

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2022.018590
ARTICLE
Multi-Institutional US Experience of the Occlutech© AFR Device in Congenital and Acquired Heart Disease
1The Heart Institute, Children’s Hospital Colorado, Aurora, USA
2Pediatric Cardiology, Helen DeVos Children’s Hospital, Grand Rapids, USA
3Pediatric Cardiology, UCLA Health, Mattel Children’s Hospital, Los Angeles, USA
4Pediatric Cardiology, UCLA Medical Centre, Los Angeles, USA
5Pediatric Cardiology, Mayo Clinic, Rochester, USA
6Pediatric Cardiology, University of Mississippi Medical Center, Jackson, USA
7Pediatric Cardiology, Riley Hospital for Children at Indiana University Health, Indianapolis, USA
*Corresponding Author: Gareth Morgan. Email: drgarethjmorgan@gmail.com
Received: 04 August 2021; Accepted: 09 September 2021
Abstract: Objectives: To detail the US multi-institutional experience with the Occlutech© (Occlutech International AB, Helsingborg, Sweden) atrial flow regulator (AFR) in children and adults with acquired or congenital heart disease. Background: The creation of a long-term atrial communication is desirable in several cardiovascular disease phenotypes, most notably pulmonary arterial hypertension, disorders of increased left ventricular filling and increased cavopulmonary pressures in patients with a Fontan type circulation. Methods: Patients were identified for inclusion from the AFR device manufacturer database. Data was collected using a RedCap database following IRB approval. 8 weeks of follow up data was sought for each patient based on available data. Data was analyzed and summarized using SPSS. Results: We report the experience of 6 US centers in the implantation of AFR devices in 15 patients, across a wide age range, with different disease phenotypes and a variety of indications. Implantation was technically successful in all patients and improvement was noted in both clinical and hemodynamic parameters. There were no immediate or intermediate term complications reported. 3 patients died remote from implantation. Their deaths were not felt to be related to the AFR device or related procedural complications. Conclusion: Compassionate use of the AFR device in children and adults with congenital & acquired heart disease is technically feasible and produces beneficial short term hemodynamic and symptomatic improvement. Widespread uptake of this technique and treatment at specialist centers has the potential to provide significant benefits to a variety of complex patients with currently limited treatment options and indeterminate prognosis.
Keywords: Congenital heart disease; pulmonary hypertension; left atrial hypertension; single ventricle palliation; diastolic heart failure
The atrial flow regulator device (AFR) by Occlutech © was designed primarily to treat adult patients with symptomatic heart failure, with both preserved and reduced ejection fraction. The centrally fenestrated double disc device is designed to maintain the patency of a newly created or already existent atrial communication and allows variable levels of pressure and volume offloading across the device (Fig. 1). The device comes in a variety of sizes. The introduction of the AFR has generated interest from the congenital cardiology community, dealing with pediatric and adult patients with congenital and acquired heart disease as well as pulmonary arterial hypertension (PH). Current life expectancy for patients with pulmonary arterial hypertension, with or without congenital heart disease (CHD) is limited and this is confounded by a scarcity of pharmacological and procedural interventions available to treat those with advanced disease. The increasing survival of CHD patients has led to an increasing complexity of disease substrate requiring treatment [1,2]. An example of this is those patients surviving with complex Fontan physiology. Treatment options here are limited in both number and efficacy [3].
The AFR device from Occlutech (Occlutech International AB, Helsingborg, Sweden) (Fig. 1A) is a centrally fenestrated double disc (*) device that is implantable across the atrial septum via a long sheath (Fig. 1B). A transesophageal echocardiogram confirms satisfactory device placement with anticipated flow through the central fenestration seen on color doppler (Fig. 1C). A 3 dimensional echocardiogram demonstrates the spatial relationship of the device and its fenestration with the entire atrial septum (Fig. 1D).
Interventional approaches to palliating patients with the above conditions to improve survival or bridge to organ transplantation are limited and fraught with complexity and potential complications [4,5]. Based on studies demonstrating improved survival in patients with Eisenmenger’s syndrome compared to idiopathic PH [6] as well as the benefits of an atrial communication in patients with PH [4], it has been proposed that the creation of a long term atrial communication in patients with elevated right ventricular pressures or elevated cavo-pulmonary pressures may improve survival at the expense of some level of systemic oxygen desaturation. Additionally, some patients with large fenestrations across their Fontan circulations who are severely and symptomatically cyanosed, may benefit from downsizing of this cavoatrial fenestration, to improve systemic oxygen saturations, without removing their ability to offload their Fontan pressures and maintain cardiac output [7]. A number of case reports and case series now exist detailing the successful and beneficial implantation of devices such as the AFR in patients with CHD and PH around the world [8,9].
A number of centers in the United States who provide congenital cardiac catheterization services have implanted the AFR device on compassionate grounds in children and adults with both congenital and acquired heart disease. We have brought the US experience together as a multi-institutional report of compassionate use of the AFR device in these patients.

Figure 1: A walkthrough of the AFR implantation process
Patients were retrospectively identified for inclusion in this study based on the FDA and manufacturer registry of recorded cases of compassionate use AFR implantation here in the US. AFR implantation was proposed for all patients described on the basis of a history of complex congenital or acquired cardiac and/or pulmonary vascular disease who were symptomatic or deteriorating on maximal medical therapy. A decision to list patients for AFR implantation was taken at a multidisciplinary team meeting of Cardiologists, Interventional Cardiologists, Cardiac Anesthesiologists and Cardiothoracic Surgeons in each institution. All implantations were performed by an interventional cardiologist and attended by a congenital interventional cardiologist. Local Institutional Review Board approval was sought for all cases for the purposes of supporting the investigational device exemption (IDE) with the Food and Drug Administration (FDA) as well as to facilitate data sharing agreements. All patients had an ‘expanded access’ application processed through the FDA on the basis of requiring an IDE.
Data was collected and contributed by each individual center using a limited data collection tool (RedCap, Nashville, TN, USA). Data was available up to 8 weeks post procedurally inclusive of at least 1 follow up visit for all patients who survived to discharge. Several patients were followed in their referring centers after this point and further follow up data was not available. Data was collected and analyzed using SPSS v22 (IBM Corp, Armonk, NY, USA). Categorical data is presented as frequencies and continuous data as medians with ranges depending on the sample data. Nonparametric tests were used to compare independent samples. Correlation between continuous data was assessed using linear regression models. Statistical significance was acknowledged when p < 0.05.
Standard procedural technique involved a femoral venous approach in all cases. All procedures were performed under general anesthesia. Procedural guidance was achieved using both fluoroscopic and angiographic imaging as well as trans esophageal echocardiography. When required, an atrial septal puncture and static balloon atrial septal dilation were performed according to local protocols. For those with an existent interatrial or cavo-atrial connection, balloon sizing of the defect was performed using a semi-compliant sizing balloon. Device fenestration sizes were selected based on patient size, baseline hemodynamics, operator experience and intended hemodynamic result.
3.1 Demographics, Clinical and Procedural Data
AFR implantation was successfully completed in 15 consecutive patients at 6 centers. 1 center carried out 6 implantations, 1 center carried out 3 implantations, 3 centers carried out 2 implantations and 1 center carried out 1 implantation.
3.1.1 Pediatric Patients (n = 6)
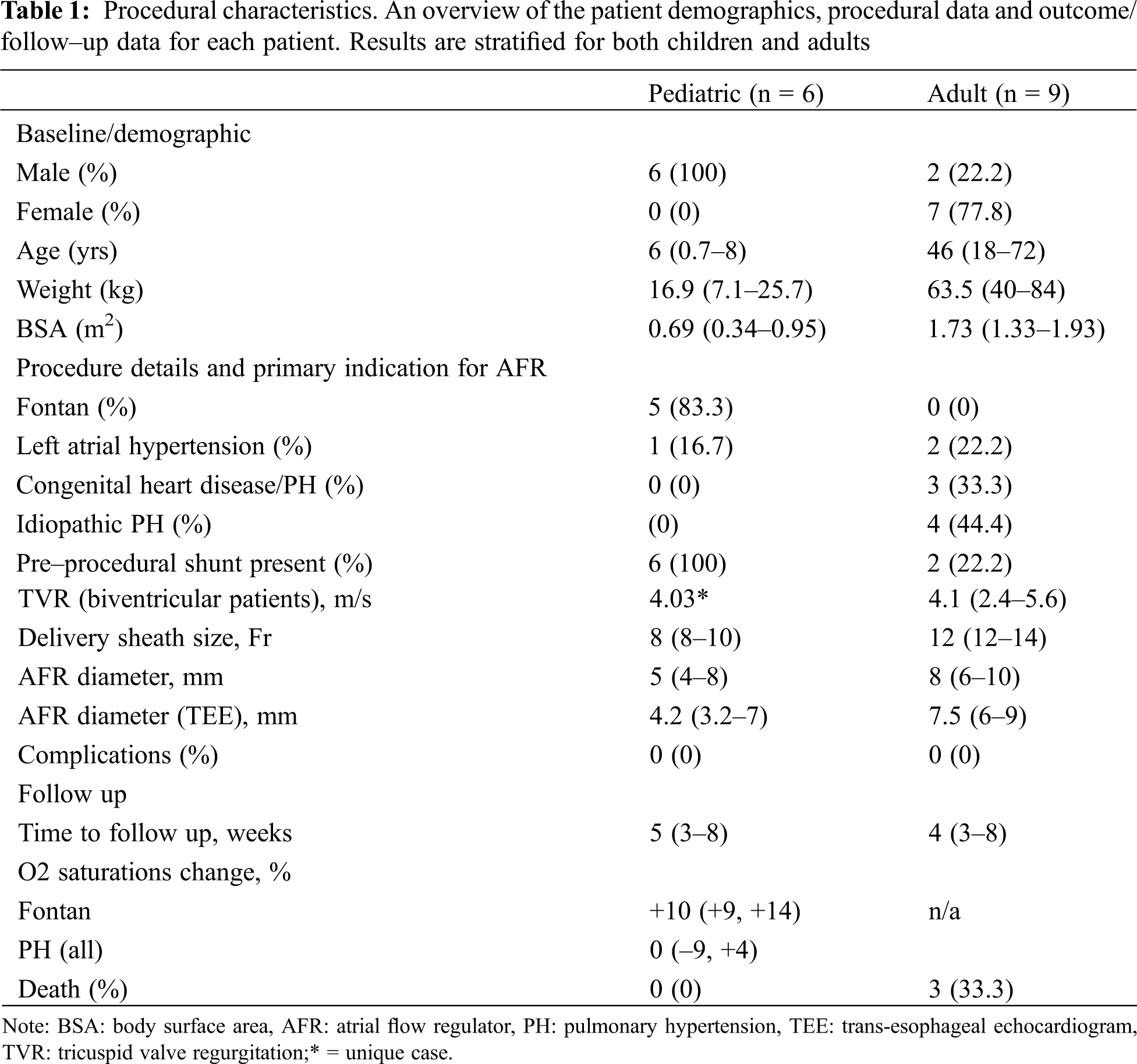
All pediatric patients were male. Five of these were for patients with failing Fontan physiology. The median age of Fontan patients was 6 years [2–8] and median weight was 16.9 kg (14.4–25.7). The median pre catheterization oxygen saturations were 79% (70–95). All Fontan patients had a pre–implantation fenestration and all patients had a right to left shunt across their fenestrations. The median fenestration diameter was 7 mm [6–8]. The median AFR implantation diameter for desaturated Fontan patients was 6 mm (4–8 mm). All implanted devices had a fenestration length of 5 mm. The median sheath size was 8 Fr (8–10 Fr). An additional patient had a history of Shone’s complex, aortic balloon valvuloplasty, an aortic valvotomy, left ventricular endocardial fibroelastosis resection, aortic arch reconstruction, bilateral PA bands and a Gore–Tex artificial duct prior to a Ross/Konno procedure. Due to left atrial hypertension and inability to wean from ventilation an AFR was implanted. This was the smallest patient (7.1 kg) to have implantation which was carried out at 8 months of age. This patient had a 5 × 6 mm AFR device implanted successfully and weaned ventilation thereafter. There was similarity between industry specified AFR diameter and the diameter measured at the time of implantation on trans esophageal echocardiogram (TEE).
The adult group of patients were more heterogeneous and made up of patients with congenital heart disease CHD PH (n = 3), idiopathic pulmonary arterial hypertension (n = 4) and left atrial hypertension (n = 2). The majority of patients were female (n = 7). 2 patients had a pre–existing atrial shunt. Further demographic details are presented in Table 1.
Patients with a subpulmonary right ventricle and pulmonary hypertension (n = 7) had a median tricuspid valve regurgitation velocity of 4.1 m/s (2.4–5.6), a pulmonary vascular resistance indexed of 12.1 WU.m2 (4.5–33) and a mean pulmonary arterial pressure of 46 mmHg (36–51). The majority (n = 5) of AFR implantations in this group were carried out at a single center. 3 patients had congenital heart disease. 2 had atrial septal defect (ASD) that was treated later in life with post–operative PH. Another patient had a diagnosis of double outlet right ventricle, transposition of the great arteries and severe PH.
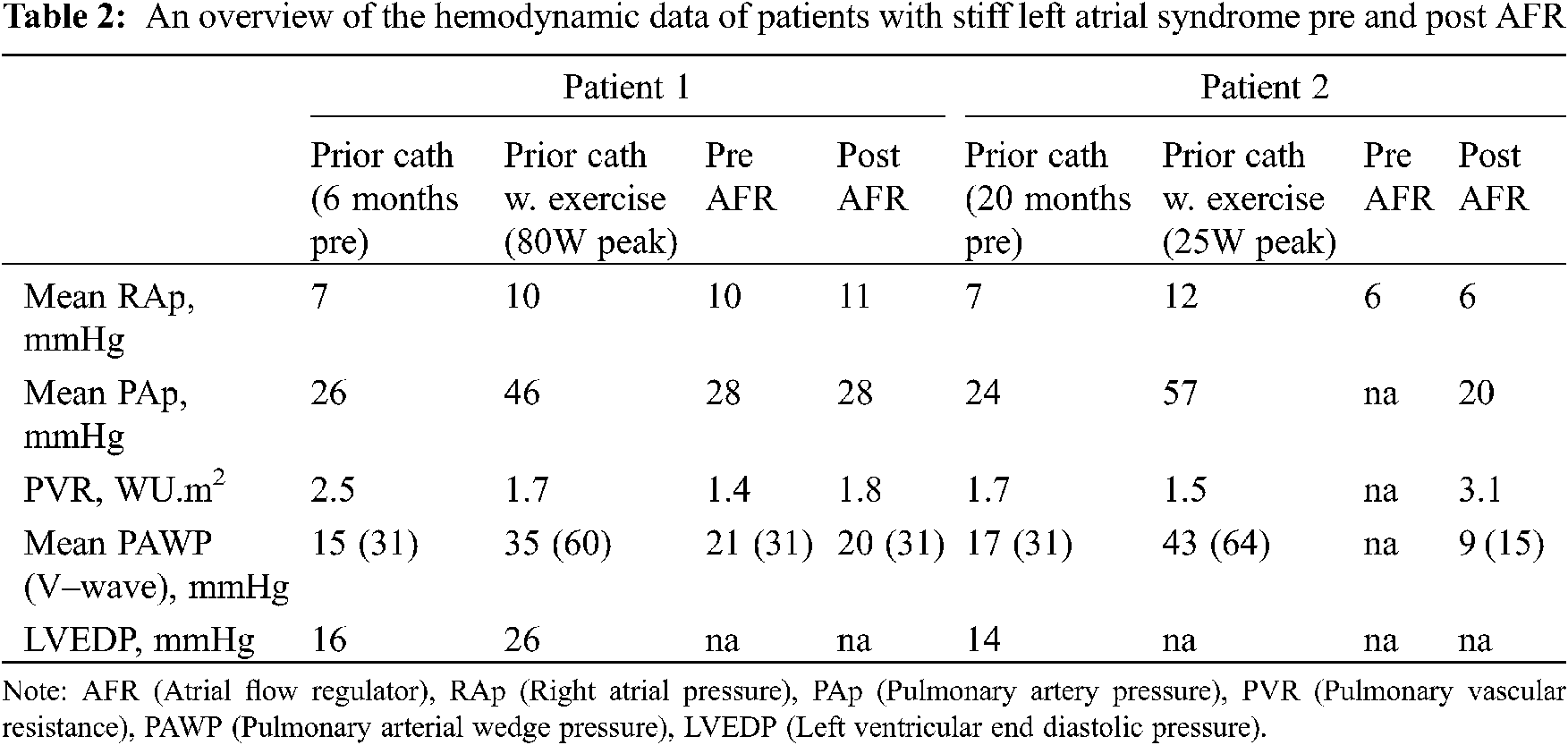
Two patients had isolated left atrial hypertension. 1 patient had a history of mediastinal irradiation as a child, and another had a history of multiple ablation procedures within the left atrium (LA) for refractory atrial fibrillation. Both had clinical and hemodynamic features of stiff LA syndrome [10]. Pertinent hemodynamic data for this subgroup is presented in Table 2.
3.2 Post Procedural Data and Follow Up (Table 3)
All pediatric patients survived without complication. Oxygen saturations increased in all Fontan patients and this increase was maintained through to follow up. NYHA classification was improved at the time of discharge from hospital and this improvement was maintained through follow up. No pediatric patients experienced complications of implantation and all patients survived to follow up and beyond.
There were no procedure related complication s associated with implantation in the adult group. All patients (n = 7) with a subpulmonary ventricle and PH had a R to L shunt through their AFR on echocardiogram at the time of discharge. 2 had bidirectional flow at the time of first follow up. A median change in systemic saturation increase of 10% (9–14) was noted in patients with Fontan circulation and 0% (−9–+4) in patients with PH after implantation. There was a preponderance toward bidirectional flow on echocardiogram and more normal resting oxygen saturations at follow up. NYHA classification had improved at the time of discharge and this improvement was maintained at first follow up.
Three patients died remote from the procedure. The median time to death was 47 days (12–54). No deaths were felt to be related to procedural complications. The 3 patients that died were female patients, treated at the same centre and all had PH. 1 had idiopathic PH and had care withdrawn after a computed tomogram (CT) proven major hemorrhagic stroke on extracorporeal membrane oxygenation. 2 further patients with CHD and PH died from complications related to right heart failure. Both died from septic complications of chronic abdominal ascites.

We report the preliminary multi-institutional experience with AFR device implantation in children and adults with congenital and acquired heart disease in the USA. There were no patients with isolated left ventricular systolic or diastolic disease in this cohort. To date a number of case reports and small series from various institutions have detailed their individual experience with the device [8,9,11,12]. The experience is promising and has propelled the off–label use of this device in patients on a compassionate use basis here in the US. Larger adult based trials on use of the device in advanced adult heart failure patients have demonstrated promising results though provide limited guidance or transferable experience for those intending to use the device off label in complex patients similar to those presented in this manuscript [13–15]. As standard sizing is often used for adult patients with left ventricular failure, attempting to size the device to accommodate the hemodynamic needs of our heterogeneous patients group requires further thought. In our study, we based device sizing on patient size, pre procedural & intraprocedural hemodynamic information and device availability. Many implanters ordered a variety of fenestration sizes for use on the day but did not have the full catalog of devices available for selection at the time of implantation.
The creation of an atrial level shunt may be favorable in some congenital and pulmonary hypertensive patients facilitating pressure and volume unloading of the hypertensive circuit, potentially providing increased systemic cardiac output at the expense of systemic desaturation which is usually well tolerated [16,17]. Pre-tricuspid shunts and shunts between the pulmonary artery and the aorta have variable indications, often determined by the function of the right ventricle [18]. Ultimately catheter based strategies to create interatrial communications are likely to be lower risk and more tolerable for these patients than surgical procedures requiring cardiopulmonary bypass and cardioplegia. None of our patients suffered any complications of implantation under anesthesia. There were no deaths directly associated with the device implantation and death secondary to cardiovascular causes happened remote from the procedure.
One major concern with traditional atrial septal interventions is the unpredictability of long term patency rates. Stenting of the atrial septum is felt to increase long term patency compared to atrial septostomy and decrease the need for recurrent re-catheterization in an at risk group [19]. Reported patency rates are acceptable in several small studies using a variety of vascular, biliary and coronary stents off–label. Potential complications include thrombus formation, stent embolization, and difficulty re-engaging the stent on re–catheterization. Benefits include the ready availability of such stents and the potential to cross and further dilate the stent to accommodate hemodynamic needs on re-catheterization. Sub-acute changes in atrial septal patency in patients with pulmonary vascular disease may have significant clinical repercussions and can result in death [4,5]. Establishing safe and specific antiplatelet and anticoagulation management for these patients is challenging due to the heterogeneity in prothrombotic substrate, anatomy and hemodynamics. Reported experience with atrial septal stenting should aid with follow up of AFR fenestration patency on echocardiography.
Patients with single ventricle palliation can develop multifactorial systemic hypoxemia months to years following Fontan completion. This can lead to a multitude of complications and can have an impact on morbidity and survival [20]. In the presence of a large Fontan fenestration with borderline, elevated or even near normal pressures, this physiology may be optimized by downsizing the fenestration size whilst maintaining its long–term patency. This may maintain safe decompression of the Fontan circuit, potentially ameliorating the development or progression of a myriad of Fontan vascular sequelae and allow patients to increase their systemic ventricular preload both at rest and during exercise at the expense of systemic oxygen saturation. Surgical intervention for the sole purposes of fenestration downsizing is high risk due to the poor pre-procedural status of many of the patients who require such intervention. The AFR device may provide the safest and most ‘controllable’ means of regulating fenestration flow in patients with this type of circulation and may delay or even prevent the evolution of complications such as protein losing enteropathy and Fontan hepatopathy. Our patients benefited from an increase in systemic oxygen saturations at the time of discharge and this was sustained through to early follow up.
A recent animal study which we published detailed post–mortem data associated with the implantation of the AFR device in normotensive swine [21]. This report demonstrated satisfactory patency rates in the implanted devices even in the absence of a significant atrial pressure differential. There were no associated thrombotic complications within the heart nor was there evidence of any significant thrombus formation within the fenestration or on the device. Endothelialization of the device on histopathological assessment was acceptable with no evidence of in situ device thrombosis and retained patency of the fenestration. We have seen no changes in fenestration patency in our patients at both discharge and early follow up. We suspect that patency rates in our patients are likely to be superior to those seen in our animal study due to the presence of significant pressure differentials across the device both at rest and during exercise. This may be further improved upon with optimization of antiplatelet and anticoagulation strategies for this group of patients. Until routine practice evolves, perioperative antiplatelet and anticoagulation management should probably mirror that employed for device occlusion of ASD and patent foramen ovale, whilst accommodating the additional anticoagulatory needs of the patients based on their underlying disease and risk profile [21–23]. This should prevent device and fenestration thrombosis as well as diminish the thromboembolic risks associated with the presence of a right to left shunt.
All US patients currently in consideration of an AFR device must undergo the rigorous process of FDA ‘expanded access’ approval, due to its limited list of indications and current status as an investigatory device. Patients must have no alternative treatment strategies and thus many are critically unwell and require an expedited application to facilitate the procedure without delay. After collecting and analyzing this reassuring data on the first 15 patients in the US to have an AFR implanted, we hope that these early data will be helpful in determining longer term appropriate use protocols through the FDA.
This was a retrospective study and the inherent biases of same are present. The sample size is small. Follow up data was limited due to the provision of these services by tertiary centers on behalf of referring institutions where patients are often followed up disparately. The device was implanted for a wide variety of indications making robust comparison of outcomes in these groups of patients challenging. There is a lack of evidence for the devices use in these disease substrates thus serial follow up data for comparison was inconsistent.
AFR implantation is a technically straight–forward procedure. This device provides the opportunity to treat life limiting illness in a small group of patients with complex disease substrate.. Short term complication rates in this series are within acceptable limits. Our preliminary data suggests that the device may provide symptomatic and hemodynamic improvement. Short term patency rates seem reassuring and acceptable when compared to alternative strategies. Further pursuit of follow up data from this group of patients, as well as expanding the number of patients undergoing implantation is likely to yield more robust data to progress this device from compassionate use to routine armamentarium.
Availability of Data and Materials: Data is stored encrypted in an institutional repository as per the COMIRB protocol. Data is available on request from the senior author.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Best, K. E., Rankin, J. (2016). Long-term survival of individuals born with congenital heart disease: A systematic review and meta-analysis. Journal of the American Heart Association, 5(6), e002846. DOI 10.1161/JAHA.115.002846. [Google Scholar] [CrossRef]
2. Ivy, D. D., Abman, S. H., Barst, R. J., Berger, R. M., Bonnet, D. et al. (2013). Pediatric pulmonary hypertension. Journal of the American College of Cardiology, 62(25), D117–D126. DOI 10.1016/j.jacc.2013.10.028. [Google Scholar] [CrossRef]
3. American College of Cardiology (2020). Evolving therapies in Fontan failure. http%3a%2f%2fwww.acc.org%2flatest–in–cardiology%2farticles%2f2020%2f07%2f15%2f13%2f12%2fevolving–therapies–in–Fontan–failure. [Google Scholar]
4. Keogh, A. M., Mayer, E., Benza, R. L., Corris, P., Dartevelle, P. G. et al. (2009). Interventional and surgical modalities of treatment in pulmonary hypertension. Journal of the American College of Cardiology, 54(1 Suppl), S67–S77. DOI 10.1016/j.jacc.2009.04.016. [Google Scholar] [CrossRef]
5. Swiston, J. R., Johnson, S. R., Granton, J. T. (2010). Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: A systematic review of the literature. Respiratory Medicine, 104(11), 1588–1607. DOI 10.1016/j.rmed.2010.08.003. [Google Scholar] [CrossRef]
6. Myers, P. O., Tissot, C., Beghetti, M. (2014). Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circulation Journal: Official Journal of the Japanese Circulation Society, 78(1), 4–11. DOI 10.1253/circj.CJ-13-1263. [Google Scholar] [CrossRef]
7. Ghanayem, N. S., Berger, S., Tweddell, J. S. (2007). Medical management of the failing Fontan. Pediatric Cardiology, 28(6), 465–471. DOI 10.1007/s00246-007-9007-0. [Google Scholar] [CrossRef]
8. Lehner, A., Schulze-Neick, I., Haas, N. A. (2018). Creation of a defined and stable Fontan fenestration with the new occlutech atrial flow regulator (AFR®). Cardiology in the Young, 28(8), 1062–1066. DOI 10.1017/S1047951118000720. [Google Scholar] [CrossRef]
9. Manuri, L., Calaciura, R. E., de Zorzi, A., Oreto, L., Raponi, M. et al. (2018). Atrial flow regulator for failing Fontan circulation: An initial European experience. Interactive Cardiovascular and Thoracic Surgery, 27(5), 761–764. DOI 10.1093/icvts/ivy165. [Google Scholar] [CrossRef]
10. Urey, M. A., Darden, D., Stoller, D., Drazner, M. H., Horn, V. et al. (2017). Stiff left atrial syndrome after multiple percutaneous catheter ablations: Role for invasive hemodynamic exercise testing. Circulation Heart Failure, 10(5), e003885. DOI 10.1161/CIRCHEARTFAILURE.117.003885. [Google Scholar] [CrossRef]
11. Bauer, A., Khalil, M., Schmidt, D., Bauer, J., Esmaeili, A. et al. (2018). Creation of a restrictive atrial communication in pulmonary arterial hypertension (PAHEffective palliation of syncope and end-stage heart failure. Pulmonary Circulation, 8(2), 2045894018776518. DOI 10.1177/2045894018776518. [Google Scholar] [CrossRef]
12. Dąbrowska-Kugacka, A., Ciećwierz, D., Żuk, G., Fijałkowski, M., Ottowicz, A. et al. (2019). Atrial flow regulator for severe drug resistant pulmonary arterial hypertension after congenital heart defect correction. Cardiology Journal, 26(1), 102–104. DOI 10.5603/CJ.2019.0016. [Google Scholar] [CrossRef]
13. Paitazoglou, C., Özdemir, R., Pfister, R., Bergmann, M., Bartunek, J. et al. (2020). The AFR–PRELIEVE trial: A prospective, non-randomised, pilot study to assess the atrial flow regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. https://eurointervention.pcronline.com/article/the-afr. [Google Scholar]
14. Guimarães, L., Del Val, D., Rodés-Cabau, J. (2019). The atrial flow regulator device: Expanding the field of interatrial shunting for treating heart failure patients. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 15(5), 398–400. DOI 10.4244/EIJV15I5A73. [Google Scholar] [CrossRef]
15. Lewicki, Ł., Sabiniewicz, R., Siebert, J., Szołkiewicz, M. (2020). Atrial flow regulator as a novel therapy for patients with chronic heart failure. Cardiology Journal, 27(3), 309–311. DOI 10.5603/CJ.a2020.0077. [Google Scholar] [CrossRef]
16. Frank, D. B., Hanna, B. D. (2015). Pulmonary arterial hypertension associated with congenital heart disease and eisenmenger syndrome: Current practice in pediatrics. Minerva Pediatrica, 67(2), 169–185. Epub 2015 Jan 21. [Google Scholar]
17. Lehner, A., Schulze-Neick, I., Fischer, M., Fernandez-Rodriguez, S., Ulrich, S. et al. (2019). The creation of an interatrial right-to-left shunt in patients with severe, irreversible pulmonary hypertension: Rationale, devices. Outcomes Current Cardiology Reports, 21(5), 31. DOI 10.1007/s11886-019-1118-8. [Google Scholar] [CrossRef]
18. Delhaas, T., Koeken, Y., Latus, H., Apitz, C., Schranz, D. (2018). Potts shunt to be preferred above atrial septostomy in pediatric pulmonary arterial hypertension patients: A modeling study. Frontiers in Physiology, 9, H1943. DOI 10.3389/fphys.2018.01252. [Google Scholar] [CrossRef]
19. Sandoval, J., Gaspar, J., Peña, H., Santos, L. E., Córdova, J. et al. (2011). Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. The European Respiratory Journal, 38(6), 1343–1348. DOI 10.1183/09031936.00072210. [Google Scholar] [CrossRef]
20. Rychik, J., Atz Andrew, M., Celermajer David, S., Deal Barbara, J., Gatzoulis Michael, A. et al. (2019). American heart association council on cardiovascular disease in the young and council on cardiovascular and stroke nursing, evaluation and management of the child and adult with Fontan circulation: A scientific statement from the american heart association. Circulation, 140(6), e234–284. DOI 10.1161/CIR0000000000000696. [Google Scholar] [CrossRef]
21. McLennan, D., Ivy, D., Morgan, G. J. (2019). Transvenous implantation of the occlutech atrial flow regulator: Preliminary results from swine models. Congenital Heart Disease, 14(5), 819–831. DOI 10.1111/chd.12816. [Google Scholar] [CrossRef]
22. Franke, A., Kühl, H. P. (2006). The role of antiplatelet agents in the management of patients receiving intracardiac closure devices. Current Pharmaceutical Design, 12(10), 1287–1291. DOI 10.2174/138161206776361309. [Google Scholar] [CrossRef]
23. American College of Cardiology (2020). Anticoagulation in the management of pulmonary arterial hypertension. https://www.acc.org/latest-in. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |