

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016396
REVIEW
Effect of Cardioplegia for Myocardial Protection in Pediatric Cardiac Surgery: A Network Meta-Analysis
1Department of Cardiac Surgery, Shengjing Hospital of China Medical University, Shenyang, China
2Department of Ultrasound, Shengjing Hospital of China Medical University, Shenyang, China
*Corresponding Author: Guang Song. Email: songg84@163.com
Received: 03 March 2021; Accepted: 26 April 2021
Abstract: Cardioplegia has been widely used to reduce myocardial injury during pediatric cardiac surgery; however, which cardioplegia solution has the best protective effect has not been established. Thus, we compared the myocardial protective effects of different cardioplegia solutions used in pediatric cardiac surgery. Seven databases were searched to identify the relevant randomized controlled trials. A network meta-analysis with a Bayesian framework was conducted. The outcomes included the following biochemical and clinical outcomes: serum concentrations of the creatine kinase-myocardial band at 6 h postoperatively; cardiac troponin I (cTnI) at 4, 12, and 24 h postoperatively; spontaneous beating after declamping; postoperative arrhythmias; inotropic support percentage and duration; mechanical ventilation hours; intensive care unit stay in days; hospital stay in days; and mortality. The group treated with cold crystalloid cardioplegia (cCCP) was chosen as the control group. The 22 studies involved 1529 patients. Six types of cardioplegia solutions were described in these studies, including cold blood cardioplegia, cCCP, del Nido, histidine-tryptophan-ketoglutarate (HTK), terminal warm blood cardioplegia, and warm blood cardioplegia (wBCP). The serum concentrations of the 24-h cTnI with wBCP (MD = −2.52, 95% CI: −4.74 to −0.27) was significantly lower than cCCP. The serum concentrations of the 24-h cTnI with HTK (MD = 4.91, 95% CI: 2.84–7.24) was significantly higher than cCCP. There was no significant difference in other biochemical and clinical outcomes when compared to cCCP. In conclusion, wBCP may have a superior myocardial protective effect with lower 24-h cTnI levels postoperatively and similar clinical outcomes after pediatric cardiac surgery.
Keywords: Cardioplegia; pediatric cardiac surgery; cardiac troponin I; meta-analysis
Approximately 100 congenital heart surgeries are performed by each pediatric cardiac surgeon in North America every year [1], and 40,000 children undergo congenital heart surgical procedures in the United States each year [2]. It is important to protect the myocardium during open heart surgery because cardiopulmonary bypass can cause myocardial ischemia and reperfusion injury, leading to impaired cardiac function. During cardiopulmonary bypass surgery, there are several methods of myocardial protection, including cardioplegia solution, hypothermia, and local cooling [3]. The most studied method, however, is the application of cardioplegia.
Cardioplegia is a chemical cardiac arrest solution that is administered to intentionally and temporarily arrest the heart. Cardioplegia decreases the myocardial metabolic demand. Cardioplegia is divided into two main categories (crystalloid- or blood-based solutions). Cold crystalloid cardioplegia (cCCP) has been the cornerstone of cardiac surgical practice since the 1950s. Cold blood cardioplegia (cBCP) was introduced in the 1970s due to the increased oxygen-carrying capacity, maintenance of oncotic pressure, and scavenging of free radicals [4]. Since the introduction of cBCP, several modified types of cardioplegia have been used in clinical applications, including del Nido (DN) and histidine-tryptophan-ketoglutarate (HTK) solutions. Although there are numerous studies comparing the effects of two or three cardioplegia solutions, there is no consensus on a cardioplegia solution that affords optimal myocardial protection. Therefore, we conducted this network meta-analysis (NMA) to systematically evaluate the myocardial protective effects of various cardioplegia solutions currently used in pediatric cardiac surgery.
We followed a reporting guideline (Preferred Reporting Items for Systematic Reviews and Meta-Analyses for NMA) to conduct this study [5]. The review protocol (number: CRD42020215431) was registered in the PROSPERO database. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, we assessed the certainty of evidence derived from the network meta-analysis results. GRADE provides a system for rating the quality of NMAs, and evaluates the quality of evidence at 4 levels (high, moderate, low, and very low).
Two examiners (KZ and XZ) independently searched for the significant studies in databases, including PubMed, MEDLINE, Web of Science, EMBASE, Scopus, ClinicalTrials.gov, and the Cochrane library on 20 January 2021. The search words were ‘cardioplegia,’ ‘pediatric,’ and ‘randomized controlled trials (RCTs).’ The details of the search strategy are shown in Tab. S1. At the same time, we read the references cited in the studies to further locate the literature which met the criteria.
Reviewers (KZ and XZ) were assigned to screen the titles and abstracts for eligibility. The third reviewer (DL) resolved any disagreement between the reviewers. Original studies were eligible if the following criteria were met: (I) RCTs of cardiac surgery; (II) full text with language restrictions; and (III) literature on assessment of the effect of myocardial protection using different cardioplegia solutions in pediatric cardiac surgery. Original studies were ineligible for the following reasons: (I) reviews, observational studies, case-control studies, abstracts, letters, or case reports; (II) patients other than children; (III) cardioplegia with adjuncts, such as nicardipine or esmolol, or leukocyte-depleted cardioplegia studies; and (IV) laboratory animal studies. If there were several publications from the same study, the study with the most cases and relevant information was included.
For eligible studies, the first author (year of publication), region, median age of participants, gender, cardioplegia type and number of participants in each group, temperature, delivery method, aortic cross-clamp time, cardiopulmonary bypass time, and outcomes were extracted by two independent authors (WW and SL). Numeric data were gathered directly from tables, or when presented in graphs only, were inferred by digitizing the figure with GetData Graph Digitizer 2.26 [6].
Researchers generally used biochemical and/or clinical outcomes to evaluate the myocardial protective effects. In this NMA, the outcomes included biochemical and clinical outcomes. Biochemical outcomes were the serum concentrations of the creatine kinase-myocardial band (CK-MB) 6 h postoperatively (IU/L) and cardiac troponin I (cTnI) 4, 12, and 24 h postoperatively (ng/ml). The clinical outcomes included spontaneous beating after declamping, postoperative arrhythmias, inotropic support (%), inotropic duration hours, mechanical ventilation hours, intensive care unit (ICU) stay in days, hospital stay in days, and risk of postoperative mortality.
Before analyzing the data, the risk of trial bias of the included studies was assessed using the Cochrane Collaboration’s Tool. Mean differences (MDs) and 95% confidence intervals (CIs) were used to report the 6-h CK-MB, 4-h cTnI, 12-h cTnI, 24-h cTnI, inotropic duration hours, mechanical ventilation hours, ICU stay in days, and hospital stay in days. Odds ratios (ORs) were used to report the risk of spontaneous beating after declamping, postoperative arrhythmias, inotropic support percentage, and mortality.
We evaluated the myocardial protection of various cardioplegia solutions using NMA. In the Bayesian NMA, random effects and consistency models were used for analyzing data and carrying out the network meta-analysis (4 chains, 50,000 iterations, and 20,000 per chain). We assessed inconsistency using the node-splitting method, and the inconsistency was reported by the Bayesian P value. The publication bias was evaluated using a comparison-adjusted funnel plot [7]. All analyses were conducted using the “gemtc” package of R (version 4.0.2; R Foundation) and Stata (version 16.0; StataCorp, College Station, TX, USA).
Our exhaustive search strategy retrieved 536 potentially relevant publications from 7 databases. After screening, 22 RCTs s were included in our final analysis (Fig. 1 shows the PRISMA flow-chart) [8–29].

Figure 1: Flow-chart of study selection
The 22 RCTs conducted in Europe, Asia, and the USA between 1994 and 2020 involved 1529 patients (Tab. 1). Six types of cardioplegia solutions were described in these studies, including cBCP, cCCP, DN, HTK, terminal warm blood cardioplegia, and warm blood cardioplegia (wBCP). Most cardioplegia solutions were delivered in an antegrade fashion. The temperature of cold cardioplegia solutions was 2°C–10°C. The temperature of warm cardioplegia solutions was 33°C–37°C. Additional details of the selected studies are shown in Tabs. S2–S4.
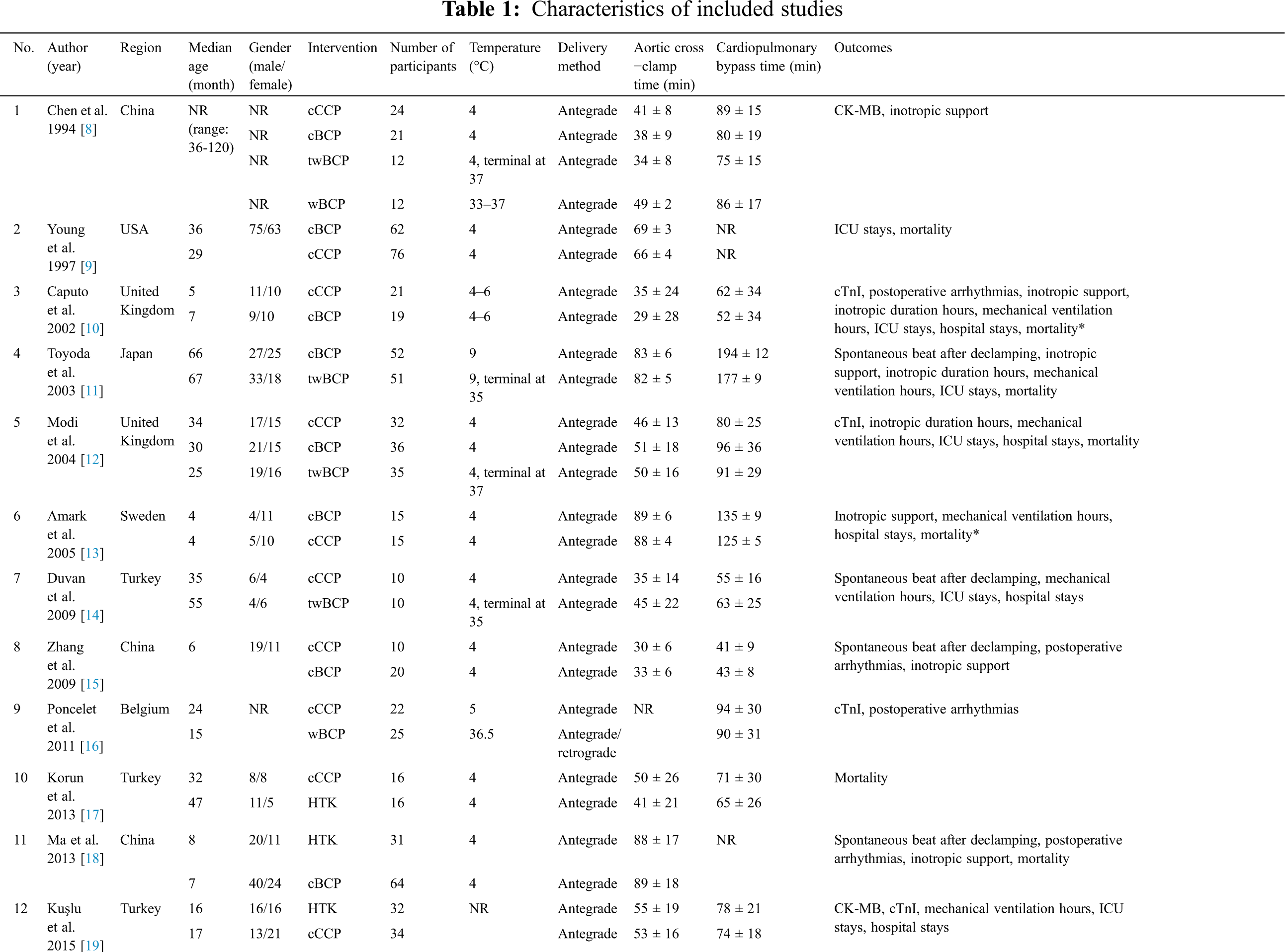
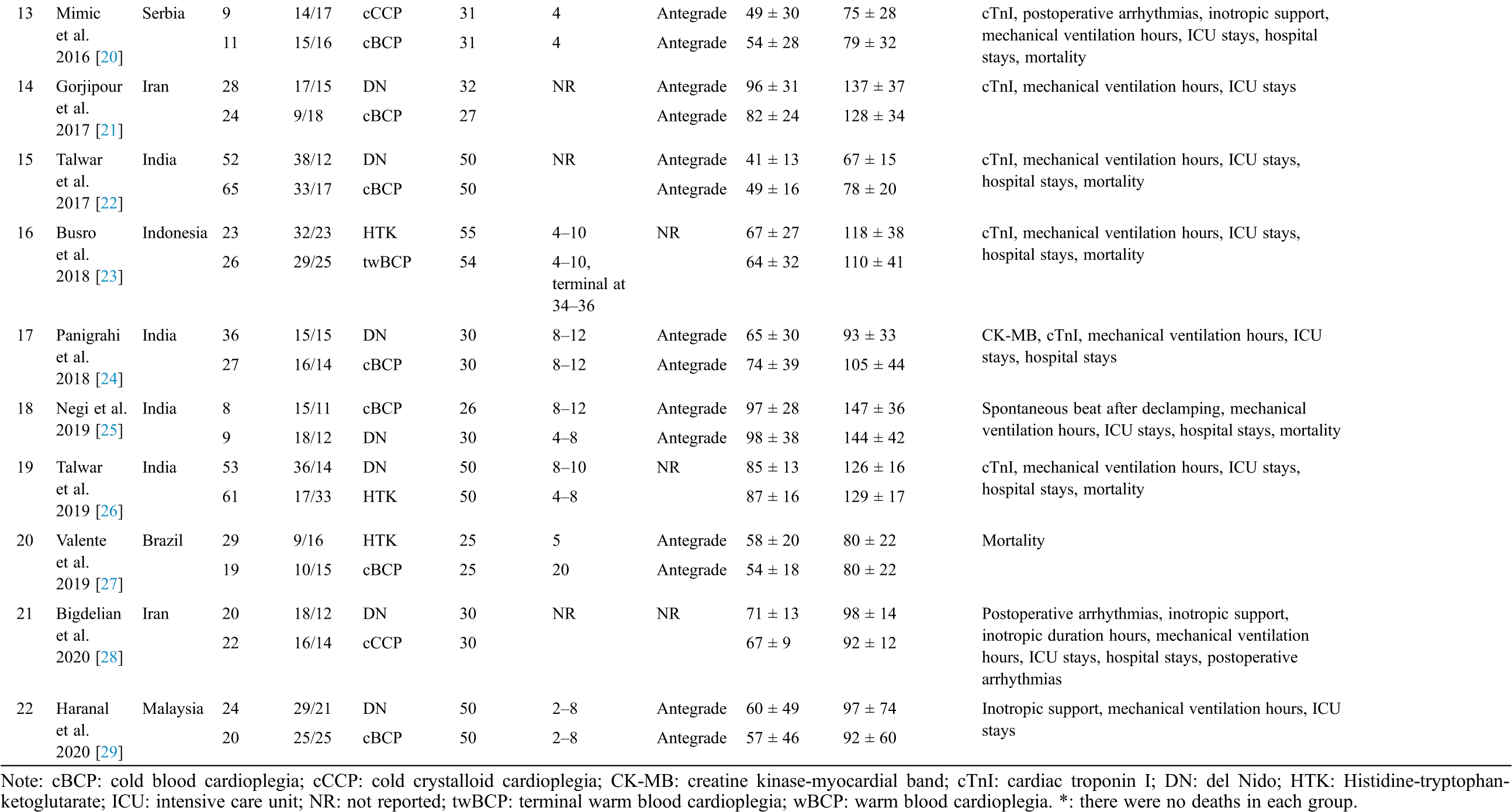
Evaluation of the bias risk for all RCTs is presented in the supplemental file (Figs. S1 and S2). Only two studies were high risk. Five studies were considered low risk and 14 studies were considered to have an unclear risk, which indicated that the selected RCTs were of good quality.
The geometry of the network is shown in Fig. S3 in the supplemental file. Ten studies with 5 types of cardioplegia solution involving the 24-h cTnI were included (Tab. 2). The serum concentration of the 24-h cTnI with wBCP (MD = −2.52, 95% CI: −4.74 to −0.27) was significantly lower than cCCP (Fig. 2). The serum concentration of the 24-h cTnI with HTK (MD = 4.91, 95% CI:2.84–7.24) was significantly higher than cCCP (Fig. 2). There was no significant difference in other biochemical outcomes among seven cardioplegia solutions (Fig. 3).
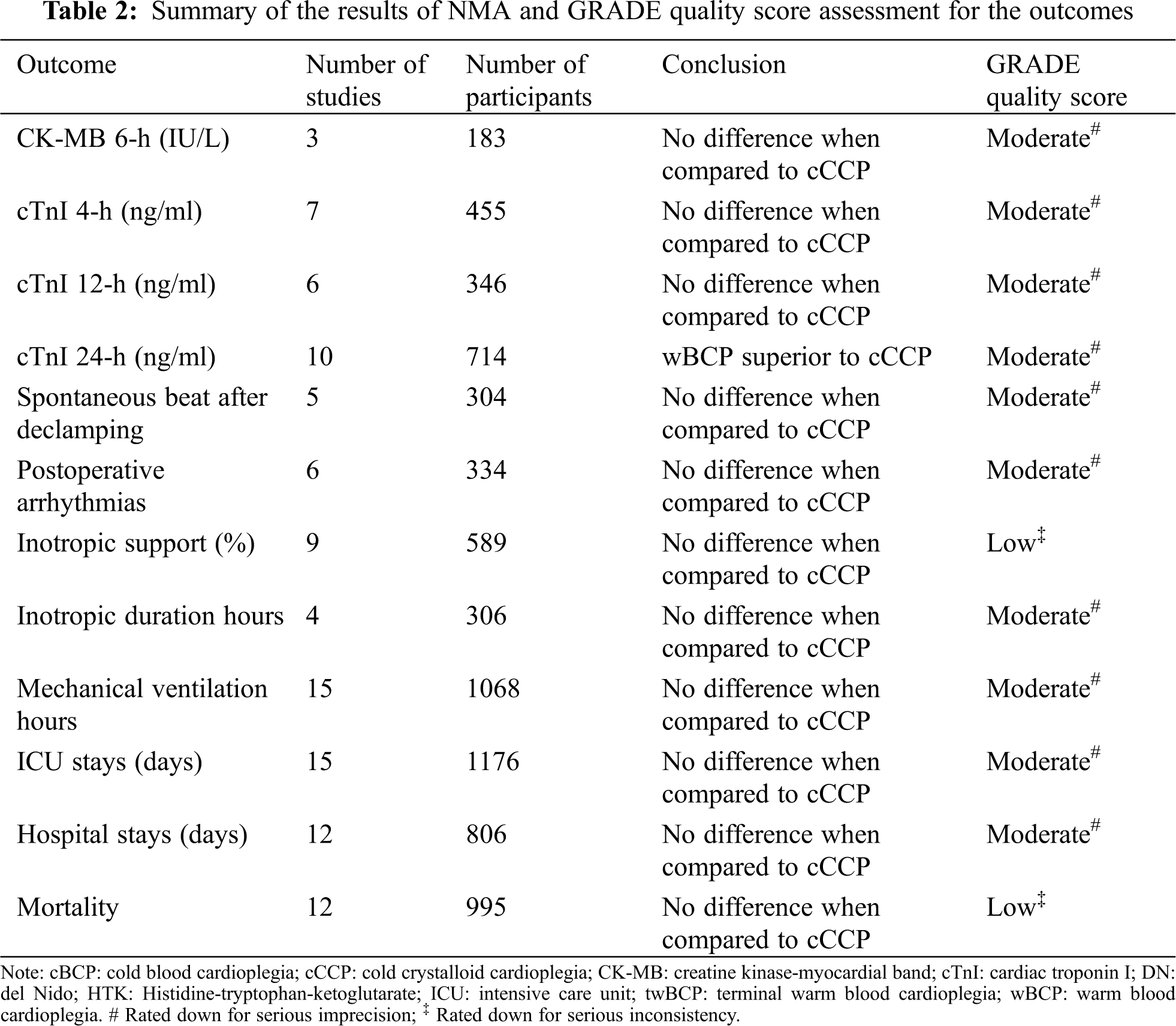

Figure 2: Forest plots of network meta-analysis of all trials for biochemical outcomes. cBCP: cold blood cardioplegia; cCCP: cold crystalloid cardioplegia; CK-MB: creatine kinase-myocardial band; cTnI: cardiac troponin I; DN: del Nido; HTK: Histidine-tryptophan-ketoglutarate; twBCP: terminal warm blood cardioplegia; wBCP: warm blood cardioplegia

Figure 3: Forest plots of network meta-analysis of all trials for clinical outcomes. cBCP: cold blood cardioplegia; cCCP: cold crystalloid cardioplegia; DN: del Nido; HTK: Histidine-tryptophan-ketoglutarate; ICU: intensive care unit; twBCP: terminal warm blood cardioplegia; wBCP: warm blood cardioplegia
There was no significant difference in spontaneous beating after declamping, postoperative arrhythmias, inotropic support percentage, inotropic duration hours, mechanical ventilation hours, ICU stay in days, hospital stay in days, or mortality of the five cardioplegia solutions when compared with cCCP. The head-to-head comparisons of each outcome are shown in Tabs. S5–S16.
The results of evaluating inconsistencies for all outcomes are presented in Figs. S4–S12 in the supplemental file. We noted a significance level of P > 0.05 for most of cases, which indicated that inconsistency was not present for most comparisons. After comparison results were obtained, we used the GRADE system to evaluate the certainty of evidence (Tab. 2). No significant asymmetry was detected in the funnel plots of primary and secondary outcomes (Fig. S13).
Based on this Bayesian NMA, cTnI measurements indicated that wBCP may afford better myocardial protection with lower cTnI levels 24 h postoperatively after cardiac surgery in children. The clinical outcomes were similar for various cardioplegia solutions. Indeed, this NMA is the first to compare the myocardial protective effects of various cardioplegia solutions during pediatric cardiac surgery.
There are several systematic reviews and meta-analyses that have compared cardioplegia during cardiac surgery over the years [30–33]. Fang et al. [30] compared cCCP and cBCP/wBCP in 5 RCTs and found that the 4-, 12-, and 24-h cTnI levels postoperatively, duration of mechanical ventilation, and ICU stays were not significantly different between groups. Mylonas et al. [31] concluded that the 4-h cTnI level was lower in the cBCP/wBCP group compared to cCCP based on 6 RCTs and 2 retrospective studies. Moreover, the duration of mechanical ventilation, length of hospital stay, and early mortality were similar in the analysis. Drury et al. [32] reported that the most common end points of cardioplegia in pediatric cardiac surgery were biomarkers of myocardial injury (cTnI [42.3%] and CK-MB [30.8%]), inotropic support (57.7%), and ICU stay (42.3%) in a systematic review involving 26 RCTs. Ler et al. [33] determined that the ICU stay and early mortality rate were similar when comparing DN and cCCP in three RCTs and one retrospective study.
The cTnI, cardiac troponin T, and CK-MB levels are specific and sensitive biomarkers of myocardial (ischemic–reperfusion) injury. Cardiac troponins are more specific markers of myocardial injury in pediatric cardiac surgery than CK-MB [34]. The diagnostic value of cTnI is similar to cardiac troponin T, but compared with cardiac troponin T, cTnI has the advantage of not being influenced by renal failure [35]. Several studies have shown the same trend; specifically, cTnI peaks at 4 h postoperatively and gradually decreases at 12 and 24 h [36–38]. Therefore, we chose 4, 12, and 24 h as the time points for cTnI in this NMA. cTnI values immediately postoperatively reflect the extent of myocardial damage from both incisional injury and intraoperative factors [39]. The 24-h cTnI level is also a good predictor for clinical outcomes following pediatric cardiac surgery, and correlated with ICU and hospital stays [39–41].
The advantages of wBCP during adult cardiac surgery were demonstrated as early as 1989 [42]. Since that time, wBCP has been shown to be safe and effective based on several RCTs involving adult cardiac surgery and widely used in clinical practice [43–45]; however, wBCP needs to be proven to become the standard method in pediatric patients. Two retrospective studies [46,47] and two RCTs [8,16] focused on wBCP during pediatric cardiac surgery. Chen et al. [8] published the first Chinese report which found that wBCP has a better myocardial protective effect with higher ATP and creatine phosphate, and lower inotropic support. Durandy et al. [46] published the first English report which showed that myocardial protection with wBCP during pediatric cardiac surgery was safe and effective in 1400 patients, with advantageous results in terms of fluid balance, sinus rhythm recovery, and time-to-extubation when compared to cBCP. Pouard et al. [47] reported that wBCP has a lower 24-h cTnI level, shorter duration of mechanical ventilation, and a trend to reduce the ICU length of stay. Poncelet et al. [16] confirmed that wBCP is as safe as cCCP through clinical outcomes, cardiac metabolic, and late neurologic and neuropsychologic assessments. The advantages of wBCP are as follows: (1) improved oxygen supply and reduced myocardial edema; and (2) easier to apply and cost-effective because cooling equipment is no longer required [48]. In addition, there is a RCT involving wBCP that is ongoing which may provide evidence to support the benefits of wBCP to clinical and biochemical outcomes during and after pediatric congenital heart surgery [48].
There were several limitations in this study. First, some of the selected studies were limited sample-sized, single-centered trials that could reduce the credibility of the results and conclusions. Second, the approach of administering cardioplegia, whether antegrade, retrograde, or a combination of both, was not analyzed in this NMA because most cardioplegia solutions were delivered in an antegrade fashion. Third, despite the use of NMA, some underlying confounders, such as the surgical complexity of the selected patients, surgical competence, and surgical proficiency may not be adjustable.
We are of the opinion that wBCP may have a superior myocardial protective effect with a lower 24-h cTnI level postoperatively and similar clinical outcomes after pediatric cardiac surgery.
Authors’ Contributions: The authors’ contributions were as follows: KZ participated in data collection, data analysis, and manuscript writing. DL participated in data collection and data analysis. XZ participated in data analysis. WW participated in data analysis. SL participated in project development. GS participated in project development, data analysis, and manuscript writing. All authors have read and approved the final manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Morales, D. L., Khan, M. S., Turek, J. W., Biniwale, R., Tchervenkov, C. I. et al. (2017). Report of the 2015 society of thoracic surgeons congenital heart surgery practice survey. Annals of Thoracic Surgery, 103(2), 622–628. [Google Scholar]
2. Pasquali, S. K., Thibault, D., O’Brien, S. M., Jacobs, J. P., Gaynor, J. W. et al. (2020). National variation in congenital heart surgery outcomes. Circulation, 142(14), 1351–1360. [Google Scholar]
3. Hirata, Y. (2018). Cardiopulmonary bypass for pediatric cardiac surgery. General Thoracic and Cardiovascular Surgery, 66(2), 65–70. [Google Scholar]
4. Chambers, D. J., Fallouh, H. B. (2010). Cardioplegia and cardiac surgery: Pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacology & Therapeutics, 127(1), 41–52. [Google Scholar]
5. Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H. et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Annals of Internal Medicine, 162(11), 777–784. [Google Scholar]
6. Li, S. M., Kang, M. T., Wu, S. S., Meng, B., Sun, Y. Y. et al. (2017). Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: A meta-analysis. Ophthalmic and Physiological Optics, 37(1), 51–59. [Google Scholar]
7. Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One, 8(10), e76654. [Google Scholar]
8. Chen, R. W., Wang, Z. W., Xu, F. X. (1994). Myocardium protection using warm blood cardioplegia in corrective operation of tetralogy Fallot. Chinese Journal of Surgery, 32(8), 499–501. [Google Scholar]
9. Young, J. N., Choy, I. O., Silva, N. K., Obayashi, D. Y., Barkan, H. E. (1997). Antegrade cold blood cardioplegia is not demonstrably advantageous over cold crystalloid cardioplegia in surgery for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 114(6), 1002–1009. [Google Scholar]
10. Caputo, M., Modi, P., Imura, H., Pawade, A., Parry, A. J. et al. (2002). Cold blood versus cold crystalloid cardioplegia for repair of ventricular septal defects in pediatric heart surgery: A randomized controlled trial. Annals of Thoracic Surgery, 74(2), 530–535. [Google Scholar]
11. Toyoda, Y., Yamaguchi, M., Yoshimura, N., Oka, S., Okita, Y. (2003). Cardioprotective effects and the mechanisms of terminal warm blood cardioplegia in pediatric cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 125(6), 1242–1251. [Google Scholar]
12. Modi, P., Suleiman, M. S., Reeves, B., Pawade, A., Parry, A. J. et al. (2004). Myocardial metabolic changes during pediatric cardiac surgery: A randomized study of 3 cardioplegic techniques. Journal of Thoracic and Cardiovascular Surgery, 128(1), 67–75. [Google Scholar]
13. Amark, K., Berggren, H., Björk, K., Ekroth, A., Ekroth, R. et al. (2005). Blood cardioplegia provides superior protection in infant cardiac surgery. Annals of Thoracic Surgery, 80(3), 989–994. [Google Scholar]
14. Duvan, I., Durukan, B., Gurbuz, A., Yorgancioglu, C., Demircin, M. (2009). A comparison of different management techniques for myocardial protection in acyanotic congenital cardiac patients. Turkish Journal of Medical Sciences, 39(6), 887–893. [Google Scholar]
15. Zhang, Q., Meng, B. Y., Peng, L., Wang, T., Ma, C. et al. (2009). Myocardial protection of cold autoblood cardioplegia in infants with congenital heart disease. Chinese Journal of Contemporary Pediatrics, 11(8), 638–640. [Google Scholar]
16. Poncelet, A. J., van Steenberghe, M.,Moniotte, S., Detaille, T., Beauloye, C. et al. (2011). Cardiac and neurological assessment of normothermia/warm blood cardioplegia vs hypothermia/cold crystalloid cardioplegia in pediatric cardiac surgery: Insight from a prospective randomized trial. European Journal of Cardio-thoracic Surgery, 40(6), 1384–1390. [Google Scholar]
17. Korun, O., Özkan, M., Terzi, A., Aşkın, G., Sezgin, A. et al. (2013). The comparison of the effects of Bretschneider’s histidine-tryptophan-ketoglutarate and conventional crystalloid cardioplegia on pediatric myocardium at tissue level. Artificial Organs, 37(1), 76–81. [Google Scholar]
18. Ma, C., Shen, D. R., Zhang, Q., Meng, X. C., Wang, Y. X. et al. (2013). Protective effect of cold autologous blood cardioplegic solution on the heart of infants with cyanotic congenital heart disease. Chinese Journal of Contemporary Pediatrics, 15(6), 453–457. [Google Scholar]
19. Kuşlu, S., Camkiran, A., Zeyneloʇlu, P., Firat, A., Özkan, M. et al. (2015). A comparison of the effects of histidin-tryprtophan-ketoglutarate and conventional crystalloid cardioplegia solutions on myocardial protection during pediatric cardiac surgery. Anestezi Dergisi, 23(1), 11–19. [Google Scholar]
20. Mimic, B., Ilic, S., Vulicevic, I., Milovanovic, V., Tomic, D. et al. (2016). Comparison of high glucose concentration blood and crystalloid cardioplegia in paediatric cardiac surgery: A randomized clinical trial. Interactive Cardiovascular and Thoracic Surgery, 22(5), 553–560. [Google Scholar]
21. Gorjipour, F., Dehaki, M. G., Totonchi, Z., Hajimiresmaiel, S. J., Azarfarin, R. et al. (2017). Inflammatory cytokine response and cardiac troponin I changes in cardiopulmonary bypass using two cardioplegia solutions; del Nido and modified St. Thomas’: A randomized controlled trial. Perfusion, 32(5), 394–402. [Google Scholar]
22. Talwar, S., Bhoje, A., Sreenivas, V., Makhija, N., Aarav, S. et al. (2017). Comparison of del Nido and St Thomas cardioplegia solutions in pediatric patients: A prospective randomized clinical trial. Seminars in Thoracic and Cardiovascular Surgery, 29(3), 366–374. [Google Scholar]
23. Busro, P. W., Romolo, H., Sastroasmoro, S., Rachmat, J., Sadikin, M. et al. (2018). Role of terminal warm blood cardioplegia in complex congenital heart surgery. Asian Cardiovascular & Thoracic Annals, 26(3), 196–202. [Google Scholar]
24. Panigrahi, D., Roychowdhury, S., Guhabiswas, R., Rupert, E., Das, M. et al. (2018). Myocardial protection following del Nido cardioplegia in pediatric cardiac surgery. Asian Cardiovascular & Thoracic Annals, 26(4), 267–272. [Google Scholar]
25. Negi, S. L., Mandal, B., Singh, R. S., Puri, G. D. (2019). Myocardial protection and clinical outcomes in tetralogy of Fallot patients undergoing intracardiac repair: A randomized study of two cardioplegic techniques. Perfusion, 34(6), 495–502. [Google Scholar]
26. Talwar, S., Chatterjee, S., Sreenivas, V., Makhija, N., Kapoor, P. M. et al. (2019). Comparison of del Nido and histidine-tryptophan-ketoglutarate cardioplegia solutions in pediatric patients undergoing open heart surgery: A prospective randomized clinical trial. Journal of Thoracic and Cardiovascular Surgery, 157(3), 1182–1192.e1181. [Google Scholar]
27. Valente, A. S., Lustosa, G. P., Mota, L. A. M., Lima, A., de Mesquita, F. A. et al. (2019). Comparative analysis of myocardial protection with htk solution and hypothermic hyperkalemic blood solution in the correction of acyanogenic congenital cardiopathies—A randomized study. Brazilian Journal of Cardiovascular Surgery, 34(3), 271–278. [Google Scholar]
28. Bigdelian, H., Hosseini, A. (2020). Effect of single-dose crystalloid cardioplegic agent compared to bloody cardioplegic agent in cardiac surgery in children with Tetralogy of Fallot. ARYA Atherosclerosis, 16(1), 24–32. [Google Scholar]
29. Haranal, M., Chin, H. C., Sivalingam, S., Raja, N., Mohammad Shaffie, M. S. et al. (2020). Safety and effectiveness of Del Nido Cardioplegia in comparison to blood-based St. Thomas cardioplegia in congenital heart surgeries: A prospective randomized controlled study. World Journal for Pediatric & Congenital Heart Surgery, 11(6), 720–726. [Google Scholar]
30. Fang, Y., Long, C., Lou, S., Guan, Y., Fu, Z. (2015). Blood versus crystalloid cardioplegia for pediatric cardiac surgery: A meta-analysis. Perfusion, 30(7), 529–536. [Google Scholar]
31. Mylonas, K. S., Tzani, A., Metaxas, P., Schizas, D., Boikou, V. et al. (2017). Blood versus crystalloid cardioplegia in pediatric cardiac surgery: A systematic review and meta-analysis. Pediatric Cardiology, 38(8), 1527–1539. [Google Scholar]
32. Drury, N. E., Yim, I., Patel, A. J., Oswald, N. K., Chong, C. R. et al. (2019). Cardioplegia in paediatric cardiac surgery: A systematic review of randomized controlled trials. Interactive Cardiovascular and Thoracic Surgery, 28(1), 144–150. [Google Scholar]
33. Ler, A., Sazzad, F., Ong, G. S., Kofidis, T. (2020). Comparison of outcomes of the use of Del Nido and St. Thomas cardioplegia in adult and paediatric cardiac surgery: A systematic review and meta-analysis. Perfusion, 35(8), 724–735. [Google Scholar]
34. Taggart, D. P., Hadjinikolas, L., Wong, K., Yap, J., Hooper, J. et al. (1996). Vulnerability of paediatric myocardium to cardiac surgery. Heart, 76(3), 214–217. [Google Scholar]
35. Immer, F. F., Stocker, F. P., Seiler, A. M., Pfammatter, J. P., Printzen, G. et al. (1998). Comparison of troponin-I and troponin-T after pediatric cardiovascular operation. Annals of Thoracic Surgery, 66(6), 2073–2077. [Google Scholar]
36. Imura, H., Caputo, M., Parry, A., Pawade, A., Angelini, G. D. et al. (2001). Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation, 103(11), 1551–1556. [Google Scholar]
37. Yang, L., Wang, G., Du, Y., Ji, B., Zheng, Z. (2014). Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: A meta-analysis. Journal of Cardiothoracic and Vascular Anesthsia, 28(3), 682–689. [Google Scholar]
38. Su, J. A., Kumar, S. R., Mahmoud, H., Bowdish, M. E., Toubat, O. et al. (2019). Postoperative serum troponin trends in infants undergoing cardiac surgery. Seminars in Thoracic and Cardiovascular Surgery, 31(2), 244–251. [Google Scholar]
39. Hirsch, R., Dent, C. L., Wood, M. K., Huddleston, C. B., Mendeloff, E. N. et al. (1998). Patterns and potential value of cardiac troponin I elevations after pediatric cardiac operations. Annals of Thoracic Surgery, 65(5), 1394–1399. [Google Scholar]
40. Momeni, M., Poncelet, A., Rubay, J., Matta, A., Veevaete, L. et al. (2017). Does postoperative cardiac troponin-I have any prognostic value in predicting midterm mortality after congenital cardiac surgery? Journal of Cardiothoracic and Vascular Anesthsia, 31(1), 122–127. [Google Scholar]
41. Kojima, T., Toda, K., Oyanagi, T., Yoshiba, S., Kobayashi, T. et al. (2020). Early assessment of cardiac troponin I predicts the postoperative cardiac status and clinical course after congenital heart disease surgery. Heart and Vessels, 35(3), 417–421. [Google Scholar]
42. Lichtenstein, S. V., el Dalati, H.,Panos, A., Slutsky, A. S. (1989). Long cross-clamp time with warm heart surgery. Lancet, 1(8652), 1443. [Google Scholar]
43. Caputo, M., Dihmis, W. C., Bryan, A. J., Suleiman, M. S., Angelini, G. D. (1998). Warm blood hyperkalaemic reperfusion (‘hot shot’) prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. European Journal of Cardio-thoracic Surgery, 13(5), 559–564. [Google Scholar]
44. Mourad, F. A., Fadala, M. A., Ibrahim, A. A., Ammar, A. M., Elnahas, Y. M. et al. (2016). Myocardial protection during CABG: Warm blood versus cold crystalloid cardioplegia, is there any difference? Journal of the Egyptian Society of Cardio-Thoracic Surgery, 24(3), 215–222. [Google Scholar]
45. Ulugol, H., Aksu, U., Kocyigit, M., Kilercik, M., Karduz, G. et al. (2019). Comparative effects of blood and crystalloid cardioplegia on cellular injury and oxidative stress in cardiovascular surgery. Annals of Thoracic and Cardiovascular Surgery, 25(1), 10–17. [Google Scholar]
46. Durandy, Y., Hulin, S. (2007). Intermittent warm blood cardioplegia in the surgical treatment of congenital heart disease: Clinical experience with 1400 cases. Journal of Thoracic and Cardiovascular Surgery, 133(1), 241–246. [Google Scholar]
47. Pouard, P., Mauriat, P., Ek, F., Haydar, A., Gioanni, S. et al. (2006). Normothermic cardiopulmonary bypass and myocardial cardioplegic protection for neonatal arterial switch operation. European Journal of Cardio-thoracic Surgery, 30(5), 695–699. [Google Scholar]
48. Heys, R., Stoica, S., Angelini, G., Beringer, R., Evans, R. et al. (2020). Intermittent antegrade warm-blood versus cold-blood cardioplegia in children undergoing open heart surgery: A protocol for a randomised controlled study (Thermic-3). BMJ Open, 10(10), e036974. [Google Scholar]
Appendix


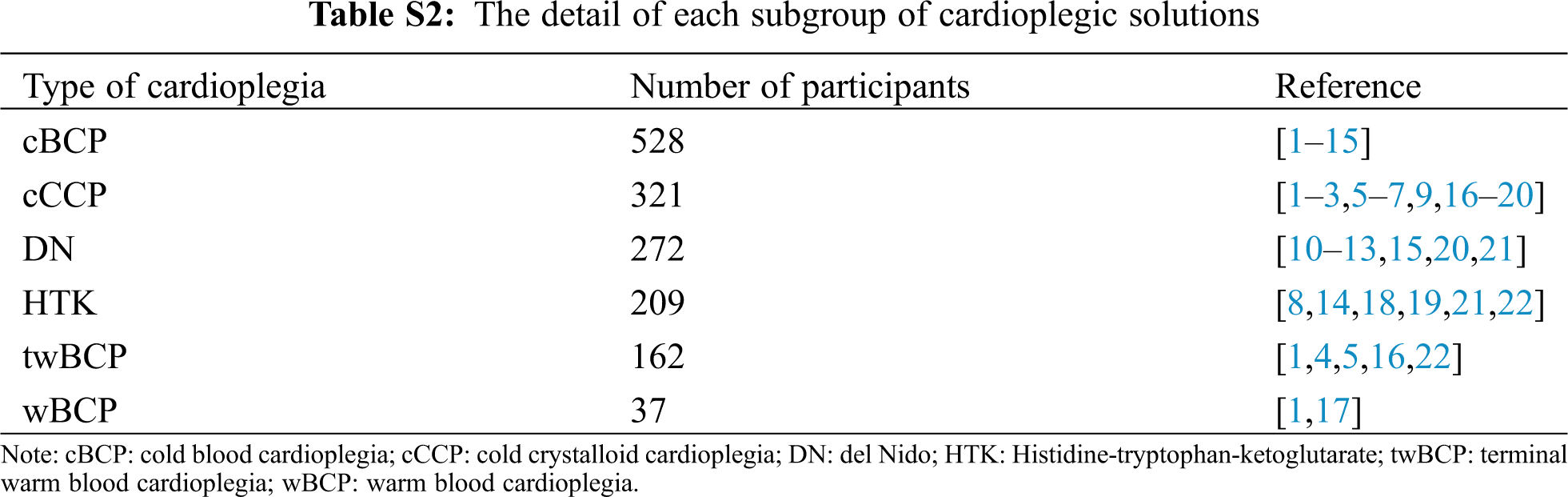
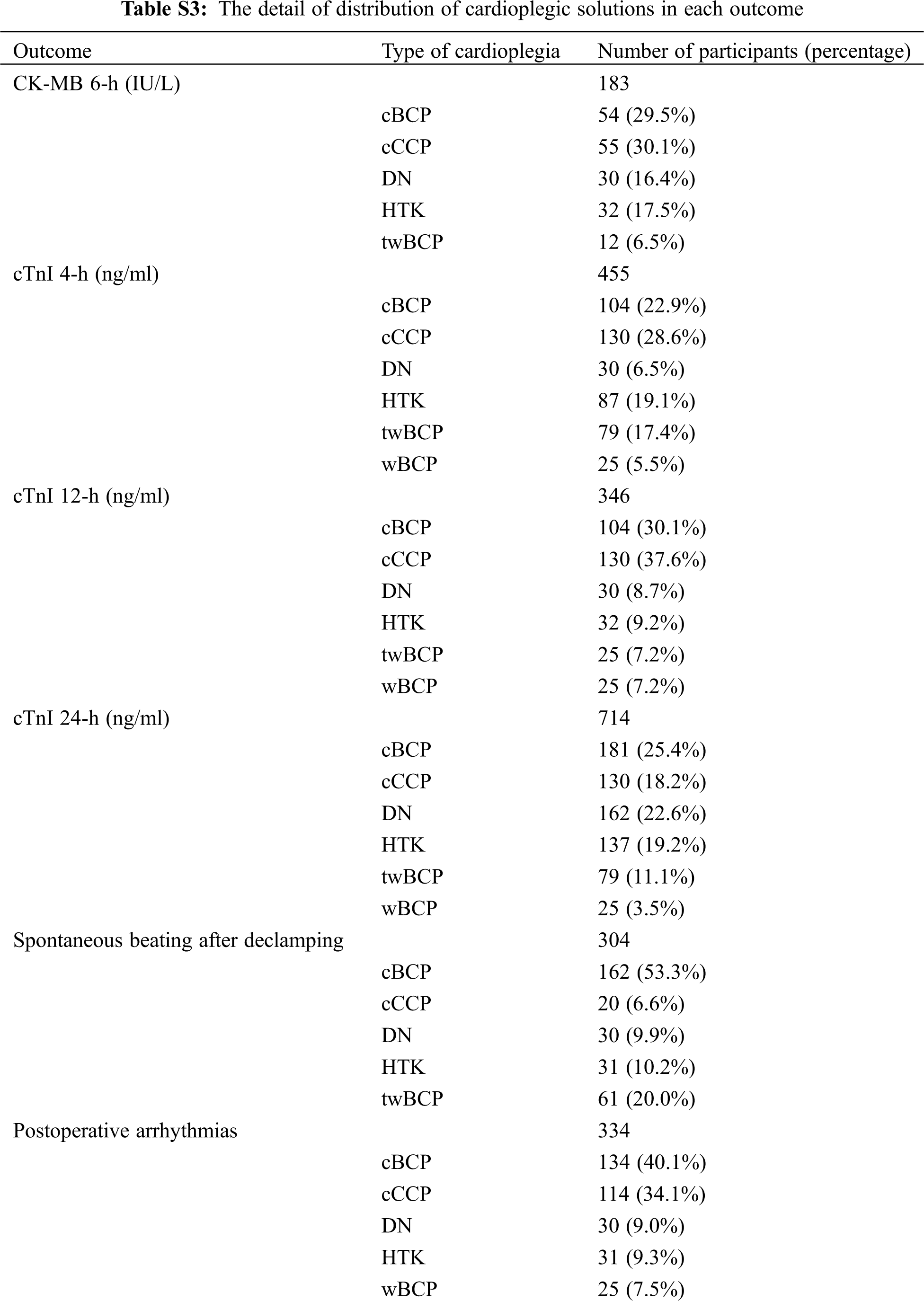

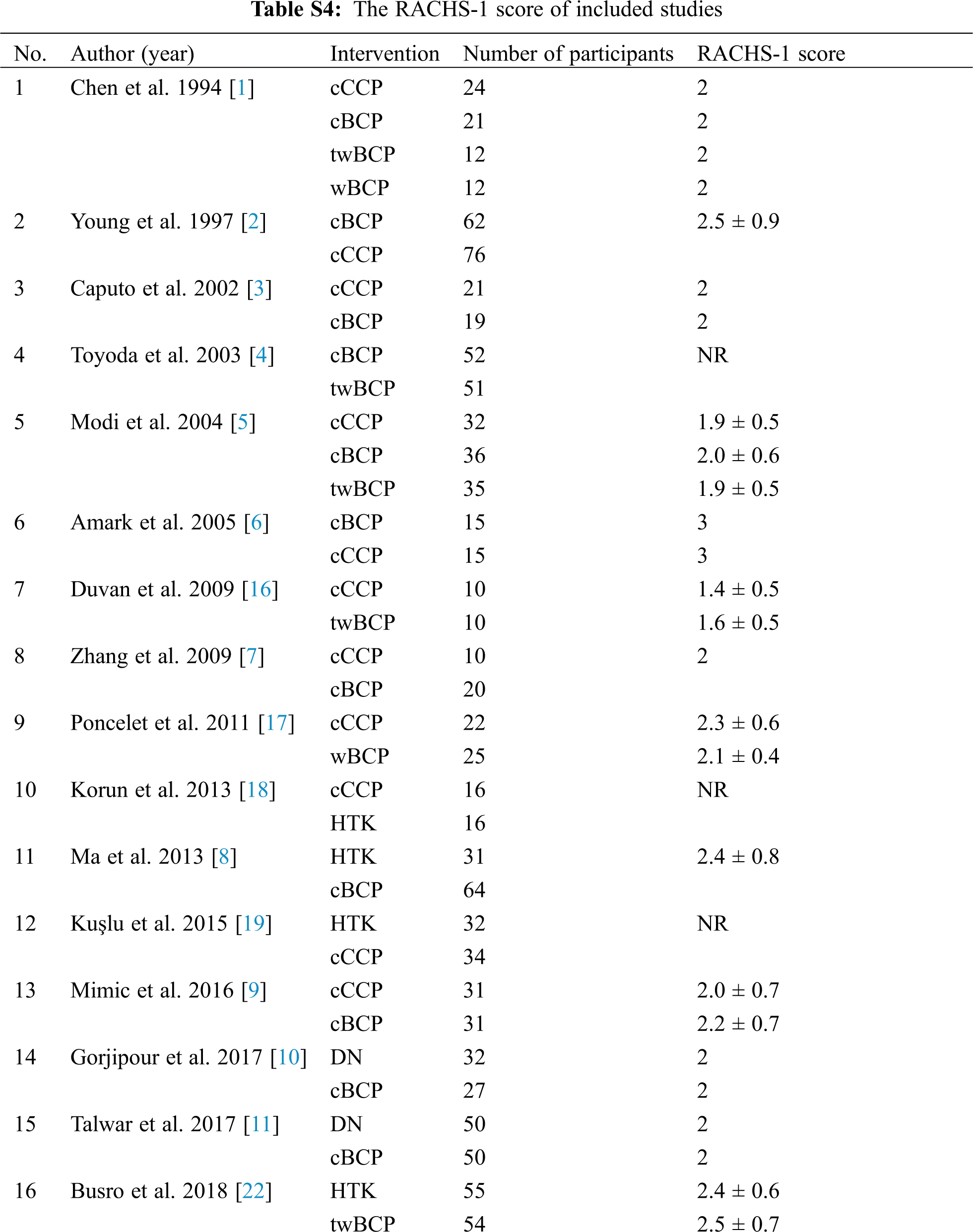
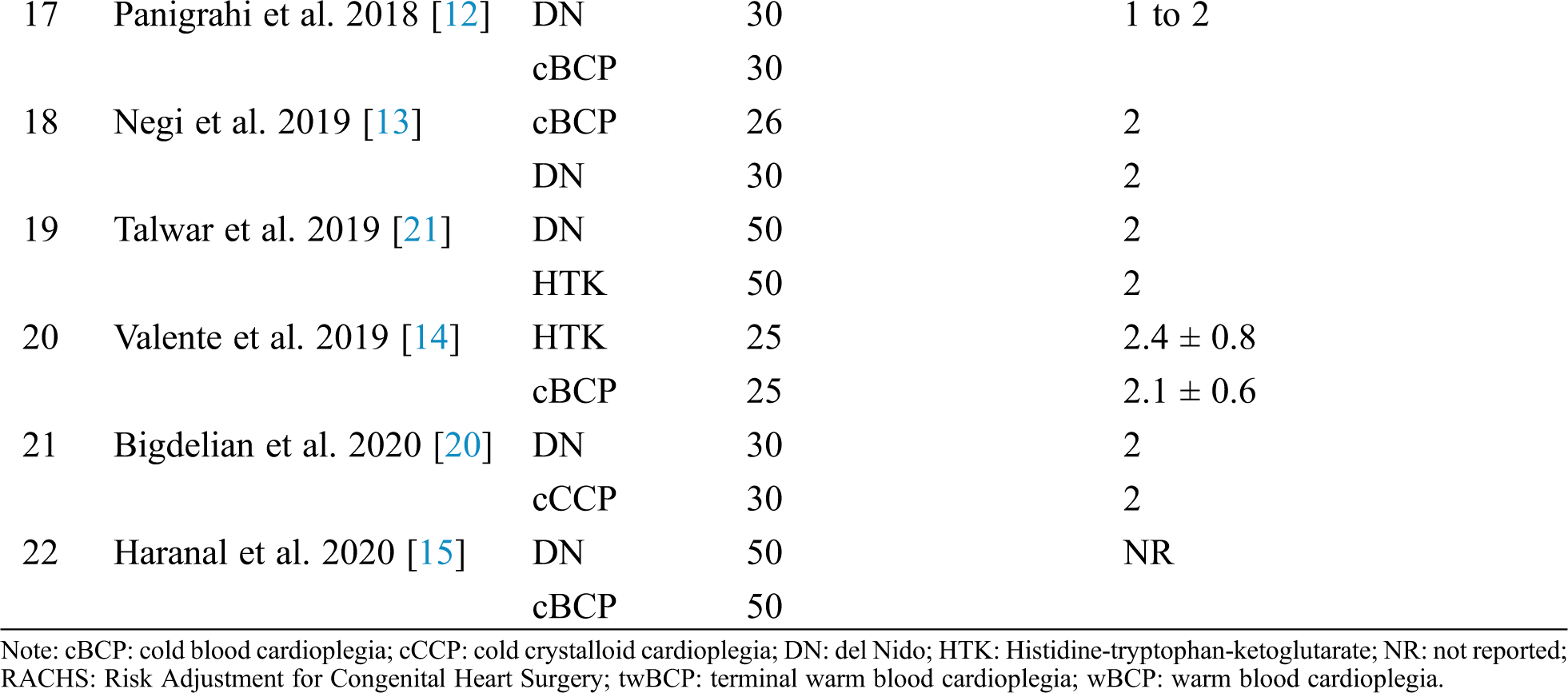
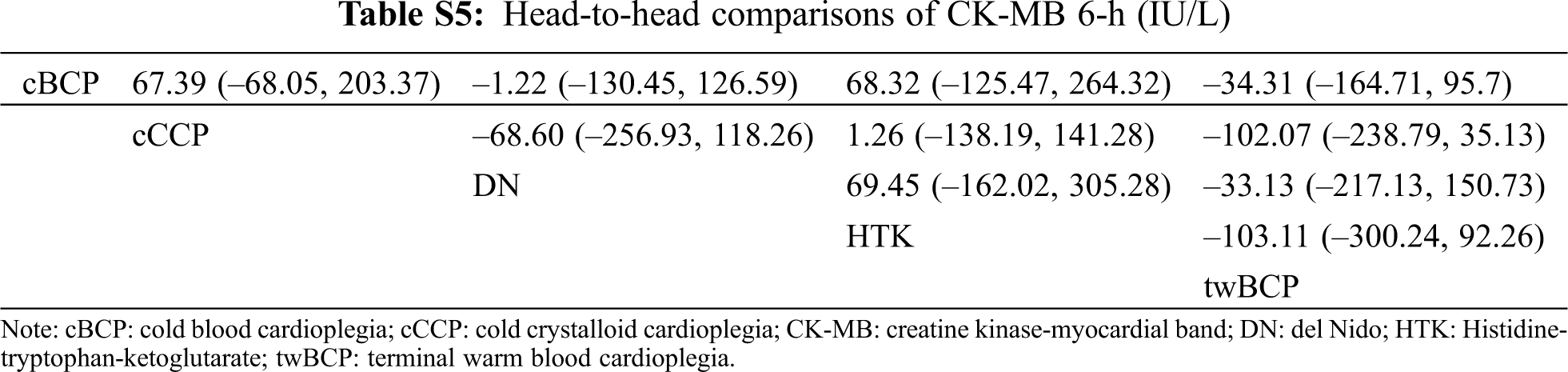

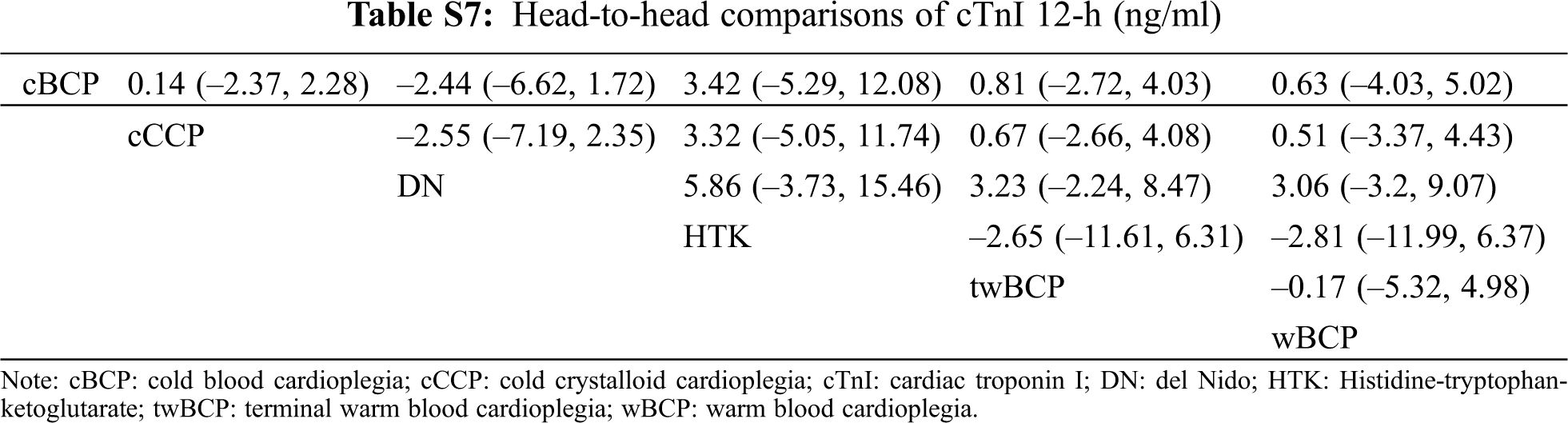
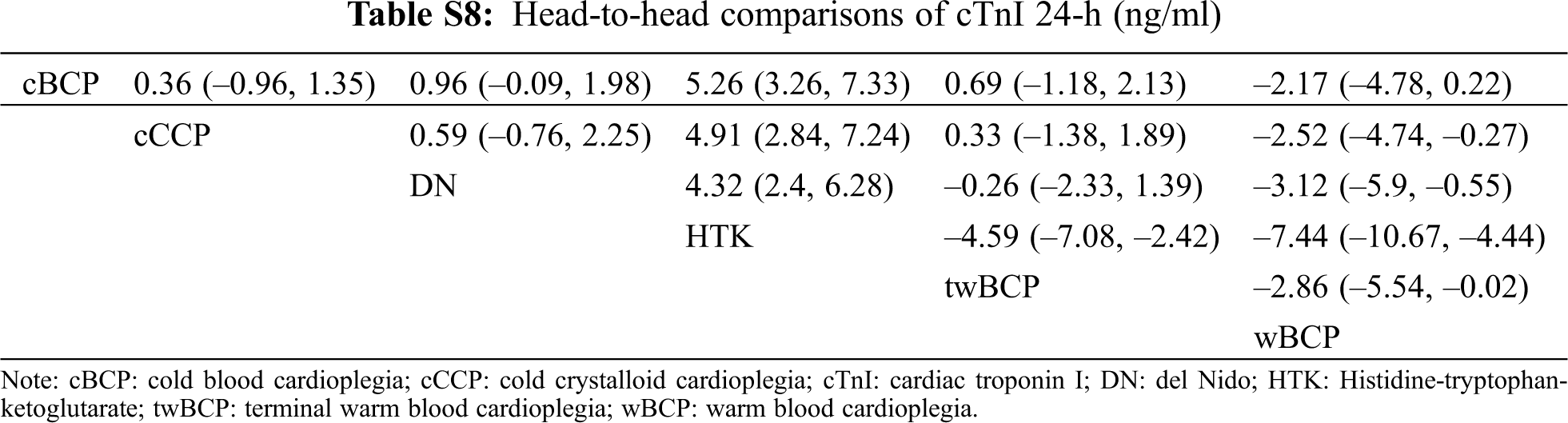
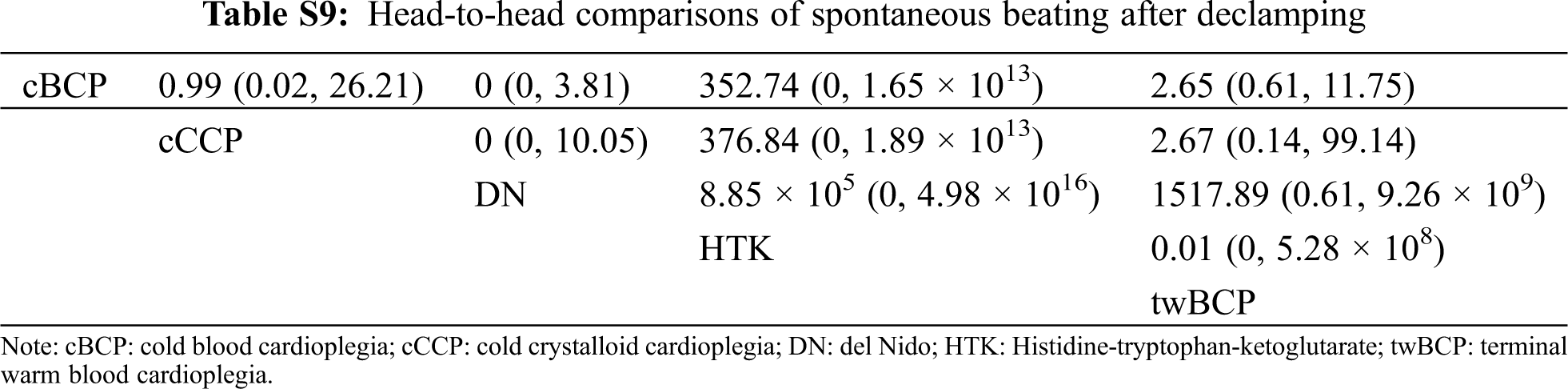
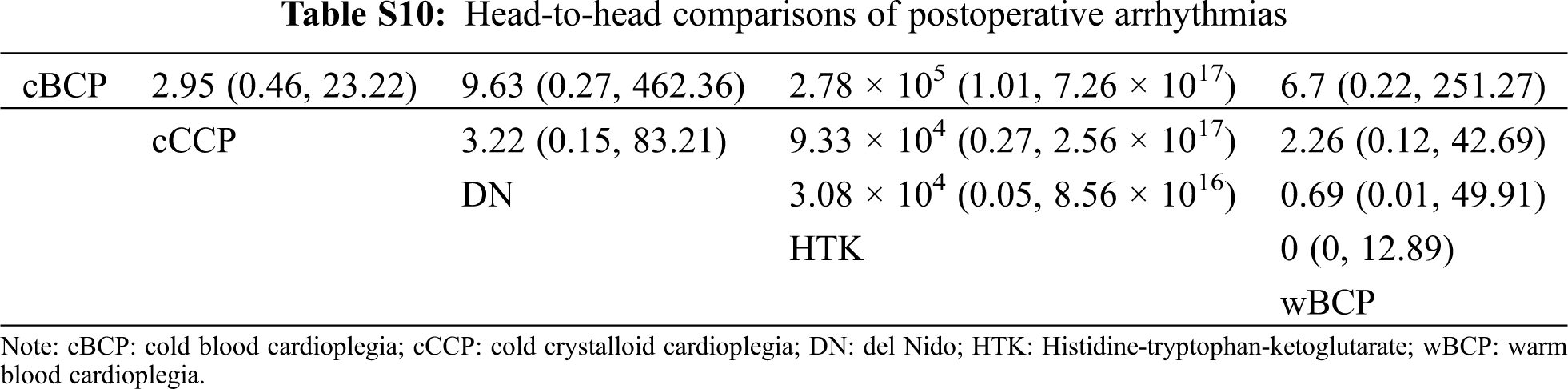



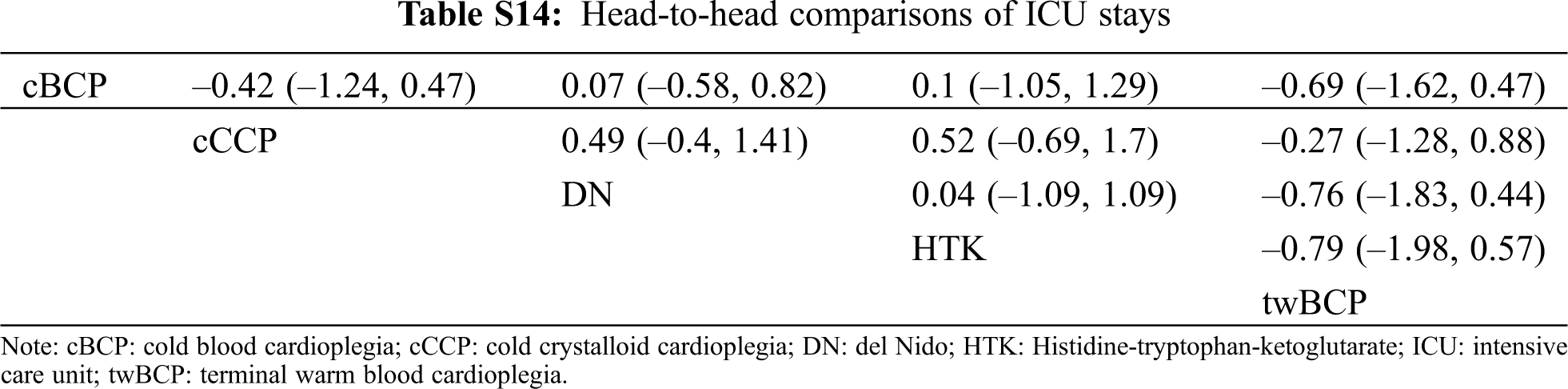



Figure S1: Risk of bias summary. +: low risk of bias; –: high risk of bias; ?: unclear risk of bias

Figure S2: Risk of bias graph

Figure S3: Geometry of the network. cBCP: cold blood cardioplegia; cCCP: cold crystalloid cardioplegia; CK-MB: creatine kinase-myocardial band; cTnI: cardiac troponin I; DN: del Nido; HTK: Histidine-tryptophan-ketoglutarate; ICU: intensive care unit; twBCP: terminal warm blood cardioplegia; wBCP: warm blood cardioplegia. Notes: circles represent the intervention as a node in the network, lines represent direct comparisons using randomized controlled trials (RCTs) and the thickness of lines corresponds to the number of RCTs included in each comparison

Figure S4: Inconsistency test of cTnI 4-h. cTnI: cardiac troponin I

Figure S5: Inconsistency test of cTnI 24-h. cTnI: cardiac troponin I

Figure S6: Inconsistency test of spontaneous beating after declamping

Figure S7: Inconsistency test of inotropic support (%)

Figure S8: Inconsistency test of inotropic duration hours

Figure S9: Inconsistency test of mechanical ventilation hours

Figure S10: Inconsistency test of ICU stays. ICU: intensive care unit

Figure S11: Inconsistency test of hospital stays

Figure S12: Inconsistency test of mortality
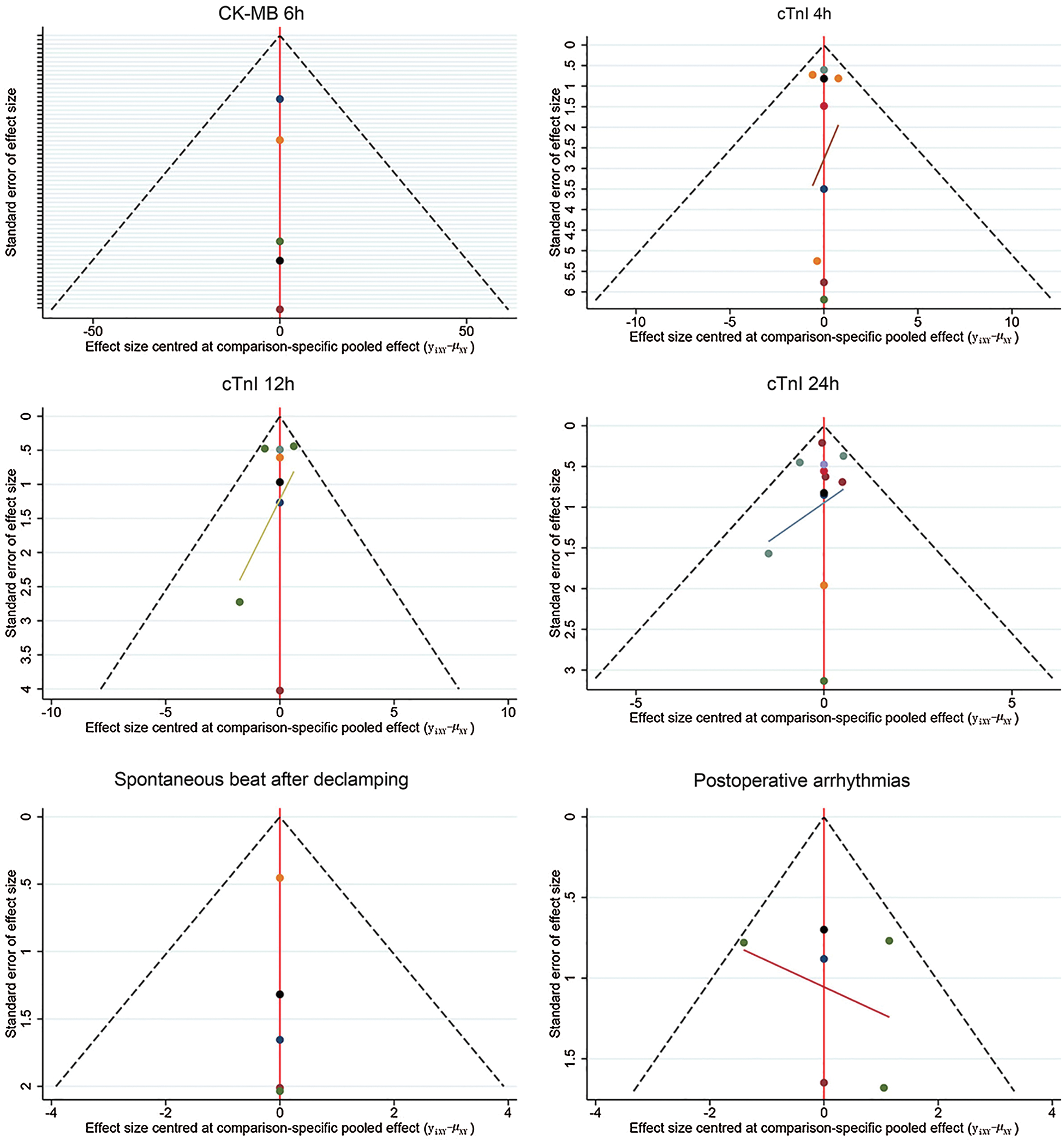
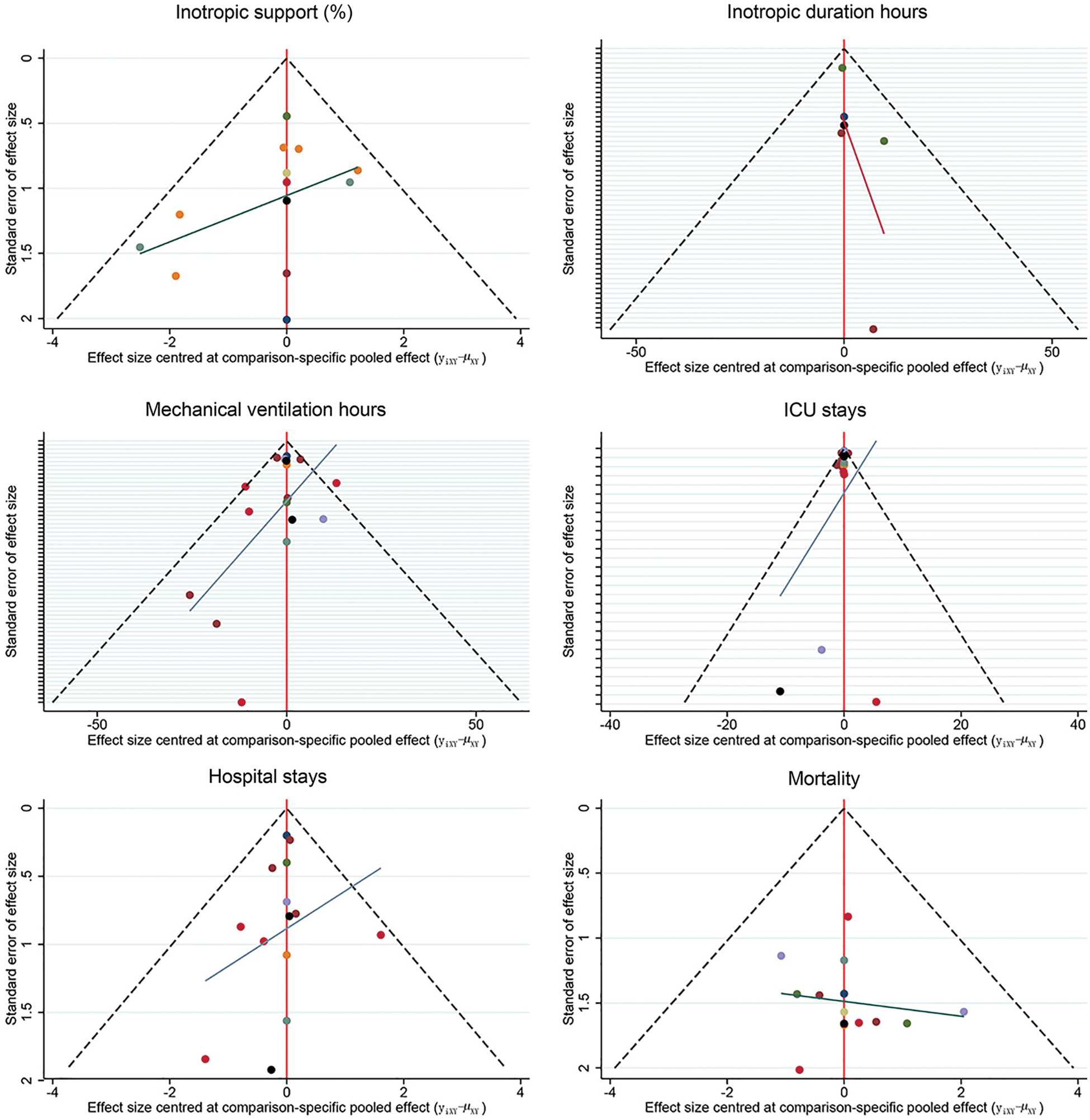
Figure S13: Funnel plot of outcomes. CK-MB: creatine kinase-myocardial band; cTnI: cardiac troponin I; ICU: intensive care unit.
e-References
1. Chen, R. W., Wang, Z. W., Xu, F. X. (1994). Myocardium protection using warm blood cardioplegia in corrective operation of tetralogy Fallot. Chinese Journal of Surgery, 32(8), 499–501.
2. Young, J. N., Choy, I. O., Silva, N. K., Obayashi, D. Y., Barkan, H. E. (1997). Antegrade cold blood cardioplegia is not demonstrably advantageous over cold crystalloid cardioplegia in surgery for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 114(6), 1002–1009.
3. Caputo, M., Modi, P., Imura, H., Pawade, A., Parry, A. J. et al. (2002). Cold blood versus cold crystalloid cardioplegia for repair of ventricular septal defects in pediatric heart surgery: A randomized controlled trial. Annals of Thoracic Surgery, 74(2), 530–535.
4. Toyoda, Y., Yamaguchi, M., Yoshimura, N., Oka, S., Okita, Y. (2003). Cardioprotective effects and the mechanisms of terminal warm blood cardioplegia in pediatric cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 125(6), 1242–1251.
5. Modi, P., Suleiman, M. S., Reeves, B., Pawade, A., Parry, A. J. et al. (2004). Myocardial metabolic changes during pediatric cardiac surgery: A randomized study of 3 cardioplegic techniques. Journal of Thoracic and Cardiovascular Surgery, 128(1), 67–75.
6. Amark, K., Berggren, H., Björk, K., Ekroth, A., Ekroth, R. et al. (2005). Blood cardioplegia provides superior protection in infant cardiac surgery. Annals of Thoracic Surgery, 80(3), 989-994.
7. Zhang, Q., Meng, B. Y., Peng, L., Wang, T., Ma, C. et al. (2009). Myocardial protection of cold autoblood cardioplegia in infants with congenital heart disease. Chinese Journal of Contemporary Pediatrics, 11(8), 638–640.
8. Ma, C., Shen, D. R., Zhang, Q., Meng, X. C., Wang, Y. X. et al. (2013). Protective effect of cold autologous blood cardioplegic solution on the heart of infants with cyanotic congenital heart disease. Chinese Journal of Contemporary Pediatrics, 15(6), 453–457.
9. Mimic, B., Ilic, S., Vulicevic, I., Milovanovic, V., Tomic, D. et al. (2016). Comparison of high glucose concentration blood and crystalloid cardioplegia in paediatric cardiac surgery: A randomized clinical trial. Interactive Cardiovascular and Thoracic Surgery, 22(5), 553–560.
10. Gorjipour, F., Dehaki, M. G., Totonchi, Z., Hajimiresmaiel, S. J., Azarfarin, R. et al. (2017). Inflammatory cytokine response and cardiac troponin I changes in cardiopulmonary bypass using two cardioplegia solutions; del Nido and modified St. Thomas’: A randomized controlled trial. Perfusion, 32(5), 394–402.
11. Talwar, S., Bhoje, A., Sreenivas, V., Makhija, N., Aarav, S. et al. (2017). Comparison of del Nido and St Thomas cardioplegia solutions in pediatric patients: A prospective randomized clinical trial. Seminars in Thoracic and Cardiovascular Surgery, 29(3), 366–374.
12. Panigrahi, D., Roychowdhury, S., Guhabiswas, R., Rupert, E., Das, M. et al. (2018). Myocardial protection following del Nido cardioplegia in pediatric cardiac surgery. Asian Cardiovascular & Thoracic Annals, 26(4), 267–272.
13. Negi, S. L., Mandal, B., Singh, R. S., Puri, G. D. (2019). Myocardial protection and clinical outcomes in Tetralogy of Fallot patients undergoing intracardiac repair: a randomized study of two cardioplegic techniques. Perfusion, 34(6), 495–502.
14. Valente, A. S., Lustosa, G. P., Mota, L. A. M., Lima, A., de Mesquita, F. A. et al. (2019). Comparative analysis of myocardial protection with htk solution and hypothermic hyperkalemic blood solution in the correction of acyanogenic congenital cardiopathies—A randomized study. Brazilian Journal of Cardiovascular Surgery, 34(3), 271–278.
15. Haranal, M., Chin, H. C., Sivalingam, S., Raja, N., Mohammad Shaffie, M. S. et al. (2020). Safety and effectiveness of Del Nido cardioplegia in comparison to blood-based St. Thomas cardioplegia in congenital heart surgeries: A prospective randomized controlled study. World Journal for Pediatric & Congenital Heart Surgery, 11(6), 720–726.
16. Duvan, I., Durukan, B., Gurbuz, A., Yorgancioglu, C., Demircin, M. (2009). A comparison of different management techniques for myocardial protection in acyanotic congenital cardiac patients. Turkish Journal of Medical Sciences, 39(6), 887–893.
17. Poncelet, A. J., van Steenberghe, M., Moniotte, S., Detaille, T., Beauloye, C. et al. (2011). Cardiac and neurological assessment of normothermia/warm blood cardioplegia vs hypothermia/cold crystalloid cardioplegia in pediatric cardiac surgery: Insight from a prospective randomized trial. European Journal of Cardio-Thoracic Surgery, 40(6), 1384–1390.
18. Korun, O., Özkan, M., Terzi, A., Aşkın, G., Sezgin, A. et al. (2013). The comparison of the effects of Bretschneider’s histidine-tryptophan-ketoglutarate and conventional crystalloid cardioplegia on pediatric myocardium at tissue level. Artificial Organs, 37(1), 76–81.
19. Kuşlu, S., Camkiran A., Zeynelolu, P., Firat, A., Özkan, M. et al. (2015). A comparison of the effects of histidin-tryprtophan-ketoglutarate and conventional crystalloid cardioplegia solutions on myocardial protection during pediatric cardiac surgery. Anestezi Dergisi, 23(1), 11–19.
20. Bigdelian, H., Hosseini, A. (2020). Effect of single-dose crystalloid cardioplegic agent compared to bloody cardioplegic agent in cardiac surgery in children with Tetralogy of Fallot. ARYA Atherosclerosis, 16(1), 24–32.
21. Talwar, S., Chatterjee, S., Sreenivas, V., Makhija, N., Kapoor, P. M. et al. (2019). Comparison of del Nido and histidine-tryptophan-ketoglutarate cardioplegia solutions in pediatric patients undergoing open heart surgery: A prospective randomized clinical trial. Journal of Thoracic and Cardiovascular Surgery, 157(3), 1182–1192.e1181.
22. Busro, P. W., Romolo, H., Sastroasmoro, S., Rachmat, J., Sadikin, M. et al. (2018). Role of terminal warm blood cardioplegia in complex congenital heart surgery. Asian Cardiovascular & Thoracic Annals, 26(3), 196–202.
PRISMA NMA Checklist of Items to Include When Reporting a Systematic Review: Involving a Network Meta-Analysis



 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |