

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.017366
CASE REPORT
Pregnancy in Patients with Shone Complex: A Single-Center Case Series
1Department of Cardiology, Cleveland Clinic, Cleveland, 44195, USA
2Department of Obstetrics, Cleveland Clinic, Cleveland, 44195, USA
3Department of Cardiology, Mayo Clinic, Phoenix, 85054, USA
*Corresponding Author: David Majdalany. Email: Majdalany.David@mayo.edu
Received: 05 May 2021; Accepted: 23 July 2021
Abstract: Background: There is limited literature written on the course and outcomes for pregnant mothers with Shone complex. Methods: We describe a case series of five pregnancies in four women with Shone complex within a multidisciplinary cardio-obstetrics clinic from 2016–2018. Results: Maternal age ranged from 21–39 years. Three patients had preserved left ventricular function while one had moderately decreased function. Gestational age at presentation ranged from 6–15 weeks. There were three successful pregnancies (mean gestational age = 37 weeks, range 35–39 weeks) with one patient accounting for two unsuccessful pregnancies. All infants were delivered via Cesarean section. One infant required a NICU stay, but all other infants delivered were healthy. Conclusion: Patients with Shone complex can have successful pregnancies although complications can occur for both the mother and the baby. Comprehensive prenatal care, coordinated and consistent management during pregnancy, and tertiary care support can promote positive maternal and fetal outcomes.
Keywords: Shone complex; pregnancy; cardiac disease in pregnancy; women’s cardiovascular health
| Abbreviations | |
| Ao: | Aorta |
| LA: | Left atrium |
| LV: | Left ventricle |
| RV: | Right ventricle |
| WHO: | World health organization |
| LVEF: | Left ventricular ejection fraction |
| NYHA: | New York Heart Association Classification |
| EKG: | Electrocardiogram |
| AV: | Atrioventricular |
| MRI: | Magnetic resonance imaging |
| INR: | International normalized ratio |
| LVEF: | Left ventricular ejection fraction |
| RVEF: | Right ventricular ejection fraction |
| BMI: | Body mass index |
| ICU: | Intensive care unit |
| NICU: | Neonatal intensive care unit |
| ACHD: | Adult congenital heart disease |
Pregnancy for patients with complex congenital heart diseases can be fraught with danger and uncertainty, yet for many women it remains a highly desired experience. Shone complex has an estimated prevalence of <1% among adults with congenital heart disease. Consequently, there is scarce literature to guide caregivers in the expectations and management of pregnancy in this population. The handful of case reports that exist suggest that pregnancy may be successfully tolerated in the right candidates with additional careful management.
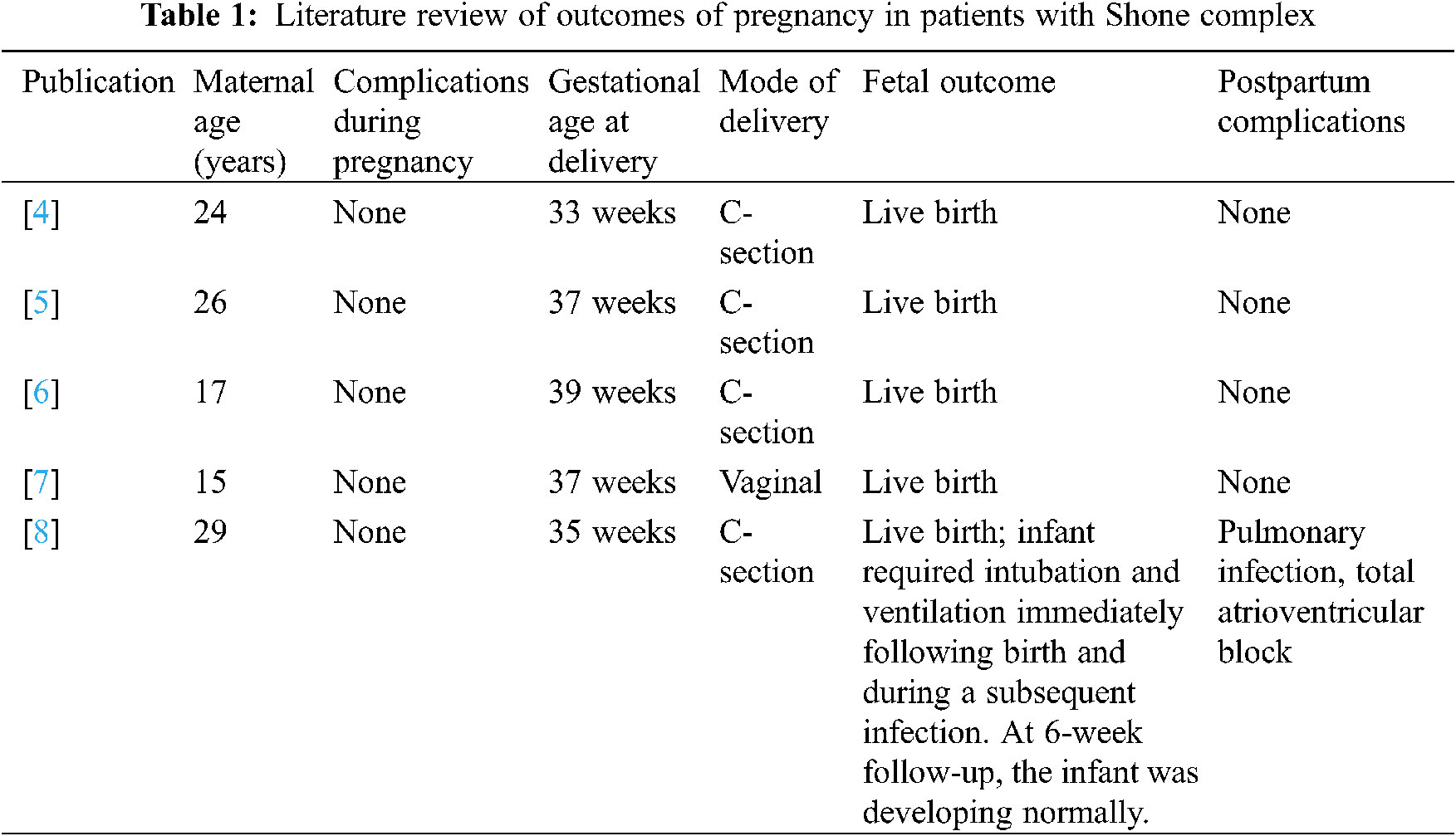
Shone complex (Fig. 1) is a rare congenital disorder of multiple left-sided, obstructive cardiac abnormalities consisting of a parachute mitral valve, a supravalvular mitral ring, subvalvular and valvular aortic stenosis, and aortic coarctation [1].

Figure 1: Shone complex series (From Jain, C. C., Warnes, C. A., Egbe, A. C., Cetta, F., DuBrock, H. M. et al. (2020). Hemodynamics in adults with the shone complex. The American Journal of Cardiology, 130, 137–142 [2]; used with the permission of Mayo Foundation for Medical Education and Research, all rights reserved).
Panel A: Depiction of the complete Shone complex including a supravalvular mitral ring (black arrow), a parachute mitral valve (red arrow), subaortic stenosis (green arrow), and aortic coarctation (blue arrow).
Panel B: 3D reconstruction of aortic coarctation. The arrow indicates residual narrowing in the proximal descending thoracic aorta in a patient with a prior coarctation repair.
Panel C: Echocardiography of aortic lesions in a patient with Shone complex. The thick white arrow indicates a subaortic membrane. The thin white arrow indicates doming of bicuspid aortic valve cusps.
Panel D: Echocardiography of a patient with a parachute mitral valve. The arrow indicates the single functional left ventricular papillary muscle.
Panel E: Echocardiography of a patient with a parachute mitral valve. The arrow indicates a supravalvular mitral ring. Dysplatic mitral valve leaflets can be visualized.
Partial forms of Shone complex consist of at least one left ventricular inflow lesion and one outflow lesion [3]. When presenting as adults, patients with Shone complex can display wide variability in symptom burden related to lesion severity and surgical history. Common symptoms may include shortness of breath, reduced functional capacity, palpitations, chest discomfort, and syncope. Complete Shone complex is categorized as a World Health Organization (WHO) Classes III–IV lesion, making pregnancy a relative to absolute contraindication with significant risk of severe morbidity and mortality. Yet of the five case reports we identified describing pregnant patients with Shone complex, four of these described successful pregnancies with no apparent symptom exacerbation (Table 1).
The aim of this paper is to expand current knowledge on Shone complex by reporting a case series of patients seen at a multidisciplinary cardio-obstetrics clinic dedicated to managing pregnancy in patients with cardiovascular disease. The case series is accompanied by a brief literature review and our recommendations.
We describe the outcomes of five pregnancies in four patients between 2016 and 2018. Baseline patient characteristics including lesion descriptions, surgical history, and functional status are summaried in Table 2.
Patient 1 was diagnosed with complete Shone complex at six months of age and subsequently underwent mechanical aortic valve replacement at age 13, myomectomy and aortic coarctation repair at age 19, and mechanical mitral valve replacement at age 20.
Complete records for Patient 1 were not available. Details of her care before being seen in our cardio-obstetrics clinic, including those from her early first trimester, were included when possible (Table 3).
Before her pregnancy, patient 1 was NYHA Class I with intermittent episodes of atrial fibrillation with rapid ventricular response. She was given flecainide, metoprolol, digoxin, and warfarin, as sotalol had previously failed, and had required direct-current cardioversion (DCCV) once with successful conversion to sinus rhythm. She followed up with a local cardiologist, with whom she consulted for preconception counseling. Her cardiac status at that time included mild mitral regurgitation, moderate tricuspid regurgitation, and a left ventricular ejection fraction (LVEF) of 50% with normal aortic valve function and no residual aortic coarctation. A baseline electrocardiogram (EKG) showed sinus rhythm with first degree atrioventricular (AV) block and a left bundle branch block. Immediately before her first pregnancy, she began intramuscular progesterone therapy, and her anticoagulation regimen was changed to low-molecular-weight heparin.
At age 39, Patient 1 became pregnant but had an early first trimester pregnancy loss.
Between pregnancies, Patient 1 continued to have follow-up with her local cardiologist. Cardiac magnetic resonance imaging (MRI) from this period showed borderline left ventricular enlargement with an ejection fraction of 40%, moderate mitral regurgitation, and no aortopathy or aortic coarctation. One month before her second conception, she again required DCCV for an episode of symptomatic atrial fibrillation.
Patient 1 became pregnant again in early 2017. She immediately noted an increase in her palpitation burden. Her digoxin and flecainide were discontinued around 9 weeks of gestation by her cardiologist over concerns for lack of efficacy and teratogenicity. She maintained therapy with metoprolol and enoxaparin with close follow-up planned.
She continued to experience worsening palpitations and shortness of breath and at 10 weeks gestational age was found to be in atrial fibrillation with rapid ventricular response (Fig. 2). Electrical cardioversion was not attempted due to a recent change in her enoxaparin dose, and she subsequently had respiratory failure requiring intubation and intensive care, at which point she was transferred to a tertiary care center.
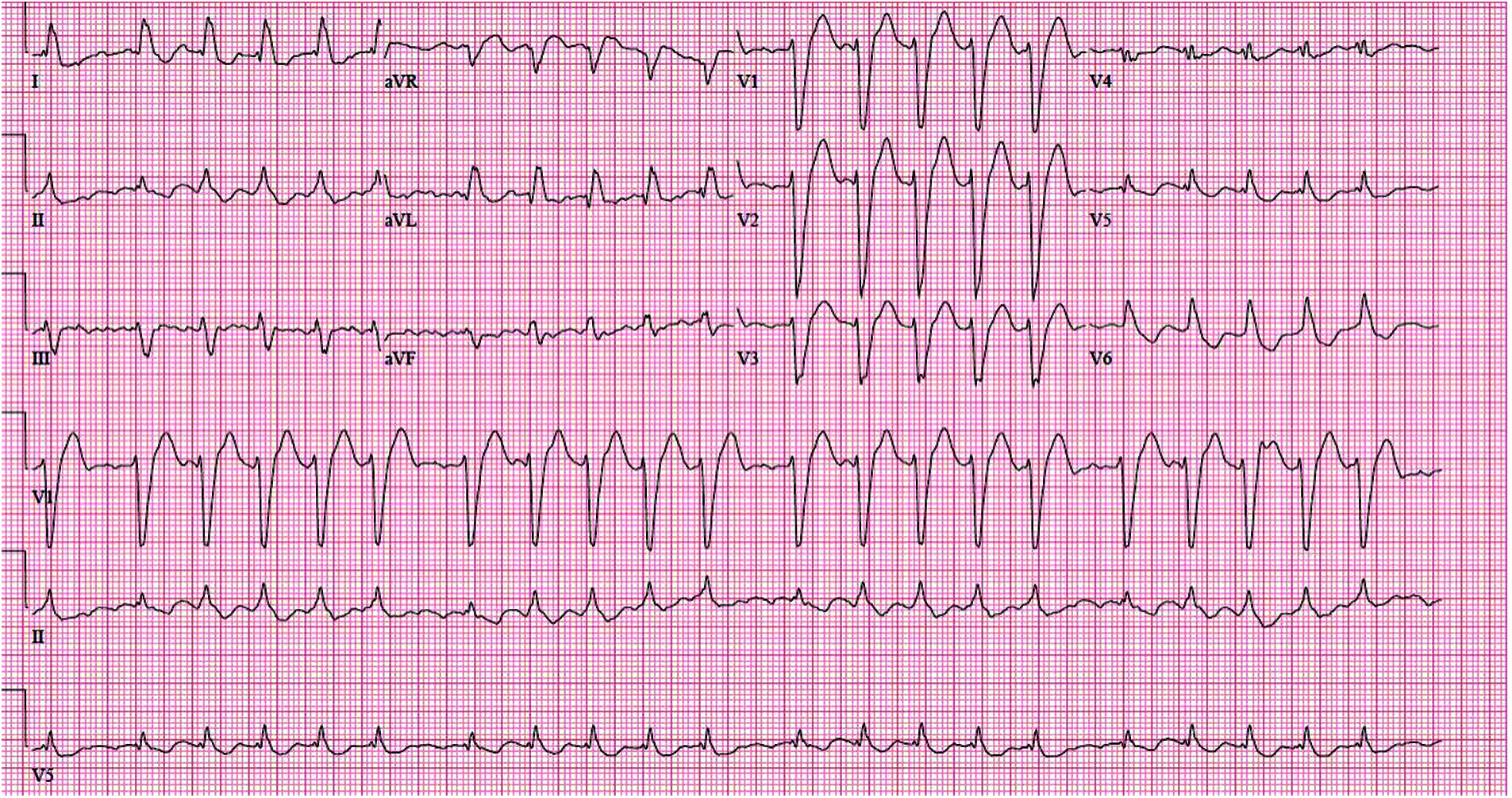
Figure 2: Electrocardiogram of Patient 1 in her early first trimester pregnancy demonstrating atrial fibrillation with rapid ventricular response along with a left bundle branch block
Following her transfer, Patient 1 remained in the intensive care unit (ICU) for three days. She improved with chemical cardioversion, diuresis, and re-initiation of flecainide. She was discharged five days after transfer in sinus rhythm taking a regime of flecainide, metoprolol, and enoxaparin. She was instructed to establish herself at our cardio-obstetrics clinic.
Patient 1 came for an appointment at our cardio-obstetrics clinic approximately one month later at 15 weeks gestation. At this time, she was NYHA Class I and appeared well compensated on examination. Echocardiographic findings from her intensive care stay were reviewed and were remarkable for an elevated mitral valve gradient, which had improved from a mean of 28 to 12 mmHg; mild aortic regurgitation and stenosis with a mean gradient of 10 mmHg; moderate tricuspid regurgitation; and right ventricular systolic pressure of 57 mmHg. A 48-h Holter monitor showed sinus rhythm with one transient episode of atrial fibrillation. She was determined to be in WHO Class III. Her anticoagulation regimen was changed to warfarin and follow up was planned for every 2 to 4 weeks.
Patient 1 continued to experience episodes of symptomatic atrial fibrillation with rapid ventricular response and required cardioversion twice more at 15 and 18 weeks of gestation. On both occasions she was discharged in sinus rhythm. Between episodes, she felt well and reported minimal cardiac symptoms.
At 20 weeks and 4 days, Patient 1 went to the cardio-obstetrics clinic with abdominal cramping and was found to have cervical shortening with funneling of membranes to the external os. She was admitted for bridging of warfarin in anticipation of cerclage placement but experienced preterm premature rupture of the membranes. She then desired termination in the context of expected fetal demise. Dilation and evacuation were pursued to minimize further alterations to her anticoagulation regimen. Her postpartum course was complicated by several admissions for sustained hemorrhage and international normalized ratio (INR) monitoring. At one month postpartum, she underwent dilation and curettage for retained products of conception. During this time, she experienced another episode of sustained atrial fibrillation with rapid ventricular response, which responded to an amiodarone bolus. Prior to discharge, her mean mitral gradient was 15 mmHg, her mean aortic gradient was 12 mmHg, and her LVEF was 50%. She had no further complications and was discharged on a regimen of digoxin, flecainide, metoprolol, warfarin, aspirin, and megestrol with close follow-up planned.
Patient 1 subsequently complied with close cardiac follow up and did not require any further admissions or cardioversions. At six months postpartum, she was NYHA Class I with an LVEF of 45% and stable valve status. She did not desire future pregnancy and took progesterone-only oral contraception.
Patient 2 had a medical history of a Shone complex variant consisting of aortic coarctation, a bicuspid aortic valve (right-left cusp fusion), and both atrial and ventral septal defects. She had a surgical history of a complete coarctation repair with an atrial and ventricular septal defect repair at three days of age, an aortic valvotomy at five years, and a Ross-Konno operation with the placement of a pulmonary homograft at 19 years of age. Additional past medical history included sickle cell trait.
Before her pregnancy, she was NYHA Class I with an LVEF of 50% and a borderline dilated neo-aortic root (mid-3 cm), mild insufficiency of her neo-pulmonary and neo-aortic valves, and no residual aortic coarctation, as well as a dilated left main coronary artery (11 mm). Following her Ross-Konno operation, she was given lisinopril and aspirin. She did not have regular cardiac follow-up.
At 21 years of age, Patient 2 sought care at our cardio-obstetrics clinic; she was at six weeks gestation with an unplanned pregnancy attributed to a lapse in medroxyprogesterone acetate usage. She was NYHA Class I and appeared well-compensated on examination. She had mild hypertension attributed to her discontinuation of cardiac medications upon learning she was pregnant. On echocardiography, she had normal biventricular function with no significant valvular disease or aortopathy. Labetalol and aspirin were started, and follow-up was planned for every 1 to 2 months. Her echocardiographic findings were stable throughout her early pregnancy despite intermittent adherence to cardiac medications.
At 27 weeks of gestation, she came to the clinic with new dyspnea on exertion and a blood pressure of 165/80 mmHg. Her echocardiogram showed mild right ventricular enlargement with moderate regurgitation of both the neo-aortic and neo-pulmonary valves but preserved biventricular systolic function. Her antihypertensive regimen was uptitrated. A non-contrast cardiac MRI was ordered to assess ventricular volumes, but it was not completed because of insurance denial. She returned to the clinic at 31 weeks of gestation with reports of stable exertional dyspnea and moderately elevated blood pressures (because of medication noncompliance). The echocardiographic findings at this time were unchanged. She continued to feel well and did not have any other cardiac symptoms.
At 32 weeks, Patient 2 sought care for decreased fetal movement and was subsequently admitted for preeclampsia without severe features. She was discharged following a reactive non-stress test and improvement in her blood pressures. There were no changes to her medications. She was scheduled for non-stress tests with close cardiac follow-up planned.
At 37 weeks, she was again admitted for preeclampsia, at which point her baby was found to be in the breech position. Subsequently, a Cesarean section and tubal ligation were performed resulting in a healthy 3.2 kg baby with APGARs of 9/9. Mother and baby recovered well and were discharged on post-operative day three. Mom was sent home on a regimen of labetalol and aspirin.
Following discharge, Patient 2 experienced worsening shortness of breath and was readmitted on postoperative day five for acute decompensated heart failure with an NT-proBNP value of 4,400, a normal troponin level, and an LVEF of 40%. She improved with diuresis and was discharged on a regimen of carvedilol, lisinopril, furosemide, and aspirin. At her two-month follow-up, she was asymptomatic and well-compensated on examination, although her LVEF was 30% on repeat echocardiography. Her cardiomyopathy regimen was adjusted at multiple follow up visits.
She experienced no more acute exacerbations and required no further admissions for heart failure. At one year postpartum, her LVEF was 35% and she was rated as NYHA Class II. A cardiac MRI was performed showing a severely dilated left ventricle with an LVEF of 33%, a moderately dilated right ventricle with a right ventricular ejection fraction (RVEF) of 42%, an unobstructed left ventricular outflow tract (after a previous Ross-Konno procedure), mild to moderate neo-aortic regurgitation, and moderate prosthetic pulmonary regurgitation. She was set up for a consultation with the heart failure team and the electrophysiology team for follow-up of her cardiomyopathy and consideration for insertion of a defibrillator.
Patient 3 is a 31-year-old woman with a medical history notable for a partial Shone complex after coarctation repair with resection and end-to-end anastomosis, beta thalassemia minor, and one prior ectopic pregnancy. In 2014, she pursued preconception counseling at an outside tertiary care center. There she was determined to be NYHA Class II with an LVEF of 60%, a bicuspid/unicuspid aortic valve with trace aortic regurgitation and severe aortic stenosis, and a well-functioning parachute mitral valve (Fig. 3). A stress test performed that year showed a multi-factorial primary cardiovascular limitation to exercise (chronotropic incompetence and impaired forward stroke volume) without ischemic changes or arrhythmia on EKG. Peak VO2 was 13.5 mL/kg/min (47% of predicted) and 75% of predicted maximal heart rate was achieved. A cardiac MRI performed at that time showed a stenotic unicuspid/bicuspid aortic valve, a well-functioning parachute mitral valve, and no significant re-coarctation or aortopathy.

Figure 3: Cardiac imaging of Patient 3 with Shone complex showcasing abnormalities of her aortic valve
Panel A: Tomographic imaging demonstrating fusion and thickening of the aortic cusps in a patient with a suspected unicuspid aortic valve.
Panel B: Tomographic imaging in the coronal axis demonstrating thickening of the aortic cusps.
Panel C: Echocardiography (parasternal long axis image) demonstrating thickening of the aortic cusps and eccentric closure of the parachute mitral valve.
Panel D: Continuous wave Doppler across the stenotic aortic valve revealing a peak aortic jet velocity of 3.9 m/s and a mean gradient of 34 mmHg.
Patient 3 presented to our cardio-obstetrics clinic during her second pregnancy at 12 weeks gestation. At this time, she was asymptomatic, well-compensated on examination, and not taking cardiac medications prior to her pregnancy. She was determined to be NYHA Class II. Given this data, a discussion was held with the patient regarding the options of maintaining a high-risk pregnancy or a termination. The patient opted to maintain the pregnancy with close cardiac follow up.
As she lived out of state, monthly follow-ups with her local cardiologist were arranged and follow up was planned at our institution every trimester. She elected not to continue to travel to our institution in her third trimester, which limited our access to her complete records thereafter.
Her pregnancy was complicated by migraines; however, from a cardiac standpoint, she remained NYHA Class II with normal biventricular systolic function. She had a severely stenotic aortic valve with a mean aortic gradient of 40–50 mmHg on serial echocardiograms. Her pregnancy was complicated by intrauterine growth restriction, and at 35 weeks of gestation, she underwent an elective Cesarean section at her local institution. Her infant remained in the neonatal intensive care unit (NICU) for one week. At two weeks postpartum, both mother and infant were doing well.
Patient 4 is a 31-year-old gravida 1 para 0 woman with a history of partial Shone complex after coarctation resection and end-to-end anastomosis at three years of age, obesity, and Grave’s disease following radioactive iodine ablation at age seven.
Patient 4 consistently followed with her local cardiologist, with whom she consulted for preconception counseling approximately six months before becoming pregnant. At this time, she was NYHA Class I with a body mass index (BMI) of 41 kg/m2. She had known moderate aortic stenosis, mild stenosis of a parachute mitral valve, and mild aortic hypoplasia without residual coarctation. There was concern for worsening of her aortic stenosis and subsequently received a stress test and a heart catheterization in preparation for pregnancy. Her stress test showed submaximal aerobic capacity (4.8 METs) with normal cardiopulmonary response. Her hemodynamic heart catheterization showed progression of her aortic stenosis with a peak gradient of >60 mmHg, and the decision was made to proceed with balloon valvuloplasty. Her postprocedure echocardiogram showed mild aortic regurgitation with a mean aortic gradient of 25 mmHg.
Approximately six weeks after the valvuloplasty, Patient 4 had acute neurologic deficits and brain MRI findings were consistent with an acute ischemic event. Her symptoms resolved completely within 24 h, and workup for an embolic source was inconclusive. She had a multidisciplinary evaluation and was subsequently treated for presumed endocarditis.
On follow-up echocardiography, Patient 4 was noted again to have significant aortic stenosis with a mean gradient of 45 mmHg, trivial mitral stenosis with a mean gradient of 4 mmHg, and normal left ventricular size and function. Her medications included aspirin, thyroid hormone replacement, and a prenatal vitamin.
Patient 4 came to the clinic in February 2017 at six weeks gestation. She was determined to be NYHA class I, but WHO class III due to her asymptomatic severe aortic stenosis. A hypercoagulability panel and a Holter monitor, obtained as part of her neurological workup, were unremarkable.
Her pregnancy proceeded uneventfully with monthly cardio-obstetrics follow-up, which increased to every two weeks during her third trimester. On serial echocardiograms, her aortic and mitral gradients increased, as expected, but she remained asymptomatic and was able to accomplish her activities of daily living throughout. In her late third trimester, her gradients increased to a mean aortic valve gradient of 50 mmHg with 1 − 2+ regurgitation and a mean mitral valve gradient of 10 mmHg (Fig. 4). Her ventricular function remained stable on serial echocardiograms. She had no obstetric complications except gestational diabetes, managed with dietary modifications.
A vaginal delivery with a shortened second stage of labor and postpartum monitoring in the ICU was planned. A multidisciplinary team involving cardiac anesthesia, interventional cardiology, the intensive care team, cardiothoracic surgery, and pediatric cardiology were peripherally consulted regarding her peripartum management.

Figure 4: Echocardiography of patient 4 demonstrating her stenotic parachute mitral valve during the third trimester of gestation
Panel A: A parasternal short axis image revealing a dominant posteromedial papillary muscle, which is commonly noted in patients with a parachute mitral valve.
Panel B: An apical image revealing a parachute mitral valve with the chordae tendinae attached to a single papillary muscle.
Panel C: Color flow imaging revealing flow acceleration across the stenotic parachute mitral valve.
Panel D: Doppler echocardiography of the stenotic parachute mitral valve showing a diastolic mean pressure gradient of 10 mmHg (heart rate 77 bpm).
At 39 weeks and 3 days of gestation, Patient 4 was admitted for induction of labor. She had an arrest of labor and subsequently delivered a healthy 4.3 kg infant via Cesarean section. APGARs were 9/9. Her recovery was complicated by a postpartum hemorrhage requiring transfusion and dilation and curettage, which necessitated a brief stay in the ICU. The mother and baby were discharged on postpartum day three with Patient 4 opting for progesterone-only oral contraception for birth control.
Patient 4 had no cardiac concerns at her postpartum follow-up. At one year postpartum, she was NYHA Class I with moderately severe aortic stenosis (mean gradient 38 mmHg), mild mitral stenosis (mean gradient 5 mmHg), an LVEF of 65%, and no residual coarctation or aortopathy. She continued to take progesterone-only contraceptives with no further plans for pregnancy.
Shone complex, first diagnosed in 1963, is a rare congenital disorder consisting of four left-sided, obstructive cardiac abnormalities that is categorized as WHO Classes III–IV [1]. In this case series, we described five pregnancies in four women aged 21–39 years with repaired Shone complex. Three patients had preserved left ventricular function (LVEF > 50%) at their initial visit to our cardio-obstetrics clinic, whereas one had mild to moderately decreased function (LVEF = 44%). The gestational age at their initial visit ranged from 6 to 15 weeks. Patients visited our cardio-obstetrics clinic on average every 6.5 weeks (range 4.3–8.8 weeks) with more frequent visits in the later stages of pregnancy. After their initial visit, patients were followed with serial echocardiograms (mean = 4 studies, range 0–7 studies). After delivery, the mean duration of follow-up was eight months postpartum (range 0–12 months). One patient was cared for out of state with no follow-up at our institution.
In this series, there were three successful pregnancies (mean gestational age 37 weeks, range 35–39 weeks). One patient accounted for the two unsuccessful pregnancies: the first ended in an early first trimester miscarriage; the second ended in a therapeutic abortion following preterm premature rupture of membranes. There was one additional case of severe intrauterine growth restriction. For all the women, the initial birth plan was for vaginal delivery. Despite this, all infants were delivered via Cesarean section. Of the two deliveries we have records for, the indications were breech presentation and arrested labor, respectively. One infant required a brief NICU stay, but, following delivery, all infants were born healthy.
All mothers in this series survived, but all experienced either cardiac or obstetric complications. One patient had recurrent atrial fibrillation requiring several direct-current cardioversions, as well as cervical insufficiency followed by postpartum hemorrhage; the second had preeclampsia; and the final patient had gestational diabetes and postpartum hemorrhage. One patient received warfarin and aspirin anticoagulation because of a mechanical valve prosthesis and atrial fibrillation. Two patients received aspirin anticoagulation alone. Of the three patients who followed up in our clinic, none desired future pregnancies.
Regarding maternal cardiac status, two patients experienced a decline in cardiac function during the study period. The first patient experienced a decline in NYHA class in the context of acute decompensated heart failure post-partum (LVEF 44% to 30%) with possible peripartum cardiomyopathy. The second patient had atrial fibrillation with rapid ventricular response (LVEF 50% to 45%). The other two patients had no observed cardiac function decline during their pregnancies. None of the patients experienced any thrombotic events or required any cardiac intervention.
Three of four patients in this series sought preconception counseling prior to pregnancy; the fourth had an unplanned pregnancy. Of the three who sought clinical advice, only one received it at a subspecialty center dedicated to cardio-obstetric care. The remaining two patients were cared for through their early pregnancies by their local cardiologists.
This case series showed that women with repaired Shone complex can have pregnancies with favorable outcomes, although there were risks for both the mother and the baby. Being evaluated in a collaborative clinic with adult congenital heart disease (ACHD) and maternal fetal medicine expertise was vital in optimizing these women’s care.
We recommend a pre-conception visit because a full work up can help physicians to assess risks, optimize maternal status, and select pregnancy-safe medications before conception. We also recommend consistent follow-up in a tertiary care center, with more frequent visits depending on the complexity and the stage of pregnancy. We observed that patients who regularly saw their cardiologists from pre-conception through delivery had better overall outcomes than patients who sporadically followed up with their cardiologists. We also appreciate the importance of integrated care between specialties, as issues within any one of these specialties can lead to risk of maternal or fetal harm. Post-pregnancy follow-up is also important for reassessing maternal status after the hemodynamic and physiologic changes of pregnancy have resolved and for reviewing the patient’s plan for contraception. Moreover, these patients should have lifelong followup with an ACHD cardiologist to monitor the long-term sequelae of their repaired congenital heart disease.
This case series demonstrated that Shone complex patients can have successful pregnancies, although complications can occur in both the mother and the baby. Comprehensive prenatal care, coordinated and consistent management during pregnancy, and tertiary care level support can promote positive maternal and fetal outcomes. Although this is a small case series that requires more data to be generalizable, our conclusions are consistent with observations reported in the literature.
Acknowledgement: We would like to acknowledge the dedication and hard work of all of the staff involved in the care of these patients.
Ethical Statement: This study was approved by the Cleveland Clinic Institutional Review Board (No. #16-357). It was determined to be of minimal risk and informed consent was waived.
Data Sharing: No further individual data will be shared beyond what is included in the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Shone, J. D., Sellers, R. D., Anderson, R. C., Adams, P., Jr, Lillehei, C. W. et al. (1963). The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. The American Journal of Cardiology, 11(6), 714–725. DOI 10.1016/0002-9149(63)90098-5. [Google Scholar] [CrossRef]
2. Jain, C. C., Warnes, C. A., Egbe, A. C., Cetta, F., DuBrock, H. M. et al. (2020). Hemodynamics in adults with the shone complex. The American Journal of Cardiology, 130(Suppl B), 137–142. DOI 10.1016/j.amjcard.2020.06.024. [Google Scholar] [CrossRef]
3. Grimaldi, A., Vermi, A. C., Ho, S. Y., Pappalardo, F., Castiglioni, A. et al. (2012). Surgical outcome of partial Shone complex. Interactive Cardiovascular and Thoracic Surgery, 14(4), 440–444. DOI 10.1093/icvts/ivr169. [Google Scholar] [CrossRef]
4. Bhatia, K., Eccles, J., Meessala, D. K. (2021). Anesthetic management of a parturient with shone’s syndrome–A case report with review of literature. Korean Journal of Anesthesiology, 74(4), 342–349. DOI 10.4097/kja.20181. [Google Scholar] [CrossRef]
5. Naz, A., Dasgupta, S., Bandyopadhyay, B. K., Shirazee, H. H. (2016). Graded epidural anesthesia for cesarean section in a parturient with shone’s syndrome: A case study. Southern African Journal of Anaesthesia and Analgesia, 22(1), 33–36. DOI 10.1080/22201181.2015.111167. [Google Scholar] [CrossRef]
6. Sachse, K., Hannallah, M. (2008). The anesthetic management for cesarean delivery in a patient with shone’s syndrome. Anesthesia and Analgesia, 107(5), 1652–1654. DOI 10.1213/ane.0b013e3181864d6e. [Google Scholar] [CrossRef]
7. Goswami, N. J., Wen, T. S., Freeman, G. L. (2003). An unusual presentation of congenital heart disease. Texas Heart Institute Journal, 30(3), 214–217. [Google Scholar]
8. Koelble, N., Weiss, B. M., Wisser, J., Jenni, R., Turina, J. et al. (2001). Shone’s anomaly complicated by ascending aortic aneurysm in a pregnant woman. Journal of Cardiothoracic and Vascular Anesthesia, 15(1), 84–87. DOI 10.1053/jcan.2001.20281. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |