly in patients with a low-weigh [4]. In our study, a large-diameter sheath was placed; this was associated with a lower complication rate in the CC group than in the femoral puncture group.
Underweight infants are susceptible to hypothermia after removal from the incubator, and their body temperature should be kept constant [21]. Otherwise, metabolic acidosis and peripheral circulatory disturbance may develop rapidly. Therefore, it is important to ensure a short procedure duration in these patients. In addition, rapid vascular access may be life-saving in hemodynamically unstable patients who require urgent intervention, such as patients with critical aortic stenosis/coarctation, mBT shunt thrombosis, and/or ductal stent thrombosis [22]. Pediatric patients undergoing cardiac catheterization have increased risks of vascular complications associated with small body weight and delayed vascular puncture [1]. A study comparing percutaneous carotid and femoral artery approaches in patients with systemic-to-pulmonary artery shunts reported that the procedure time, time to reach the target artery, and anesthesia time were shorter in patients who underwent the carotid approach [23]. Similarly, in our study, the procedure times (durations of anesthesia, procedure, and vascular access) were shorter in the CC group than in the femoral puncture group.
Renal complications can be avoided by minimizing the volume of contrast material administered to neonatal patients, who exhibit low glomerular filtration capacity [24]. A previous study reported that an excessive quantity of contrast material was used in newborns with low birth weight (<2,500 g) [25]. In the present study, there was no significant difference between groups in terms of the quantity of contrast material used.
Thirteen of the 40 patients in the CC group had previously undergone several failed femoral approach attempts, and vascular access was achieved with the CC approach. Furthermore, femoral intervention was not attempted in patients with low birth weight, vertical duct requiring ductal intervention, near-atretic aortic valve, and/or coarctation; in these patients, the carotid route was preferred.
In this study, we presented our experience with CC, compared with the femoral puncture method. We found that the carotid approach was superior to the femoral approach and involved fewer complications. We performed carotid color Doppler ultrasounds on all CC patients at 24 h and 6 months after the procedure; we found no instances of carotid stenosis or decreased blood flow.
The limitations of this study were its retrospective design, small number of cases, lack of a percutaneous carotid puncture group, and short follow-up duration.
The procedure time was shorter, vascular complication rate was lower, and procedural success rate was higher in the CC group, compared with the traditional femoral route group. Although CC is an invasive procedure, it can be used successfully in centers with experienced pediatric vascular surgeons who are able to perform sophisticated techniques that involve a meticulous approach and close patient monitoring. Our study suggests that CC can be used as the primary catheterization route in selected cases, such as patients with aortic arch pathologies or small body weight, as well as patients who require rapid vascular access or stenting of the vertical duct. However, studies investigating long-term follow-up results are needed to corfirm whether CC is safe for cardiac catheterization in the pediatric population.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Roushdy, A. M., Abdelmonem, N., El Fiky, A. A. (2012). Factors affecting vascular access complications in children undergoing congenital cardiac catheterization. Cardiology in the Young, 22(2), 136–144. DOI 10.1017/S1047951111000989. [Google Scholar] [CrossRef]
2. Uçar, B., Kılıç, Z., Karataş, Z. (2012). Vascular complications belonging to catheter insertion in children who underwent cardiac catheterization. Turkish Journal of Pediatric Disease, 6, 23–30. [Google Scholar]
3. Davenport, J. J., Lam, L., Whalen-Glass, R., Nykanen, D. G., Burke, R. P. et al. (2008). The successful use of alternative routes of vascular access for performing pediatric interventional cardiac catheterization. Catheterization and Cardiovascular Interventions, 72(3), 392–398. DOI 10.1002/ccd.21621. [Google Scholar] [CrossRef]
4. Glatz, A. C., Shah, S. S., McCarthy, A. L., Geisser, D., Daniels, K. et al. (2013). Prevalence of and risk factors for acute occlusive arterial injury following pediatric cardiac catheterization: A large single-center cohort study. Catheterization and Cardiovascular Interventions, 82(3), 454–462. DOI 10.1002/ccd.24737. [Google Scholar] [CrossRef]
5. Justino, H., Petit, C. J. (2016). Percutaneous common carotid artery access for pediatric interventional cardiac catheterization. Circulation: Cardiovascular Interventions, 9(4), S22. DOI 10.1161/CIRCINTERVENTIONS.115.003003. [Google Scholar] [CrossRef]
6. Fischer, D. R., Ettedgui, J. A., Park, S. C., Siewers, R. D., Del Nido, P. J. (1990). Carotid artery approach for balloon dilation of aortic valve stenosis in the neonate: A preliminary report. Journals of the American College of Cardiology, 15(7), 1633–1636. DOI 10.1016/0735-1097(90)92839-T. [Google Scholar] [CrossRef]
7. Steinberg, C., Weinstock, D. J., Gold, J. P., Notterman, D. A. (1992). Measurements of central blood vessels in infants and children: Normal values. Catheterization and Cardiovascular Diagnosis, 27, 197–201. DOI 10.1002/(ISSN)1097-0304. [Google Scholar] [CrossRef]
8. Azzolina, G., Eufrate, S. A., Allella, A. (1973). New approach to catheterization of the heart in infants and children. British Heart Journal, 35(6), 643–646. DOI 10.1136/hrt.35.6.643. [Google Scholar] [CrossRef]
9. Borghi, A., Agnoletti, G., Poggiani, C. (2001). Surgical cutdown of the right carotid artery for aortic balloon valvuloplasty in infancy: Midterm follow-up. Pediatric Cardiology, 22(3), 194–197. DOI 10.1007/s002460010202. [Google Scholar] [CrossRef]
10. Robinson, B. V., Brzezinska-Rajszys, G., Weber, H. S., Ksiazyk, J., Fricker, F. J. et al. (2000). Balloon aortic valvotomy through a carotid cutdown in infants with severe aortic stenosis: Results of the multi-centric registry. Cardiology in the Young, 10(3), 225–232. DOI 10.1017/S104795110000915X. [Google Scholar] [CrossRef]
11. Tadphale, S., Yohannan, T., Kauffmann, T., Maller, V., Agrawal, V. et al. (2020). Accessing femoral arteries less than 3 mm in diameter is associated with increased incidence of loss of pulse following cardiac catheterization in infants. Pediatric Cardiology, 41(5), 1058–1066. DOI 10.1007/s00246-020-02357-4. [Google Scholar] [CrossRef]
12. Feltes, T. F., Bacha, E., BeekmanIII, R. H., Chetham, J. P., Feinstein, J. A. et al. (2011). Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation, 123(22), 2607–2652. DOI 10.1161/CIR.0b013e31821b1f10. [Google Scholar] [CrossRef]
13. Brotschi, B., Hug, M. I., Kretschmar, O., Rizzi, M., Albisetti, M. (2015). Incidence and predictors of cardiac catheterisation-related arterial thrombosis in children. Heart, 101(12), 948–953. DOI 10.1136/heartjnl-2014-306713. [Google Scholar] [CrossRef]
14. Varan, B., Tokel, N. K., Yakut, K., Erdoğan, İ., Özkan, M. (2019). The results of interventional catheterization in infants weighing under 2,000 g. Turkish Journal of Thoracic and Cardiovascular Surgery, 27(3), 304–313. DOI 10.5606/tgkdc.dergisi.2019.17229. [Google Scholar] [CrossRef]
15. Kim, J., Sun, Z., Benrashid, E., Southerland, K. W., Lawson, J. H. (2017). The impact of femoral arterial thrombosis in paediatric cardiac catheterisation: A national study. Cardiology in the Young, 27(5), 912–917. DOI 10.1017/S104795111600161X. [Google Scholar] [CrossRef]
16. Gasparella, M., Milanesi, O., Biffanti, R., Cerruti, A., Sabatti, M. et al. (2003). Carotid artery approach as an alternative to femoral access for balloon dilation of aortic valve stenosis in neonates and infants. The Journal of Vascular Access, 4, 146–149. DOI 10.1177/112972980300400403. [Google Scholar] [CrossRef]
17. Choudhry, S., Balzer, D., Murphy, J., Nicolas, R., Shahanavaz, S. (2016). Percutaneous carotid artery access in infants <3 months of age. Catheterization and Cardiovascular Interventions, 87, 757–761. DOI 10.1002/ccd.26310. [Google Scholar] [CrossRef]
18. McMahon, C. J., Price, J. F., Salerno, J. C., El-Said, H., Taylor, M. et al. (2003). Cardiac catheterization in infants weighing less than 2500 grams. Cardiology in the Young, 13, 117–122. DOI 10.1017/s1047951103000246. [Google Scholar] [CrossRef]
19. Polat, T. B. (2020). Use of percutaneous carotid artery access for performing pediatric cardiac interventions: Single‐center study. Annals of Pediatric Cardiology, 13, 16–24. DOI 10.4103/apc.APC_26_19. [Google Scholar] [CrossRef]
20. Crombleholme, T. M., Adrzick, N. S., de Lorimier, A. A., Longaker, M. T., Harrison, M. R. et al. (1990). Carotis artery reconstruction following extracorporeal membrane oxigenation. The American Journal of Diseases of Children, 144, 872–874. DOI 10.1001/archpedi.1990.02150320036021. [Google Scholar] [CrossRef]
21. Baum, D., Mullins, G. (1965). Core temperature in infants undergoing cardiac catheterization. Pediatrics, 36(1), 88–93. DOI 10.1542/peds.36.1.88. [Google Scholar] [CrossRef]
22. Bonnet, M., Petit, J., Lambert, V., Brenot, P., Riou, J. Y. et al. (2015). Catheter-based interventions for modified Blalock-Taussig shunt obstruction: A 20-year experience. Pediatric Cardiology, 36(4), 835–841. DOI 10.1007/s00246-014-1086-0. [Google Scholar] [CrossRef]
23. Ligon, R. A., Ooi, Y. K., Kim, D. W., Vincent, R. N., Petit, C. J. (2017). Intervention on surgical systemic‐to‐pulmonary artery shunts: Carotid versus femoral access. JACC: Cardiovascular Interventions, 10(17), 1738–1744. DOI 10.1016/j.jcin.2017.05.023. [Google Scholar] [CrossRef]
24. Cantais, A., Hammouda, Z., Mory, O., Patural, H., Stephan, J. L. et al. (2016). Incidence of contrast-induced acute kidney injury in a pediatric setting: A cohort study. Pediatric Nephrology, 31(8), 1355–1362. DOI 10.1007/s00467-016-3313-9. [Google Scholar] [CrossRef]
25. Mobley, M. M., Stroup, R. E., Kaine, S. F. (2013). Comparative risk of cardiac catheterisations performed on low birth weight neonates. Cardiology in the Young, 23(5), 722–726. DOI 10.1017/S1047951112002247. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |