

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016623
ARTICLE
3D Non-Fluoroscopic Cryoablation of Right-Sided Accessory Pathways in Children: Monocentric Study and Literature Review
Pediatric Cardiology and Cardiac Arrhythmias Unit, Department of Pediatric Cardiology and Cardiac Surgery, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
*Corresponding Author: Fabrizio Drago. Email: fabrizio.drago@opbg.net
#European Reference Network for Rare and Low Prevalence Complex Disease of the Heart (ERN GUARD-Heart)
Received: 12 March 2021; Accepted: 29 April 2021
Abstract: Background: Cryoablation of accessory pathways (APs) is effective and very safe in children, as previously reported by our group. The aim of this retrospective study was to evaluate the current efficacy of 3D non-fluoroscopic cryoablation of right sided APs in children, comparing results obtained with the Ensite Velocity™ and the more recent Ensite Precision™ 3D mapping systems. Methods and Results: From January 2016 to December 2019, 102 pediatric patients [mean age 12.5 ± 2.8, 62 males (61% of total cohort)] with right APs underwent 3D non-fluoroscopic transcatheter cryoablation at our Institution. Fifteen (14.7%) patients had previously undergone catheter ablation. Acute procedural success rate was 95.1% (n = 97). No significant differences were detected in acute success rates achieved with Ensite Velocity™ or Ensite Precision™ systems nor between manifest (94%) and concealed APs (100%). No permanent complications occurred. During follow-up (428 ± 286 days, median 396 days [interquartile range 179-713]), 19 patients (19.6%) had recurrences. Recurrences were more frequent for parahissian/anterior APs compared to midseptal/posterior and lateral APs (p = 0.043). Recurrences were not related to the Ensite system used. A redo ablation procedure was attempted in 13 cases, 11 cryoablation and 2 radiofrequency ablations: the former was successful in 10 cases out of 11 (90.9%). Conclusion: 3D cryoablation of right-sided APs is associated with a very high acute success rate with limited use of fluoroscopy, resulting in great benefit to the children. Recurrence rates are not high and patients can be retreated with cryo-energy with higher success rates.
Keywords: 3D Mapping; accessory pathway; children; cryoablation; pediatric tachyarrhythmias
Catheter ablation for the treatment of pediatric tachyarrhythmias was first introduced in the early 1990s. Since then, its use as an alternative to antiarrhythmic drugs for patients with accessory pathways (APs) has been gradually increasing.
Very recently, the MAP-IT (Multicenter Pediatric and Adult Congenital EP Quality) Task Force [1] showed that acute success rates, fluoroscopy and procedural times in pediatric ablation have improved over the last 25 years, although there have been no major improvements in long-term success rates. In particular, despite multiple approaches and different energy modalities, ablation of right anterior-septal and midseptal APs still remains challenging, with recurrence rates approaching 20% (anterior-septal 18%, midseptal 19%) [2].
The efficacy and role of cryoablation as opposed to radiofrequency ablation for APs in children is still a matter of debate within the scientific community. In our previous study [3] about conventional cryoablation of right APs in children, it was effective and very safe, with better acute results achieved in manifest APs (acute success rate 100%) compared with concealed APs (acute success rate 80%, p < 0.05). However, none of the ablation parameters considered at that time was able to predict the long-term outcome. The learning curve only affected fluoroscopy time with a significant decrease (mean time 28.4 min, range 19.7–44.6, p < 0.033) in relation to the increase of the institutional experience.
At the same time, for arrhythmia treatment, more and more consideration has been given to the necessity of reducing radiation exposure in children because of their longer life expectancy [4,5].
In our preliminary experience [6] about the use of Ensite Velocity 3D system compared with the conventional fluoroscopy-guided method for cryoablation of right septal APs in children, we reported a significant reduction in patient radiation dose without an increase in success rate using 3D system. This result was recently confirmed by Tseng et al. [7]. On the basis of our long-lasting experience [3,6], the aim of this study was: (I) to evaluate the current efficacy of 3D non-fluoroscopic cryoablation of right sided APs in children, (II) to compare results obtained with the use of Ensite Velocity™ and those with the use of the more recent Ensite Precision™ 3D mapping systems.
This is a retrospective cohort study performed at Bambino Gesù Children’s Hospital of Rome, Italy. The study was approved by the Committee on Clinical Investigation as part of the Research of the Italian Ministry of Health RRC-2020-23669481.
From January 2016 to December 2019, all patients who underwent 3D transcatheter cryoablation for right manifest or concealed APs were enrolled in this study.
According to the current guidelines, patients underwent ablation if they presented symptoms or short AP refractory period and/or inducible atrioventricular reentrant tachycardia (AVRT) during risk stratification using trans-oesophageal atrial pacing [8–11].
Written informed consent was obtained from the parents of all patients prior to the procedure. Patients were divided into three groups according to the site of the AP: parahissian/anterior-septal/anterior APs (Group RA), midseptal/posterior-septal/posterior APs (Group RS), and posterolateral/lateral/anterolateral APs (Group RL).
2.2 3D Electroanatomical Mapping and Electrophysiological Study
The procedure was performed under general anesthesia, induced with sevoflurane or propofol and maintained with sevoflurane. A thermal mattress was used to maintain normal body temperature. All antiarrhythmic drugs were discontinued for at least five half-lives before the procedure to ensure complete pharmacological washout.
Surface electrocardiogram (ECG) leads and endocardial potentials (EGM) were recorded and stored on a multichannel recorder (Bard Electrophysiology, Billerica, MA, USA). The bipolar bandwidth filter was set at a range of 30–300 Hz.
The EnSite Velocity™ system 4.0.2 (Abbott Medical Italia SRL, Sesto San Giovanni, Milano, Italy) 3D mapping system was used in 47 procedures. From January 2018, the EnSite Precision™ system EE3000 v.2.0 (Abbott Medical Italia SRL, Sesto San Giovanni, Milano, Italy) was used in the other procedures. This latter, differently from EnSite Velocity™ system, combines impedance and magnetics.
A quadripolar catheter (Inquiry™ or Livewire™, St Jude Medical, St Paul, MN, USA), with 2 mm electrodes and inter-electrodes space of 2 mm, was inserted into a femoral vein and advanced toward the right atrium.
The catheter was gently moved first to acquire a precise geometrical reconstruction of the caval venous system, right atrium, coronary sinus and tricuspid annulus and then to establish the target site of ablation.
Programmed stimulation with atrial or ventricular single, double and triple premature extrastimuli, as well as incremental pacing, were used to induce AVRT. The same stimulation protocol was repeated under isoproterenol infusion (0.04–0.08 μg/kg/min in incremental doses) if tachycardia was not inducible at baseline.
Pre-excitation was mapped during sinus rhythm for manifest APs, targeting the earliest V signal compared to delta wave on surface ECG, the presence, amplitude, precocity of AP potential and unipolar QS signal recording from the mapping catheter.
In case of concealed APs, mapping of the earliest retrograde atrial electrogram was performed during AVRT.
The cryoablation system consists of a central console (Cryo Console, CryoCath Technologies Inc., Montreal, Canada) and a steerable 7 Fr catheter (Freezor, CryoCath Technologies Inc., Montreal, Canada) using N2O as a refrigerant [3]. After 3D electroanatomical mapping with a steerable quadripolar diagnostic catheter, a 7 Fr cryoablation catheter was advanced to the site of interest from the right femoral vein and/or right jugular vein. Four- or 6-8-mm tip electrode was preferably used in patients <25 or >25 kg, respectively.
The cryoablation procedure was performed as previously described in detail elsewhere [12–15]. Briefly:
• In manifest APs: during cryomapping, tip temperature was progressively reduced to –30°C or step-by-step by 10°C every 10 s. Cryomapping was considered positive when loss of ventricular pre-excitation was observed, and cryoablation was delivered to create a permanent irreversible lesion (−75/80°C for 480 s).
• In concealed APs: step-by-step cryomapping was performed at this site during tachycardia and, if a sudden retrograde interruption of AVRT was observed, cryoablation was done to create a permanent lesion.
After a successful cryoablation, extra lesions following the initially successful lesion were placed at the site and immediately adjacent to the site on both sides in order to consolidate the lesion.
During cryomapping and ablation, ECG and endocavitary signals were continuously monitored. Cryoablation delivery was stopped if signs of atrioventricular conduction impairment or ineffective results were detected.
In all patients, a post-ablation electrophysiological study at baseline and during isoproterenol infusion was performed immediately and after 30 min to demonstrate complete and persistent interruption of retrograde and anterograde conduction over the AP and/or non-inducibility of AVRT.
After the procedure all patients were carefully monitored. A standard ECG was performed 24 h post-procedure in all patients.
After discharge, patients were checked at 1, 6, 12, 18 months after cryoablation by clinical evaluation with standard ECG and ECG Holter monitoring. An exercise stress test was also performed in cooperative patients every 6 months.
Recurrence was defined as ECG-documented tachycardia, relapse of pre-excitation (delta wave), or return of clinical symptoms identical to those before cryoablation.
As safety endpoint, transient and permanent complications were considered.
Data distribution was assessed by the Kolmogorov-Smirnov test, and data were reported as mean ± standard deviation (SD) or median (interquartile range [IQR]) if they followed a normal or non-normal distribution, respectively. Continuous variables among the three study groups (anterior/parahisian, midseptal/posterior, and lateral APs) were compared with ANOVA (Analysis of Variance test) followed by Bonferroni correction for comparison among groups or Kruskal-Wallis test, as appropriate. Categorical variables were compared using the chi-square or Fisher’s exact tests. A p-value <0.05 was required for statistical significance. All the analyses were performed using the SPSS 25.0 software (SPSS Inc., Chicago, IL, USA).
From January 2016 to December 2019, 102 pediatric patients [mean age 12.5 ± 2.8, 62 males (61% of total cohort)] with right APs underwent 3D non-fluoroscopic transcatheter cryoablation at our Institution.
Overall, 113 cryoablation procedures were performed due to a second cryoablation procedure in 11 patients.
A total of 64 patients (62.7%) had Wolff-Parkinson-White syndrome, 18 (17.6%) had asymptomatic ventricular pre-excitation, 18 (17.6%) had AVRT due to concealed right APs, 1 (0.9%) had a Mahaim fiber, and 1 (0.9%) had permanent junctional reciprocating tachycardia.
Four patients presented structural heart diseases: Ebstein’s anomaly, dilated cardiomyopathy, ventricular septal defect status-post surgical closure, heart rhabdomyomas.
Clinical and electrophysiological characteristics according to AP site are summarized in Tab. 1.


Interestingly, male gender was more prevalent in Group RS than in Groups RA and RL (p = 0.015), and symptoms were more common in Group RA than in Groups RS and RL (p = 0.015).
Acute success rate was 95.1% (97/102). There were no significant differences in acute success rates between manifest (94%) and concealed APs (100%), nor in acute success rates achieved in all groups using the Ensite Velocity™ or Ensite Precision™ systems.
In all groups, a catheter tip size of 6 mm was more frequently used compared to catheter tip sizes of 4 mm and 8 mm (p = 0.002). A significantly higher number of lesions was performed in Group RL than in Groups RA and RS (p = 0.023).
A superior approach introducing the cryoablation catheter through a jugular vein was used in 15 patients and was associated with procedural success in all cases (100%). The superior approach was more frequently used in Group RA than in Groups RS and RL as well as in concealed than manifest APs (27.8% vs. 9.8%, p = 0.04) (Fig. 1).

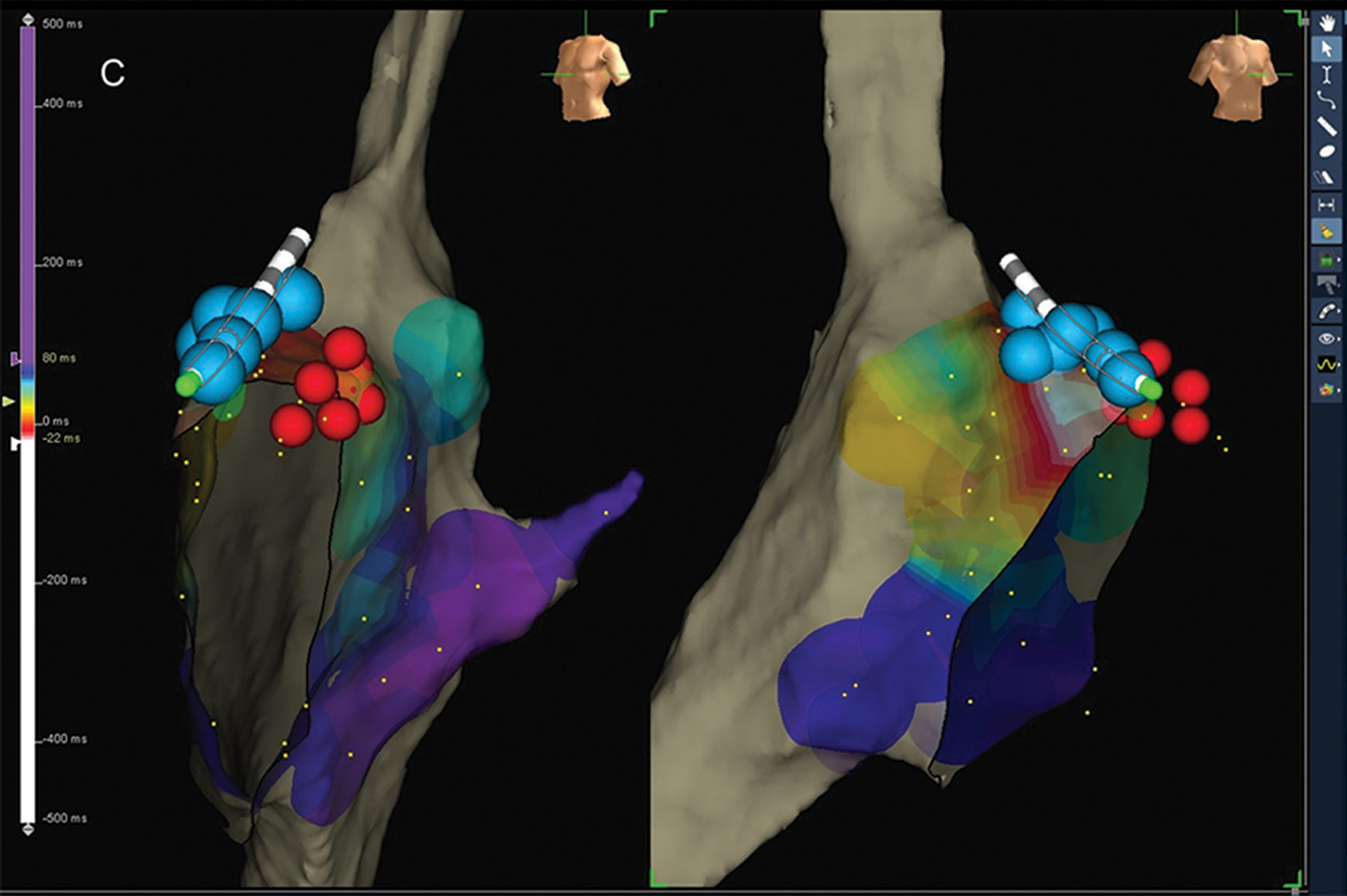
Figure 1: Cryoablation of anterior-septal AP using retrograde trans-jugular approach: (A) ablation site with the earliest ventricular electrogram compared to delta wave; (B) cryo-energy delivery on the ablation site (green dot) with the disappearance of delta wave. The distortion of the cryo-catheter is due to freezing (this appears both with impedance and magnetic field and this characteristic does not alter the procedure). this characteristic does not alter the procedure); (C) multiple extra cryo-applications (blue dots) to consolidate the lesion. Red dots represent the sites of His-bundle recording.
Transient complications during cryoablation delivery occurred in 3 patients (2.9%): 1 accelerated junctional rhythm, 1 PR interval prolongation, 1 right bundle branch block.
Limited use of fluoroscopy was performed in all patients with no differences among study groups (Tab. 1). Fluoroscopy was used exclusively to check vascular access in very young children.
Mean follow-up was 428 ± 286 days [median 396 days (IQR 179-713)]. There were no permanent complications.
The recurrence rate was 19.6% (19/97 patients) and was significantly higher in Group RA (Tab. 1).
Moreover, there were no significant differences in recurrence rate detected in all groups after use of the Ensite Velocity™ or the Ensite Precision™ systems.
For all types of APs, recurrences occurred after a mean period of 89 ± 117 days.
After recurrences, 5 patients were lost to follow-up and 1 underwent pharmacological treatment. Among the remaining 13 patients with recurrences, 2 patients with lateral APs underwent successful radiofrequency catheter ablation; in 11 of them, cryo-energy was used (Fig. 2).
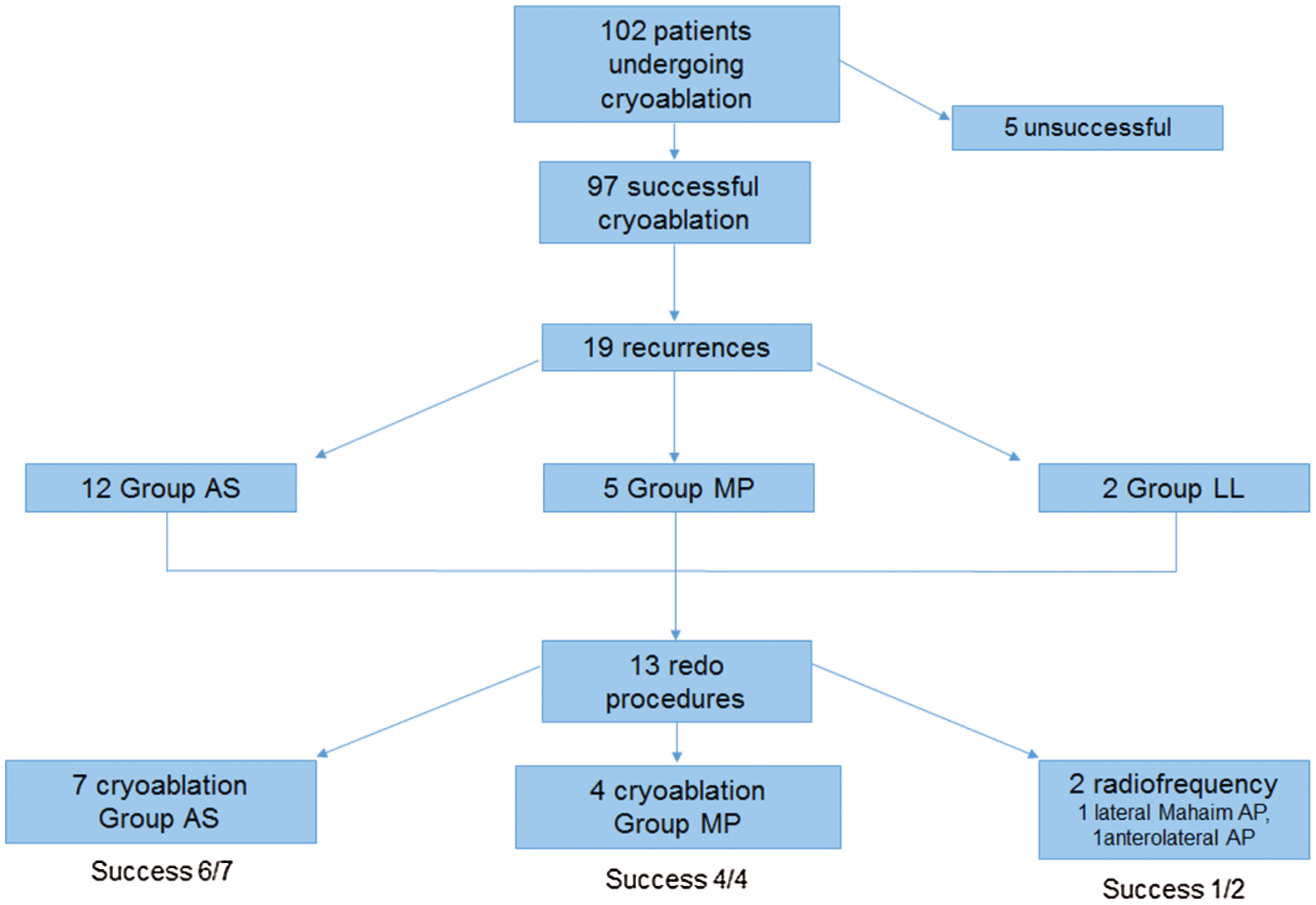
Figure 2: Cryoablation and redo procedures. AP, accessory pathway
Ten out of 11 (90.9%) redo cryoablation procedures were acutely successful. Thus, the total success rate of cryoablation procedures performed during the study period was 88.3% (83/94 patients regularly followed up) with no significant differences according to the AP site (p = 0.439; Tab. 1).
The efficacy and role of cryo-energy as opposed to radiofrequency for ablation of right-sided APs in children are still a matter of debate. In this regard, however, the use of cryomapping that allows transcatheter ablation of APs very close to the conduction system with no risk of permanent atrioventricular block, is completely in favor of cryoenergy [15]. Moreover, the use of 3D electroanatomical mapping systems seems to be crucial for increasing the efficacy of cryoablation in addition to decreasing fluoroscopic exposure [6,16].
In this study, we report our most recent experience (last 4 years) with cryoablation of right-sided APs, which was performed using only non-fluoroscopic 3D mapping. In this 4-year time period, 102 pediatric patients were enrolled and, as previously reported by Kovach et al. [2], symptoms were more common in those with parahissian/anterior-septal/anterior APs (Group RA).
Acute success rate of cryoablation was 95% confirming the high quality of 3D mapping. This success rate is similar to that we previously reported using conventional mapping (97%) and in our initial experience with 3D mapping (93.7%) [6]. In contrast, it seems to be better than those reported by Kovach et al. (87%) using both cryoablation and/or radiofrequency ablation, and very similar to Karadeniz et al. (93%) using cryoablation and non-fluoroscopic 3D mapping [2,17].
Regarding AP sites, in this experience as in other studies, there were no statistically significant differences (p = 0.558) in the acute success rate [1,2,18].
In most cases, as in other our experiences, cryoablation was more frequently performed using a 6 mm tip catheter (75.5%), whereas the 8 mm tip catheter was used only for posterior/posterior-septal and lateral APs which, as well known, are larger and deeper. Mapping and targeting the best ablation site were performed, instead, using the 2-2-2 mm quadripolar deflectable diagnostic catheter which is more precise and accurate than the 4-6-8 mm tip cryoablation catheter.
Differently from our previous experience with conventional mapping [3], the acute success rate for concealed APs was increased by the use of non-fluoroscopic 3D mapping (100% vs. 80%), though the success rate for manifest APs did not change significantly (94% vs. 100%). This finding could be due to the fact that 3D mapping enables to achieve better cryocatheter stability on the target site during tachycardia and to re-navigate the cryocatheter in the target site both during tachycardia and sinus rhythm. In this regard, however, both types of 3D mapping systems did not show different efficacy, despite that Ensite Precision™ enhances model creation using both impedance- and magnetic-field and improves stability.
Our study revealed a recurrence rate of 19.6% after successful ablation. This finding is very similar to the results reported by Kovach et al. (18%), where a higher recurrence rate for cryoablation compared to radiofrequency ablation was described regardless of the type of approach used (trans-jugular vs. trans-femoral approach) [2].
In our study, most recurrences (58%) were observed during the first 30 days after the procedure. Moreover, recurrence rate was significantly higher for parahissian/anterior-septal/anterior APs compared to other types of APs, although cryoablation of this type of APs had the highest acute success rate. Innovation in the 3D system used did not change the recurrence rate.
This experience confirms the safety profile of cryoablation given that no patient in our series experienced permanent complications and that transient complications occurred in only 2% of the study population. In particular, atrioventricular block did not develop in any patient with septal or parahissian APs, whereas recently, using both cryoablation and radiofrequency ablation, iatrogenic atrioventricular block was reported in 1.2% and 0.1% of patients by Kovach et al. [2,18] and the MAP-IT registry, respectively.
In general, we obtained much better results than those reported in a recent European multicenter study [19] on ablation of APs very close to the atrioventricular junction, showing an acute success rate of 77%, a complication rate of 0.7% and a recurrence rate of 14.1% with a final success rate of 62%. In our study, after taking into consideration redo ablations, the final success rate was much higher (88.3%).
As regards the use of fluoroscopy, near-zero fluoroscopy was adopted in most procedures (mean fluoroscopy dose 0.33 mGy) and zero-fluoroscopy was achieved in 9 procedures. This result is really different from our previous experience with cryoablation of right-sided APs and confirms, once again, that 3D mapping is absolutely necessary for the long-term safety of the ablation procedure in children.
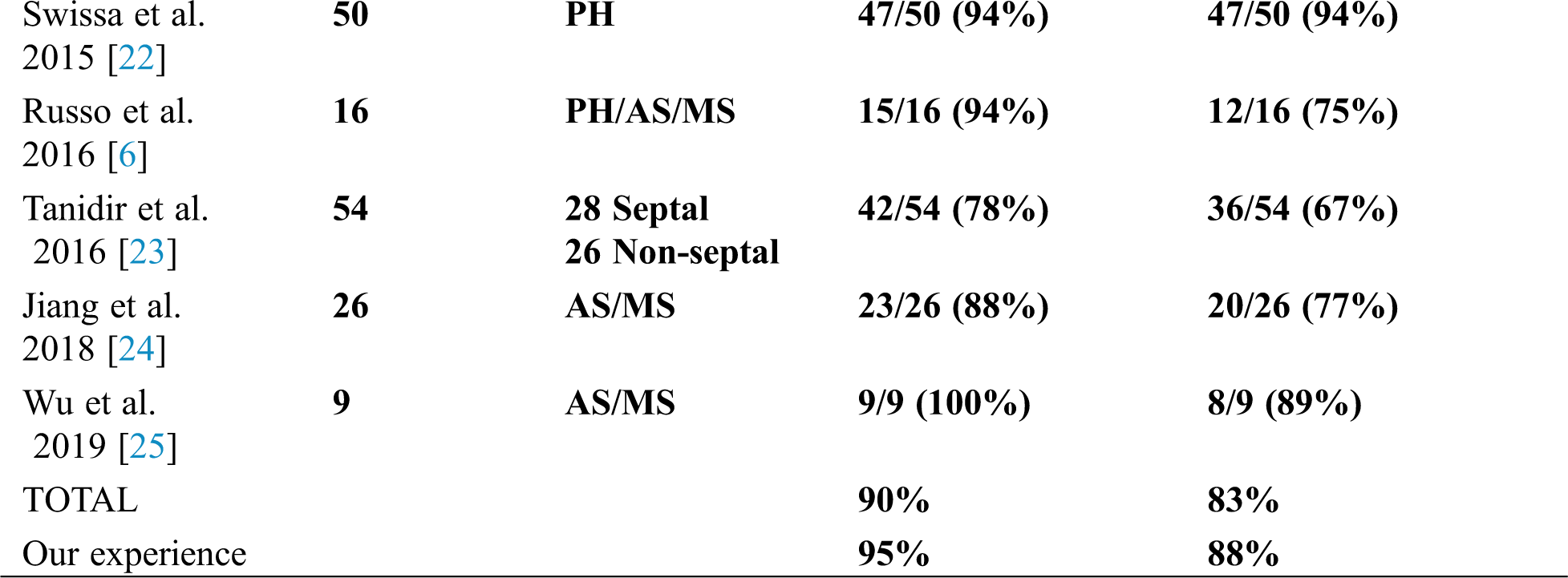
3D non-fluoroscopic cryoablation of right APs in children has been the object of a limited number of studies published in the last decade on PubMed. Globally, considering the pooled data of 8 selected specific studies including 261 patients, initial total procedural success rate was 90% (range 78%–100%), whereas total chronic success rate was 83% (range 67%–100%).
In Tab. 2, acute and chronic success rates of all these published papers are summarized and compared with the results of our study, in which the highest number of patients treated was included.

On the basis of our finding, we can state that: 3D transcatheter cryoablation of right-sided APs is associated with a very high acute success rate; complications can be completely prevented; recurrence rates are not high and similar when compared to radiofrequency ablation; recurrences can be re-treated with cryo-energy with a higher success rate and use of fluoroscopy can be avoided.
Acknowledgement: The authors would like to thank Dr. Elisa Del Vecchio for editorial revision and Cardioenglish for English language services.
Data Availability: The data underlying this article will be shared on reasonable request to the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Seslar, S. P., Kugler, J., Batra, A. S., Collins, K. K., Crosson, J. et al. (2013). The Multicenter Pediatric and Adult Congenital EP Quality (MAP-IT) initiative-rationale and design: Report from the pediatric and congenital electrophysiology society’s MAP-IT taskforce. Congenital Heart Disease, 8(5), 381–392. [Google Scholar]
2. Kovach, J. R., Mah, D. Y., Abrams, D. J., Alexander, M. E., Cecchin, F. et al. (2020). Outcomes of catheter ablation of anteroseptal and midseptal accessory pathways in pediatric patients. Heart Rhythm, 17(5), 759–767. [Google Scholar]
3. Drago, F., Righi, D., Placidi, S., Russo, M. S., Di Mambro, C. et al. (2013). Cryoablation of right-sided accessory pathways in children: report of efficacy and safety after 10-year experience and follow-up. Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing. Arrhythmias and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 15(11), 1651–1656. [Google Scholar]
4. Gaita, F., Guerra, P. G., Battaglia, A., Anselmino, M. (2016). The dream of near-zero X-rays ablation comes true. European Heart Journal, 37(36), 2749–2755. [Google Scholar]
5. Baysson, H., Réhel, J. L., Boudjemline, Y., Petit, J., Girodon, B. et al. (2013). Risk of cancer associated with cardiac catheterization procedures during childhood: A cohort study in France. BMC Public Health, 13(1), 1–8. [Google Scholar]
6. Russo, M. S., Drago, F., Silvetti, M. S., Righi, D., Di Mambro, C. et al. (2016). Comparison of cryoablation with 3D mapping versus conventional mapping for the treatment of atrioventricular re-entrant tachycardia and right-sided paraseptal accessory pathways. Cardiology in the Young, 26(5), 931–940. [Google Scholar]
7. Tseng, W. C., Wu, M. H., Lu, C. W., Wu, K. L., Wang, J. K. et al. (2019). Zero fluoroscopy during ablation of right-sided supraventricular tachycardia substrates in a pediatric population-Initial experience in Taiwan. Acta Cardiologica Sinica, 35(5), 476–483. [Google Scholar]
8. Saul, J. P., Kanter, R. J., Abrams, D., Asirvatham, S., Bar-Cohen, Y. et al. (2016). PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: Developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAPthe American Heart Association (AHAand the Association for European Pediatric and Congenital Cardiology (AEPC). Heart Rhythm, 13, e251–e289. [Google Scholar]
9. Brugada, J., Blom, N., Sequella-Brugada, G., Blomstrom-Lundqvist, C., Deanfield, J. et al. (2013). Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 15(9), 1337–1382. [Google Scholar]
10. Brugada, J., Katritsis, D. G., Arbelo, E., Arribas, F., Bax, J. J. et al. (2020, 2019). ESC Guidelines for the management of patients with supraventricular tachycardia the task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). European Heart Journal, 41(5), 655–720. [Google Scholar]
11. Katritsis, D. G., Boriani, G., Cosio, F. G., Hindricks, G., Jaïs, P. et al. (2017). European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRSAsia-Pacific Heart Rhythm Society (APHRSand Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 19(3), 465–511. [Google Scholar]
12. Drago, F., De Santis, A., Grutter, G., Silvetti, M. S. (2005). Transvenous cryothermal catheter ablation of re-entry circuit located near the atrioventricular junction in pediatric patients: Efficacy, safety, and midterm follow-up. Journal of the American College of Cardiology, 45(7), 1096–1103. [Google Scholar]
13. Drago, F., Silvetti, M. S., De Santis, A., Grutter, G., Andrew, P. (2006). Lengthier cryoablation and a bonus cryoapplication is associated with improved efficacy for cryothermal catheter ablation of supraventricular tachycardias in children. Journal of Interventional Cardiac Electrophysiology: An International Journal of Arrhythmias and Pacing, 16(3), 191–198. [Google Scholar]
14. Drago, F., Russo, M. S., Silvetti, M. S., De Santis, A., Onofrio, M. T. (2009). ‘Time to effect’ during cryomapping: A parameter related to the long-term success of accessory pathways cryoablation in children. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 11(5), 630–634. [Google Scholar]
15. Drago, F. (2008). Paediatric catheter cryoablation: Techniques, successes and failures. Current Opinion in Cardiology, 23(2), 81–84. [Google Scholar]
16. Strauss, K. J., Kaste, S. C. (2006). ALARA in pediatric interventional and fluoroscopic imaging: Striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients--A white paper executive summary. Journal of the American College of Radiology, 3(9), 686–688. [Google Scholar]
17. Karadeniz, C., Akdeniz, C., Turan, O., Tuzcu, V. (2014). Cryoablation of septal accessory pathways in children: Midterm results. Pacing and Clinical Electrophysiology, 37(9), 1095–1099. [Google Scholar]
18. Dubin, A. M., Jorgensen, N. W., Radbill, A. E., Bradley, D. J., Silva, J. N. et al. (2019). What have we learned in the last 20 years? A comparison of a modern era pediatric and congenital catheter ablation registry to previous pediatric ablation registries. Heart Rhythm, 16(1), 57–63. [Google Scholar]
19. Kubuš, P., Vít, P., Gebauer, R. A., Zaoral, L., Peichl, P. et al. (2014). Long-term results of paediatric radiofrequency catheter ablation: A population-based study. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 16(12), 1808–1813. [Google Scholar]
20. Ergul, Y., Tola, H. T., Kiplapinar, N., Akdeniz, C., Saygi, M. et al. (2013). Cryoablation of anteroseptal accessory pathways in children with limited fluoroscopy exposure. Pediatric Cardiology, 34(4), 802–808. [Google Scholar]
21. Ozturk, E., Ergul, Y., Tanidir, I. C., Akdeniz, C., Tola, H. T. et al. (2015). Electroanatomic mapping guided cryoablation of Mahaim pathways in children with limited fluoroscopy exposure. Pacing and Clinical Electrophysiology, 38(3), 362–367. [Google Scholar]
22. Swissa, M., Birk, E., Dagan, T., Fogelman, M., Einbinder, T. et al. (2015). Cryotherapy ablation of parahisian accessory pathways in children. Heart Rhythm, 12(5), 917–925. [Google Scholar]
23. Tanidir, I. C., Ergul, Y., Ozturk, E., Dalgic, F., Kiplapinar, N. et al. (2016). Cryoablation with an 8-mm-Tip catheter for right-sided accessory pathways in children. Pacing and Clinical Electrophysiology, 39(8), 797–804. [Google Scholar]
24. Jiang, H., Li, X. (2018). Cryoablation of the right anteroseptal or midseptal accessory pathways in children: A 2-year single-center experience. Pacing and Clinical Electrophysiology, 41(9), 1123–1128. [Google Scholar]
25. Wu, K. L., Chiu, S. N., Lu, C. W., Tseng, W. C., Wu, M. H. (2019). Acute outcomes for cryoablation in pediatric patients with perinodal Tachyarrhythmia: Single center report. Acta Cardiologica Sinica, 35(2), 134–143. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |