

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016189
ARTICLE
What Is the Relation between Aerobic Capacity and Physical Activity Level in Adults with Congenital Heart Disease?
1FPCEE-Blanquerna, Ramon Llull University, Barcelona, Spain
2Cardiology Department, Santa Creu and Sant Pau Hospital, IIB Sant Pau, Barcelona, Spain
3Cors Units Foundation, Barcelona, Spain
4Zoophysiology, Department of Biology, Aarhus University, Aarhus, Denmark
5Institute of Nursing and Health Research, Ulster University, Jordanstown, Northern Ireland
*Corresponding Author: Kelly Ferri. Email: kellyprisciladf@blanquerna.url.edu
Received: 15 February 2021; Accepted: 01 April 2021
Abstract: Background: Aerobic capacity (AC) in adults with congenital heart disease (CHD) is often reduced, mainly due to low confidence levels towards physical activity (PA). The main objective of this study was to estimate the association between PA level and AC (measured as peak of oxygen consumption, VO2peak) in adults with CHD. Methods: A total of 183 individuals (83 women and 100 men; mean (SD) age 36.9 (11.0) years old) from Vall d’Hebron Hospital, Barcelona-Spain in 2019, participated in this cross-sectional study. The AC was assessed by cardiopulmonary exercise testing (CPET) using a treadmill ramp protocol. Considering values of metabolic equivalent of task (MET, MET-min·week−1) obtained by the short International Physical Activity Questionnaire (IPAQ), participants were divided into three categories of PA: health-enhancing PA (HEPA), minimally active, and inactive. Results: Median (SD) PA was 2737.2 (2835.7) MET-min·week−1, with 60 participants (32.8%) reporting HEPA, 91 (49.7%) minimally active, and 32 (17.5%) inactivity. Participants demonstrated a mean VO2peak of 28.9 (8.8) mL·Kg−1·min−1, showing AC values on average 13% lower than expected in a healthy population. Overall, PA and AC were positively associated. Adjusting for sex and age, an increase of 1000 MET-min·week−1 was associated with an increase in VO2peak of 0.8 units (95% CI 0.4–1.2; p < 0.001). There were no differences in the degree of increase between sexes (p = 0.427). Conclusion: These findings suggest that an increase in PA in patients with CHD significantly improves their AC, and hence, could be recommended when the goal is to improve their physical condition.
Keywords: Physical activity level; aerobic capacity; congenital heart disease
The relationship between physical activity (PA) level and the aerobic capacity (AC) of adults with congenital heart disease (CHD) is subject of interest in PA and health research. Some authors demonstrate that this population of adults follows the general guidelines of PA, while others highlight low levels of daily PA [1–6]. However, there is limited information about the effect of PA on the AC of adults with CHD.
The AC of people with CHD has been described to be lower than among people from a healthy population [7]. When evaluating adults with CHD, common findings in maximum tests of cardiopulmonary exercise testing (CPET) include a reduced peak oxygen consumption (VO2peak), resulting in a compromised physical condition with anomalies that primarily affect the heart, the blood vessels, the lungs and the muscles [8]. The capability to carry out daily living activities in people with CHD is often linked to the quality of the post-surgical results [9]. However, their AC-an approximation of physical condition-can be also related to the anatomical defect and the PA level [10].
A low AC in adults with CHD can be attributed to different reasons, such as low confidence levels towards physical exercise, disease symptoms, exercise restrictions or childhood overprotection [11]. In some cases, these aspects can result in a poor development of psychomotor skills, a limited fitness level, fear to participate in physical exercise, or fear of sudden death. Nevertheless, data confirms that adults with CHD are willing to engage in physical exercise following appropriate advice and under suitable supervision [12].
Current guidelines featuring recommendations of PA for people with CHD have traditionally been very useful to make informed decisions related to their participation in competitive sports based on heart injuries [9,13]. However, there are limited guidelines for leisure or daily PA. In these cases, the recommendations could be more practical and objective when the prescription of PA for these patients has, as a reference, the CPET. The CPET has proved to be an essential tool that can objectively evaluate the functional cardiovascular ability of the patients, and identify pathological mechanisms, as circulatory failure, shunt and/or pulmonary hypertension [8], as well as help to prescribe an individualized program of physical exercise when applicable, and promote daily PA [14].
In a study of the risks and benefits of exercise training in adults with CHD, Chaix et al. [15] proposed a model of assessment of PA, including current exercise (type, intensity, duration, frequency), before exercise prescription that it is suggested to help to increase confidence and encourage patients towards the practice of PA. However, it remains unknown whether an increase in PA level has an effect on the AC of adults with CHD. The main objective of this study was to evaluate the association between PA level and AC in adults with CHD and assess if increasing levels of PA could improve the AC of adults with different CHD.
2.1 Study Design and Participants
This study followed a cross-sectional design. Participants were all adults recruited from the CHD Unit of Vall d’Hebron Hospital in Barcelona (Spain). All of them presented a CHD, and were referred to the CPET department of Santa Creu i Sant Pau Hospital in Barcelona (Spain) from September 2018 to April 2020. A total of 212 individuals received a detailed information sheet regarding this study and were invited to participate. However, 29 of them were excluded based on the following exclusion criteria: two were pregnant, 25 were younger than 18 years, and two were unable to provide informed consent due to language barriers. The remaining 183 participants accomplished with the inclusion criteria and agreed to volunteer taking part in the study providing their informed consent duly signed.
Considering the multiplicity of diagnoses of CHD and the different surgeries performed, we classified the residual state of heart disease in three categories according to its severity: simple, moderate and complex. These categories are shown in Tab. 1, and were obtained based on the criteria of a previous study by Serra-Grima et al. [11] including (1) clinical, surgical, echocardiographic data, (2) result of the surgery, presence of residual injuries, sequel or possible complications, (3) the hemodynamic condition, (4) functional class and the possibility of hospital admission due to heart failure. An expert cardiologist performed this classification without direct relationship with the participants, and without knowledge of the CPET results to avoid expectation and maintain a blind classification.
The study protocol was approved by the FPCEE-Blanquerna institutional research board (Protocol No. 1718005D) and follows the Helsinki guidelines for ethical behavior [16].

2.2.1 Anthropometric Measurements
Height was measured to the nearest 0.1 cm using a stadiometer (Seca 225, Seca, Hamburg, Germany). Weight was measured to the nearest 0.1 kg on a digital scale (Seca 861, Hamburg, Germany) with the subject wearing lightweight clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2) [17].
2.2.2 Cardiopulmonary Exercise testing (CPET)
All tests were conducted during the afternoon at a room temperature of 22°C–24°C and relative physical humidity between 55% and 65%. The CPET was performed on a treadmill (MTM–1500 MED by Schiller™ España SA, Madrid, Spain) using a ramp protocol. The participants started walking at 3 km/h for two minutes, after which the speed increased 0.3 km/h and 1.4% grade every minute to a maximum of 12% until exhaustion of participants. Participants were asked every 2/3 min to tell if symptoms (as dyspnea, angina, general discomfort or nausea) appeared, and were verbally encouraged to push themselves [14].
Heart rate was obtained using a 12-lead electrocardiogram (Cardiovit® CS–200 by Schiller™ España S.A, Madrid, Spain) and blood pressure was measured using a sphygmomanometer (Sphygmomanometer model Big Ben round® by Riester™, Jungigen, Germany) at rest, at the end of each stage, at peak exercise, and during recovery (at the first, third and fifth minute after finishing the exercise).
Relative VO2peak (mL·Kg−1·min−1), peak ventilation VE (L·min−1), and respiratory exchange ratio (RER) were obtained breath-by-breath with an automatic gas analysis system (PowerCube®-Ergo by Ganshorn™ Medizini Electronic GmbH, Niedlauer, Germany).
The expected VO2peak (mL·Kg−1·min−1) for men and women was obtained with the automatic gas analysis system (PowerCube®-Ergo by Ganshorn™ Medizini Electronic GmbH, Niedlauer, Germany). The system calculated this parameter based on the equation published by Wassermann et al. [18] to take into account various influencing factors, including weight (measured and expected), mode of exercise (treadmill) and sex. We compared the VO2peak with the expected VO2peak through a percentage of difference between the observed and the expected. For example, a participant with a value of 20% has a VO2peak 20% higher than the expected based on their sex and age; alternatively, a participant with a value of −10% has a VO2peak 10% lower than expected.
2.2.3 International Physical Activity Questionnaire (IPAQ)
The IPAQ [19] short form was used to assess PA levels within the last seven days. With three questions related to vigorous, moderate or walking activities, the questionnaire distinguishes the activity levels in three categories: health-enhancing PA (HEPA), minimally active, and inactive [20]. The IPAQ shows an acceptable reliability and validity, and has been shown to be a valid measurement tool for assessing PA levels in individuals with CHD [21,22].
Descriptive statistics were calculated for all variables. Continuous variables were expressed as mean and standard deviation (SD), or median and inter-quartile range (IQR, 3rt quartile–1st quartile) when variables were not normally distributed. Categorical variables were described as frequency and percentage.
The relation between PA, VO2peak and expected VO2peak was assessed by linear regression analysis and adjusting for sex (men/women) and exact age (as a continuous variable) of the participants. With 11, 71 and 101 cases of simple, moderate and complex CHD, respectively, we did not have a sample large and balanced enough to investigate potential differences in the association between PA and AC between CHD severities. Residuals of the models were checked for normality and heteroscedasticity.
Statistical analyses were conducted using R software (version 3.6.1) [23]. Statistical significance was set at an alpha level of 0.05.
A total of 100 men and 83 women were included in this study, with a mean (SD) age of 36.9 (11.0) years, mean height and weight of 167.8 cm and 69.0 kg, respectively, and mean BMI of 24.4 kg/m2. Of the 183 participants and according to the WHO BMI criteria, 13 (7.1%) were classified as underweight, 95 (51.6%) as normal weight, 58 (31.5%) as overweight, and 17 (9.2%) as obese (Tab. 2).
Symptom-limited CPET were performed successfully and without complications in all patients (mean (SD) respiratory exchange ratio of 1.1 (0.1)).
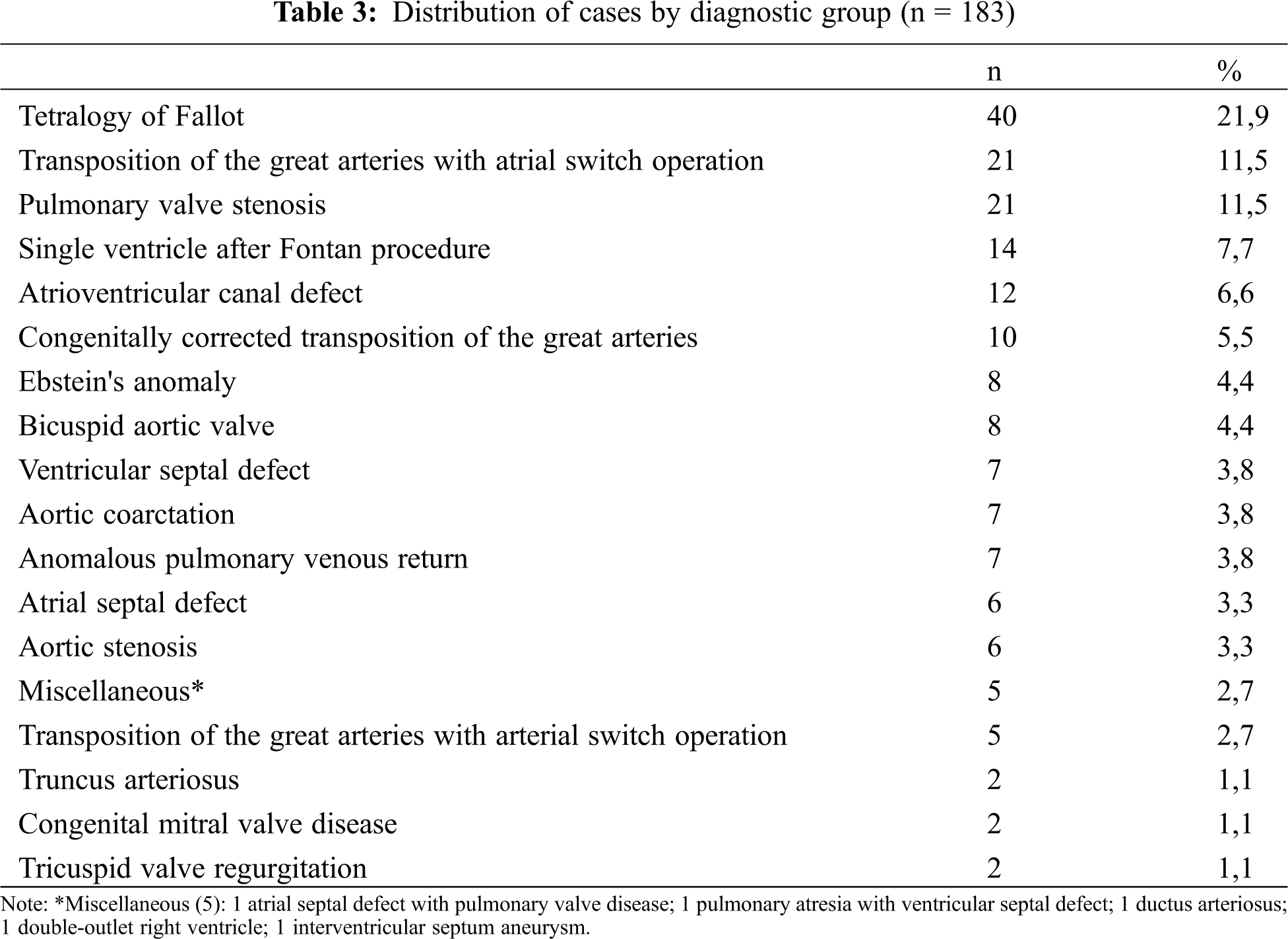
The clinical diagnosis distribution of the participants is presented in Tab. 3. The most frequent diagnosis related to severity category was the complex CHD presented in 101 participants (55.2%), followed by moderate CHD seen in 71 (38.8%) and simple CHD in 11 participants (6%).
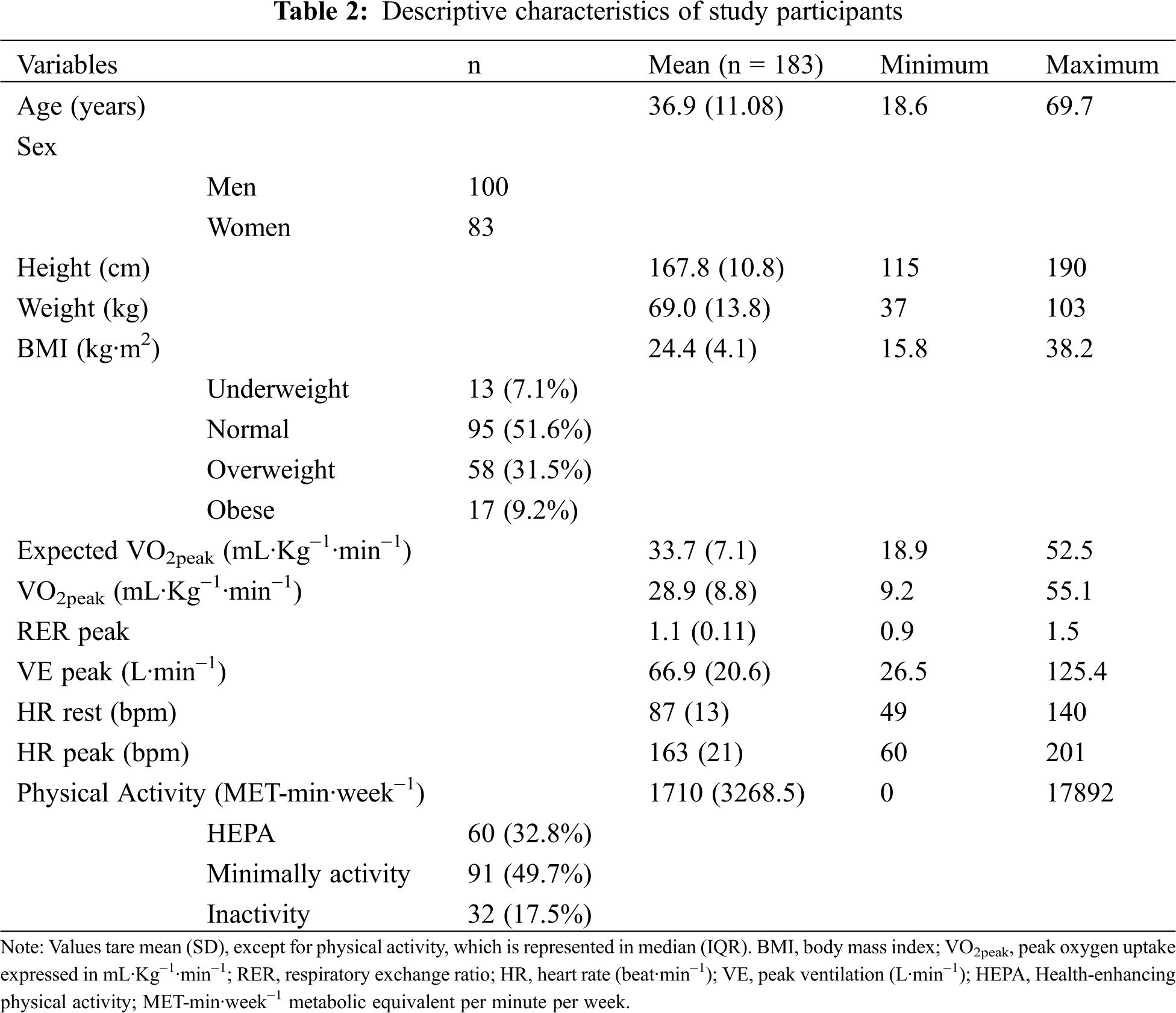
Median PA reports as assessed by IPAQ were 1710 (IQR 3268.5) MET-min·week−1, oscillating between 0 and 17892.0 MET-min·week−1 (Tab. 2). From 183 participants that answered the IPAQ questionnaire, 60 (32.8%) reported HEPA, 91 (49.7%) reported minimally active, and 32 (17.5%) reported inactivity (Tab. 2).
3.2 Peak Oxygen Uptake in Relation to Aerobic Capacity
The mean (SD) value of VO2peak for all participants was 28.9 (8.8) mL·Kg−1·min−1, ranging from 9.2 to 55.1 mL·Kg−1·min−1 (Tab. 2). On average, VO2peak of the participants was 13% lower than expected for healthy individuals, with values ranging from 58% lower to 53% higher. Even when excluding from the analysis the group of individuals with complex CHD (n = 82), results showed that participants with simple and moderate CDH still presented a reduced VO2peak compared to a healthy population (10% lower, ranging from 56% lower to 53% higher).
The AC of the participants (estimated by VO2peak) was linearly associated with PA reported by IPAQ, as shown in Fig. 1. After adjusting for sex and age, an increase of 1000 MET-min·week−1 was associated with an increase in VO2peak of 0.8 units (95% CI 0.4−1.2; p < 0.001). The association between PA and VO2peak was similar in men and women (p = 0.427) (Fig. 1). Results were very similar when focusing on participants without complex CHD (0.8 units of increase per 1000 MET-min·week−1 [95% CI 0.2−1.3; p = 0.008]; Fig. 1B.
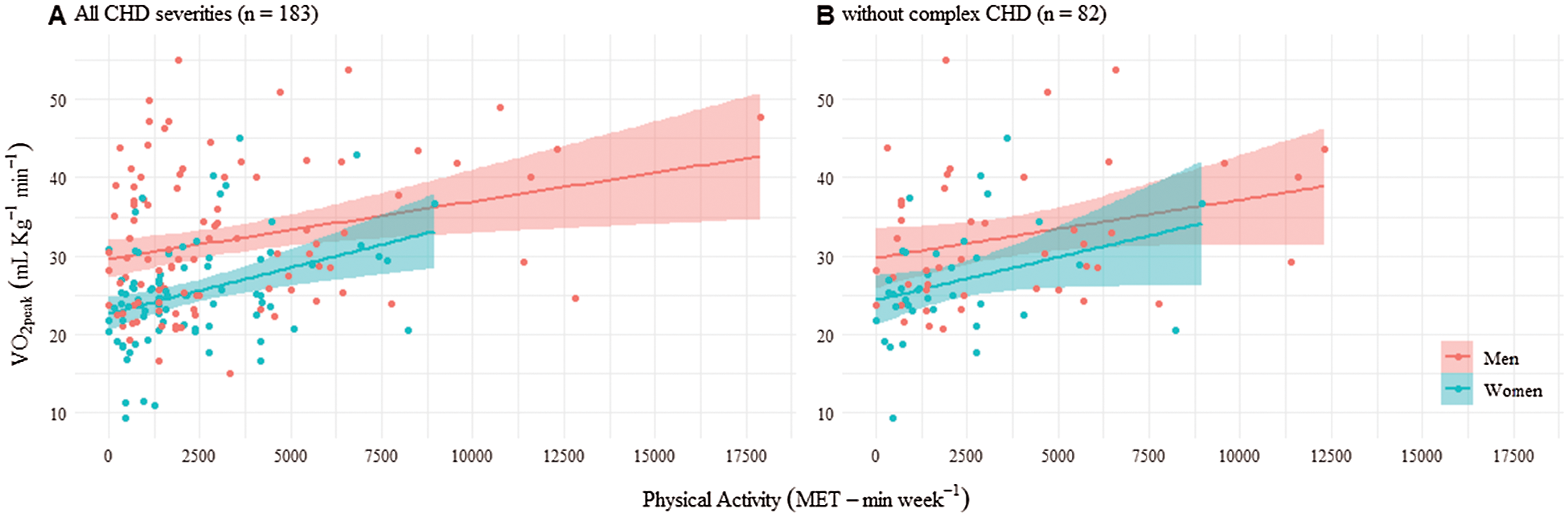
Figure 1: Aerobic capacity (VO2peak) depending on physical activity (MET-min·week−1) in men and women
Similarly, most individuals had an observed VO2peak lower than the expected according to their sex and age. However, the difference between observed and expected VO2peak was smaller as PA reported by IPAQ increased. An increase of 1000 MET-min·week−1 was associated with a 2.1% (95% CI 1.0–3.1%; p = 0.001) improvement in the value of VO2peak compared to the expected VO2peak in a healthy population (Fig. 2). Results were similar when considering only participants without complex CHD (1.5% improvement in the value of VO2peak compared to a healthy population [95% CI 0.01−3.0; p = 0.048] (Fig. 2B).
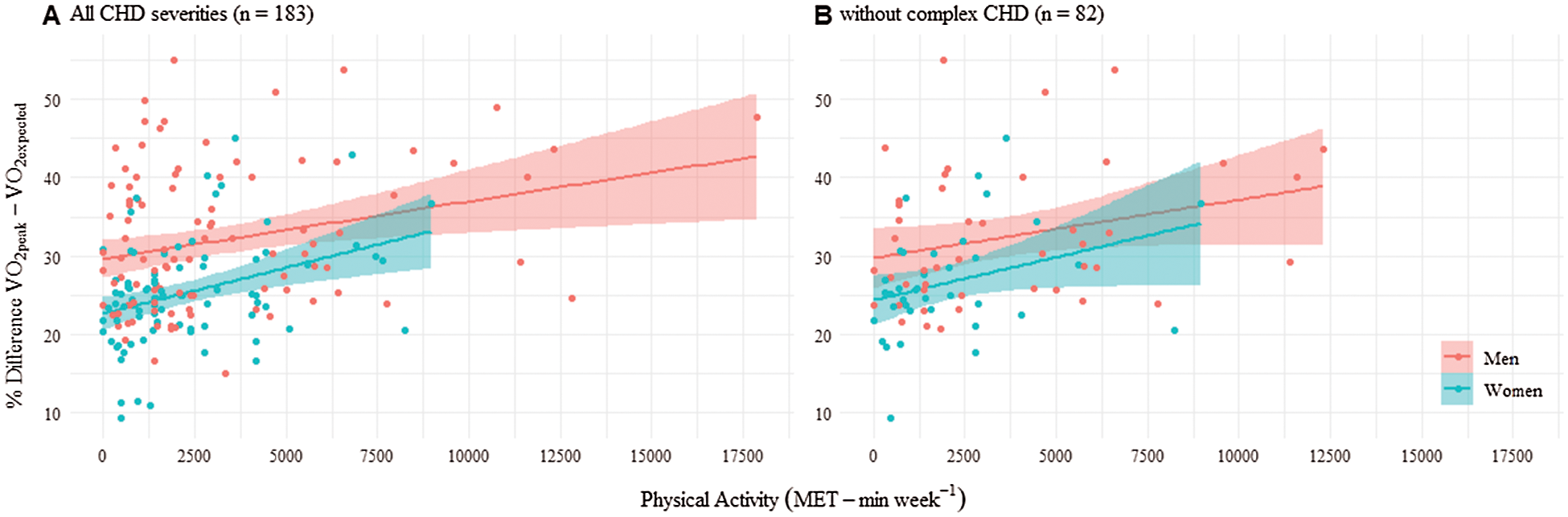
Figure 2: Percentage of differences between observed and expected VO2peak depending on physical activity (MET-min·week−1) in men and women
This study describes the association between the self-reported PA questionnaire (IPAQ-short form) and the objectively measured CPET in a cohort of adults, including both women and men, with CHD from Catalonia (Spain). The objective of the study was to evaluate the association between PA level and AC in adults with CHD and assess if increasing levels of PA could improve the AC of adults with different CHD.
Previous studies have pointed out the importance of CPET as a gold standard test to provide information such as AC, hemodynamic parameters, blood pressure and electrocardiographic parameters, as the information obtained in this test is a prognostic factor in CHD [14]. The cardiopulmonary parameter VO2peak has been demonstrated to be valuable in complementing other clinical information to optimize risk stratification for cardiac transplantation, medical device therapy (e.g., implantable cardioverter-defibrillator and cardiac resynchronization therapy) and for a variety of pre-surgical evaluations [24].
A study conducted by Diller et al. [25] assessed a group of 335 adults with CHD and suggested a VO2peak of 15.5 mL·Kg−1·min−1 as a cut-off point for predicting cardiac events. In our sample, only four female participants had VO2peak lower than the suggested cut-off point. These four participants had VO2peak between 9.2 and 11.3 mL·Kg−1·min−1. Our other participants, despite showing a generally lower VO2peak than a healthy population, they individually showed a higher value than the cut-off reported by Diller et al. [25].
Importantly, despite the reported lower VO2peak, the AC of people with CHD improved with increasing levels of PA, suggesting that VO2peak can reach values similar to the estimated for healthy people of the same age and sex at the highest levels of PA. These results are in agreement with Müller et al. [21] who demonstrated that adults with CHD that reported HEPA are less likely to present diminished exercise capacity. In total 80.2% of his sample were physically active, while in our sample 82.5% reported PA meeting the recommended activity per day to remain healthy (minimum of at least 600 MET-min·week−1). In our study, we demonstrate a positive linear association between PA and AC that emphasizes that increasing the practice of PA may improve the physical condition of people with CHD, even at lower AC levels.
According to Hambrecht et al. [26], to reduce the risk for cardiovascular disease in a healthy population, including improvement of endothelial function and reduction of lipid accumulation in the arterial wall, it is necessary a minimum of 1500 MET-min·week−1. In our study, 32.8% of the participants that reported HEPA were above this threshold of PA level for cardiovascular prevention. The relatively high median PA (1710 (IQR 3268.5) MET-min·week−1) in our population of study suggests that the adults with CHD may be receptive to advice about meeting the minimum recommendations for physical activity and regardless of the complexity of the CHD, the functional impact of the residual heart injury is less significant in terms of daily PA. However, while PA was on average higher than the threshold for cardiovascular prevention, AC was low compared to the expected VO2peak based on the Wasserman equation for healthy individuals [18], showing that, even at higher than recommended levels of PA, people with CHD tend to present reduced AC (even when only considering the group of simple and moderate CHD).
There are some limitations in this study. On one hand, these findings could be affected by the subjective nature of the instrument for measuring PA (i.e., self-reported questionnaires). An earlier study by Dua et al. [3] with 61 adults with CHD comparing self-reported with accelerometer-based measurements of PA levels describes that most participants had relatively low levels of PA, but that these low activity levels were not mirrored in the self-reported PA questionnaire. A possible explanation for this result could be that the final classification of PA level by self-report PA questionnaires (for example, IPAQ) use the total amount of MET-min·week–1. Therefore, high levels of PA could be reached at the expenses of light activities, such as walking, and not due to engagement in moderate to vigorous intensity PA activities. Such characteristics make it more difficult to interpret the effect of PA, and particularly those who are minimally active, on the AC of people with CHD. Another possible limitation of the study is the unbalanced number of participants classified with different CHD severities. Given the much lower number of patients diagnosed with a simple CHD, we could not adjust our model for CHD severity. The association between PA and AC could differ between CHD severities: for instance, it could be hypothesized that AC of people with simple CHD could improve faster with increasing PA. We argue, therefore, that this should be considered in future research.
The study emphasizes the importance of sports science professionals as a team with the health professionals when making decisions about the prescription and monitoring of PA in adults with CHD. It is very important to note that higher levels of PA in adults with CHD might improve their AC, and hence, PA prescription should be taken into consideration. Encouraging this collective to an active lifestyle, including PA to improve AC is the most important goal of prescription. However, despite the apparent capability of PA to improve the AC of people with CHD, it is important to highlight that (1) the prescription of PA aimed at adults with CHD should be individualized and tailored with controlled intensity when the objective is to improve AC and cardiovascular prevention; (2) the control of weight (under, overweight and obesity) in this population is part of their cardiovascular prevention, and could influence the CPET results, considering the relative VO2peak.
Finally, we emphasize that (1) the association between PA reported by IPAQ and AC estimated through VO2peak highlights that the combination between the self-reported questionnaire IPAQ and CPET can provide important clinical information that can be used to prescribe moderate or vigorous exercise, depending on the CHD, before beginning an exercise program, and (2) the IPAQ is an useful and valid instrument for estimating PA in clinical practice when there are no objective instruments available. According to the Guidelines for the Management of Adults with Congenital Heart Disease [27], daily PA should be monitored and controlled in order to improve the physical condition of this population.
AC in adults with CHD was generally low compared to the expected VO2peak values in a healthy population. However, we demonstrated higher levels of VO2peak associated with increasing levels of PA. These findings suggest that when the goal is to improve AC among adults with CHD, an increase in PA should be recommended. Future studies are needed to determine the intensity of PA appropriate to improve AC in adults with CHD of different severities.
Acknowledgement: We would like to thank Cors Units Foundation for the support in all the cardiopulmonary exercise testing, Dr. Gloria Navarro for and Dr. Oleguer Plana-Ripoll for providing feedback on the manuscript statistical analysis and review.
Author Contribution: KF and RS contributed to the conception or design of the study and drafted the manuscript. MD, MP contributed to the acquisition of data and critical revision of the article. GRO and MG contributed to the interpretation of data and critical revision of the article. LR contributed to the analysis, and interpretation of the data for the current study. NB critically reviewed the manuscript. All authors critically revised the manuscript, gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Data Sharing: Data sharing of anonymized data may be possible upon request to the corresponding author.
Funding Statement: Kelly Ferri is supported by a PhD grant by SUR of DEC Generalitat de Catalunya and European Union 2019FI_BI 00168. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The present study complies with the current laws of the country in which it was performed.
Conflicts of Interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this study.
1. Caruana, M., Grech, V. (2016). Lifestyle habits among adult congenital heart disease patients in Malta. Congenital Heart Disease, 11(4), 332–340. DOI 10.1111/chd.12366. [Google Scholar] [CrossRef]
2. Dean, P. N., Gillespie, C. W., Greene, E. A., Pearson, G. D., Robb, A. S. et al. (2015). Sports participation and quality of life in adolescents and young adults with congenital heart disease. Congenital Heart Disease, 10(2), 169–179. DOI 10.1111/chd.12221. [Google Scholar] [CrossRef]
3. Dua, J. S., Cooper, A. R., Fox, K. R., Graham, S. A. (2016). Physical activity levels in adults with congenital heart disease. European Journal of Cardiovascular Prevention & Rehabilitation, 14(2), 287–293. DOI 10.1097/HJR.0b013e32808621b9. [Google Scholar] [CrossRef]
4. Dua, J. S., Cooper, A. R., Fox, K. R., Graham, S. A. (2010). Exercise training in adults with congenital heart disease: Feasibility and benefits. International Journal of Cardiology, 138(2), 196–205. DOI 10.1016/j.ijcard.2009.01.038. [Google Scholar] [CrossRef]
5. Müller, J., Hess, J., Hager, A. (2012). Daily physical activity in adults with congenital heart disease is positively correlated with exercise capacity but not with quality of life. Clinical Research in Cardiology, 101(1), 55–61. DOI 10.1007/s00392-011-0364-6. [Google Scholar] [CrossRef]
6. Sandberg, C., Pomeroy, J., Thilen, U., Gradmark, A., Wadell, K. et al. (2016). Habitual physical activity in adults with congenital heart disease compared with age- and sex-matched controls. Canadian Journal of Cardiology, 32(4), 547–553. DOI 10.1016/j.cjca.2015.08.021. [Google Scholar] [CrossRef]
7. Fredriksen, P. M., Veldtman, G., Hechter, S., Therrien, J., Chen, A. et al. (2001). Aerobic capacity in adults with various congenital heart diseases. American Journal of Cardiology, 87(3), 310–314. DOI 10.1016/S0002-9149(00)01364-3. [Google Scholar] [CrossRef]
8. Mantegazza, V., Apostolo, A., Hager, A. (2017). Cardiopulmonary exercise testing in adult congenital heart disease. Annals of the American Thoracic Society, 14(1), S93–S101. DOI 10.1513/AnnalsATS.201611-876FR. [Google Scholar] [CrossRef]
9. Baumgartner, H., Bonhoeffer, P., de Groot, N. M. S., de Haan, F., Deanfield, J. E. et al. (2010). ESC Guidelines for the management of grown-up congenital heart disease. European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249. [Google Scholar] [CrossRef]
10. Amedro, P., Picot, M. C., Moniotte, S., Dorka, R., Bertet, H. et al. (2016). Correlation between cardio-pulmonary exercise test variables and health-related quality of life among children with congenital heart diseases. International Journal of Cardiology, 203, 1052–1060. DOI 10.1016/j.ijcard.2015.11.028. [Google Scholar] [CrossRef]
11. Serra-Grima, R., Doñate, M., Borrás, X., Rissech, M., Puig, T. et al. (2011). Cardiopulmonary stress testing in children who have had congenital heart disease surgery: Physical exercise recommendations during school hours. Revista Española de Cardiologia, 64(9), 780–787. DOI 10.1016/j.recesp.2011.05.007. [Google Scholar] [CrossRef]
12. Swan, L. (2000). Exercise prescription in adults with congenital heart disease: A long way to go. Heart, 83(6), 685–687. DOI 10.1136/heart.83.6.685. [Google Scholar] [CrossRef]
13. Bredy, C., Ministeri, M., Kempny, A., Alonso-Gonzalez, R., Swan, L. et al. (2018). New York Heart Association (NYHA) classification in adults with congenital heart disease: Relation to objective measures of exercise and outcome. European Heart Journal-Quality of Care & Clinical Outcomes, 4(1), 51–58. DOI 10.1093/ehjqcco/qcx031. [Google Scholar] [CrossRef]
14. Serra-Grima, R. (2015). Cardiología en el deporte: Revisión de casos clínicos basados en la evidencia. 3rd. ed. España: Elsevier. [Google Scholar]
15. Chaix, M. A., Marcotte, F., Dore, A., Mongeon, F. P., Mondésert, B. et al. (2016). Risks and benefits of exercise training in adults with congenital heart disease. Canadian Journal of Cardiology, 32(4), 459–466. DOI 10.1016/j.cjca.2015.12.007. [Google Scholar] [CrossRef]
16. Word Medical Association (2013). WMA Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. DOI 10.1001/jama.2013.281053. [Google Scholar] [CrossRef]
17. World Health Organization (2016). Obesity and overweight: Fact sheet. WHO Media. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
18. Wasserman, K., Hansen, J., Sue, D. (2004). Principles of exercise testing and interpretation. 4th. ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
19. International Physical Activity Questionnaire (2005). Guidelines for data processing and analysis of the international physical activity questionnaire (ipaqShort and long forms. [Google Scholar]
20. Craig, C. L., Marshall, A. L., Sjöström, M., Bauman, A. E., Booth, M. L. et al. (2003). International physical activity questionnaire: 12-Country reliability and validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. DOI 10.1249/01.MSS.0000078924.61453.FB. [Google Scholar] [CrossRef]
21. Müller, J., Amberger, T., Berg, A., Goeder, D., Remmele, J. et al. (2017). Physical activity in adults with congenital heart disease and associations with functional outcomes. Heart, 103(14), 1117–1121. DOI 10.1136/heartjnl-2016-310828. [Google Scholar] [CrossRef]
22. Voss, C., Dean, P. H., Gardner, R. F., Duncombe, S. L., Harris, K. C. (2017). Validity and reliability of the Physical Activity Questionnaire for Children (PAQ-C) and Adolescents (PAQ-A) in individuals with congenital heart disease. PLoS One, 12(4), e0175806. DOI 10.1371/journal.pone.0175806. [Google Scholar] [CrossRef]
23. The R Development Core Team (2019). R: A language and environment for statistical computing. 2020. [Google Scholar]
24. Guazzi, M., Adams, V., Conraads, V., Halle, M., Mezzani, A. et al. (2012). Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation, 126(18), 2261–2274. DOI 10.1161/CIR.0b013e31826fb946. [Google Scholar] [CrossRef]
25. Diller, G. P., Dimopoulos, K., Okonko, D., Li, W., Babu-Narayan, S. V. et al. (2005). Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation, 112(6), 828–835. DOI 10.1161/CIRCULATIONAHA.104.529800. [Google Scholar] [CrossRef]
26. Hambrecht, R., Niebauer, J., Marburger, C., Grunze, M., Kälberer, B. et al. (1993). Various intensities of leisure time physical activity in patients with coronary artery disease: Effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. Journal of the American College of Cardiology, 22(2), 468–477. DOI 10.1016/0735-1097(93)90051-2. [Google Scholar] [CrossRef]
27. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). AHA/ACC Guideline for the management of adults with congenital heart disease. Journal of the American College of Cardiology, 73(12), e81–e192. DOI 10.1016/j.jacc.2018.08.1029. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |