

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.018350
CASE REPORT
A Rare Case of Infective Mediastinitis after Melody Valve Implantation
1Department of Cardiovascular and Thoracic Surgery, Cliniques Universitaires Saint-Luc, Brussels, Belgium
2Department of Pediatric Cardiology, Cliniques Universitaires Saint-Luc, Brussels, Belgium
*Corresponding Author: Alain Poncelet. Email: alain.poncelet@uclouvain.be
Received: 18 July 2021; Accepted: 11 September 2021
Abstract: Pulmonary valve implant is frequently necessary in children and adults with congenital heart disease. Infective endocarditis represents a rare but life-threatening complication after transcatheter pulmonary valve implantation. There are various treatments for native or prosthetic valve endocarditis. Surgical intervention, combined with intravenous antibiotic treatment, is of paramount importance, in case of concomitant mediastinal infection, in order to ensure the radical debridement of all infected tissue, avoiding any recurrent endocarditis. In this report, we describe a rare case of mediastinitis, associated with an infected endocarditis, occurring 8 months after Melody (Medtronic®, Minneapolis, USA) valve implant, successfully treated with the implantation of a homograft to reconstruct the right ventricular outflow tract.
Keywords: Transcatheter valve prosthesis; infective endocarditis; cardiac surgery; congenital heart disease; homograft
Over the last 20 years transcatheter pulmonary valve implantation has proven excellent hemodynamic results, alleviating the risks of repeated surgical procedure in patients with congenital heart disease. As such, it became an important therapeutic tool for the management of right ventricular outflow tract (RVOT) dysfunctions.
However, there is a 3–8% incidence of infective endocarditis following Melody (Medtronic®, Minneapolis, USA) valve implant. Re-interventions (endovascular or surgical) are often required, but clear guidelines are lacking on the optimal choice of treatment [1].
In this report we describe a rare case of concomitant mediastinitis, and infective endocarditis of a Melody valve implanted 8 months earlier in an 18-year-old patient with stenosis of a Contegra® conduit (Medtronic®, Minneapolis, USA). At the age of 7 months, he had undergone pulmonary atresia repair, with a 16-mm Contrega® for RVOT reconstruction.
The patient presented with a massive vegetation on the pulmonary valve prosthesis as well as localized mediastinitis. He was operated urgently because of hemodynamic deterioration and mediastinal infection. The operative approach combined debridement surgery as well as the use of a pulmonary homograft to reconstruct the right ventricular outflow tract.
After an initial diagnosis of pulmonary atresia with ventricular septal defect, the patient was palliated in neonatal period (Modified Blalock-Taussig shunt) and a complete repair was performed at the age of 7 months. A 16-mm Contrega conduit (Medtronic®) was used to reconstruct the right ventricular outflow tract (RVOT).
At age of 18, while asymptomatic, follow-up transthoracic echocardiography showed a severe pulmonary valve disease, with a peak gradient of 64 mmHg and moderate pulmonary valve regurgitation. Right ventricular pressure was >75% of systemic pressure. Balloon valvuloplasty was performed followed by 3 consecutive stents implantations in the calcified Contrega conduit during the same procedure.
One month later, the patient underwent Melody valve implantation (Medtronic®, Minneapolis, USA) mounted on a 20-mm Ensemble delivery system. Peri-procedural antibiotic prophylaxis was administered. He made an uneventful recovery and was discharged on day 1. He was regularly followed in our adult congenital heart disease clinic.
Eight months later, the patient was admitted to the hospital, suffering from headaches, and vomiting with hyperpyrexia for three days. Due to rapid hemodynamic deterioration, he was transferred to our intensive care unit, and intravenous norepinephrine was started.
Serial blood cultures grew positive for Methicillin-sensitive Staphylococcus Aureus (MSSA) microorganism and cardiac echocardiography revealed a stenotic pulmonary valve (peak gradient 43 mmHg, pulmonary valve area 0.9 cm2) with severe regurgitation and clear images of endocarditis of the pulmonary valve. The right ventricle appeared dilated.
The diagnosis of endocarditis was confirmed by a PET-SCAN with suspicion of an abscess between the upper part of the conduit and the sternum. In addition, it revealed secondary septic localizations with multiple pulmonary lesions suggestive of pulmonary emboli, as well as a right hip arthritis (Fig. 1).
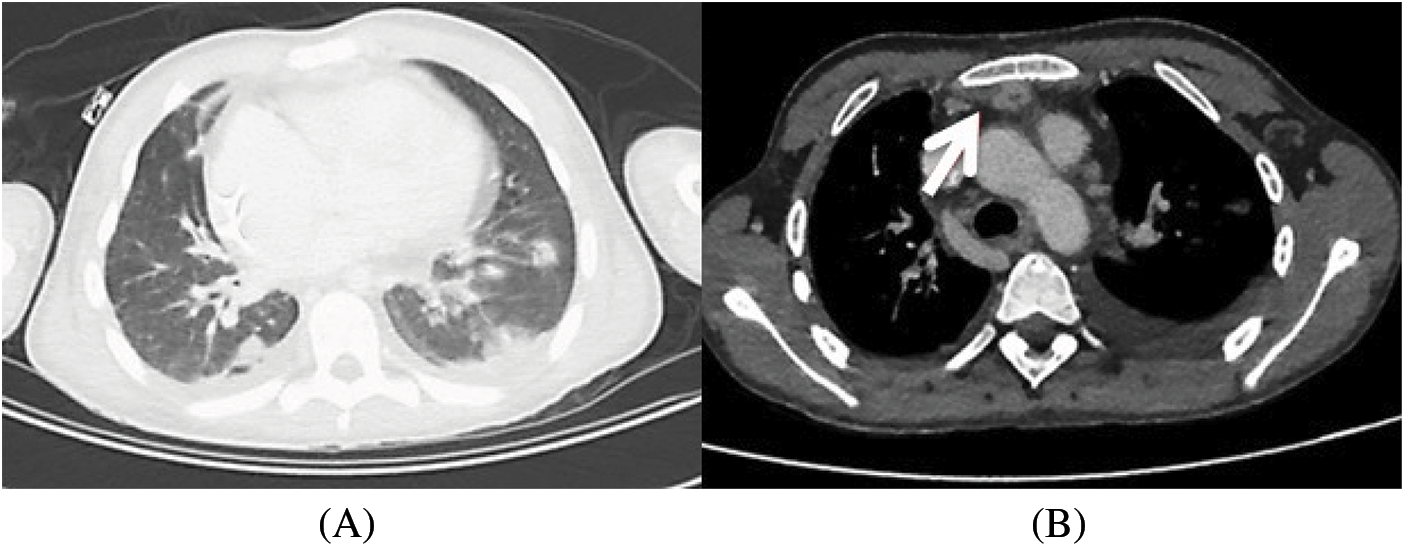
Figure 1: CT scan that shows pulmonary embolism (1A) and mediastinal collection (1B)
In the context of mediastinal abscess complicating right-sided valve endocarditis, the decision was made to proceed with surgery while parenteral antibiotics (Flucloxacillin and Ceftazidime) were administered.
At surgery, after an uneventful repeat sternotomy, the diagnosis of mediastinitis was confirmed, with purulent material at the level of the old Gore-tex membrane used 18 years earlier to close the pericardium. This infection was adjacent to the anterior part of the Contrega Conduit (Fig. 2).
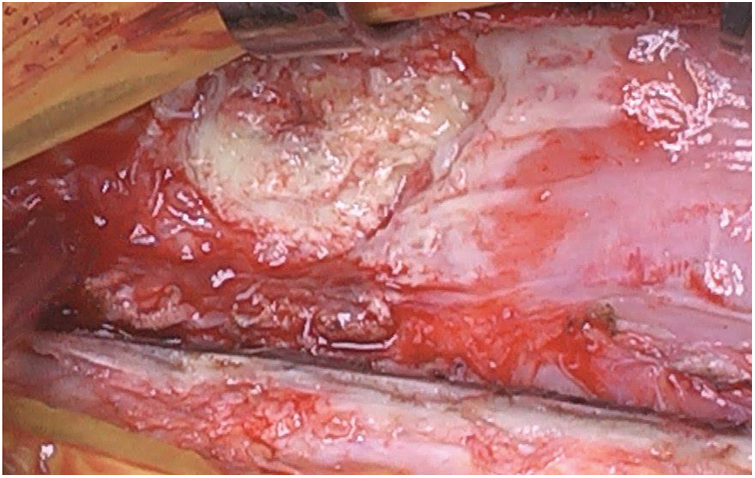
Figure 2: The infection on the Gore-tex membrane
The foreign body material was removed, the area of purulent material debrided, and the anterior mediastinum was irrigated with povidone-iodine solution.
Following the debridement, peripheral cardiopulmonary bypass (CPB) was initiated via the femoral vein and artery. On a beating heart, the right ventricle infundibulum was opened, the previously placed Contrega conduit was resected “en-bloc” together with the 3 stents and the Melody valve (Fig. 3). The prosthesis appeared calcified with large vegetations. A 26-mm pulmonary homograft (European Homograft Bank, St-Jean Clinic, Brussels) was used for RVOT reconstruction.
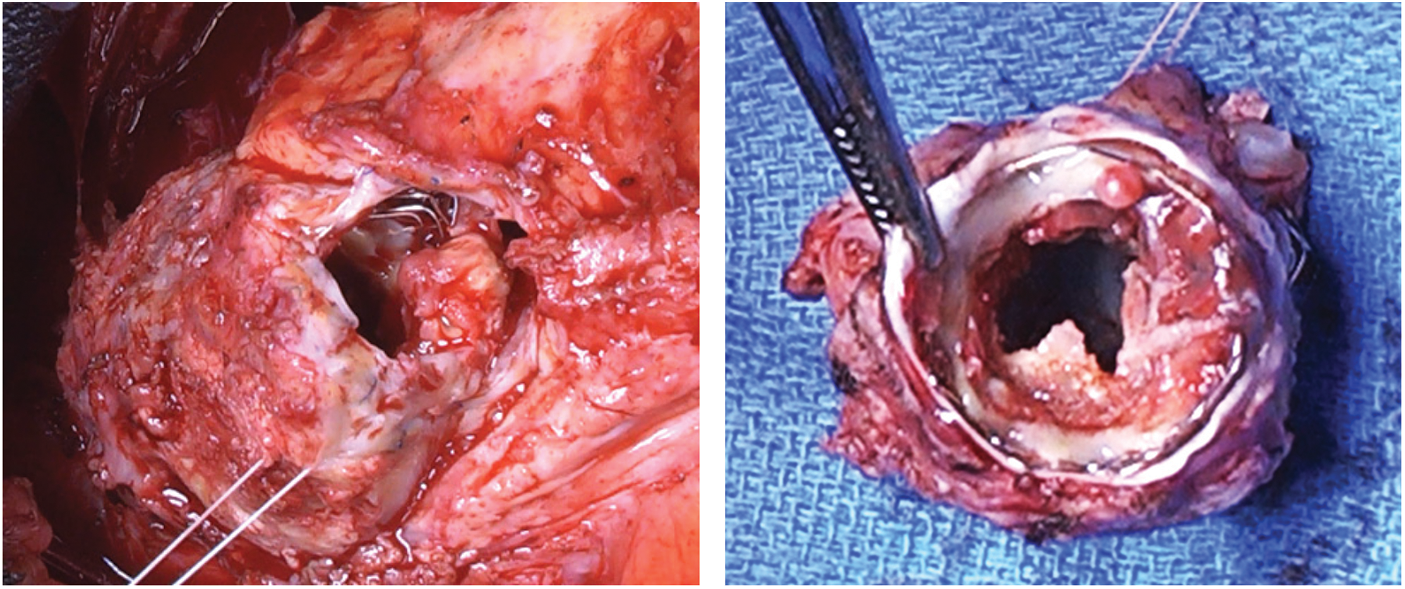
Figure 3: The large vegetation of the valve
Total cardiopulmonary bypass (CPB) time was 77 min without cross-clamping. The sternum was primarily closed. Two mediastinal drains were left in place, one in pericardial cradle and one in the anterior mediastinum, as usual. Another Gorethex membrane was not placed to close the pericardium, in order to avoid the use of any prothesic material.
Peri-operative echocardiography confirmed a normal functioning pulmonary valve homograft. The patient underwent an uneventful post-operative course. He was extubated the same day and was transferred on post-operative day 10 to a rehabilitation facility to pursue his 6-week course of intravenous antibiotic treatment.
The perioperative cultures resulted negative, thanks to the effectiveness of the antibiotic treatment that was ongoing at the time of the surgery (targeted antibiotic treatment started ten days before surgery).
As the blood cultures showed a good sensitivity to antibiotics, we did not carry out more extensive tests, like PCR, on the surgical material.
Since its first description in 2000 [2], percutaneous bio-prosthetic pulmonary valve implantation has received great credit in previously operated patients with congenital heart disease as well as in patients with acquired valvular disease alleviating the risks of repeated surgical procedure [3].
Various studies [4,5] confirmed their effectiveness in restoring valve competence. However, there is still a 3–8% risk of infective endocarditis (IE), in particular, following Melody valve implantation [6–9].
Prosthetic valve infective endocarditis (PVE) are life-threatening conditions. In a recent study reporting a post-Melody PVE rate of 2.4% patient-year, Bos et al. [10] underscored that 20% of those patients presented either with severe sepsis and/or heart failure, and 30% with severe RVOT obstruction [10]. Similarly in his recent large-scale multi-centric study, McElhinney et al. [11] reported an annualized incidents of 2.2% patient-year. Endocarditis was severe in 44% of the patients, resulting in an overall mortality rate of 6.6%. Nevertheless, with regard to the right sided PVE, current guidelines are not clear as outlined by Gierlinger et al. [6], especially concerning the pulmonary valve. With regard to IE in congenital heart diseases, the 2015 guidelines state that cardiac surgery is appropriate when a) medical therapy fails, b) serious haemodynamic complications arise or c) there is a high risk of septic embolism [1]. For right-sided IE especially, Habib et al. [1] highlighted that intravenous antibiotics are the cornerstone of treatment: a 2-week regiment may be sufficient for MSSA infection in the absence of PVE, whereas a 4–6-week regimen must be used in the context of a) vegetations larger than 20 mm, b) acute respiratory failure, c) septic systemic emboli or d) cardiac complications. However, those guidelines did not address the type of valved conduit to select for replacement in the context of pulmonary valve endocarditis.
For PVE, the 2015 guidelines underscore the need for prolonged antibiotic therapy and early radical surgery with radical debridement for the criteria cited above, together with the presence of staphylococcal species. This more aggressive management does not comment on the respective role of valvular homograft or stentless xenografts in this setting [1]. In our patient, based on guidelines recommendations for IE (PVE, and septic emboli), together with the presence of mediastinitis, specific parenteral antibiotic treatment was maintained for six weeks following surgery.
When deciding on the best therapeutic options for RVOT dysfunction, it is necessary to keep in mind that congenital heart disease patients are often young. Therefore, the cumulative risk of IE is higher due to their expected lifespan. In addition, the presence of more prosthetic material, can lead to recurrent endocarditis, chronic infection and it can later on increase the perioperative risk profile [6].
Our patient developed an “early” PVE (8 months after Melody valve implantation). Mediastinitis is likely to have occurred secondary to one of the two percutaneous interventions, as a loco-regional spread from the active infection site. We hypothesize that it could be due to a covered rupture of the previously placed Contrega conduit following stents or valve deployment.
Even though this is a very rare complication after Melody valve implantations, it becomes central when deciding on the surgical procedure. In fact, any prosthetic material adjacent to the infected valve has a greater risk to become infected too. In his series of 20 patients with right-sided PVE, Gierlinger et al. [6] identified only a single case of concomitant mediastinitis (5%): debridement, excision of all infected material and pulmonary homograft implantation was successfully performed as in our patients [6].
When pulmonary valve replacement is indicated for native IE and/or PVE, the use of pulmonary homograft is reported to have the lowest rate of endocarditis, when compared to the use of Melody or Contrega valves or even the Hancock® Bioprosthetic Valved Conduit (Medtronic) [7,12–14].
Right-sided prosthetic valve infective endocarditis is not an uncommon complication and can be life-threatening. Concomitant mediastinal infection has rarely been reported but should be routinely searched for as it represents a clear indication for surgical intervention together with a long-course of antimicrobial therapy.
Homograft remains the best surgical substitute in the context of right-sided prosthetic valve infective endocarditis.
Ethical Statement: The patient gave us the written informed consent to publish the article.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Habib, G., Lancellotti, P., Antunes, M. J., Bongiorni, M. G., Casalta, J. P. et al. (2015). 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the european society of cardiology (ESC). Endorsed by: European association for cardio-thoracic surgery (EACTSthe European Association of Nuclear Medicine (EANM). European Heart Journal, 36(44), 3075–3128. DOI 10.1093/eurheartj/ehv319. [Google Scholar] [CrossRef]
2. Bonhoeffer, P., Boudjemline, Y., Saliba, Z., Merckx, J., Aggoun, Y. et al. (2000). Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet, 356(9239), 1403–1405. DOI 10.1016/S0140-6736(00)02844-0. [Google Scholar] [CrossRef]
3. Davidson, W. R., Jr., Stefanescu Schmidt, A. C. (2018). Transcatheter pulmonic valve replacement: Progress and pitfalls. Journal of the American College of Cardiology, 72(22), 2729–2731. DOI 10.1016/j.jacc.2018.09.040. [Google Scholar] [CrossRef]
4. Nordmeyer, J., Ewert, P., Gewillig, M., AlJufan, M., Carminati, M. et al. (2019). Acute and midterm outcomes of the post-approval MELODY registry: A multicentre registry of transcatheter pulmonary valve implantation. European Heart Journal, 40(27), 2255–2264.DOI 10.1093/eurheartj/ehz201. [Google Scholar] [CrossRef]
5. Chatterjee, A., Bajaj, N. S., McMahon, W. S., Cribbs, M. G., White, J. S. et al. (2017). Transcatheter pulmonary valve implantation: A comprehensive systematic review and meta-analyses of observational studies. Journal of the American Heart Association, 6(8), e006432. DOI 10.1161/JAHA.117.006432. [Google Scholar] [CrossRef]
6. Gierlinger, G., Sames-Dolzer, E., Kreuzer, M., Mair, R., Zierer, A. et al. (2021). Surgical therapy of infective endocarditis following interventional or surgical pulmonary valve replacement. European Journal of Cardio-Thoracic Surgery, 59(6), 1322–1328. DOI 10.1093/ejcts/ezab086. [Google Scholar] [CrossRef]
7. Abdelghani, M., Nassif, M., Blom, N. A., van Mourik, M. S., Straver, B. et al. (2018). Infective endocarditis after melody valve implantation in the pulmonary position: A systematic review. Journal of the American Heart Association, 7(13), e008163. DOI 10.1161/JAHA.117.008163. [Google Scholar] [CrossRef]
8. Lehner, A., Haas, N. A., Dietl, M., Jakob, A., Schulze-Neick, I. et al. (2019). The risk of infective endocarditis following interventional pulmonary valve implantation: A meta-analysis. Journal of Cardiology, 74(3), 197–205. DOI 10.1016/j.jjcc.2019.04.007. [Google Scholar] [CrossRef]
9. Cools, B., Brown, S., Budts, W., Heying, R., Troost, E. et al. (2018). Up to 11 years of experience with the melody valved stent in the right ventricular outflow tract. EuroIntervention, 14(9), e988–e994. DOI 10.4244/EIJ-D-18-00054. [Google Scholar] [CrossRef]
10. Bos, D., de Wolf, D., Cools, B., Eyskens, B., Hubrechts, J. et al. (2021). Infective endocarditis in patients after percutaneous pulmonary valve implantation with the stent-mounted bovine jugular vein valve: Clinical experience and evaluation of the modified Duke criteria. International Journal of Cardiology, 323, 40–46. DOI 10.1016/j.ijcard.2020.08.058. [Google Scholar] [CrossRef]
11. McElhinney, D. B., Zhang, Y., Aboulhosn, J. A., Morray, B. H., Biernacka, E. K. (2021). Multicenter study of endocarditis after transcatheter pulmonary valve replacement. Journal of the American College of Cardiology, 78(6), 575–589. DOI 10.1016/j.jacc.2021.05.044. [Google Scholar] [CrossRef]
12. Sharma, A., Cote, A. T., Hosking, M. C. K., Harris, K. C. (2017). A systematic review of infective endocarditis in patients with bovine jugular vein valves compared with other valve types. Journal of the American College of Cardiology Cardiovascular Interventions, 10(14), 1449–1458. DOI 10.1016/j.jcin.2017.04.025. [Google Scholar] [CrossRef]
13. Gröning, M., Borg Tahri, N., Sondergaard, L., Helvind, M., Ersboll, M. K. et al. (2019). Infective endocarditis in right ventricular outflow tract conduits: A register-based comparison of homografts, Contegra grafts and Melody transcatheter valves. European Journal of Cardio-Thoracic Surgery, 56(1), 87–93. DOI 10.1093/ejcts/ezy478. [Google Scholar] [CrossRef]
14. van Dijck, I., Budts, W., Cools, B., Eyskens, B., Boshoff, D. E. et al. (2015). Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart, 101(10), 788–793. DOI 10.1136/heartjnl-2014-306761. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |