

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.018779
ARTICLE
Pulmonary Perfusion Asymmetry in Patients after Repair of Tetralogy of Fallot: A 4D Flow MRI-Based Study
1Unité Médico-Chirurgicale de Cardiologie Congénitale et Pédiatrique, Centre de Référence des Maladies Cardiaques Congénitales Complexes, Hôpital Universitaire Necker-Enfants Malades, Université de Paris, Paris, France
2Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
3Catholic University of the Sacred Heart, Rome, Italy
4Pediatric Cardiology Department, Hospital de Santa Cruz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal
5Centre de Cardiologie Evecquemont, Paris, France
6Pediatric Radiology Unit, Hôpital Universitaire Necker-Enfants Malades, Université de Paris, Paris, France
7Decision and Bayesian Computation, Computation Biology Department, Neuroscience Department, Paris, France
8School of Biomedical Engineering & Imaging Sciences, King’s College London, London, UK
*Corresponding Author: Francesca Raimondi. Email: francesca.raimondi@gmail.com
#The two authors contributed equally as first author
Received: 17 August 2021; Accepted: 27 October 2021
Abstract: Background: Repaired Tetralogy of Fallot (rTOF) patients may have residual lesions such as main (MPA) and branch pulmonary artery stenosis (BPAS). While MPA stenosis is well studied, few data are available on BPAS in rTOF. We aimed to describe pulmonary perfusion in a large paediatric cohort of rTOF and its impact on right ventricular and outflow-tract hemodynamics using 4D flow CMR. Methods: 130 consecutive patients (mean age at CMR 14.3 ± 4.6 years) were retrospectively reviewed. 96 patients had transannular patch without valve preservation while 34 patients had conserved annulus or valved conduit. A pulmonary blood flow ratio (right pulmonary artery (RPA)/left pulmonary artery (LPA)) between 0.75 and 1.56 was considered normal. Results: Asymmetric pulmonary perfusion was present in 59/130 patients (45%), with 54/59 (91%) having left lung hypoperfusion (blood flow ratio >1.56). RPA/LPA perfusion ratio in the whole cohort was independently associated with the LPA Z-score (−0.053, p = 0.007), the RPA regurgitant fraction (RF) (0.013, p = 0.011) and previous LPA stenting (0.648, p = 0.004). Decreasing LPA % perfusion (and conversely increasing RPA % perfusion) was significantly associated with higher MPA diameter Z-score (−0.06, p = 0.007). On multivariate analysis, MPA Z-score was independently associated with pulmonary RF (0.48, p < 0.001) and with right ventricular indexed volumes (coefficient 3.6, p = 0.023). In patients with transannular patch repair, asymmetric pulmonary flow was an independent predictor of right ventricular ejection fraction (RVEF) (−3.66, p = 0.04). Conclusions: Pulmonary perfusion asymmetry is frequent in rTOF and is associated with abnormal right ventricular and outflow-tract hemodynamics, including MPA dilatation and decreased RVEF in patients after transannular patch.
Keywords: Tetralogy of fallot; cardiac MRI; 4D flow MRI; pulmonary perfusion
| Abbreviations | |
| TOF | Tetralogy of Fallot |
| rTOF | Repaired Tetralogy of Fallot |
| BPAS | Branch pulmonary artery stenosis |
| CHD | Congenital Heart Disease |
| RVOT | Right ventricular outflow tract |
| PVR | Pulmonary valve replacement |
| MPA | Main pulmonary artery |
| PA | Pulmonary artery |
| RV | Right ventricle |
| PR | Pulmonary regurgitation |
| EF | Ejection fraction |
| RVEF | Right ventricular ejection fraction |
| LPA | Left pulmonary artery |
| RPA | Right pulmonary artery |
| RF | Regurgitation fraction |
| PRF | Pulmonary regurgitation fraction |
| LVEDV | Left ventricle end diastolic volume |
Tetralogy of Fallot (TOF) is the most common cyanotic heart defect (CHD), accounting for 3%–5% of all infants born with CHD [1]. The advent of cardiac surgery has led to improved survival to adulthood with about 17 per 100,000 adults currently living with repaired TOF (rTOF) [2,3]. Adult survivors are, however, not cured, and many have residual hemodynamic lesions such as pulmonary regurgitation (PR), residual right ventricular outflow tract (RVOT) or main pulmonary artery (MPA) stenosis, and branch pulmonary artery stenosis (BPAS). Residual stenosis of the MPA is well studied and is associated with abnormal pulmonary artery (PA) growth, leading to adverse right ventricle (RV) remodelling and dysfunction, and chronic ventilation-perfusion mismatch [4,5] with consequent increased RV afterload.
In contrast, very few data are available on the burden of unilateral residual BPAS in rTOF patients. Past investigations found that patients with significant residual unilateral BPAS seem to have reduced exercise tolerance compared to other rTOF patients [6–10]. These studies also suggested that BPAS may exacerbate pulmonary regurgitation (PR).
Pulmonary regurgitation is an important determinant not only of RV dilatation and remodelling but also of exercise capacity in rTOF patients [9,11,12]. PR in these patients has been shown to relate to the use of a transannular patch to reconstruct the RVOT. Furthermore, transannular patching and/or aggressive infundibulectomy predispose to RVOT aneurysms or akinetic regions. Although well tolerated in the short term, PR is an important determinant of late outcome in rTOF patients, being associated with progressive ventricular dysfunction, arrhythmia, exercise intolerance and late sudden death [2–5].
Frigiola et al. [13] demonstrated that in patients with rTOF over 35 years of age and free from pulmonary valve replacement (PVR), normal exercise capacity was associated with unobstructed branch pulmonary arteries, mild residual RVOT obstruction and pulmonary annulus diameter Z-score < 0.5. Interestingly, Petit et al. [14] showed that relief of unilateral BPAS reduces PR and improves RV systolic function in an animal model. In humans, limited data suggest that successful balloon angioplasty of unilateral BPAS can improve exercise capacity, ventilatory efficiency and symptoms in rTOF patients [15,16], but the majority of clinical research has been focused on the successful performance of percutaneous procedures, assuming clinical benefit rather than confirming it.
Furthermore, severity of isolated unilateral BPAS is often underestimated because pulmonary arteries are often poorly visualized by echocardiography and finding “normal right ventricular pressure” may be misleading. Additionally, the belief that PA development has been completed in young patients may contribute to the underestimating unilateral BPAS so that intervention may never be undertaken [16].
4D flow MRI is an accurate, non-invasive method to evaluate flow hemodynamics in rTOF patients [17,18] that does not involve ionizing radiation. It allows evaluation of PA flow and estimation of differential lung perfusion, even after stent implantation, allowing direct measurement of distal branch PA flow, often not possible with conventional 2D phase contrast MRI because of stent artifacts.
The aims of our study are 1) to describe the prevalence of asymmetrical pulmonary perfusion in a large paediatric cohort of rTOF patients; 2) to assess the potential impact of pulmonary perfusion asymmetry on PR, MPA diameter, RV volume and systolic function.
We retrospectively included all consecutive patients with a history of surgical repair of TOF (with pulmonary stenosis or atresia or absent pulmonary valve) who were admitted to one tertiary hospital for their scheduled follow-up from May 2017 to July 2020 and underwent cardiac MRI with 4D flow acquisition. Patients were divided in two groups according to previous surgical treatment: Group 1-transannular patch without valve preservation; Group 2-surgical repair with conserved valve function (conserved annulus or valved RV-PA conduit). The study complied with European GRPD law on retrospective studies (MR004 conformity, registration n° 20200509173610).
Exclusion criteria were: Complex associated cardiac anomalies, presence of major aorto-pulmonary collateral flow, associated respiratory pathologies, or PVR (Fig. 1).
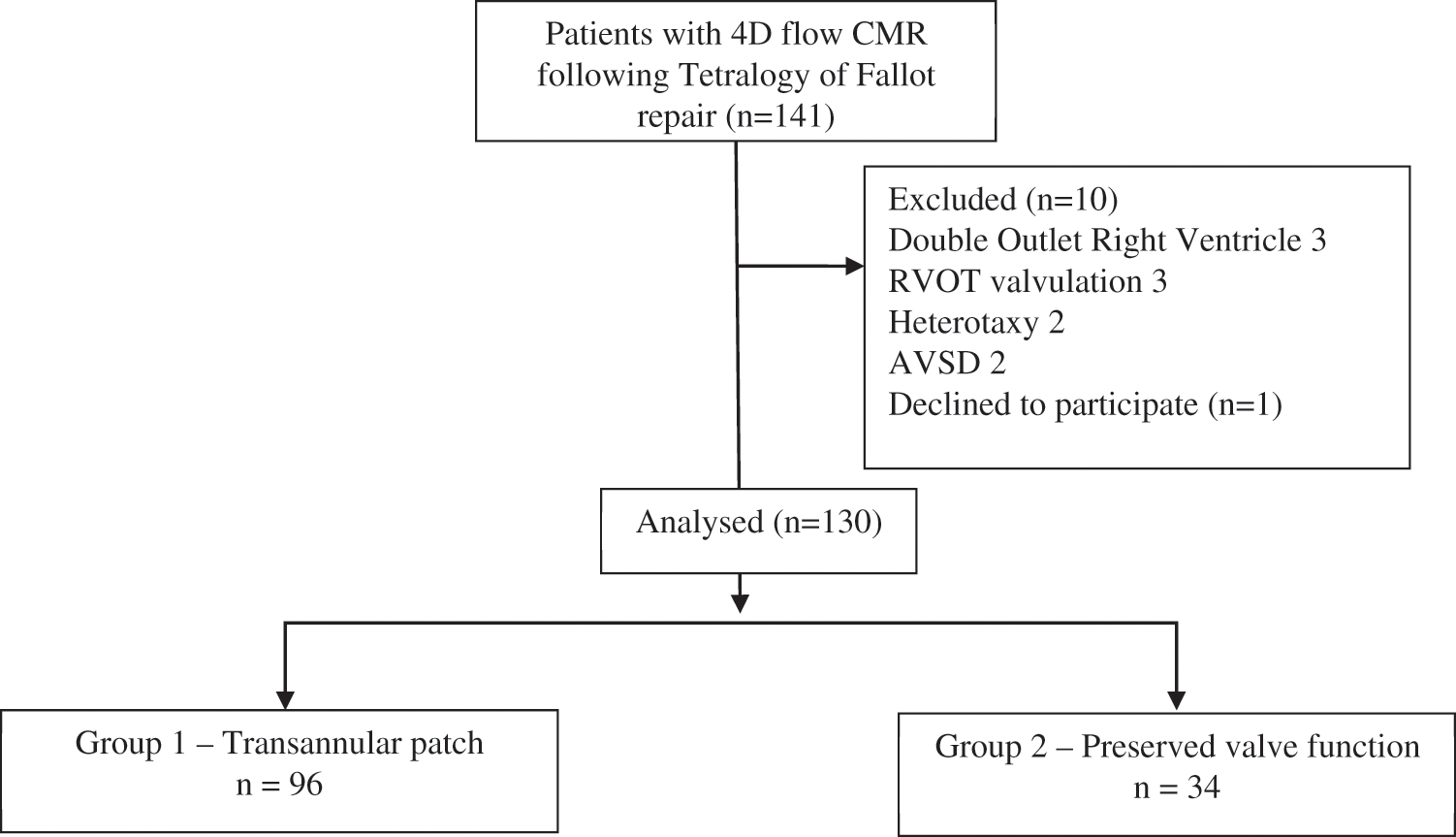
Figure 1: CONSORT flow chart of study cohort
Cardiac magnetic resonance was performed using a 1.5 Tesla magnet (MR450 GE Medical systems, Milwaukee, USA). Images were acquired with a 32-channel phased-array cardiac coil and a vector electrocardiogram for R wave triggering using a standard CMR imaging protocol. The images were acquired during a breath hold if possible, or during free-breathing in patients younger than 6 years of age. RV dimensions and function were obtained from a steady-state free precession short-axis stack (FIESTA), as well as 2 and 4 chamber views.
All 4D CMR flow data were acquired using a gadolinium-based contrast agent (Gadovist 1 mmol/mL, Bayer, Mijdrecht, The Netherlands) and the same 1.5-T Discovery MR450 machine. The images were acquired during free-breathing, using semi-retrospective ECG gating to produce a three-dimensional volume covering the entire heart. The parameters were as follows: TR/TE, 2.7/2.2 ms; flip angle, 10°; acquisition voxel size-2.1 × 2.1 × 2.4 mm; reconstructed voxel size-1.4 × 1.4 × 1.2 mm; 30 phases reconstructed during one average cardiac cycle. The velocity encoding value was individually adapted to yield images without aliasing artefacts (200–550 cm/s).
2.4 Data Analysis and Measurement
The images were read by two experienced paediatric cardiologists (13 and 5 years of experience in congenital heart disease imaging, respectively). Quantification of ventricular volumes and function were performed using dedicated software (Qmass 7, 2, Medis, Leiden, The Netherlands).
Definitions of RV dilatation and normal RV ejection fraction (EF) were based on reported normal values [19].
4D flow raw data were processed using dedicated software (Arterys Inc., San Francisco, CA, USA).
4D flow quantifications were assessed using cloud-based image reconstruction after data correction (Fig. 2, supplementary Video 1).
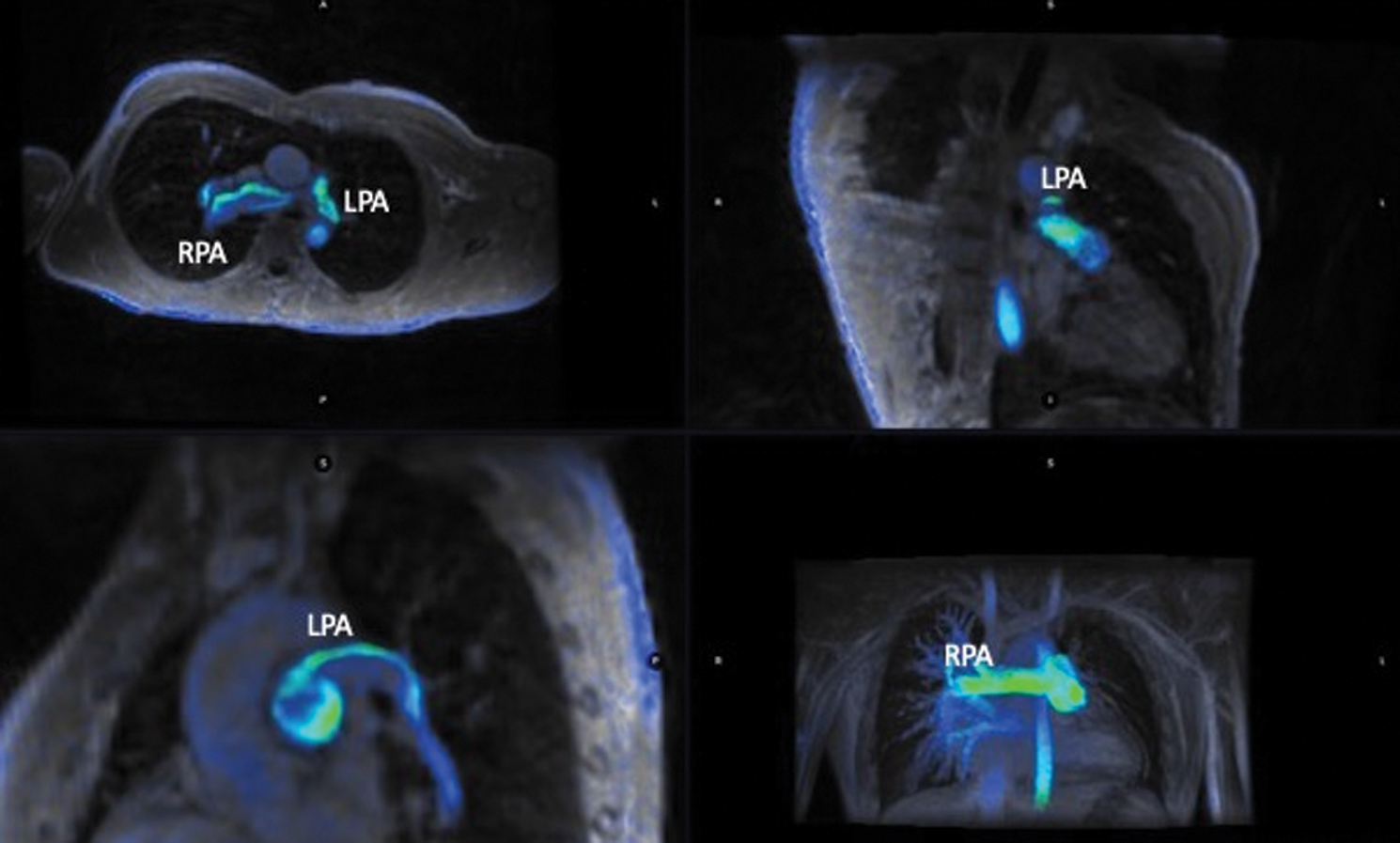
Figure 2: Multiplanar view of 4D flow acquisition in a patient with left lung hypoperfusion. RPA: Right pulmonary artery; LPA: Left pulmonary artery
The pulmonary valve was identified within the volumetric data set on the double-oblique cross-sectional views.
MPA and branch PA diameters were measured during diastole at proximal pulmonary trunk level and proximal part of the branch PAs, respectively. The Z-score was calculated using algorithms reported by Knobel et al. [20].
The following parameters were measured or calculated for MPA, left (LPA) and right (RPA) branches: Forward flow volume; regurgitant flow volume; net forward flow volume; PR fraction (PRF); net forward flow to right lung [as % of total net pulmonary flow], net forward flow to left lung [as % of total net pulmonary flow] and blood flow ratio to the right and left lungs (net forward flow to right lung [%]/net forward flow to left lung [%]). A blood flow ratio (R/L) between 43/57 (0.75) and 61/39 (1.56) was considered normal as proposed by Lin et al. [21]. Asymmetric flow was defined as a blood flow ratio >1, 56 or <0.75.
The relationship between 4D flow CMR parameters and PRF, MPA Z-score, RV ejection fraction (RVEF) and RV end-diastolic volume (RVEDV) was examined to explore the impact of flow abnormalities on right heart hemodynamics.
Continuous data were described as mean ± SD when normally distributed or as median (ICR–interquartile range) if not, and categorical data as number (%). Distribution normality was checked using the Shapiro-Wilk test. The Chi2 test was used to compare categorical variables and Student’s t-test to compare continuous variables. A median regression model was used to identify variables independently associated. Values of p below 0.05 were taken to indicate significant differences. The statistical analyses were performed using JMP 9.1 software (SAS Institute Inc., Cary, USA).
130 consecutive patients (mean age at CMR 14.3 ± 4.6 years) were reviewed. Clinical and CMR characteristics are shown in Table 1. 96 patients had transannular patch without valve preservation (Group 1) while 34 patients had surgical repair with conserved valve function (conserved annulus or valved RV-PA conduit, Group 2). 3/130 (3%) patients had previous RPA stenting, 19/130 (14%, 6%) had previous LPA stenting.
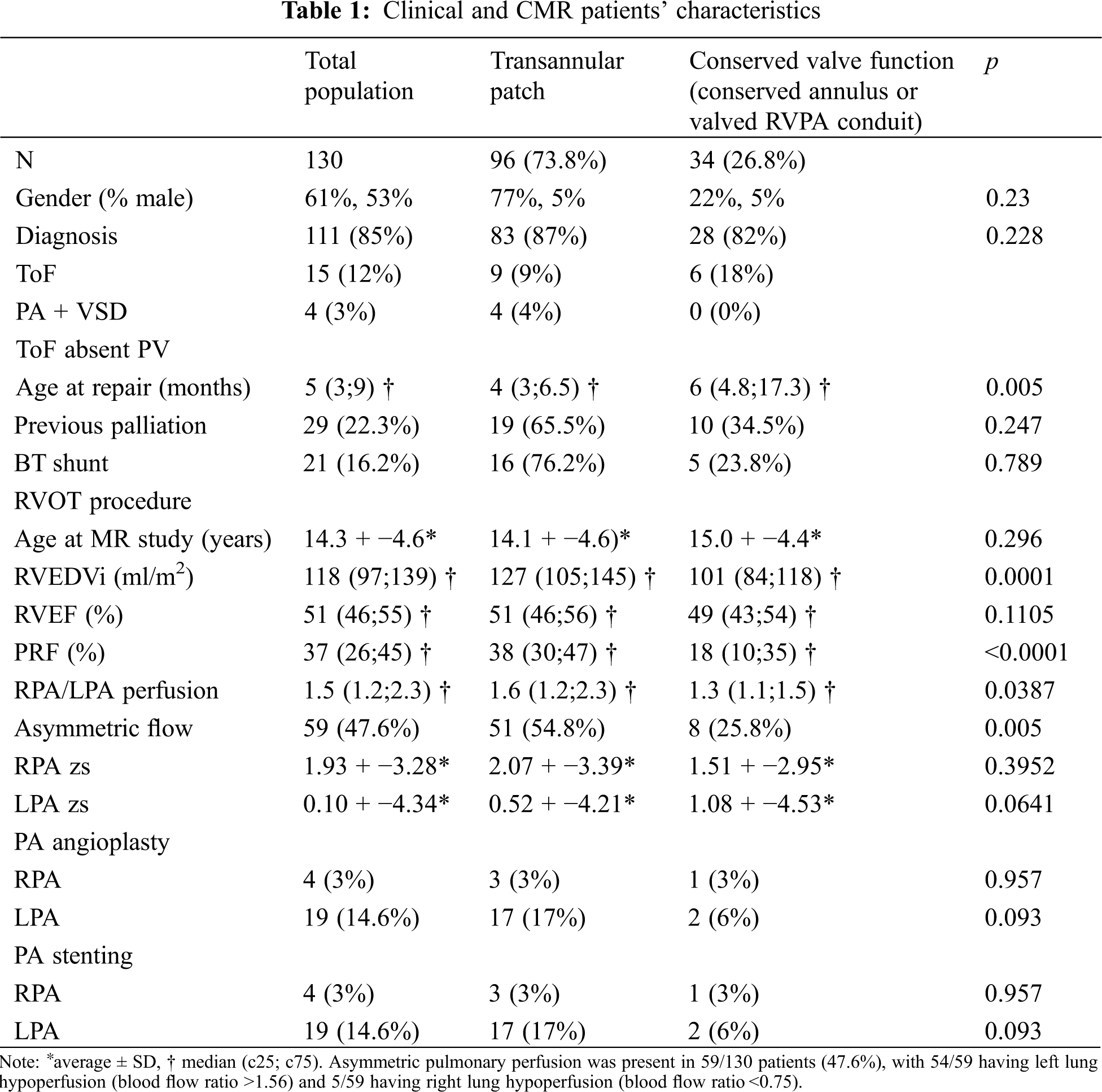
As shown in Table 1, median PRF was 37% (ICR 26–45%) with a statistically significant difference between Group 1 and Group 2. Median RVEDVi was 118 ml/m2 (ICR 97–139 ml) with a significant difference between Groups 1 and 2.
Pulmonary perfusion ratio was significantly different between Groups 1 and 2 with higher prevalence of asymmetric flow in Group 1 than in Group 2.
3.2 Predictors of RPA/LPA Perfusion Ratio and Asymmetric Flow
On multivariate analysis, the RPA/LPA perfusion ratio in the whole cohort was independently associated with the LPA Z score, the regurgitant fraction of the RPA (respectively, coefficient −0.053 p = 0.007 and coefficient 0.013 p = 0.011) and previous LPA stenting (coefficient 0.648, p = 0.004). Indeed, a higher RPA RF and presence of a stent in the LPA were associated with a higher likelihood of asymmetric pulmonary blood flow (respectively OR 1.03, p = 0.037; OR 4.3, p = 0.02).
In a subgroup analysis of group 1, independent associations of RPA/LPA perfusion ratio were LPA Z-score (coefficient −0.06, p = 0.02) and LPA stenting (coefficient 0.625, p = 0.035), while no associations were found for Group 2. A lower LPA Z-score was also associated with overall asymmetric pulmonary perfusion (OR 0.84, p = 0.04).
Additionally, in the full cohort, decreasing LPA % perfusion (and conversely increasing RPA % perfusion) was significantly associated with higher MPA Z-score (coefficient −0.06, p = 0.007, Supplementary Fig. 1).
On multivariate analysis MPA Z-score was independently associated with PRF (coefficient 0.48, p < 0.001) in the whole cohort. Subgroup analysis showed independent association of PRF with LPA stenting (coefficient 3.2, p < 0.01), but only in the group with transannular patch (Group 1).
3.4 Predictors of RVEDVi and RVEF
In the whole cohort, PRF was the only independent predictor of RVEDVi (0.98, p = 0.039). However, in subgroup analysis (Fig. 3), MPA Z-score was also associated with the RVEDVi independently of PRF (coefficient 3.6, p = 0.023) in the patients with transannular patch (Group 1).
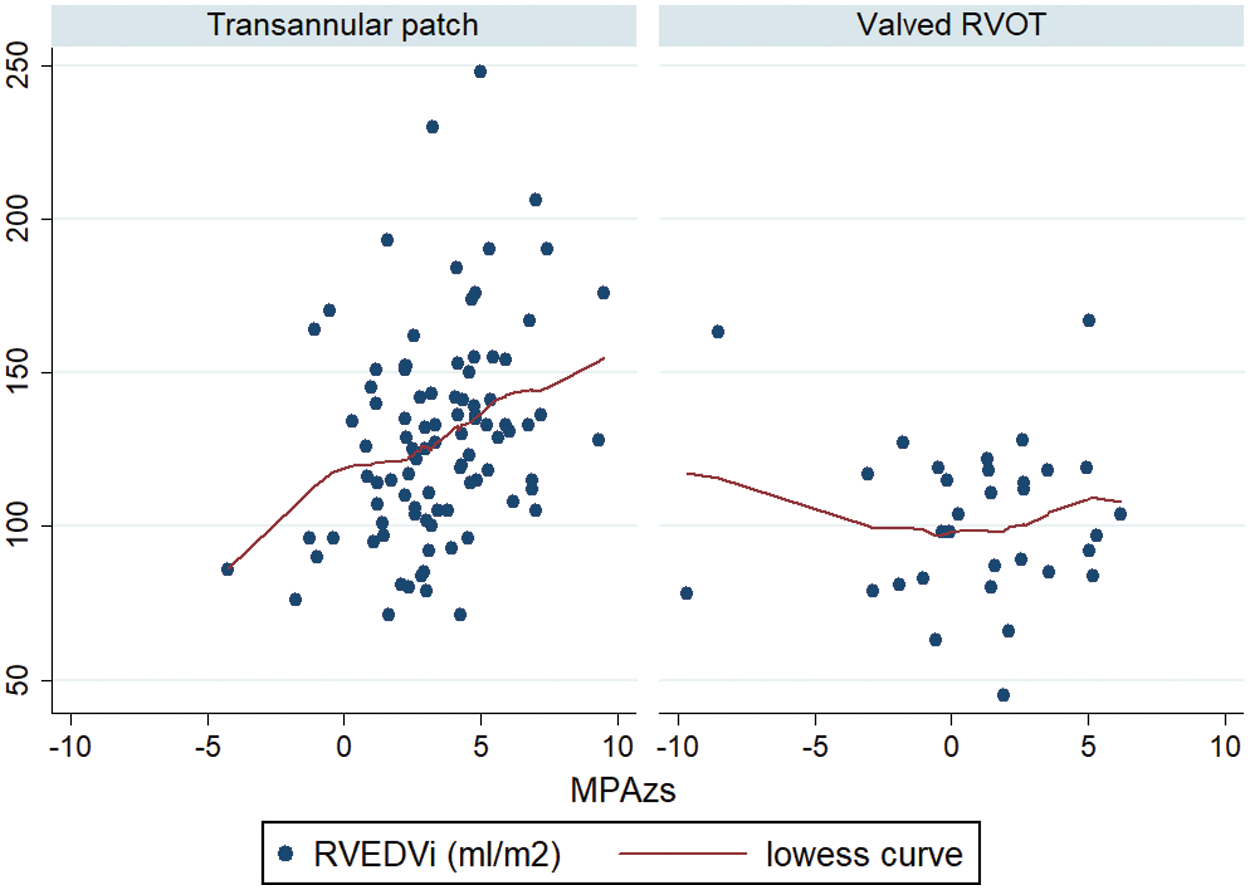
Figure 3: Correlation between MPA Z-score and RVEDVi in Group 1 and Group 2. MPA: Main pulmonary artery; RVEDVi: Right ventricular end diastolic volume indexed for BSA
The only significant independent predictor of RVEF identified was asymmetric pulmonary flow in Group 1 (−3.66, p = 0.04), as displayed in Fig. 4. No other predictors were identified in either the full cohort or Group 2.
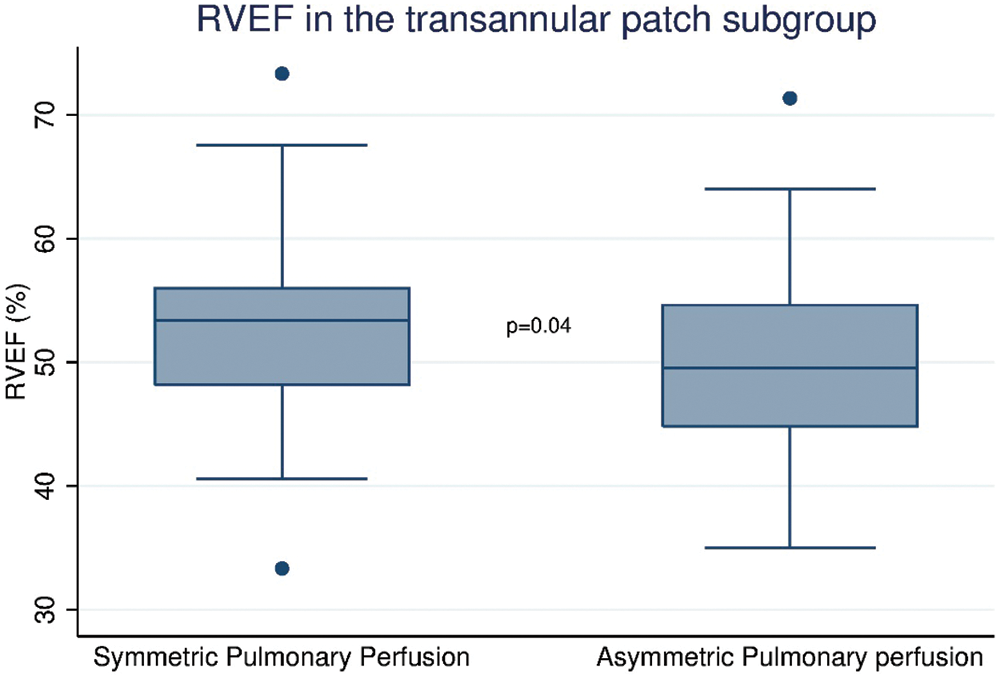
Figure 4: Correlation between presence or absence of asymmetric pulmonary perfusion and RVEF % in Group 1. RVEF: Right ventricular ejection fraction
This is a retrospective, cross-sectional observational study, with no longitudinal data. Consequently, progression of flow dynamics and right heart hemodynamics cannot be evaluated.
Longitudinal studies are needed to explore the long-term role of predictors identified in our study, by repeating MRI 4D flow study regularly in the same cohort of patients.
To the best of our knowledge this is the first study analysing pulmonary perfusion distribution and its impact on right heart hemodynamics in a large cohort of paediatric patients. Pulmonary perfusion asymmetry is often underestimated, because echocardiography cannot show the anatomy of the distal branch pulmonary arteries and cannot measure relative pulmonary flow.
Previous studies have suggested that pulmonary flow maldistribution may have an adverse impact on exercise performance and general clinical status of the patient [6–10]. For this reason, we wanted to explore pulmonary flow distribution in a large cohort of paediatric patients after repair of TOF.
Our results show that pulmonary blood flow is often asymmetric (48% of all our cohort) and that the most prevalent form of asymmetry is left lung hypoperfusion with a prevalence of 42% (54/130) in our population. Asymmetric flow is also more prevalent after transannular patch without valve preservation. We confirmed that this group of patients is also more prone to RV dilation and higher PRF.
Moreover, we demonstrated that in patients without valve preservation (Group 1), RPA/LPA perfusion ratio is strongly associated with the dimension of the LPA and the presence of a LPA stent and not with branch PA RF. Interestingly, asymmetric pulmonary flow is the only independent predictor of lower RV ejection fraction that we found in this patient group.
Furthermore, decreasing LPA % perfusion (and conversely increasing RPA % perfusion) is associated with a higher MPA Z-score, an independent predictor of both PRF and RV dilatation. We speculate that unbalanced pulmonary flow adversely influences right heart hemodynamics, leading to dilatation of the pulmonary trunk and progression of PRF, RV dilatation and decreased of RV EF in patients repaired without valve preservation (Group 1).
In patients with preserved valve or valved conduit (Group 2), we found no evidence of an impact of asymmetry on right heart hemodynamics. Our findings are consistent with the hemodynamic characteristics of a more rigid system with a preserved valve or surgical conduit.
Identifying better predictors of right heart hemodynamics is important for decision-making in patient after TOF repair. We know that chronic PR adversely affects prognosis [2–5], leading to RV dysfunction, reduced exercise capacity, arrhythmias and sudden death. Severe PR and RV dilatation are also included in the decision algorithm for PVR. The current indications for PVR in rTOF with hemodynamically significant PR, according to the most recent guidelines, are based on the presence of symptoms (Class I) [22]. In asymptomatic patients, PVR performed prior to a specific ventricular size is associated with normalization of RV volumes, but not improved morbidity or mortality [23,24].
A recent study from Boston Children’s Hospital found no evidence that mild or even moderate RV dilatation after PVR—based on end-diastolic volume criteria alone—is associated with adverse clinical outcomes [25].
No extensive study has been performed exploring the role of unbalanced pulmonary perfusion in patients after TOF repair before or after PVR. Here, we demonstrate that pulmonary perfusion maldistribution is common in patients after TOF repair. In our cohort this condition has a clear association with impaired right heart hemodynamics, independent of PRF, in the presence of a transannular patch (Supplementary Fig. 2).
Another recent study from our group has demonstrated that in patients with severe PR, no resting MRI measurement predicts poor exercise performance [26]. We speculate that other MRI parameters, such as pulmonary perfusion asymmetry, should be investigated as predictors of exercise performance. If our findings are further clarified and confirmed, routine study of pulmonary perfusion symmetry should be added to follow up MRI protocols and the presence of unbalanced perfusion should be taken into account in management decisions.
Pulmonary perfusion asymmetry, in particular LPA hypoperfusion, is common in rTOF patients, and is strongly associated with branch PA hypoplasia. Perfusion asymmetry is associated with MPA dilatation and RV dysfunction in the presence of a transannular patch. Assessment of pulmonary flow distribution should be part of routine follow-up in rTOF patients. Longitudinal studies are needed to study hemodynamic and clinical impact of pulmonary flow maldistribution over time.
Authors contribution:
Elena Panaioli: Data collection, manuscript redaction
Duarte Martins: Manuscript conception, Data analysis, manuscript redaction
Marc Antoine Isorni: Manuscript conception, Data analysis
Diala Khraiche: Data collection, manuscript review
Antoine Legendre: Data collection, manuscript review
Nathalie Boddaert: manuscript review
Damien Bonnet: manuscript review
Filippo Crea: manuscript review
Francesca Raimondi: Manuscript conception, data collection, data analysis, manuscript redaction.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The corresponding author Dr. Francesca Raimondi is a member of the Editorial Board of Congenital Heart Disease. And Dr. Francesca Raimondi was not involved in any review sessions for this article. This submission is subject to the exact same review process as any other manuscript.
1. Apitz, C., Webb, G. D., Redington, A. N. (2009). Tetralogy of fallot. The Lancet, 374(9699), 1462–1471. DOI 10.1016/S0140-6736(09)60657-7. [Google Scholar] [CrossRef]
2. Hickey, E. J., Veldtman, G., Bradley, T. J., Gengsakul, A., Manlhiot, C. et al. (2009). Late risk of outcomes for adults with repaired tetralogy of fallot from an inception cohort spanning four decades. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 35(1), 156–164. DOI 10.1016/j.ejcts.2008.06.050. [Google Scholar] [CrossRef]
3. Marelli, A. J., Mackie, A. S., Ionescu-Ittu, R., Rahme, E., Pilote, L. (2007). Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation, 115(2), 163–172. DOI 10.1161/CIRCULATIONAHA.106.627224. [Google Scholar] [CrossRef]
4. Gatzoulis, M. A., Balaji, S., Webber, S. A., Siu, S. C., Hokanson, J. S. et al. (2000). Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of fallot: A multicentre study. The Lancet, 356(9234), 975–981. DOI 10.1016/S0140-6736(00)02714-8. [Google Scholar] [CrossRef]
5. Harrison, D. A., Harris, L., Siu, S. C., MacLoghlin, C. J., Connelly, M. S. et al. (1997). Sustained ventricular tachycardia in adult patients late after repair of tetralogy of fallot. Journal of the American College of Cardiology, 30(5), 1368–1373. DOI 10.1016/S0735-1097(97)00316-1. [Google Scholar] [CrossRef]
6. Rhodes, J., Dave, A., Pulling, M. C., Geggel, R. L., Marx, G. R. et al. (1998). Effect of pulmonary artery stenoses on the cardiopulmonary response to exercise following repair of tetralogy of fallot. The American Journal of Cardiology, 81(10), 1217–1219. DOI 10.1016/s0002-9149(98)00095-2. [Google Scholar] [CrossRef]
7. Dowdle, S. C., Human, D. G., Mann, M. D. (1990). Pulmonary ventilation and perfusion abnormalities and ventilation perfusion imbalance in children with pulmonary atresia or extreme tetralogy of fallot. Journal of Nuclear Medicine, 31(8), 1276–1279. [Google Scholar]
8. Rosenthal, G. L. (2008). Exercise capacity after pulmonary artery angioplasty of TOF. AAP Grand Rounnds 32008, 19(6), 65. DOI 10.1542/gr.19-6-659. [Google Scholar] [CrossRef]
9. Rowe, S. A., Zahka, K. G., Manolio, T. A., Horneffer, P. J., Kidd, L. (1991). Lung function and pulmonary regurgitation limit exercise capacity in postoperative tetralogy of fallot. Journal of the American College of Cardiology, 17(2), 461–466. DOI 10.1016/S0735-1097(10)80116-0. [Google Scholar] [CrossRef]
10. Wessel, H. U., Cunningham, W. J., Paul, M. H., Bastanier, C. K., Muster, A. J. (1980). Exercise performance in tetralogy of fallot after intracardiac repair. The Journal of Thoracic and Cardiovascular Surgery, 80(4), 582–593. [Google Scholar]
11. Marx, G. R., Hicks, R. W., Allen, H. D., Goldberg, S. J. (1988). Noninvasive assessment of hemodynamic responses to exercise in pulmonary regurgitation after operations to correct pulmonary outflow obstruction. The American Journal of Cardiology, 61(8), 595–601. DOI 10.1016/0002-9149(88)90771-0. [Google Scholar] [CrossRef]
12. Bouzas, B., Kilner, P. J., Michael, A. (2005). Gatzoulis. Pulmonary regurgitation: Not a benign lesion. European Heart Journal, 26, 433–439. DOI 10.1093/eurheartj/ehi091. [Google Scholar] [CrossRef]
13. Frigiola, A., Hughes, M., Turner, M., Taylor, A., Marek, J. et al. (2013). Physiological and phenotypic characteristics of late survivors of tetralogy of fallot repair who are free from pulmonary valve replacement. Circulation, 128(17), 1861–1868. DOI 10.1161/CIRCULATIONAHA.113.001600. [Google Scholar] [CrossRef]
14. Petit, C. J., Gillespie, M. J., Harris, M. A., Seymour, T. L., Liu, T. Y. et al. (2009). Relief of branch pulmonary artery stenosis reduces pulmonary valve insufficiency in a swine model. The Journal of Thoracic and Cardiovascular Surgery, 138(2), 382–389. DOI 10.1016/j.jtcvs.2009.02.030. [Google Scholar] [CrossRef]
15. Sutton, N. J., Peng, L., Lock, J. E., Lang, P., Marx, G. R. et al. (2008). Effect of pulmonary artery angioplasty on exercise function after repair of tetralogy of fallot. American Heart Journal, 155(1), 182–186. DOI 10.1016/j.ahj.2007.08.019. [Google Scholar] [CrossRef]
16. Hiremath, G., Qureshi, A. M., Prieto, L. R., Nagaraju, L., Moore, P. et al. (2019). Balloon angioplasty and stenting for unilateral branch pulmonary artery stenosis improve exertional performance. JACC: Cardiovascular Interventions, 12(3), 289–297. DOI 10.1016/j.jcin.2018.11.042. [Google Scholar] [CrossRef]
17. Isorni, M. A., Martins, D., Ben Moussa, N., Monnot, S., Boddaert, N. et al. (2020). 4D flow MRI vs. conventional 2D for measuring pulmonary flow after tetralogy of fallot repair. International Journal of Cardiology, 300, 132–136. DOI 10.1016/j.ijcard.2019.10.030. [Google Scholar] [CrossRef]
18. Isorni, M. A., Moisson, L., Moussa, N. B., Monnot, S., Raimondi, F. et al. (2020). 4D flow cardiac magnetic resonance in children and adults with congenital heart disease: Clinical experience in a high volume center. International Journal of Cardiology, 320, 168–177. DOI 10.1016/j.ijcard.2020.07.021. [Google Scholar] [CrossRef]
19. Kawel-Boehm, N., Maceira, A., Valsangiacomo-Buechel, E. R., Vogel-Claussen, J., Turkbey, E. B. et al. (2015). Normal values for cardiovascular magnetic resonance in adults and children. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 17(1), 29. DOI 10.1186/s12968-015-0111-7. [Google Scholar] [CrossRef]
20. Knobel, Z., Kellenberger, C. J., Kaiser, T., Albisetti, M., Bergsträsser, E. et al. (2011). Geometry and dimensions of the pulmonary artery bifurcation in children and adolescents: Assessment in vivo by contrast-enhanced MR-angiography. The International Journal of Cardiovascular Imaging, 27(3), 385–396. DOI 10.1007/s10554-010-9672-6. [Google Scholar] [CrossRef]
21. Lin, C. Y. (1971). Lung scan in cardiopulmonary disease: I. Tetralogy of fallot. The Journal of Thoracic and Cardiovascular Surgery, 61(3), 370–379. DOI 10.1016/S0022-5223(19)42220-4. [Google Scholar] [CrossRef]
22. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2018). AHA/ACC guideline for the management of adults with Congenital Heart Disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation, 139(14), e698–e800. DOI 10.1016/j.jacc.2018.08.1029. [Google Scholar] [CrossRef]
23. Geva, T. (2014). Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease? Circulation. Cardiovascular Imaging, 7(1), 190–197. DOI 10.1161/CIRCIMAGING.113.000553. [Google Scholar] [CrossRef]
24. Oosterhof, T., van Straten, A., Vliegen, H. W., Meijboom, F. J., van Dijk, A. P. et al. (2007). Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of fallot using cardiovascular magnetic resonance. Circulation, 116(5), 545–551. DOI 10.1161/CIRCULATIONAHA.106.659664. [Google Scholar] [CrossRef]
25. Pastor, T. A., Geva, T., Lu, M., Duarte, V. E., Drakeley, S. et al. (2020). Relation of right ventricular dilation after pulmonary valve replacement to outcomes in patients with repaired tetralogy of fallot. The American Journal of Cardiology, 125(6), 977–981. DOI 10.1016/j.amjcard.2019.12.017. [Google Scholar] [CrossRef]
26. Karsenty, C., Khraiche, D., Jais, J. P., Raimondi, F., Ladouceur, M. et al. (2021). Predictors of low exercise cardiac output in patients with severe pulmonic regurgitation. Heart (British Cardiac Society), 107(3), 223–228. DOI 10.1136/heartjnl-2020-317550. [Google Scholar] [CrossRef]
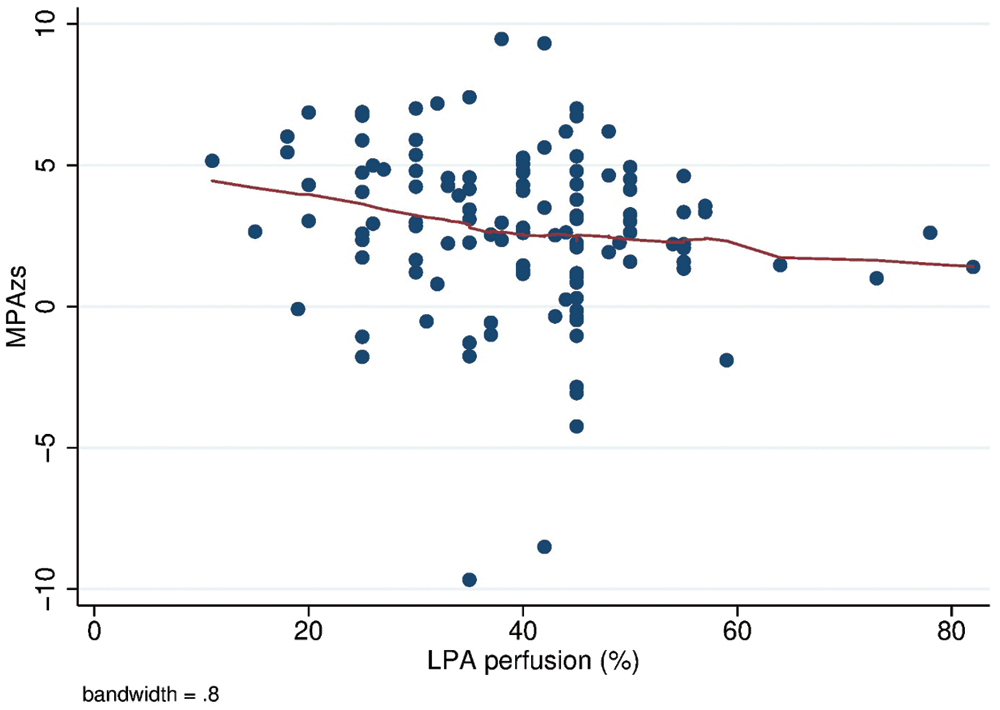
Supplementary Figure 1: Correlation between % of left pulmonary perfusion and MPA Z-score. MPA zs: Main pulmonary artery Z-score; LPA: Left pulmonary artery
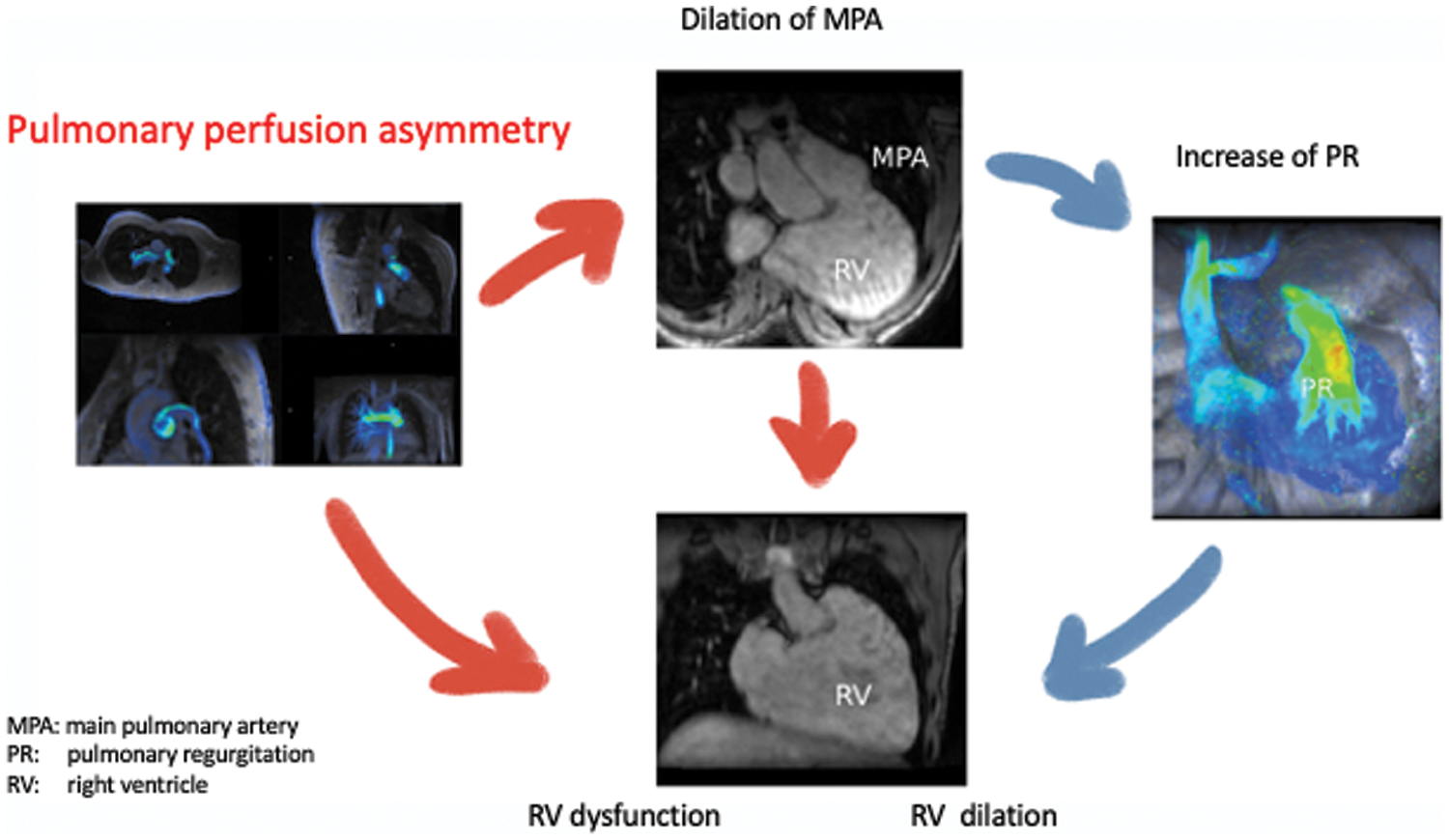
Supplementary Figure 2: Graphical abstract about physiopathological hypothesis of the impact of pulmonary perfusion asymmetry on right ventricular dilation and dysfunction, independently from pulmonary regurgitation
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |