

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2022.016332
REVIEW
Ductus Arteriosus Stent Compared with Surgical Shunt for Infants with Ductal-Dependent Pulmonary Blood Flow: A Systematic Review and Meta-Analysis
Heart Center, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao, 266034, China
*Corresponding Author: Silin Pan. Email: silinpan@126.com
Received: 01 March 2021; Accepted: 27 May 2021
Abstract: The aim of this study was to perform a systematic review and meta-analysis to evaluate the safety and efficacy of ductus arteriosus stent (DAS) compared with surgical systemic-pulmonary artery shunt (SPS) in patients with ductal-dependent pulmonary blood flow. A literature search was conducted in PubMed, Embase, and the Cochrane Library databases from their inception to December 2020. Two reviewers independently screened the articles, evaluated the quality of the articles, and collected the data. Meta-analyses were conducted using fixed and random effects models. We used the I-square (I2) test to examine heterogeneity and the funnel plot Egger’s test was used to test for publication bias. We analyzed nine studies including 842 patients were included in the present study (DAS: n = 295; SPS: n = 547). There was a benefit in favor of DAS group for medium-term mortality (RR, 0.63; 95% CI, [0.40, 0.99]; P = 0.91, I2 = 0%). DAS group demonstrated a reduced risk for complications compared with SPS (RR, 0.46; 95% CI, [0.29, 0.72]; P = 0.78, I2 = 0%). There was an increased risk for unplanned reintervention for DAS (RR, 1.77; 95% CI, [1.42, 2.20]; P = 0.61, I2 = 0%). DAS demonstrated shorter mean intensive care unit length of stay (MD, –5.12; 95% CI, [–7.33, –2.91]; P = 0.005, I2 = 76%). There was also demonstrated higher postprocedure oxygen saturation for SPS over DAS (MD, 1.78; 95% CI, [0.92, 2.64]; P = 0.46, I2 = 0%). There was no difference between the two groups in terms of mortality within 30 days, Nakata Index, and hospital length of stay. Conclusions: In terms of initial palliative surgical in the ductal-dependent pulmonary blood flow, DAS demonstrated a lower risk of medium-term mortality, lower risk of complications, higher risk of unplanned reintervention, shorter ICU length of stay, and higher postprocedure oxygen saturation compared with SPS.
Keywords: Surgical shunt; Blalock-Taussig shunt; ductus arteriosus; stents; infants; meta-analysis
Congenital heart abnormalities with ductal-dependent pulmonary blood flow are the complex the congenital heart diseases types. After birth, the pulmonary blood flow of infants depends on the arterial duct. An initial palliative procedure to provide a more stable source of pulmonary blood flow is often required prior to undergoing a more definitive repair. The surgical systemic-pulmonary artery shunt (SPS) has been widely used in initial palliative surgery for the ductal-dependent pulmonary blood flow, but the morbidity and mortality of the corrective surgery may be raised by shunt-related complications such as shunt occlusion, shunt stenosis, distortion of the pulmonary arteries, pulmonary hypertension, differential growth of the right and left pulmonary arteries, as well as surgical adhesion [1]. Since firstly reported in 1992, transcatheter ductus arteriosus stent (DAS) was descript as one of the early palliative treatments for congenital heart lesions with ductal-dependent pulmonary blood flow in high-risk patients suitable for primary repair [2]. DAS possesses advantageous on minimally invasive procedures, thereby avoiding a surgical thoracotomy and cardiopulmonary bypass. And it can make the pulmonary blood flow evenly distributed after the operation, which is beneficial to the growth of pulmonary arteries for future definitive repairs [3]. However, there are some potential complications of DAS additionally, such as spontaneous closure of the stented duct as a result of progressive intimal hyperplasia, stent dislodgement or acute stent thrombosis. Moreover, the alternative of an initial palliative procedure to provide a more stabilized provenance of pulmonary blood flow remains controversial [4–6]. For sake of achieving preferable testaments of evidence-based medicine, we performed a systematic review and meta-analysis to evaluate the safety and efficacy of DAS compared with SPS in patients with ductal-dependent pulmonary blood flow.
2.1 Data Sources and Search Strategies
We performed our study according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [7]. The review has been registered in PROSPERO with an identification number of CRD42020221480. Two independent reviewers (GL and NA) identified all eligible articles through a search of the PubMed, Embase, and the Cochrane Library databases (From inception to December 2020). The search terms were as follows: (Ductus Arteriosus) OR (Ductus) OR (Patent Ductus Arteriosus) OR (Stents) OR (Ductus arteriosus stent) AND (Blalock-Taussig Procedure) OR (Surgical Shunt) OR (surgical systemic-pulmonary artery shunt) OR (Modified Blalock-Taussig Shunt). Reference lists were systematically searched for relevant articles using a “cited by” function in Google Scholar to identify additional relevant studies. We also constructed a PRISMA diagram to illustrate the study selection process (Fig. 1).
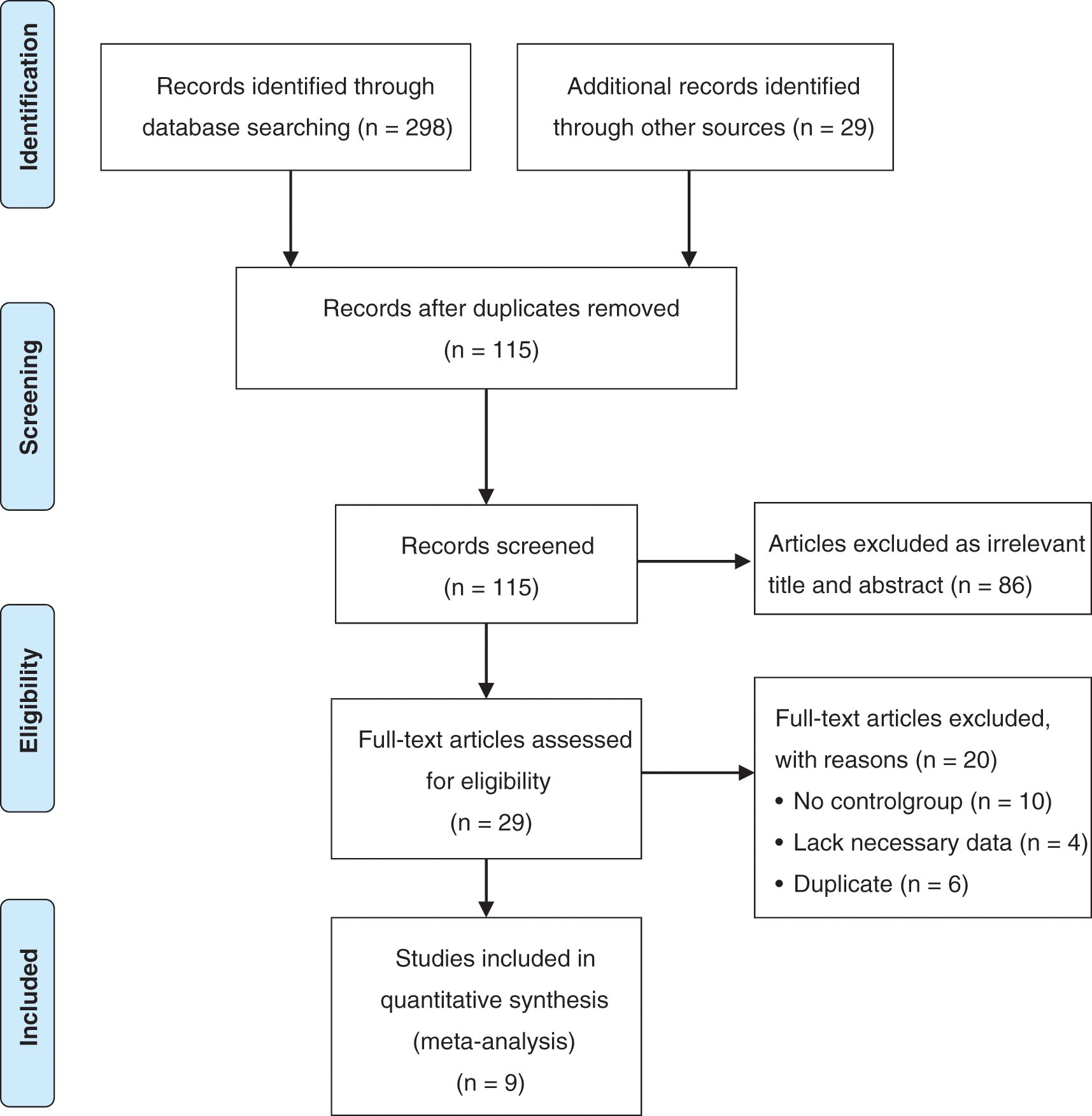
Figure 1: Flow chart of the selection process of search and selection of articles
2.2 Inclusion Criteria and Exclusion Criteria
Inclusion criteria included randomized controlled trials, non-randomized prospective studies, and retrospective clinical studies comparing DAS compared with SPS in patients with ductal-dependent pulmonary blood flow. No language, or publication status was imposed. Exclusion criteria included animal and in vitro studies, overlapped datasets, data that could not be reliably extracted, conference papers, case reports, review articles, editorials, and letters.
Two authors independently and then cross-checked for the full papers of eligible studies. All disagreements were resolved with discussion and the final decision was reached by consensus with a third reviewer. The following data were extracted: study characteristics, patient demographics, and baseline symptomatology, mortality within 30 days, medium-term mortality, unplanned reintervention (incidence of unintended interventions before next-stage surgery), procedural complications, hospital length of stay, length of intensive care unit (ICU) stay, postprocedure oxygen saturation, Nakata index (Indexed pulmonary artery cross-sectional area was considered indicative of pulmonary artery growth) [8].
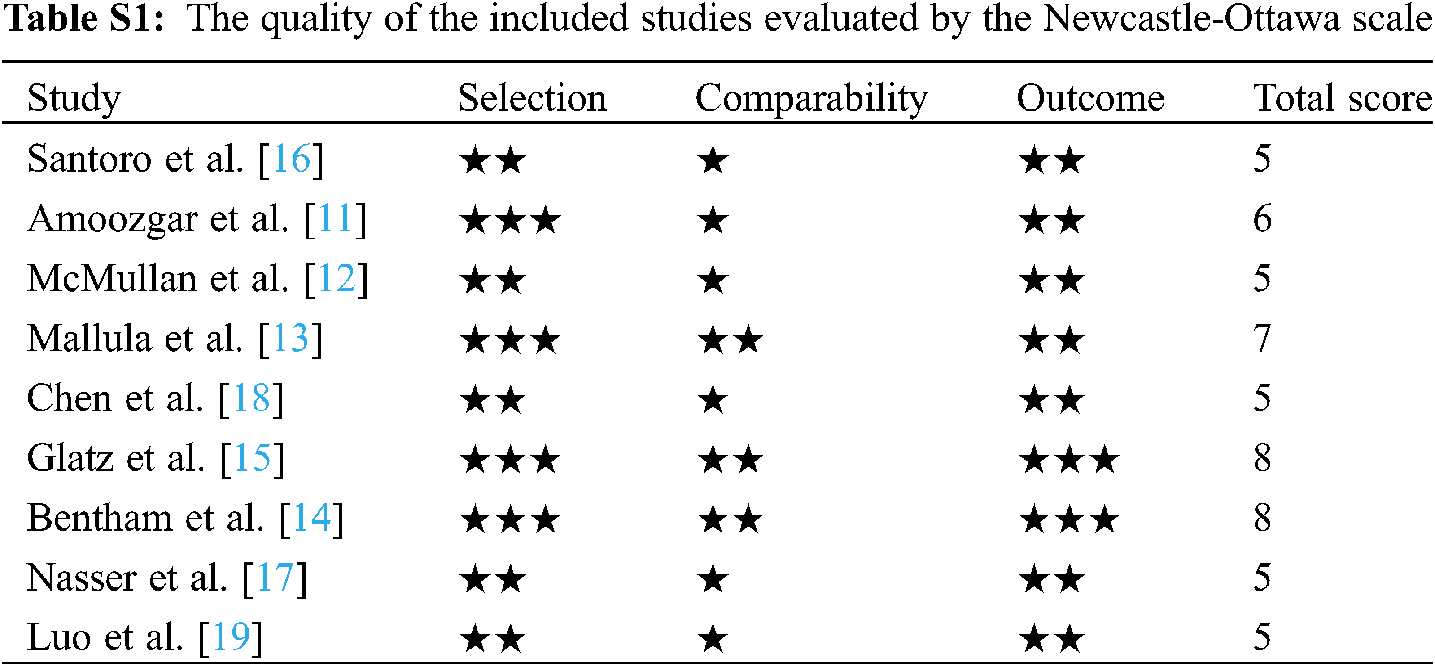
We assessed the methodological quality using the Newcastle-Ottawa Scale (NOS) [9]. The NOS contains eight items, categorized into three dimensions including selection (0–4 points), comparability (0–2 points), and depending on the study type outcome or exposure (0–3 points). Three reviewers assessed the methodological quality of each study independently, and any discrepancy was resolved by discussion. High-quality projects were awarded a corresponding number of stars. In the 9 studies, 4 of the studies scored 6 or higher, and 5 of the studies scored 5. Quality evaluation results for each study are reported in Supporting materials: Table S1.
High heterogeneity was confirmed with a significance level of P < 0.10 and I-square (I2) value of ≥50%. The random effects model (Der Simonian-Laird) was used to calculate pooled effect estimates when high heterogeneity was encountered. The fixed-effects (Mantel-Haenszel) model was applied when low heterogeneity was encountered. Sensitivity analyses were performed to find the origin of the heterogeneity by removing each study one at a time. Funnel plots were created for visual inspection for publication bias Egger’s test was performed to statistically test for publication bias [10]. Statistical analysis was performed using Stata Version 15 (Stata Corp, College Station, TX). The level of statistical significance was a priori set at P < 0.05 and all P-values were two-sided. For continuous outcomes, the mean and standard deviation (SD) reported in each study were collected and analyzed, and presented as mean differences (MD) along with 95% confidence intervals (CI). For categorical outcomes, data were extracted for each intervention group for analysis and presented as risk ratio (RR) with 95% CI. Subgroup analyses were performed according to sample size (≤100, >100).
3.1 Characteristics of Eligible Studies
The initial literature search yielded 327 potentially relevant records. After screening titles and abstracts, 29 reports were retrieved for full-text evaluation. Ultimately, 9 studies were included in our systematic review [11–19]. In total, 842 patients were included in the present study (DAS: n = 295; SPS: n = 547). The characteristic of these studies is summarized in Table 1. All studies were published from 2009 to 2020. There were three studies from America, two studies from China, one study from the United Kingdom, one study from Italy, one study from Iran, and one study from Saudi Arabia. Two studies were multicenter researches, and seven were single-center researches. Seven studies suffered from inadequate follow-up and lack of matching of the exposed and unexposed groups. Two studies performed analyses adjusted for patient covariates. Detailed demographics of the included studies are summarized in Table 1.
Patients in the DAS group experienced a reduced risk of death before repair compared with patients in the SPS group (RR, 0.63; 95% CI, [0.40,0.99]; P = 0.91, I2 = 0%; Fig. 2). DAS demonstrated a reduced risk for procedural complications compared with SPS (RR, 0.46; 95% CI, [0.29,0.72]; P = 0.78, I2 = 0%; Fig. 3). There was an increased risk for unplanned reintervention for DAS (RR, 1.77; 95% CI, [1.42,2.20]; P = 0.61, I2 = 0%; Fig. 4). Meta-analysis for hospital length of stay demonstrated no difference between SPS and DAS (MD, –6.68; 95% CI, [–13.96, 0.60]; P < 0.01, I2 = 98%; Fig. 5). However, the forest plot demonstrated that the length of ICU stay of the DAS group was significantly lower than that of the control group (MD, –5.12; 95% CI, [–7.33, –2.91]; P = 0.005, I2 = 76%; Fig. 6). The meta-analysis also demonstrated statistically higher postprocedure oxygen saturation for SPS over DAS (MD, 1.78; 95% CI, [0.92,2.64]; P = 0.46, I2 = 0%; Fig. 7). There was no difference between the two groups in terms of mortality within 30 days, and Nakata Index (Figs. 8 and 9). Neither visual inspection of the funnel plot nor Egger regression test found publication bias of platelet count (Fig. 10, Table 2). Sensitivity analyses analysis demonstrated that exclude each study at a time did not change in outcome for 6 outcomes (Supporting Materials: Fig. S1).
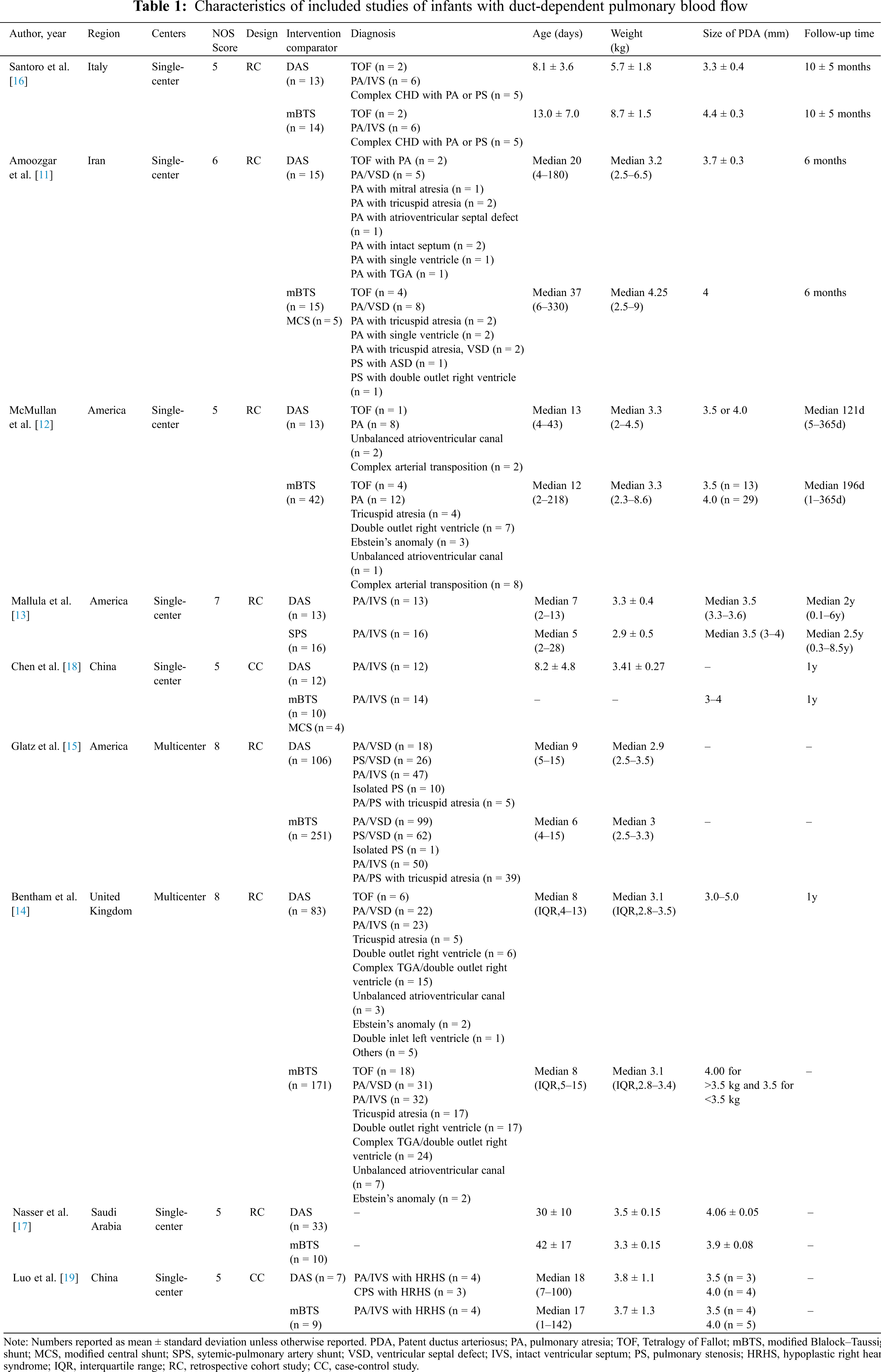
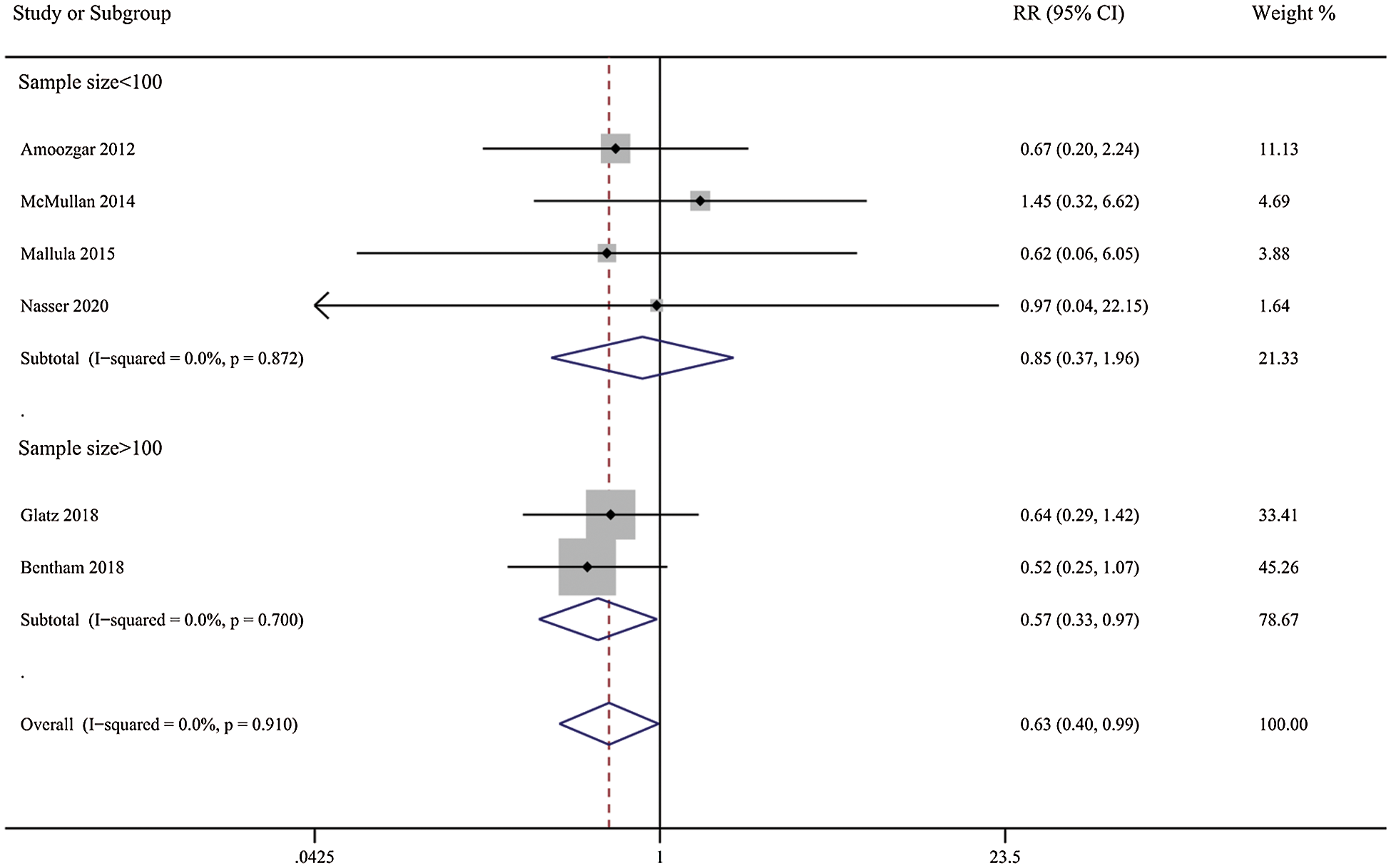
Figure 2: Forest plots showing meta-analysis of DAS compared with SPS for outcome of medium-term mortality. Meta-analysis demonstrates decreased risk of medium-term mortality with DAS compared with SPS
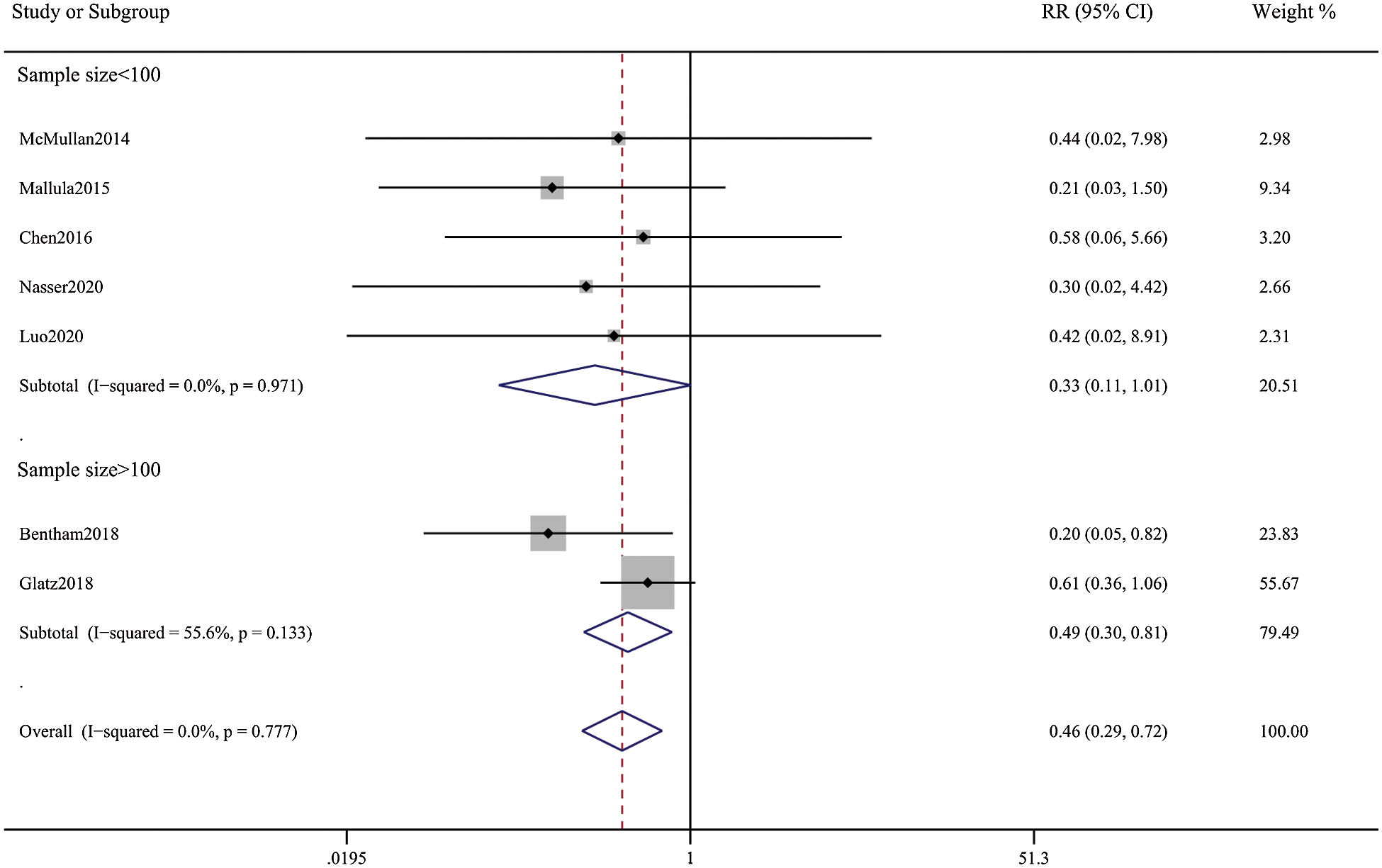
Figure 3: Forest plots showing meta-analysis of DAS compared with SPS for outcome of procedural complications. Meta-analysis demonstrates decreased risk of complications with DAS compared with SPS
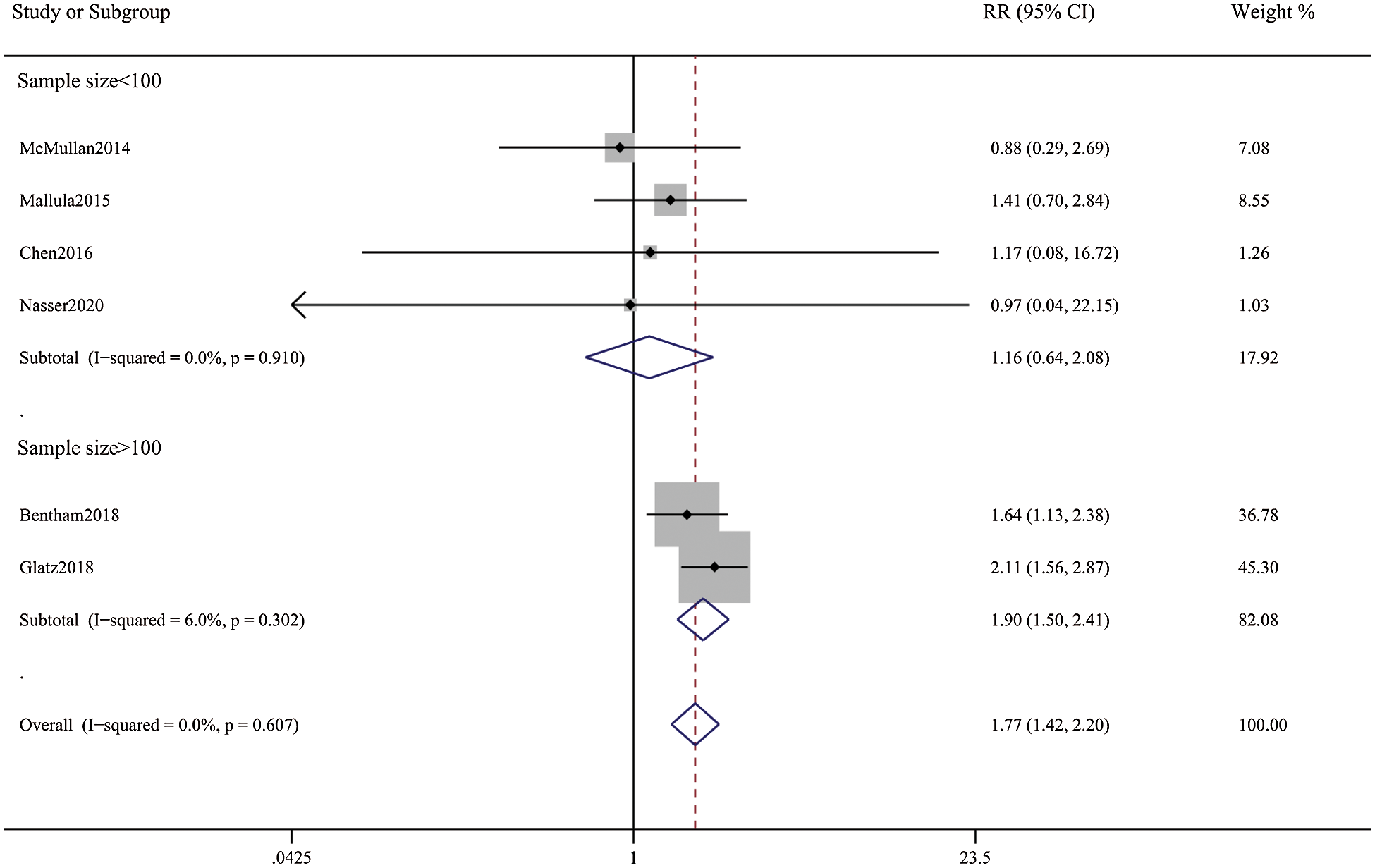
Figure 4: Forest plots showing meta-analysis of DAS compared with SPS for outcome of unplanned reintervention. Meta-analysis demonstrates increased risk of Reintervention with DAS compared with SPS
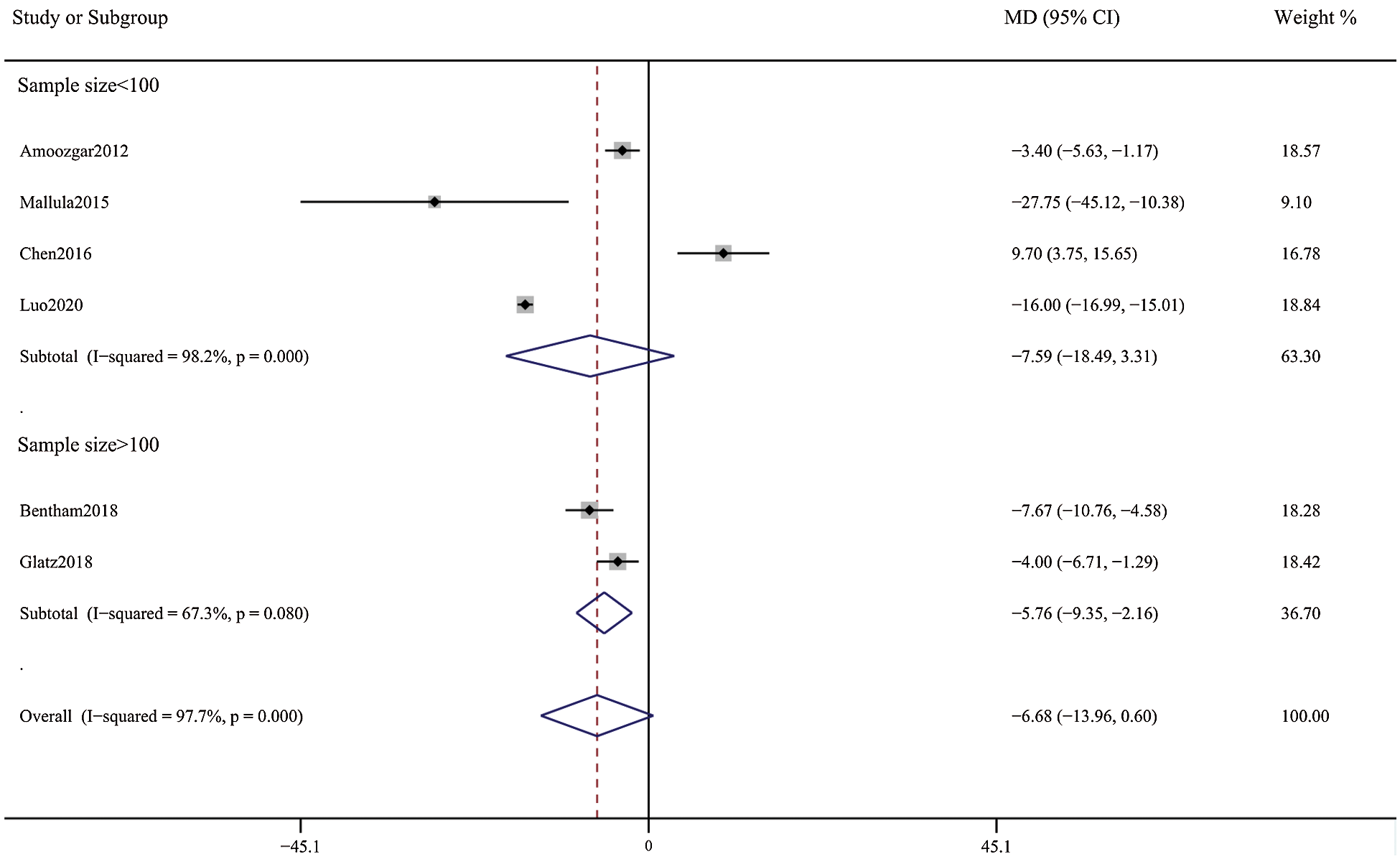
Figure 5: Forest plots showing meta-analysis of DAS compared with SPS for outcome of hospital length of stay. There was no significant difference between the 2 groups
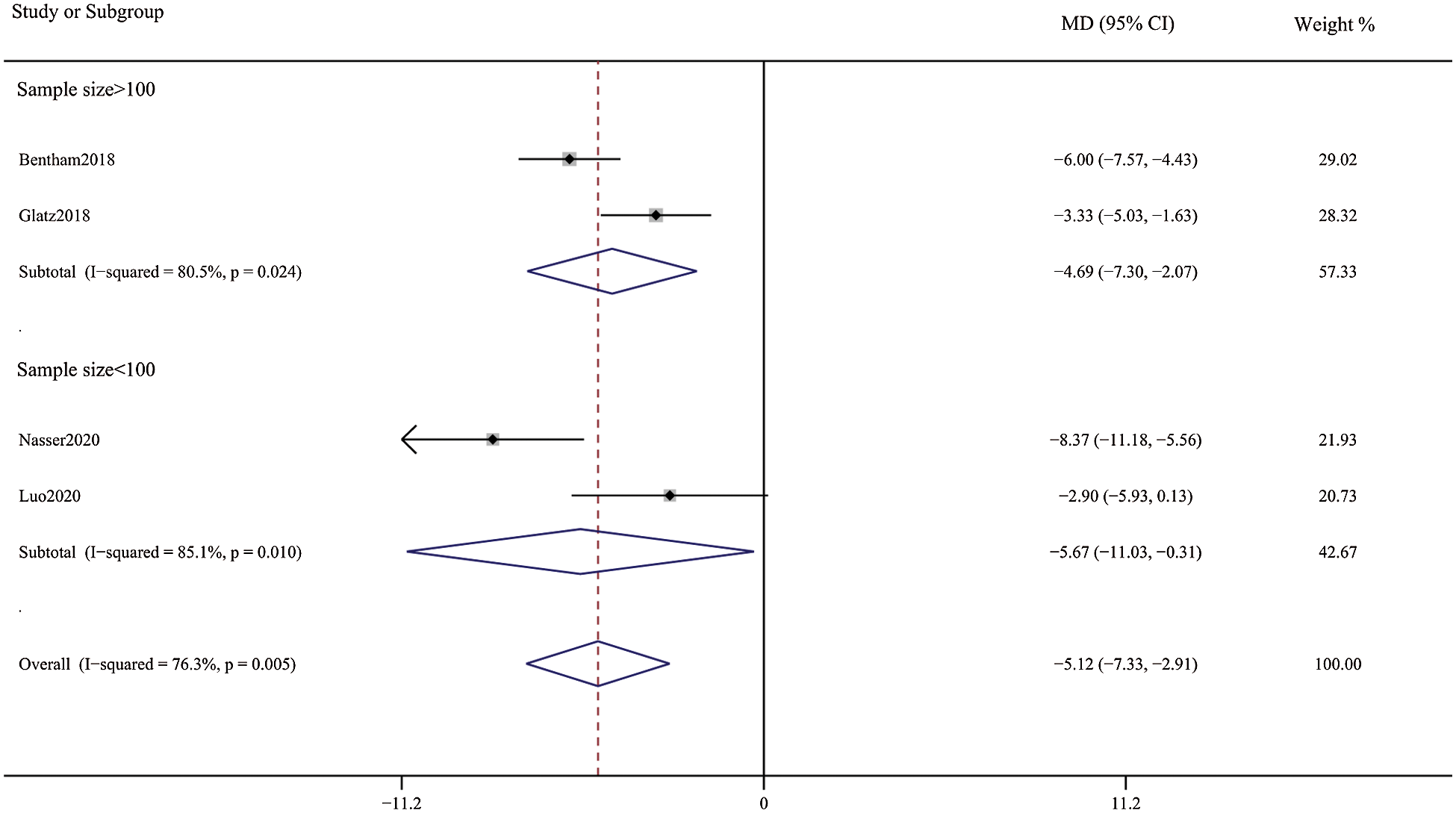
Figure 6: Forest plots showing meta-analysis of DAS compared with SPS for length of intensive care unit stay. Meta-analysis demonstrates shorter intensive care unit length of stay with DAS compared with SPS
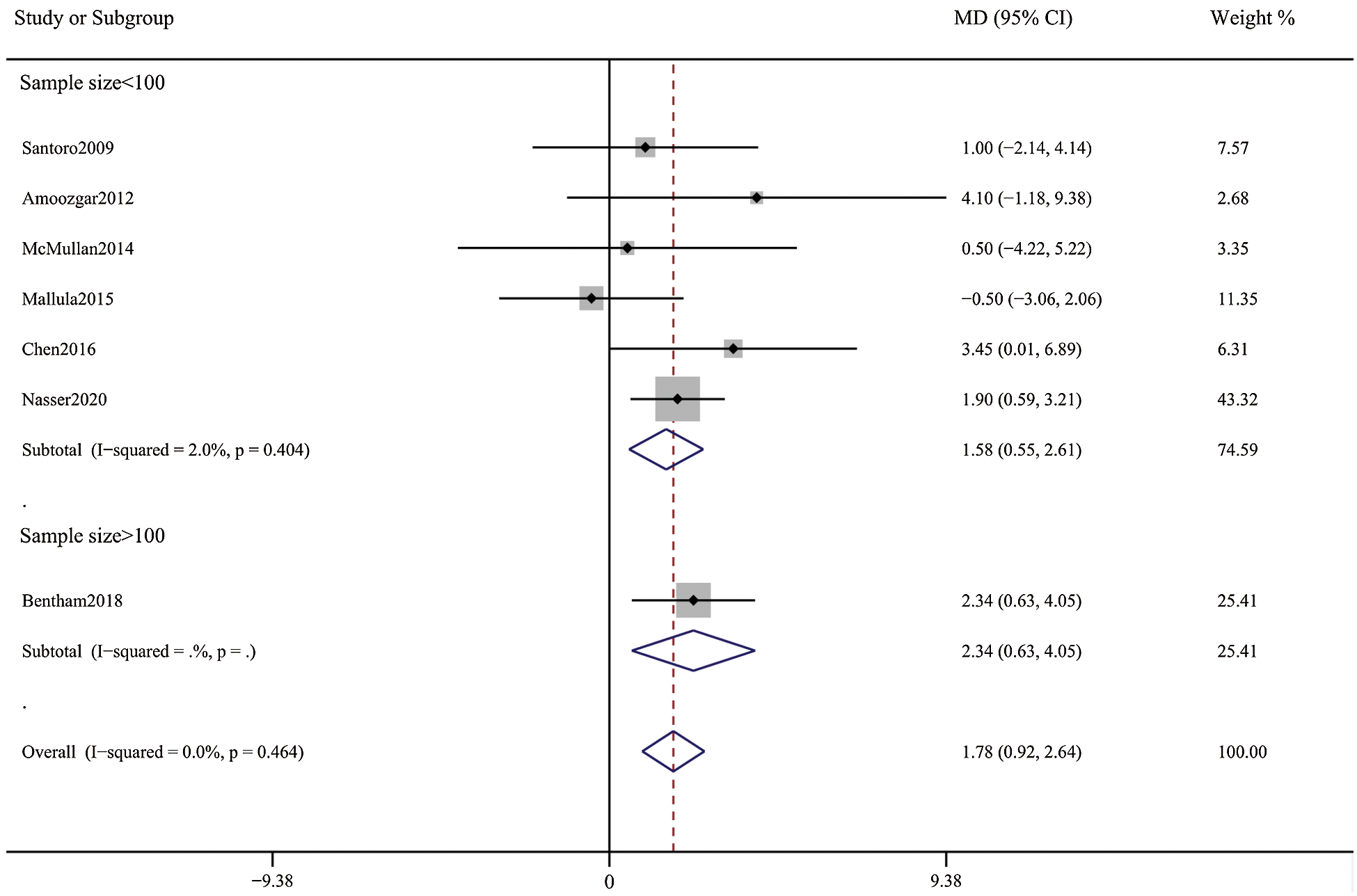
Figure 7: Forest plots showing meta-analysis of DAS compared with SPS for post-procedure oxygen saturation. Meta-analysis demonstrates higher post-procedure oxygen saturation with DAS compared with SPS
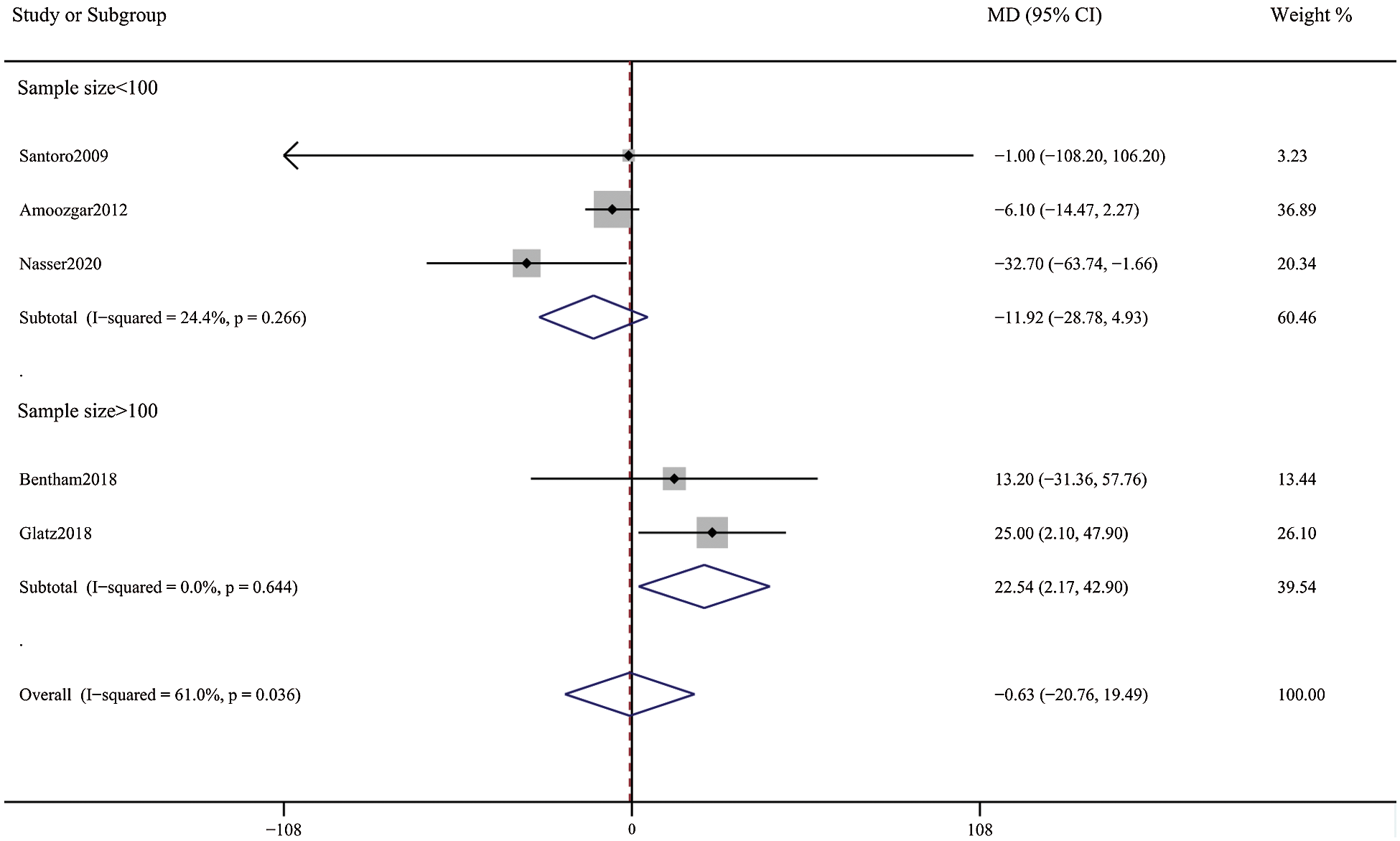
Figure 8: Forest plots showing meta-analysis of DAS compared with SPS for Nakata index. There was no significant difference between the 2 groups
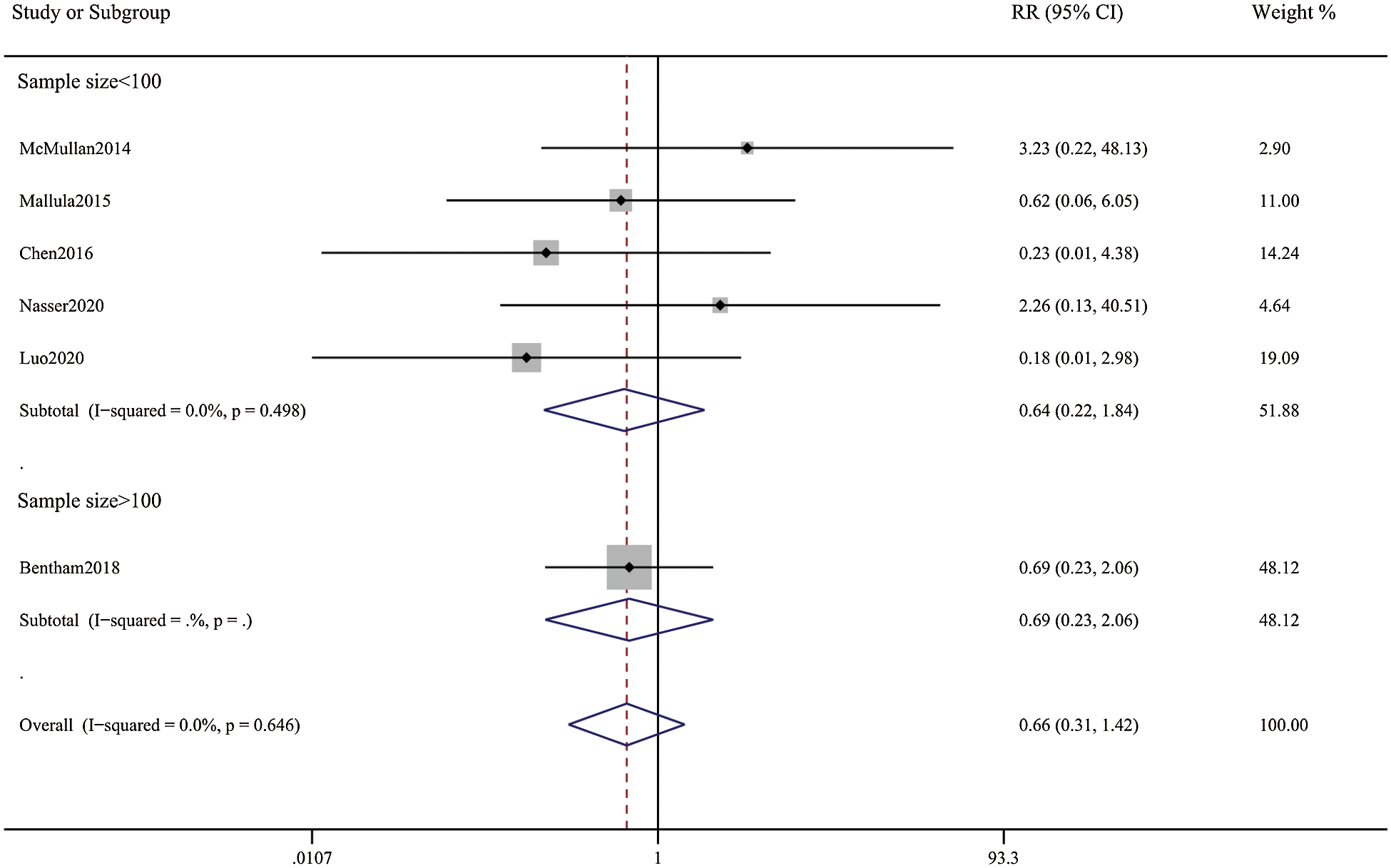
Figure 9: Forest plots showing meta-analysis of DAS compared with SPS for outcome of mortality within 30 day. There was no significant difference between the 2 groups
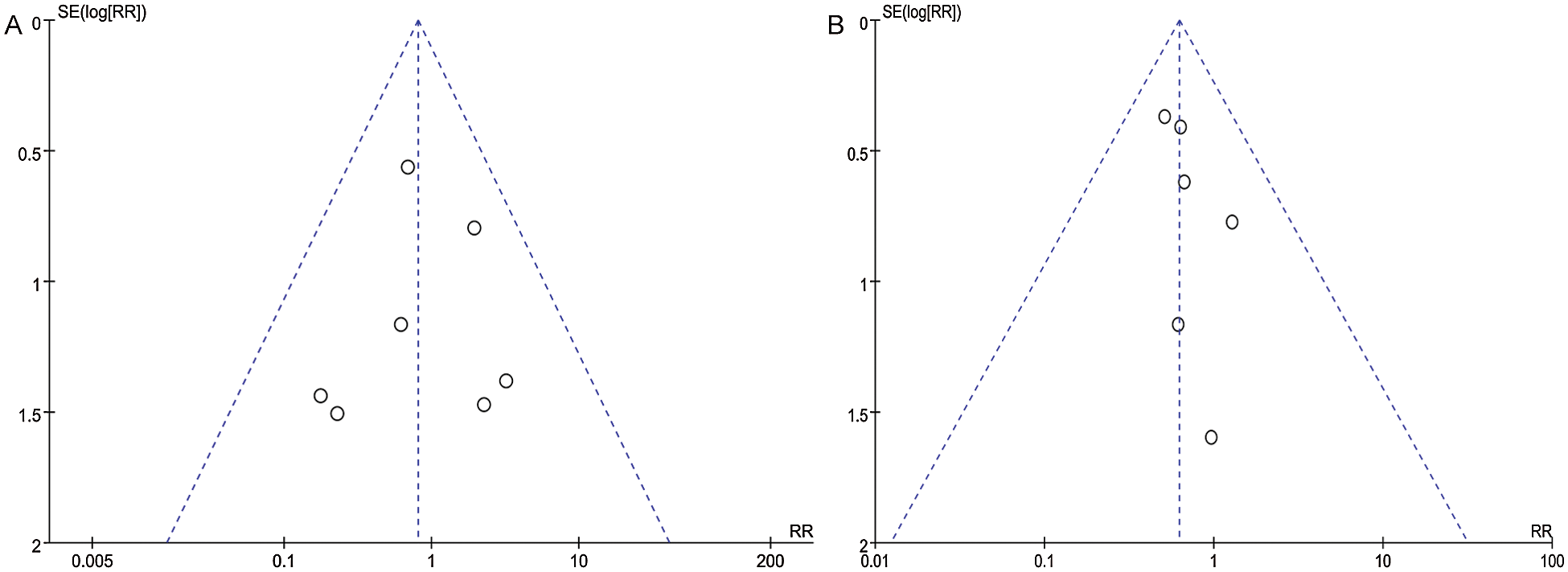
Figure 10: Funnel plot for publication bias for outcome of (A) mortality within 30 days, (B) medium-term mortality. No funnel plot asymmetry on visual inspection
For congenital heart abnormalities with ductal-dependent pulmonary blood flow, initial palliative treatment is normally needed to provide a more stabilized source of pulmonary blood flow before undergoing more definitive palliation or repair [20]. Some notable developments have been made in the placement of PDA stent in late years. DAS is a technically feasible, well-tolerated, and effective efficient palliation for high-incidence congenital heart lesions with duct-dependent pulmonary blood flow, and it is proposed to be put into practice when short-term pulmonary blood flow support is anticipated or when patients scheme to carry out the early surgical restoration [15,21]. At the mid-term follow-up, DAS facilitates spontaneous physiological amelioration or promotes significant pulmonary artery growth. In addition, compared with SPS, it ensures more homogeneous pulmonary blood flow, which makes the development of the pulmonary artery bed more equilibrated [3,22]. Previous reports suggested that the DAS strategy was not deemed to be sufficiently plausible to justify a randomized study in all patients with ductal-dependent pulmonary blood flow. Even with initial palliative treatment to provide a source of pulmonary blood flow at least half of patients with ductal-dependent pulmonary blood flow require a systemic to pulmonary artery shunt [4,23].
Alsagheir et al. [24] have already performed a systematic review and meta-analysis to evaluate DAS versus modified Blalock–Taussig surgical shunt in patients with duct-dependent pulmonary blood flow. For all that, we have added other three studies in different regions. Furthermore, our Meta-analysis demonstrates higher post-procedure oxygen saturation with patent duct stenting compared with surgical systemic-pulmonary artery shunt. Here the subgroup analyses of the sample size are made, and meta-regression analysis shows no significant incidence of sample size, different countries, and whether it is multicenter research, which makes the result that more credible. Our meta-analysis demonstrates a significant difference in medium-term mortality between DAS and SPS. Procedural complications are also in favor of DAS. Whereas patients undergoing DAS experienced procedural complications such as arrhythmia and access-related vascular injury, procedural complications in patients undergoing SPS included shunt-related bleeding, arrhythmia, and cardiac arrest [22]. This meta-analysis demonstrates an obvious risk reduction in unplanned reintervention in favor of SPS. A shorter length of ICU-stay, and higher postoperative oxygen saturation for DAS are also announced. Nevertheless, this meta-analysis illustrates no significant difference in mortality within 30 days, pulmonary vascular development, and hospital length of stay. The reason may be various unknown patient covariates that likely render the treatment groups prognostically different. For example, due to the failed attempt of DAS or needing a late shunt because of inadequate initial stenting that resulted in abnormally low saturation, the patients are included in the SPS group. Therefore, the conclusion is not uniformly identified. And some studies have confirmed that measured by the Nakata index, there was no difference in overall pulmonary artery growth between the two groups, while the growth of pulmonary artery was significant in both groups. However, more symmetric growth of the pulmonary was observed in the DAS group as measured by the left pulmonary artery/right pulmonary artery diameter ratio. Less use of diuretics at discharge and shorter hospital length of stay are exhibited in the DAS group, as well as larger and more symmetrical pulmonary arteries at later follow-up [14,25,26]. But virtually, the failure to achieve adequate pulmonary artery growth is a major obstacle to undergoing surgical repair for patients with cyanotic congenital heart disease [27].
DAS has a tendency to develop neointimal proliferation over time, which may affect its patency and durability, and thus patients may require reintervention in the form of pulmonary arteries [6,28]. But in many cases, for infants who are not suitable for primary repair, or whose oxygen saturation are anticipated to improve spontaneously with the decrease of pulmonary vascular resistance, maintaining the arterial duct patency is better than undergoing systemic pulmonary artery shunt. Future developments in stent technology, for example, more flexible stents, may expand the applications to very tortuous ducts and extent the scope of palliative options for augmenting pulmonary blood flow in duct-dependent pulmonary blood flow.
It is worth mentioning that this meta-analysis has certain limitations. Firstly, in the present meta- analysis, a noticeable difference of sample size exists between treatment groups. The included studies are not randomized trials and they all are subject to serious potential sources of bias, notably confounding by indication. The attempt to statistically adjust patient covariates of outcomes is merely seen in two studies, and unknown patient-specific factors may render the treatment groups different prognostically. In addition, some studies lack random assignment of surgical procedures, which may contribute to the reduction in efficiency of the analysis. All eligible articles are retrospective design. There is a risk of a degree of inherent selection bias presented in our study, as it is impossible to thoroughly assess the outcomes of all patients who may have been considered for DAS but ultimately did not undergo this procedure due to anatomic concerns, or technical failure.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Williams, J. A., Bansal, A. K., Kim, B. J., Nwakanma, L. U., Patel, N. D. et al. (2007). Two thousand Blalock-Taussig shunts: A six-decade experience. Annals of Thoracic Surgery, 84(6), 2070–2075. [Google Scholar]
2. Gibbs, J. L., Rothman, M. T., Rees, M. R., Parsons, J. M., Blackburn, M. E. et al. (1992). Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. British Heart Journal, 67(3), 240–245. [Google Scholar]
3. Santoro, G., Gaio, G., Capozzi, G., Giugno, L., Palladino, M. T. et al. (2015). Fate of hypoplastic pulmonary arteries after arterial duct stenting in congenital heart disease with duct-dependent pulmonary circulation. JACC Cardiovascular Interventions, 8(12), 1626–1632. [Google Scholar]
4. Santoro, G., Gaio, G., Russo, M. G. (2018). Letter by Santoro et al., Regarding articles, “Duct stenting versus modified blalock-taussig shunt in neonates with duct-dependent pulmonary blood flow: Associations with clinical outcomes in a multicenter national study” and “Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: Insights from the congenital catheterization research collaborative”. Circulation, 138(4), 432–433. [Google Scholar]
5. Bentham, J. R. (2018). Response by Bentham to Letter Regarding article, “Duct stenting versus modified Blalock-Taussig shunt in neonates with duct-dependent pulmonary blood flow: Associations with clinical outcomes in a multicenter national study”. Circulation, 138(4), 434–435. [Google Scholar]
6. Boucek, D. M., Qureshi, A. M., Goldstein, B. H., Petit, C. J., Glatz, A. C. (2019). Blalock-Taussig shunt versus patent ductus arteriosus stent as first palliation for ductal-dependent pulmonary circulation lesions: A review of the literature. Congenital Heart Disease, 14(1), 105–109. [Google Scholar]
7. Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C. et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. [Google Scholar]
8. Nakata, S., Imai, Y., Takanashi, Y., Kurosawa, H., Tezuka, K. et al. (1984). A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. Journal of Thoracic and Cardiovascular Surgery, 88(4), 610–619. [Google Scholar]
9. Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology, 25(9), 603–605. [Google Scholar]
10. Egger, M., Davey Smith, G., Schneider, M., Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. [Google Scholar]
11. Amoozgar, H., Cheriki, S., Borzoee, M., Ajami, G., Soltani, M. et al. (2012). Short-term result of ductus arteriosus stent implantation compared with surgically created shunts. Pediatric Cardiology, 33(8), 1288–1294. [Google Scholar]
12. McMullan, D. M., Permut, L. C., Jones, T. K., Johnston, T. A., Rubio, A. E. (2014). Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. Journal of Thoracic and Cardiovascular Surgery, 147(1), 397–401. [Google Scholar]
13. Mallula, K., Vaughn, G., El-Said, H., Lamberti, J. J., Moore, J. W. (2015). Comparison of ductal stenting versus surgical shunts for palliation of patients with pulmonary atresia and intact ventricular septum. Catheterization and Cardiovascular Interventions, 85(7), 1196–1202. [Google Scholar]
14. Bentham, J. R., Zava, N. K., Harrison, W. J., Shauq, A., Kalantre, A. et al. (2018). Duct stenting versus modified Blalock-Taussig shunt in neonates with duct-dependent pulmonary blood flow: Associations with clinical outcomes in a multicenter national study. Circulation, 137(6), 581–588. [Google Scholar]
15. Glatz, A. C., Petit, C. J., Goldstein, B. H., Kelleman, M. S., McCracken, C. E. et al. (2018). Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: Insights from the congenital catheterization research collaborative. Circulation, 137(6), 589–601. [Google Scholar]
16. Santoro, G., Capozzi, G., Caianiello, G., Palladino, M. T., Marrone, C. et al. (2009). Pulmonary artery growth after palliation of congenital heart disease with duct-dependent pulmonary circulation: Arterial duct stenting versus surgical shunt. Journal of the American College of Cardiology, 54(23), 2180–2186. [Google Scholar]
17. Nasser, B. A., Abdulrahman, M., Qwaee, A. A. L., Alakfash, A., Mohamad, T. et al. (2020). Impact of stent of ductus arteriosus and modified Blalock-Taussig shunt on pulmonary arteries growth and second-stage surgery in infants with ductus-dependent pulmonary circulation. Journal of the Saudi Heart Association, 32(1), 86–92. [Google Scholar]
18. Chen, Z., Fang, L., Zhai, B., Chen, Z., Wang, P. et al. (2016). Efficacy analysis on arterial catheter stents for pulmonary atresia with intact ventricular septum in newborns. Chinese Journal of Applied Clinical Pediatrics, 31(23), 1795–1798. [Google Scholar]
19. Luo, G., Liu, A., Wang, K. L., Yao, W., Ji, Z. X. et al. (2020). Application of arterial duct stent in ductus-dependent hypoplastic right heart syndrome. Zhonghua Er Ke Za Zhi, 58(4), 319–323. [Google Scholar]
20. Santoro, G., Gaio, G., Palladino, M. T., Castaldi, B., Iacono, C. et al. (2010). Arterial duct stenting: Do we still need surgical shunt in congenital heart malformations with duct-dependent pulmonary circulation? Journal of Cardiovascular Medicine, 11(11), 852–857. [Google Scholar]
21. Santoro, G., Gaio, G., Giugno, L., Capogrosso, C., Palladino, M. T. et al. (2015). Ten-years, single-center experience with arterial duct stenting in duct-dependent pulmonary circulation: Early results, learning-curve changes, and mid-term outcome. Catheterization and Cardiovascular Interventions, 86(2), 249–257. [Google Scholar]
22. Aggarwal, V., Petit, C. J., Glatz, A. C., Goldstein, B. H., Qureshi, A. M. (2019). Stenting of the ductus arteriosus for ductal-dependent pulmonary blood flow-current techniques and procedural considerations. Congenital Heart Disease, 14(1), 110–115. [Google Scholar]
23. Chubb, H., Pesonen, E., Sivasubramanian, S., Tibby, S. M., Simpson, J. M. et al. (2012). Long-term outcome following catheter valvotomy for pulmonary atresia with intact ventricular septum. Journal of the American College of Cardiology, 59(16), 1468–1476. [Google Scholar]
24. Alsagheir, A., Koziarz, A., Makhdoum, A., Contreras, J., Alraddadi, H. et al. (2021). Duct stenting versus modified Blalock-Taussig shunt in neonates and infants with duct-dependent pulmonary blood flow: A systematic review and meta-analysis. Journal of Thoracic and Cardiovascular Surgery, 161(2), 379–390. [Google Scholar]
25. Vida, V. L., Speggiorin, S., Maschietto, N., Padalino, M. A., Tessari, C. et al. (2010). Cardiac operations after patent ductus arteriosus stenting in duct-dependent pulmonary circulation. Annals of Thoracic Surgery, 90(2), 605–609. [Google Scholar]
26. Santoro, G., Capozzi, G., Giordano, M., Gaio, G., Palladino, M. T. et al. (2017). Fate of duct-dependent, discontinuous pulmonary arteries after arterial duct stenting. Pediatric Cardiology, 38(7), 1370–1376. [Google Scholar]
27. Kanter, K. R., Kogon, B. E., Kirshbom, P. M., Carlock, P. R. (2010). Symptomatic neonatal tetralogy of Fallot: Repair or shunt? Annals of Thoracic Surgery, 89(3), 858–863. [Google Scholar]
28. Benson, L., Arsdell, G. V. (2018). Comparisons between ductal stenting and Blalock-Taussig shunts for infants with ductal-dependent pulmonary circulation. Circulation, 137(6), 602–604. [Google Scholar]
Appendix
Supporting Materials
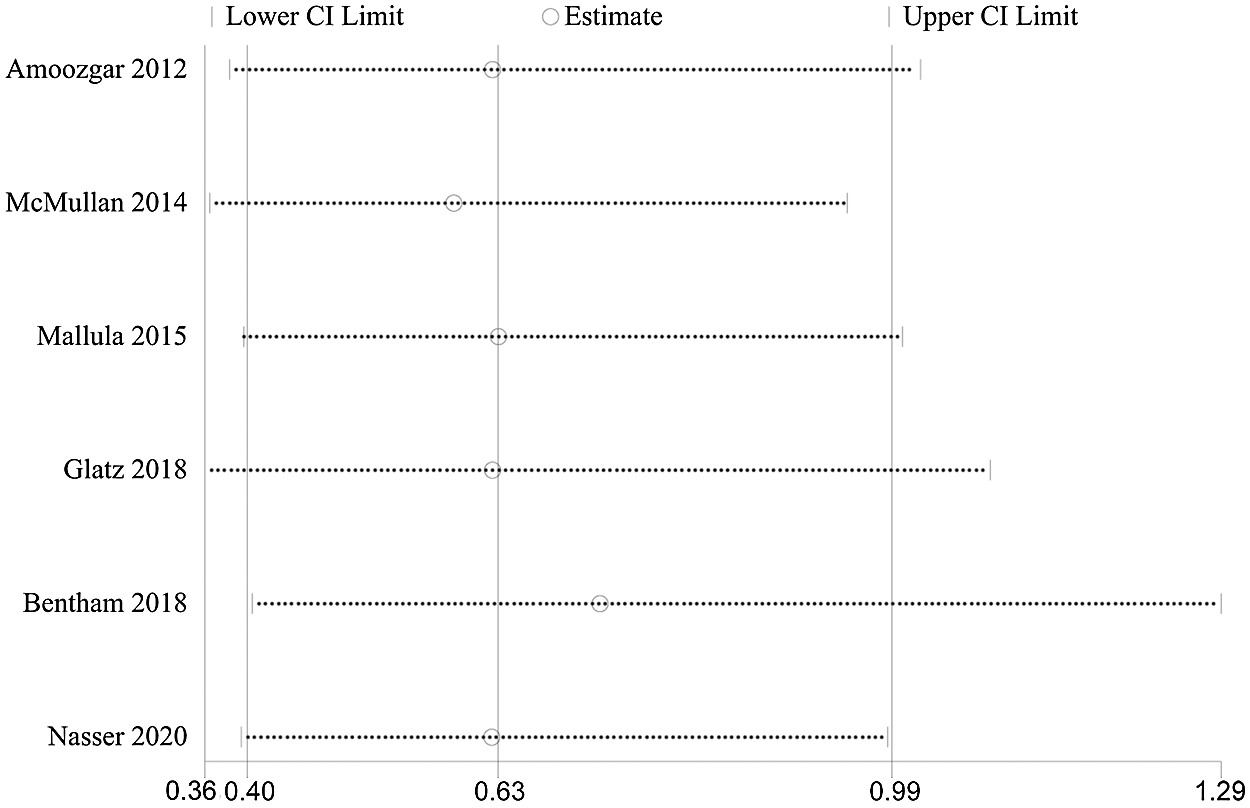
Figure S1: Influence analysis demonstrated that exclude anyone study at a time did not change the final statistical performance of medium-term mortality
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |