

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016635
ARTICLE
The Impact of a Bicuspid Aortic Valve on Aortic Geometry and Function in Patients with Aortic Coarctation: A Comprehensive CMR Study
1Department of Congenital Heart Disease and Paediatric Cardiology, University Hospital Schleswig-Holstein, Kiel, Germany
2Royal Brompton Hospital, London, UK
*Corresponding Author: Inga Voges. Email: inga.voges@uksh.de
Received: 13 March 2021; Accepted: 11 May 2021
Abstract: Background: An isolated bicuspid aortic valve (BAV) is associated with structural and functional abnormalities of the aorta and the left ventricle (LV). Although ~50% of patients with aortic coarctation (CoA) have a BAV, less is known about its impact on LV function and aortic geometry and function in CoA patients. In this cardiovascular magnetic resonance imaging (CMR) study, we analysed markers of LV and aortic function as well as aortic geometry in a large cohort of CoA patients with a BAV and compared them with CoA patients with a tricuspid aortic valve (TAV). Methods: We included 48 patients with a BAV (18.4 ± 9.3 years) and 45 patients with TAV (20.7 ± 9.9 years). LV volumes, mass and ejection fraction as well as aortic distensibilty, pulse wave velocity (PWV) were measured from standard cine CMR and phase-contrast CMR images. 2-dimensional CMR feature tracking (2D-CMR-FT) was performed to measure longitudinal, circumferential and radial strain and strain rate of the LV. Aortic arch geometry was classified as romanic, gothic and crenel. Results: LV volumes, mass and ejection fraction as well as aortic distensibility and PWV did not significantly differ between the BAV and the TAV group. There was also no significant difference for LV global longitudinal, radial and circumferential strain and strain rate between both groups. Patients with a BAV had more commonly a gothic aortic arch compared to TAV patients, but this difference was not statistically significant (22 vs. 14, p = 0.2). Ascending and descending aortic distensibility correlated with LV mass in the entire patient group (p < 0.001). Global longitudinal, circumferential and radial strain (GLS, GCS, GRS) and global longitudinal and circumferential strain rate (GLSR, GCSR) correlated with LV ejection fraction (p < 0.001). Conclusion: Our data suggest that the presence of a BAV does not adversely impact LV and aortic function in children and young adults with CoA. The correlation of global circumferential, longitudinal and radial strain values with LV ejection fraction demonstrates that 2D-CMR-FT might provide additional information related to ventricular function in CoA patients.
Keywords: Aortic coarctation; bicuspid aortic valve; cardiovascular magnetic resonance; feature tracking
Patients with aortic coarctation (CoA) are at risk for late complications associated with increased cardiovascular morbidity and mortality [1]. This not only includes a postoperative persisting arterial hypertension [2] and residual aortic obstruction but also left ventricular dysfunction [3–5].
About 50% of CoA patients have a bicuspid aortic valve (BAV) which is associated with structural and functional abnormalities of the aorta including aortic root and ascending aortic dilatation, reduced aortic elasticity and abnormal aortic blood flow patterns [6–8]. In a recent study in patients with aortic valve stenosis, it was demonstrated that patients with BAV have increased left ventricular (LV) volumes and a larger LV outflow tract diameters to patients with a tricuspid aortic valve (TAV) [9].
However, less is known about patients with both, CoA and BAV. Frandsen et al. [10] reported that CoA patients with TAV often have smaller aortic root and ascending aortic diameters compared to CoA patients with BAV. But concrete information about the influence of a BAV on aortic elasticity as well as on LV size, function and mass in CoA patients is scarce.
Cardiovascular magnetic resonance (CMR) imaging is routinely used for the follow up in repaired CoA patients and allows detailed assessment of aortic and LV function and geometry. Regional LV myocardial function and deformation can be assessd with 2-dimensional CMR feature tracking (2D-CMR-FT) [11].
The aim of this CMR study, was to compare CoA patients with a BAV to those with a TAV regarding: (1) LV size and global function, (2) LV regional function and deformation using 2D-CMR-FT, (3) aortic distensibility and pulse wave velocity (PWV) and (4) aortic geometry.
We consecutively included all patients with repaired CoA who underwent CMR imaging between 2009 and 2019. Exclusion criteria were evidence of a mitral valve stenosis (mean gradient >8 mmHg), moderate or severe aortic stenosis and more than mild aortic or mitral valve regurgitation. Clinical, demographic and surgical data were obtained from the patients’ medical records. The study was approved by the local ethics committee (Nr. D566/19, Date of Approval 09/12/2019). Informed consent from patients or parents was obtained as appropriate.
CMR studies were performed using a 3.0-Tesla or 1.5 Tesla scanner (Philips Medical Systems, Netherlands) with a dedicated coil for cardiac imaging. Standard steady-state free precession (SSFP) sequences were used to acquire short axis cine stacks, axial cine stacks, 4-chamber-views and aortic arch views.
To measure aortic PWV we applied two-dimensional phase-contrast flow imaging with through-plane velocity encoding and retrospective ECG gating at three locations as previously described [12]: 1) ascending aorta (AAo), 2) proximal descending aorta (DAo) and 3) DAo.
All CMR analyses were performed using a dedicated CMR software (Medis Suite MR, Medis Medical Imaging Systems, Leiden, The Netherlands).
LV volumetry was performed using short axis cine stacks. First, end-diastolic and end-systolic time points were defined. Second, endo- and epicardial contours in all slices showing the left ventricle at end-diastole were drawn; at end-systole only endocardial contours were defined. LV volumes (LV end-diastolic volume, LVEDV; LV end-systolic volume, LVESV; LV stroke volume, LVSV) and LV mass were automatically calculated by the software.
2D-CMR-FT was performed to measure longitudinal, circumferential and radial strain and strain rate of the LV. Global longitudinal strain (GLS) and strain rate (GLSR) as well as regional longitudinal strain values were measured from the acquired 4-chamber views. Global circumferential and radial strain and strain rate values (GCS, GCSR, GRS, GRSR) as well as regional strain and strain rate values were obtained from short axis cine images. Regional strain and strain rate values are reported according to the American Heart association 17-segment-model [13].
PWV in the aortic arch and descending thoracic aorta was measured as described before by one of the authors [12,14].
Aortic distensibility was measured from CMR cine images in the ascending aorta, aortic isthmus and descending thoracic aorta by using the following formula [14,15]:
with Amax and Amin representing the maximal and minimal cross-sectional area and Pmax and Pmin being the systolic and diastolic blood pressure.
Aortic arch shape was assessed by visual inspection of aortic arch views and classified as: (1) gothic, (2) romanesque and (3) crenel [16].
Statistical analysis was performed using MedCalc Version 19.6 (MedCalc statistical software, Mariakerke, Belgium). Data was adequately evaluated according to their distribution by T-test or Mann-Whitney-U-Test. To measure the associations between variables Spearman rank correlation was used. Chi-squared test for the comparison of proportions was performed to test for the difference in distribution of categorical variables. A p value of 0.05 was considered to be statistically significant.
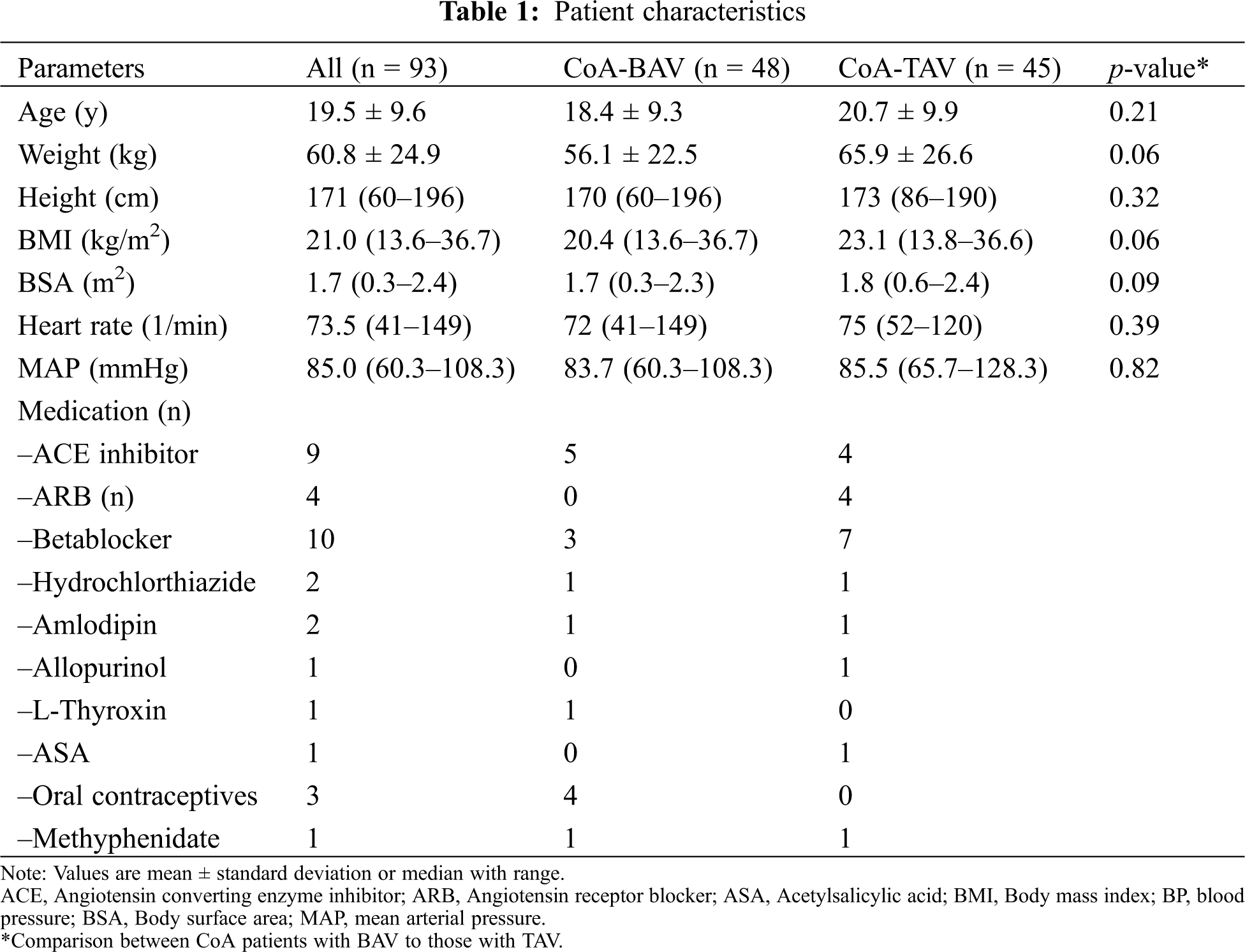
Patient characteristics are shown in Tab. 1. In total, 93 patients were included in this study of which, 48 (52%) had a BAV and 45 (48%) had a TAV.
22 patients were treated for arterial hypertension. None of the patients had more than mild aortic stenosis or regurgitation (as assessed by CMR).
3.1 Ventricular, Volumes Mass and Ejection Fraction
There was no difference in LV volumes, mass and ejection fraction between the two groups (all p > 0.05, Tab. 2). A higher LV mass was associated with a lower aortic distensibility at all three levels (ascending aorta: r = –0.38, p < 0.01; aortic isthmus: r = –0.25, p < 0.05; descending aorta: –0.44, p < 0.01; Figs. 1A and 1B). No association between blood pressure and LV mass was found.
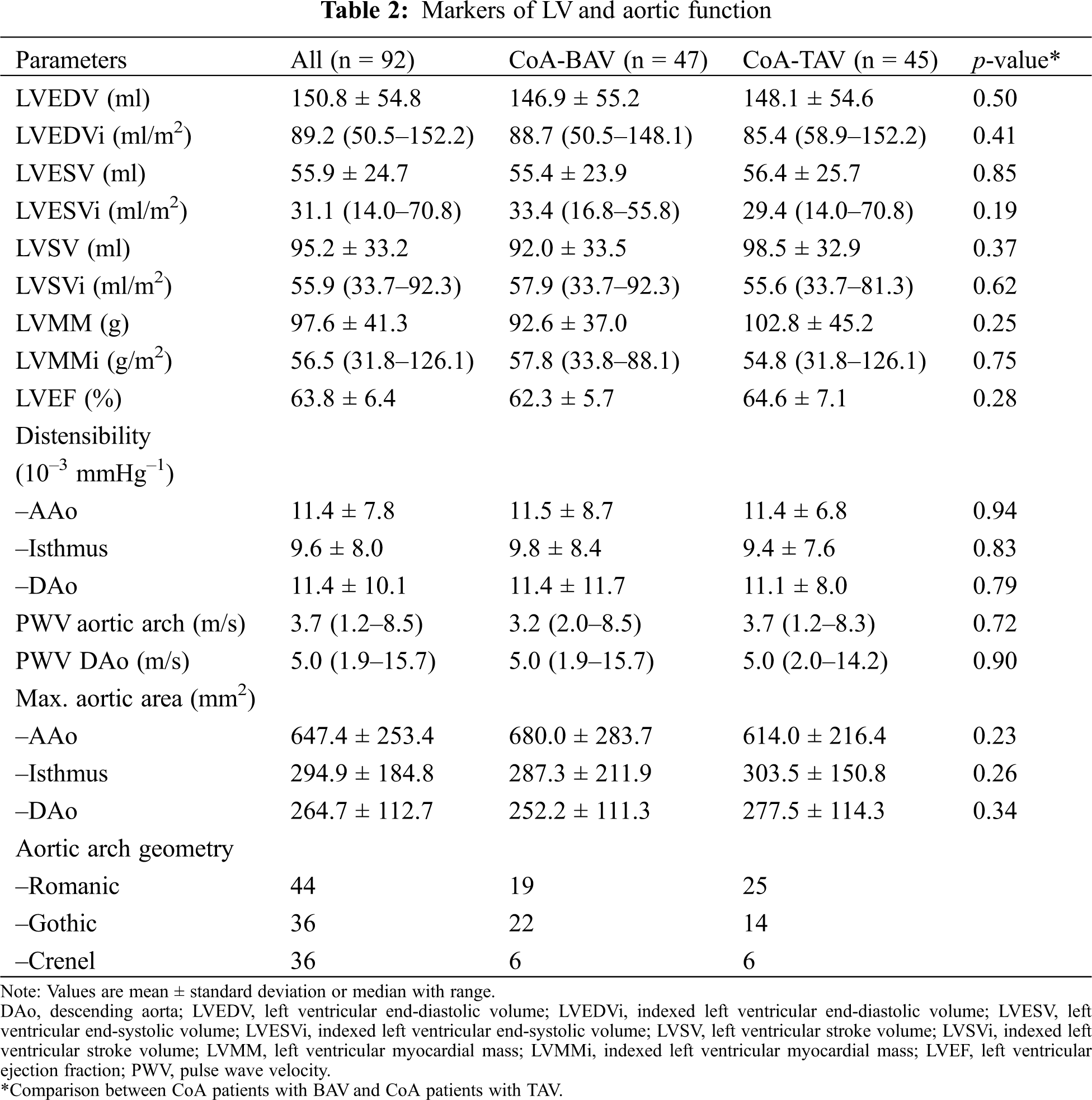
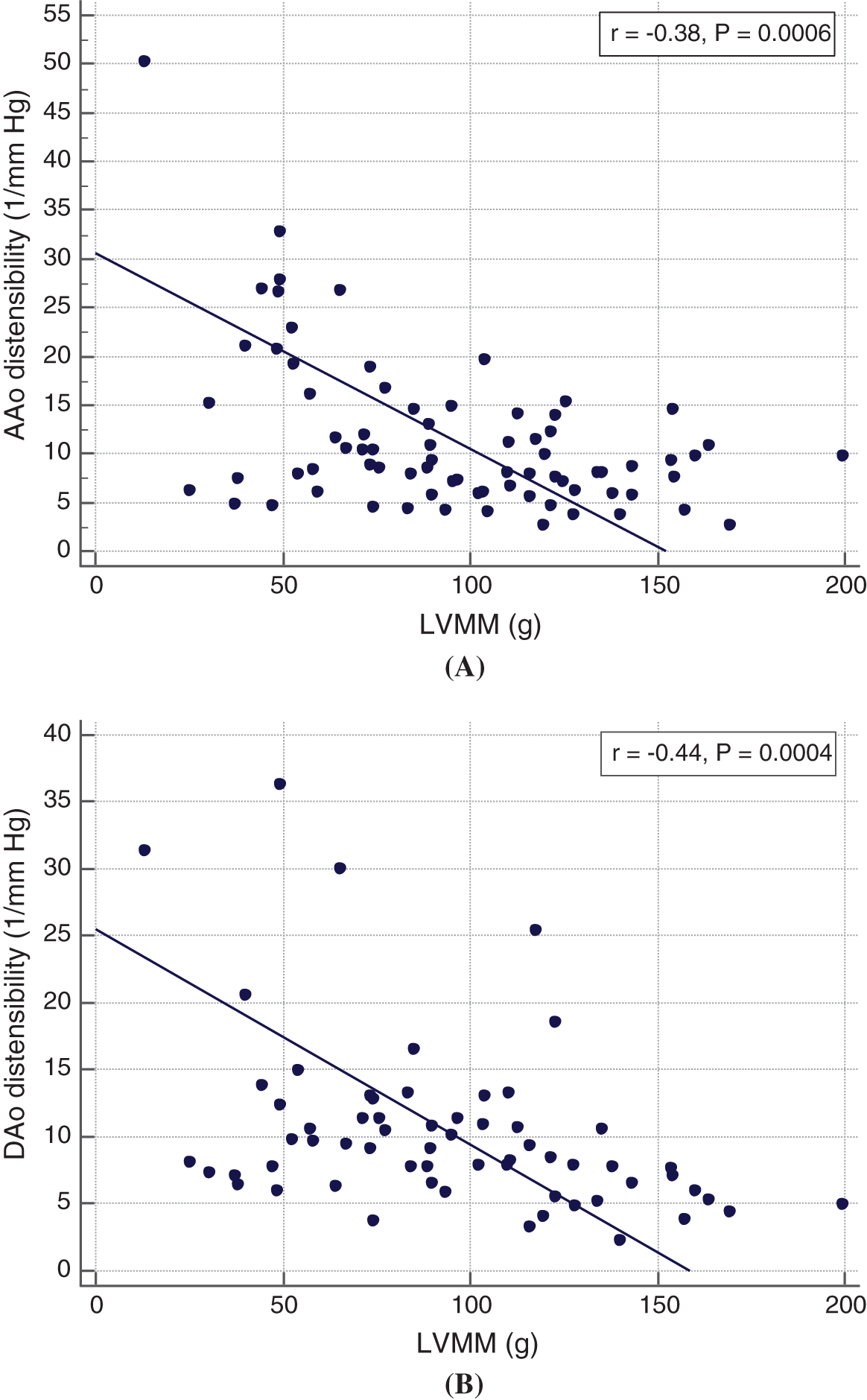
Figure 1: Relationship between LV mass (LVMM) and ascending (A) and descending (B) aortic distensibilty
Global strain and strain rate values were not different between BAV and TAV patients (Tab. 3). Global longitudinal, circumferential and radial strain (GLS, GCS, GRS) and global longitudinal and circumferential and radial strain rate (GLSR, GCSR) correlated with LV ejection fraction (r = 0.30–0.39; all p < 0.01; Figs. 2A–2C).
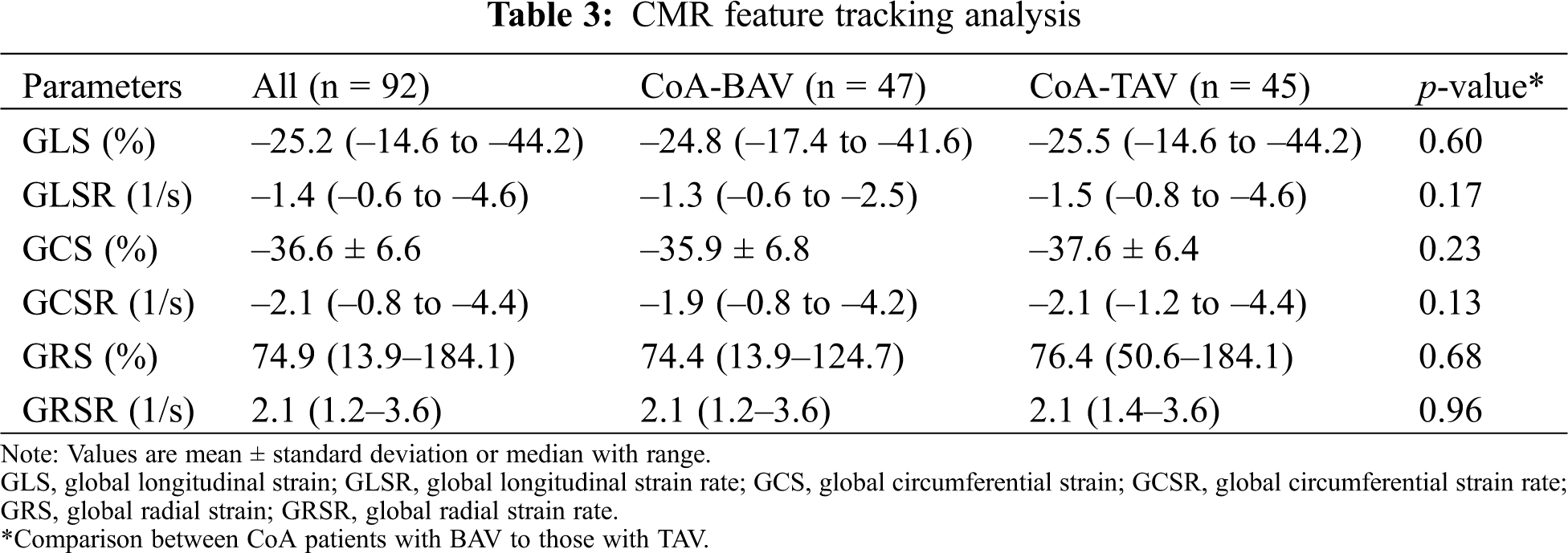
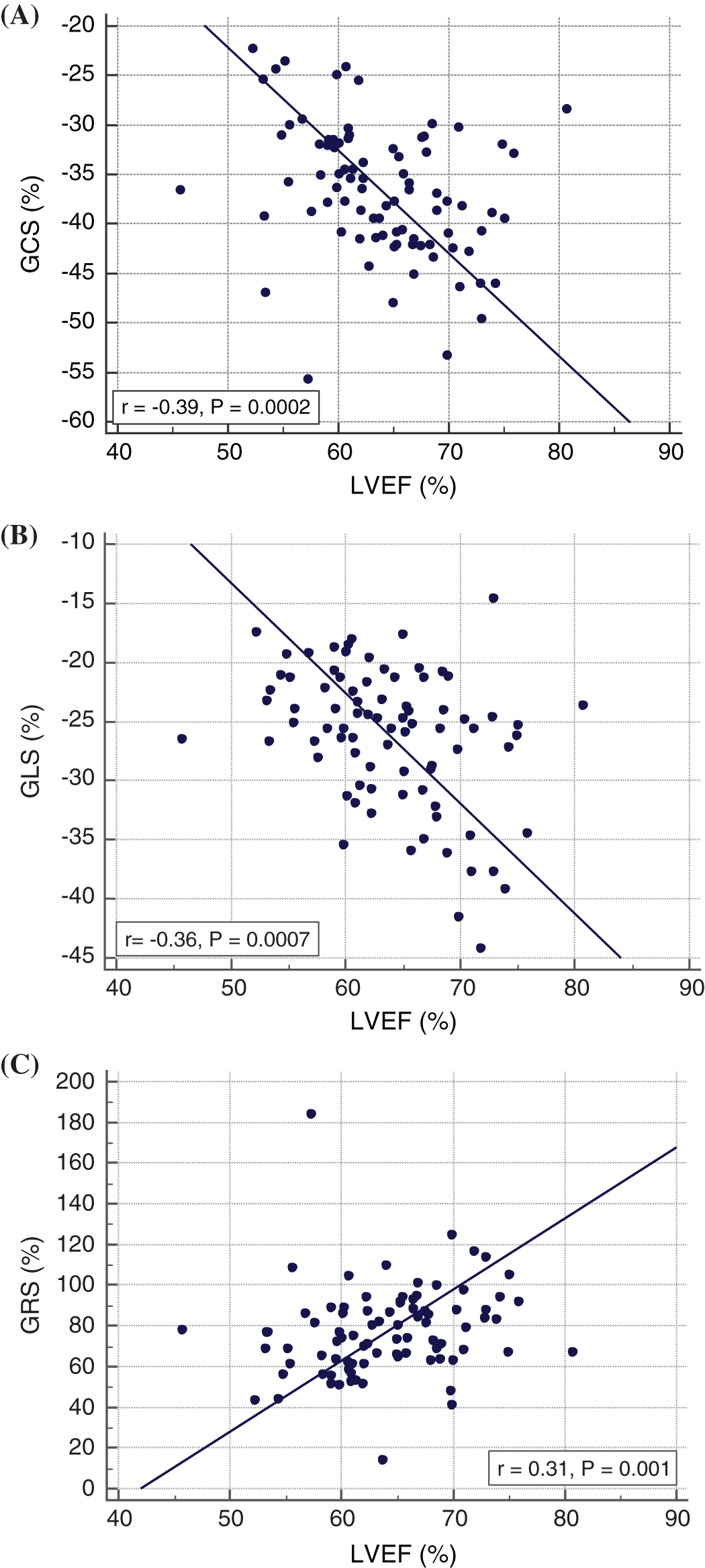
Figure 2: Relationship between LVEF and global strain values
3.3 Aortic Elasticity and Geometry
The aortic elasticity parameters PWV and distensibility did not differ between BAV and TAV patients (Tab. 2). PWV was significantly higher in the descending aorta compared to aortic arch PWV (p < 0.001). Aortic isthmus distensibility was significantly lower compared to distensibility in the ascending aorta (p = 0.025). There was a trend towards a lower isthmus distensibility compared to the descending aortic distensibility (p = 0.05).
The distribution of aortic geometry as well as aortic cross-sectional areas from distensibility measurements are shown in Tab. 2. Patients with a BAV had more commonly a gothic aortic arch compared to non-BAV patients, but this difference was not statistically significant (22 vs. 14, p = 0.2). Cross-sectional areas of the AAo were higher and cross-sectional areas of the descending aorta (isthmus and DAo) were lower in BAV patients compared to TAV patients. However, these findings were not statistically significant. In addition, in patients with a BAV cross-sectional areas of the BAV patients were more often above the 95th centile compared to TAV patients (16 BAV vs. 6 TAV patients) [17].
We assessed the influence of a bicuspid aortic valve on LV and aortic functional parameters in a relatively large cohort of CoA patients and could demonstrate:
1) LV size, mass, ejection fraction, global strain values and aortic elasticity parameters are not different between CoA patients with a BAV and a TAV.
2) Global longitudinal, circumferential and radial strain values correlate with LV ejection fraction.
To our knowledge, this is the first study comparing LV size as well as LV and aortic functional parameters between CoA patients with TAV and BAV. Previous studies in CoA patients found larger aortic diameters in BAV compared to TAV patients and this was independent from arterial hypertension. The authors concluded that their findings suggest that aortopathy in BAV patients is a reflection of the BAV but not the CoA phenotype [9]. Our results, did not demonstrate any differences in aortic elasticity between BAV and TAV patients supporting the assumption that the CoA phenotype but not the valve phenotype is mainly responsible for an increased aortic stiffness [4,18]. In particular, intrinsic structural abnormalities of the aortic wall in CoA patients might be important in this context [19].
Frandsen et al. [10] reported differences in LV size and morphology between with BAV and TAV stenosis. We evaluated LV volumes and mass and did not find any differences between the two study groups which again suggests that the valve phenotype in CoA patients does not seem to have a major influence on cardiovascular changes. However, we could demonstrate that a higher aortic distensibility is associated with a higher LV mass in the entire patient group emphasizing that arterial changes have an impact on LV morphology [20]. The differences to the study by Frandsen et al. [10] might be explained by the fact that their patient group was significantly older than our patient cohort. Furthermore, in our patient group there were no relevant aortic valve lesions with the need for intervention.
In this study we also compared ventricular function and deformation markers and did not demonstrate differences between TAV and BAV CoA patients. Our results are similar to a CMR study by Stefek et al. [21] who did not find any differences in LV volumetric indices and LV strain between pediatric BAV patients with normal valve function and pediatric TAV patients. No similar studies in CoA patients have been performed according to our knowledge. Studies in CoA patients analysing LV function and myocardial deformation compared patients with healthy controls and used CMR-FT and speckle tracking echocardiography. Kutty et al. found reduced global longitudinal and radial strain values with a normal LVEF in CoA patients compared to healthy subjects [22]. Others could demonstrate that adult CoA patients have an impaired LV GLS compared to controls whereas LV ejection fraction did not differ between patients and controls [23].
We demonstrated that LVEF correlates with GCS, GLS and GRS as well as with GLSR, GCSR and we therefore believe that 2D-CMR-FT can be helpful in assessing LV function in CoA patients. Similar relationships between EF and strain values have been shown by other groups [24,25]. However, correlations between myocardial deformation parameters and EF are controversially discussed and recently a mathematical model has been presented to describe the relationship between LVEF, GCS and GLS [26].
This study has a few limitations. Due to the retrospective nature of the study not all measures were available in some patients and this potentially could have had an impact on our study results. The retrospective study design also did not allow us to describe the detailed BAV phenotype.
We did not include a control group due to ethical reasons as this would have meant to prospectively recruit healthy subjects including children. In addition, GLS was only measured from the 4-chamber view and this might have impacted our global strain results. Finally, we did not assess in detail the influence on blood pressure on aortic and ventricular measurements.
Our results suggest that the valve phenotype in CoA patients does not have an impact on LV and aortic function as well as on LV morphology in a relatively young cohort of children and younger adults. However, thorough long-term follow up is needed as BAV patients likely have a higher risk for the development of relevant valve lesions predisposing them to morphological and functional LV changes. Global circumferential, longitudinal and radial strain values correlate with LV ejection fraction demonstrating that 2D-CMR-FT might be useful in assessing ventricular function and deformation in CoA patients.
Acknowledgement: This study was supported by Deutsches Zentrum für Herz-Kreislaufforschung e.V. We also thank Mrs Traudel Hansen for her support with patient management.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Vriend, J. W., Mulder, B. J. (2005). Late complications in patients after repair of aortic coarctation: Implications for management. International Journal of Cardiology, 101(3), 399–406. [Google Scholar]
2. de Divitiis, M., Rubba, P., Calabrò, R. (2005). Arterial hypertension and cardiovascular prognosis after successful repair of aortic coarctation: A clinical model for the study of vascular function. Nutrition, Metabolism, and Cardiovascular Diseases, 15(5), 382–394. [Google Scholar]
3. Egbe, A. C., Qureshi, M. Y., Connolly, H. M. (2020). Determinants of left ventricular diastolic function and exertional symptoms in adults with coarctation of aorta. Circulation Heart Failure, 13(2), e006651. [Google Scholar]
4. Voges, I., Kees, J., Jerosch-Herold, M., Gottschalk, H., Trentmann, F. et al. (2016). Aortic stiffening and its impact on left atrial volumes and function in patients after successful coarctation repair: A multiparametric cardiovascular magnetic resonance study. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 18(1), 56. [Google Scholar]
5. Florianczyk, T., Werner, B. (2011). Assessment of left ventricular diastolic function in children after successful repair of aortic coarctation. Clinical Research in Cardiology: Official Journal of the German Cardiac Society, 100(6), 493–499. [Google Scholar]
6. Siu, S. C., Silversides, C. K. (2010). Bicuspid aortic valve disease. Journal of the American College of Cardiology, 55(25), 2789–2800. [Google Scholar]
7. Grotenhuis, H. B., Ottenkamp, J., Westenberg, J., Bax, J. J., Kroft, L. et al. (2007). Reduced aortic elasticity and dilatation are associated with aortic regurgitation and left ventricular hypertrophy in nonstenotic bicuspid aortic valve patients. Journal of the American College of Cardiology, 49(15), 1660–1665. [Google Scholar]
8. Bissell, M. M., Hess, A. T., Biasiolli, L., Glaze, S. J., Loudon, M. et al. (2013). Aortic dilation in bicuspid aortic valve disease: Flow pattern is a major contributor and differs with valve fusion type. Circulation Cardiovascular Imaging, 6(4), 499–507. [Google Scholar]
9. Disha, K., Dubslaff, G., Rouman, M., Fey, B., Borger, M. et al. (2017). Evidence of subannular and left ventricular morphological differences in patients with bicuspid versus tricuspid aortic valve stenosis: Magnetic resonance imaging-based analysis. Interactive Cardiovascular and Thoracic Surgery, 24(3), 369–376. [Google Scholar]
10. Frandsen, E. L., Burchill, L. J., Khan, A. M., Broberg, C. S. (2018). Ascending aortic size in aortic coarctation depends on aortic valve morphology: Understanding the bicuspid valve phenotype. International Journal of Cardiology, 250, 106–109. [Google Scholar]
11. André, F., Robbers-Visser, D., Helling-Bakki, A., Föll, A., Voss, A. et al. (2016). Quantification of myocardial deformation in children by cardiovascular magnetic resonance feature tracking: Determination of reference values for left ventricular strain and strain rate. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 19(1), 8. [Google Scholar]
12. Pieper, T., Latus, H., Schranz, D., Kreuder, J., Reich, B. et al. (2019). Aortic elasticity after aortic coarctation relief: Comparison of surgical and interventional therapy by cardiovascular magnetic resonance imaging. BMC Cardiovascular Disorders, 19(1), 286. [Google Scholar]
13. Cerqueira, M. D., Weissman, N. J., Dilsizian, V., Jacobs, A. K., Kaul, S. et al. (2002). American Heart Association Writing Group on myocardial segmentation and registration for cardiac imaging, standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. International Journal of Cardiovascular Imaging, 18(1), 539–542. [Google Scholar]
14. Voges, I., Jerosch-Herold, M., Hedderich, J., Westphal, C., Hart, C. et al. (2010). Maladaptive aortic properties in children after palliation of hypoplastic left heart syndrome assessed by cardiovascular magnetic resonance imaging. Circulation, 122(11), 1068–1076. [Google Scholar]
15. Nollen, G. J., Groenink, M., Tijssen, J. G., van der Wall, E. E., Mulder, B. J. (2004). Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. European Heart Journal, 25(13), 1146–1152. [Google Scholar]
16. Ou, P., Celermajer, D. S., Mousseaux, E., Giron, A., Aggoun, Y. et al. (2007). Vascular remodeling after “successful” repair of coarctation: Impact of aortic arch geometry. Journal of the American College of Cardiology, 49(8), 883–890. [Google Scholar]
17. Voges, I., Jerosch-Herold, M., Hedderich, J., Pardun, E., Hart, C. et al. (2012). Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: A cross-sectional study. Journal of Cardiovascular Magnetic Resonance, 14(1), 77. [Google Scholar]
18. Ou, P., Celermajer, D. S., Jolivet, O., Buyens, F., Herment, A. et al. (2008). Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. American Heart Journal, 155(1), 187–193. [Google Scholar]
19. Niwa, K., Perloff, J. K., Bhuta, S. M., Laks, H., Drinkwater, D. C. et al. (2001). Structural abnormalities of great arterial walls in congenital heart disease: Light and electron microscopic analyses. Circulation, 103(3), 393–400. DOI 10.1161/01.cir.103.3.393. [Google Scholar] [CrossRef]
20. Shang, Q., Sarikouch, S., Patel, S., Schuster, A., Steinmetz, M. et al. (2017). Assessment of ventriculo-vascular properties in repaired coarctation using cardiac magnetic resonance-derived aortic, left atrial and left ventricular strain. European Radiology, 27(1), 167–177. DOI 10.1007/s00330-016-4373-8. [Google Scholar] [CrossRef]
21. Stefek, H. A., Berhane, H., Robinson, J. D., Reilly, B., Ruh, A. et al. (2019). Comprehensive MR analysis of cardiac function, aortic hemodynamics and left ventricular strain in pediatric cohort with isolated bicuspid aortic valve. Pediatric Cardiology, 40(7), 1450–1459. [Google Scholar]
22. Kutty, S., Rangamani, S., Venkataraman, J., Li, L., Schuster, A. et al. (2013). Reduced global longitudinal and radial strain with normal left ventricular ejection fraction late after effective repair of aortic coarctation: A CMR feature tracking study. International Journal of Cardiovascular Imaging, 29(1), 141–150. [Google Scholar]
23. Menting, M. E., van Grootel, R. W.,van den Bosch, A. E.,Eindhoven, J. A., McGhie, J. S. et al. (2016). Quantitative assessment of systolic left ventricular function with speckle-tracking echocardiography in adult patients with repaired aortic coarctation. International Journal of Cardiovascular Imaging, 32(5), 777–787. [Google Scholar]
24. Stokke, T. M., Hasselberg, N. E., Smedsrud, M. K., Sarvari, S. I., Haugaa, K. H. et al. (2017). Geometry as a confounder when assessing ventricular systolic function: Comparison between ejection fraction and strain. Journal of the American College of Cardiology, 70(8), 942–954. [Google Scholar]
25. Altman, M., Bergerot, C., Aussoleil, A., Davidsen, E. S., Sibellas, F. et al. (2014). Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking: A comparison between 2D and 3D echo modalities. European Heart Journal Cardiovascular Imaging, 15(3), 316–323. [Google Scholar]
26. Pedrizzetti, G., Lapinskas, T., Tonti, G., Stoiber, L., Zaliunas, R. et al. (2019). The relationship between EF and strain permits a more accurate assessment of LV systolic function. JACC Cardiovascular Imaging, 12(9), 1893–1895. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |