

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016031
ARTICLE
Cardiopulmonary Response to Exercise at High Altitude in Adolescents with Congenital Heart Disease
1Department of Cardiology, Bern University Hospital and University of Bern, Bern, Switzerland
2Center for Congenital Heart Disease, Department of Cardiology, Bern University Hospital and University of Bern, Bern, Switzerland
3Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland
4Department of Cardiology, Clinic Barmelweid, Barmelweid, Switzerland
*Corresponding Author: Jean-Paul Schmid. Email: jean-paul.schmid@barmelweid.ch
Received: 02 February 2021; Accepted: 11 March 2021
Abstract: Objective: To extend our knowledge on tolerance of acute high-altitude exposure and hemodynamic response to exercise in adolescents with congenital heart disease (AscCHD) without meaningful clinical or functional restriction. Methods: A symptom limited cardiopulmonary exercise stress test and a non-invasive cardiac output measurement during steady state exercise were performed at 540 m and at 3454 m a.s.l. Symptoms of acute mountain sickness were noted. Results: We recruited 21 healthy controls and 16 AscCHD (59% male, mean age 14.7 ± 1.1 years). Three subjects (2 controls, 1 AscCHD) presented light symptoms of acute mountain sickness (dizziness and headache). During the symptom limited exercise test at lowland, control subjects showed a significantly higher power to weight index (3.5 ± 0.6 W/kg vs. 3.0 ± 0.7 W/kg, p < 0.001), heart rate (188.8 ± 10.4 1/min vs. 179.4 ± 13.1 1/min, p < 0.050) and ventilation (92.8 ± 22.9 l/min vs. 75.4 ± 18.6 l/min, <0.050). At altitude, power to weight index only remained significantly higher in the control group (2.8 ± 0.6 W/kg vs. 2.6 ± 0.6 W/kg, p < 0.001). Pulmonary blood flow (PBF) at lowland showed no difference between the control and the AscCHD group, neither at rest (5.4 ± 0.8 l/min vs. 5.1 ± 0.9 l/min, p = 0.308), nor during the steady state test (10.6 ± 2.4 l/min vs. 10.5 ± 2.0 l/min, p = 0.825). At high altitude, PBF increased by 110% and 112%, respectively (12.8 ± 2.32 l/min vs. 12.5 ± 3.0 l/min; intergroup difference: p = 0.986). Conclusions: High altitude exposure was well tolerated in an unselected group of AscCHD. No significant difference in the cardio-pulmonary adaptation to a control group was noted during a steady state exercise. Symptoms of minor acute mountain sickness did occur, which should however not be misinterpreted as signs of hemodynamic maladaptation.
Keywords: Adolescents with congenital heart disease; high altitude; non-invasive cardiac output measurement; exercise
Spending time at high altitude for recreational activities such as skiing, ski touring, hiking or mountaineering is common practice already at young age in Switzerland. Exclusion from at least some of the less strenuous activities with their peers at high altitude may have an adverse impact on quality of life. Nevertheless, this is the case for a large number of adolescents with congenital heart disease (AscCHD), even those with simple congenital heart disease [1]. Unfortunately, the most recent recommendations for physical activity, recreational sport and exercise training in paediatric, adolescent or adult patients with congenital heart disease do not provide specific recommendations regarding activities at high altitude [2] and only few expert opinions on this topic have been published [3,4]. However, in real life, healthcare workers in mountainous regions are regularly confronted with AscCHD patients seeking advice about safety and tolerance of moderate to high altitude exposure for altitudes beyond 2,000 m above sea level (a.s.l.).
AscCHD generally suffer from reduced exercise tolerance when compared to healthy subjects of the same age [5]. One reason for this may be an altered hemodynamic response to exercise due to the underlying heart disease. However, it is known that central hemodynamic factors are not the only determinant of exercise capacity, but training status and in particular the condition of peripheral muscles play a pivotal role [6]. A frequent cause of physical deconditioning in these patients is overprotection by parents or caregivers during their childhood, sometimes leading to almost complete exercise abstinence [7]. Parents have to rely on clinicians’ advice, which sports activities their children are able to perform safely. Unfortunately, there is not much scientific evidence published for these recommendations.
Data about cardiopulmonary adaptation of AscCHD patients to exercise at high altitude are scarce [8], except for Fontan patients, which were published by our group previously [9]. The aim of this study was to extend our knowledge on tolerance of acute high altitude exposure and hemodynamic response to exercise in a group of unselected AscCHD patients.
We recruited 16 AscCHD patients with an age between 12 and 17 years in NHYA functional class I and II and 21 healthy controls. None of the patients was on medication. For classification of the severity of the disease, the definition of the 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease was used [10]. Exclusion criteria were NYHA functional class > II, cyanotic heart disease, univentricular physiology, hemodynamically relevant intracardial shunts, pulmonary hypertension, uncontrolled arterial hypertension (>180/100 mmHg at rest), valvular heart disease classified as severe, chronic obstructive pulmonary disease (FEV1 <60% of the predicted) and any disabling condition precluding the participation in the study.
The study protocol was reviewed and approved by the local Ethical Committee and complied with the ‘Declaration of Helsinki’ regarding investigations in humans. All participants provided written informed consent with the approval of their parents.
Within four weeks, all participants underwent a symptom limited cardiopulmonary exercise stress test (CPET) and a non-invasive cardiac output measurement (inert gas rebreathing method) at lowland (Bern, 540 m) and at an altitude of 3454 m (Jungfraujoch, Switzerland). The study setting has been described previously [11]. For the measurements at high altitude, patients started at 540 m by public transport and reached the ‘Jungfraujoch Research Station’ within 3 1/2 h. There, they spent four to five hours, including tests, sight-seeing and eating a meal. The tests were performed indoors within one to three hours after the arrival. Symptoms of acute mountain sickness (headache, poor appetite, nausea or dizziness/light headedness) were noted.
2.3 Symptom Limited Exercise Stress Test
The CPET with breath-by-breath gas exchange measurements was performed on a computer controlled, rotational, speed independent bicycle ergometer (Cardiovit CS-200 Ergo-Spiro; Schiller AG, Baar, Switzerland). Gases were calibrated each day taking into account the ambient barometric pressure (494 mm Hg). The flow sensor was calibrated before each test. A 12 lead ECG was recorded continuously.
The protocol consisted of a 1 min resting phase, followed by a 3 min reference phase during which patients cycled with a workload of 20 W and an incremental, symptom limited test phase with a 15 W/min. ramp protocol. Blood pressure was measured with a sphygmomanometer every 2 min. Measured gas exchange parameters were O2 uptake, CO2 output, tidal volume and breathing rate. From these data, minute ventilation and respiratory exchange ratio (CO2 output/O2 uptake) were calculated. Peak VO2 was defined as the highest VO2 achieved during the last 30 s of exercise.
2.4 Non-Invasive Cardiac Output Measurement
Cardiac output was measured by an inert gas rebreathing method using an infrared photoacoustic gas analyzer (Innocor®, Innovision A/S, Odense, Denmark), which allows to measure pulmonary blood flow. In the absence of a relevant shunt, pulmonary blood flow corresponds to cardiac output. Previous validations of the foreign gas rebreathing method showed its accuracy in the measurement of cardiac output both, at rest and during exercise [12,13].
Inert gas rebreathing measurements are sensitive to changes in barometric pressure, and the measured values at altitude needed a correction for the ambient pressure. Because cardiac output is calculated on the basis of pulmonary blood flow, assuming a capillary O2 saturation of 98%, which is not applicable at high altitude, we report the pulmonary blood flow values instead of an erroneously calculated cardiac output.
Non-invasive hemodynamic measurements were performed at rest and at a fixed workload in a steady state-situation, which was reached after 3–6 min. The predefined constant workload (Watt) was intended to lay at a moderate intensity below the anaerobic threshold (second lactate threshold) [14] and was determined by multiplying body weight (kg) by factor 1.5 for males and 1.2 for females. The same workload was applied for both measurements, at lowland and at high altitude. Heart rate, blood pressure and haemoglobin oxygen saturation were also measured.
Statistical analysis was performed using the SPSS® for Windows® software (version 25.0, SPSS® Inc., Chicago, Illinois, USA). Data are presented as mean ± SD, unless otherwise stated, categorical data are summarized as percentages. The comparison of means within and between groups were made using non-parametric tests. Significance was set at p < 0.05 for all statistical analyses.
3.1 Baseline and Patient Characteristics
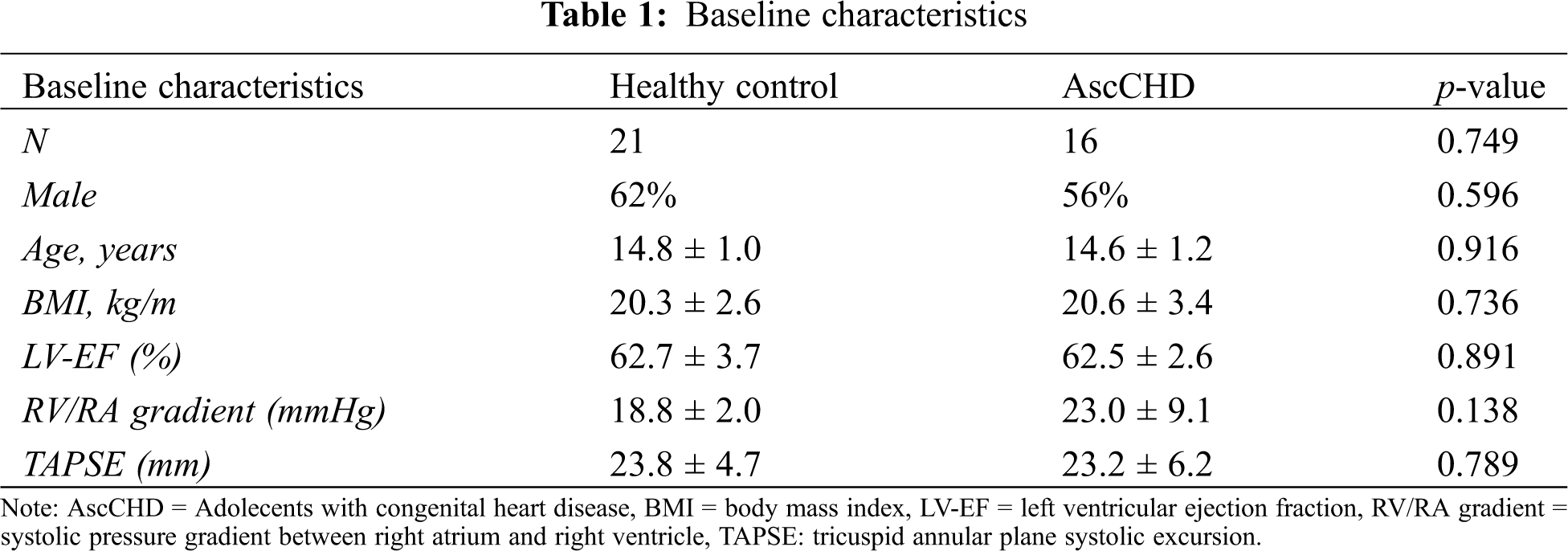
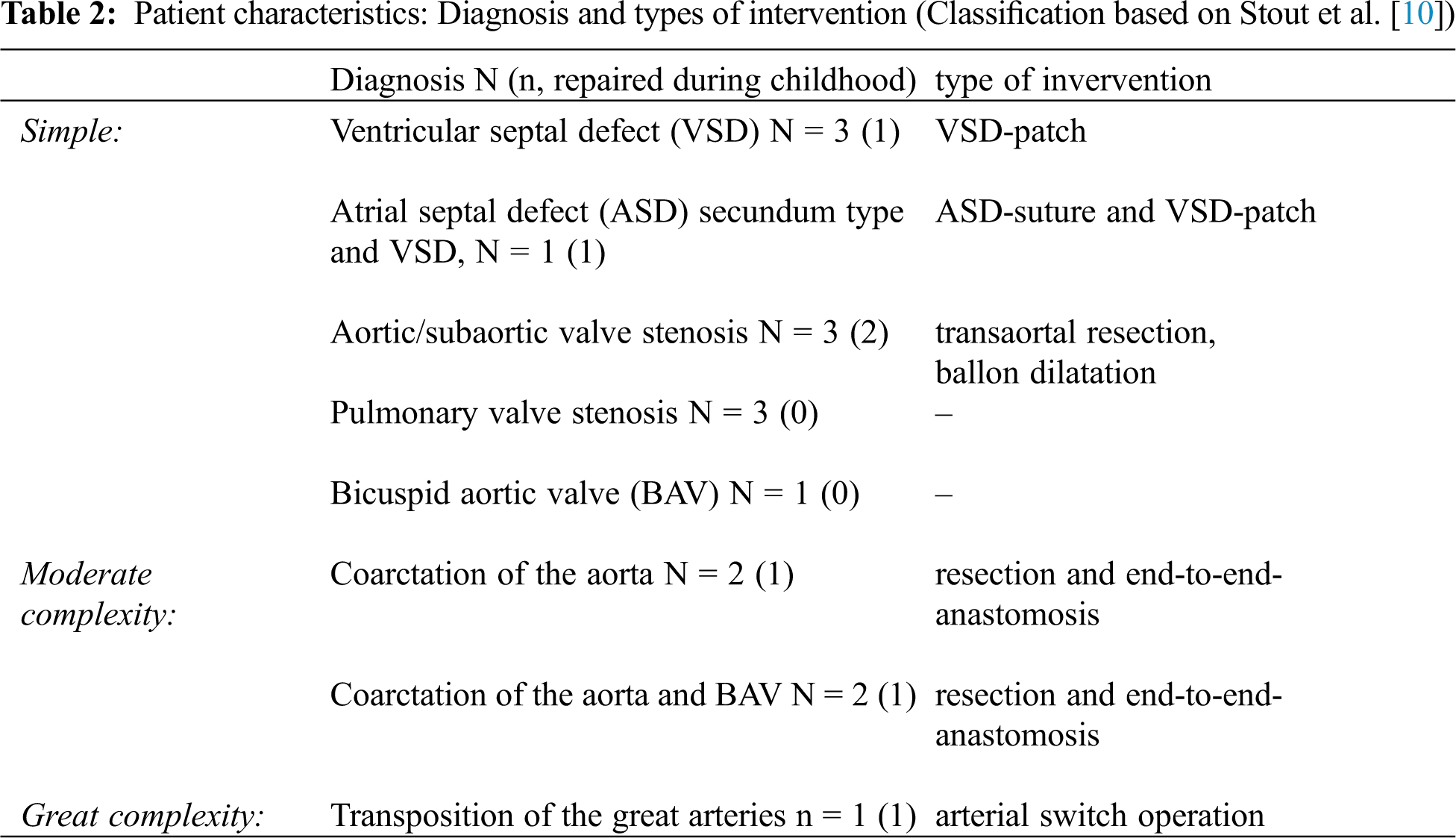
We recruited 21 healthy controls and 16 AscCHD, 59% male, mean age 14.7 ± 1.1 years (13–16 years), height 165.9 ± 9.4 cm (143–192 cm), weight 56.5 ± 10.3 kg (31.2–80.4 kg) and BMI 20.4 ± 2.9 kg/m2 (14.9–25.8 kg/m2). The baseline and patient characteristics of the two groups are summarized in Tabs. 1 and 2, respectively.
3.2 Symptoms of Acute Mountain Sickness
High altitude exposure was well tolerated. Three subjects (2 controls, 1 AscCHD) presented light symptoms of acute mountain sickness (dizziness and headache). None of the subjects, however, had to return to lowland prematurely.
3.3 Symptom Limited Exercise Stress Test
No differences for resting values were found between the control group and AscCHD patients at lowland and at high altitude (Tab. 3). Resting heart rate was significantly higher at altitude in both groups, whereas diastolic blood pressure was higher only in the control group at high altitude.
During the exercise test at lowland, peak power output in control subjects was 22% higher (p < 0.010), power to weight index 17% higher (p < 0.001), heart rate 5% higher (p < 0.050) and ventilation 23% higher (p < 0.050) compared to the AscCHD-group. No between-group differences were observed in peak VO2 at lowland. At high altitude, the same pattern was observed. The only difference which remained significant between the groups was the power to weight index (W/kg) in favor of the control group.
As expected, most parameters of exercise capacity decreased at high altitude. This included a decrease of power output (–21% in the control group, –12% in the AscCHD group), power to weight index (–20% in the control group, –13% in the AscCHD group) and peak VO2 (–19% in the control group, –11% in the AscCHD group) (Tab. 3 and Fig. 1). The same was true regarding O2-saturation. Consequently, also ventilatory efficiency was significantly lower, reflected by the higher values of VE/VCO2. Maximal ventilation showed a different behavior in the two groups, being slightly lower in the control group and higher in the AscCHD group, whereas peak heart rate was slightly higher at altitude in the two groups.
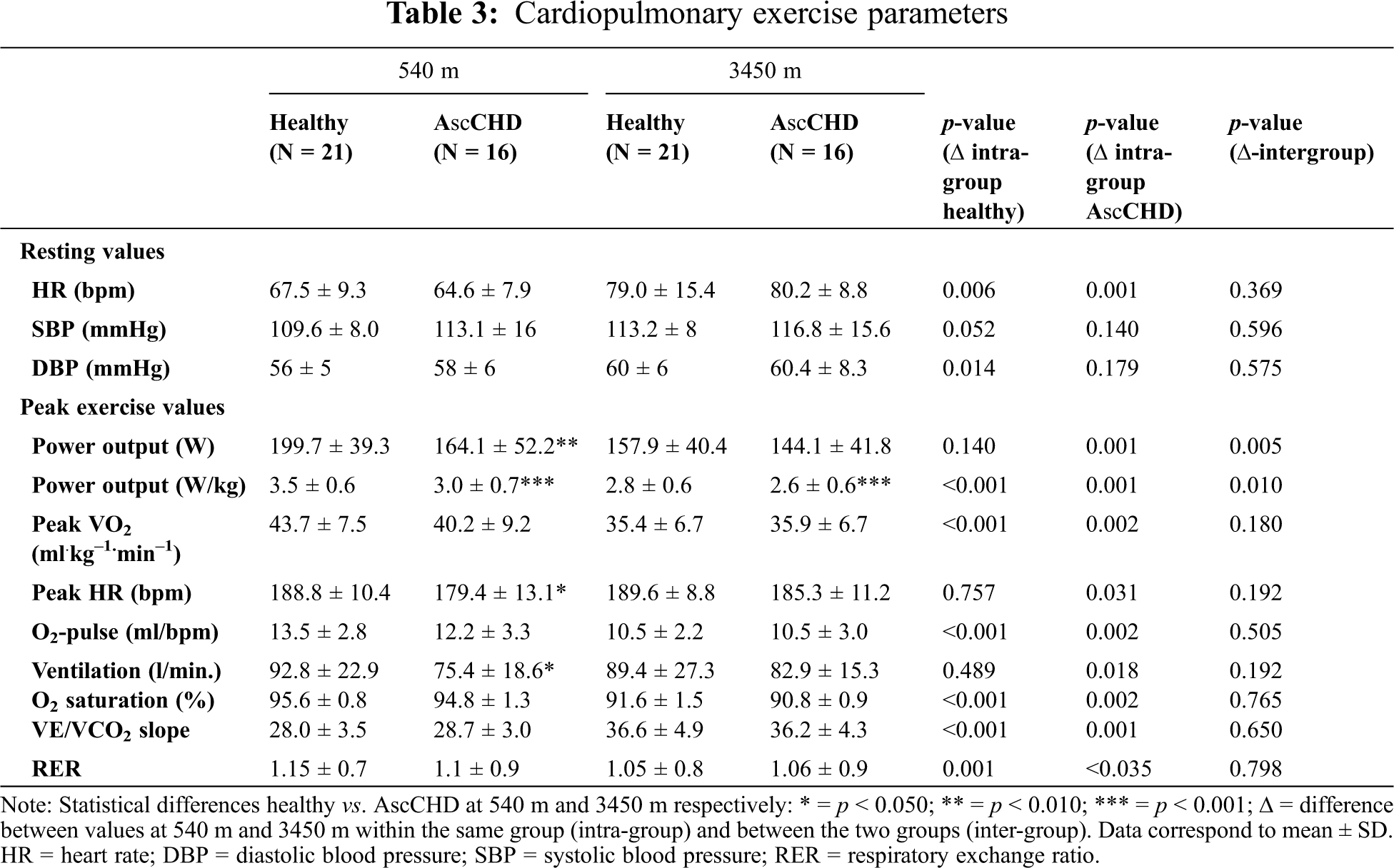
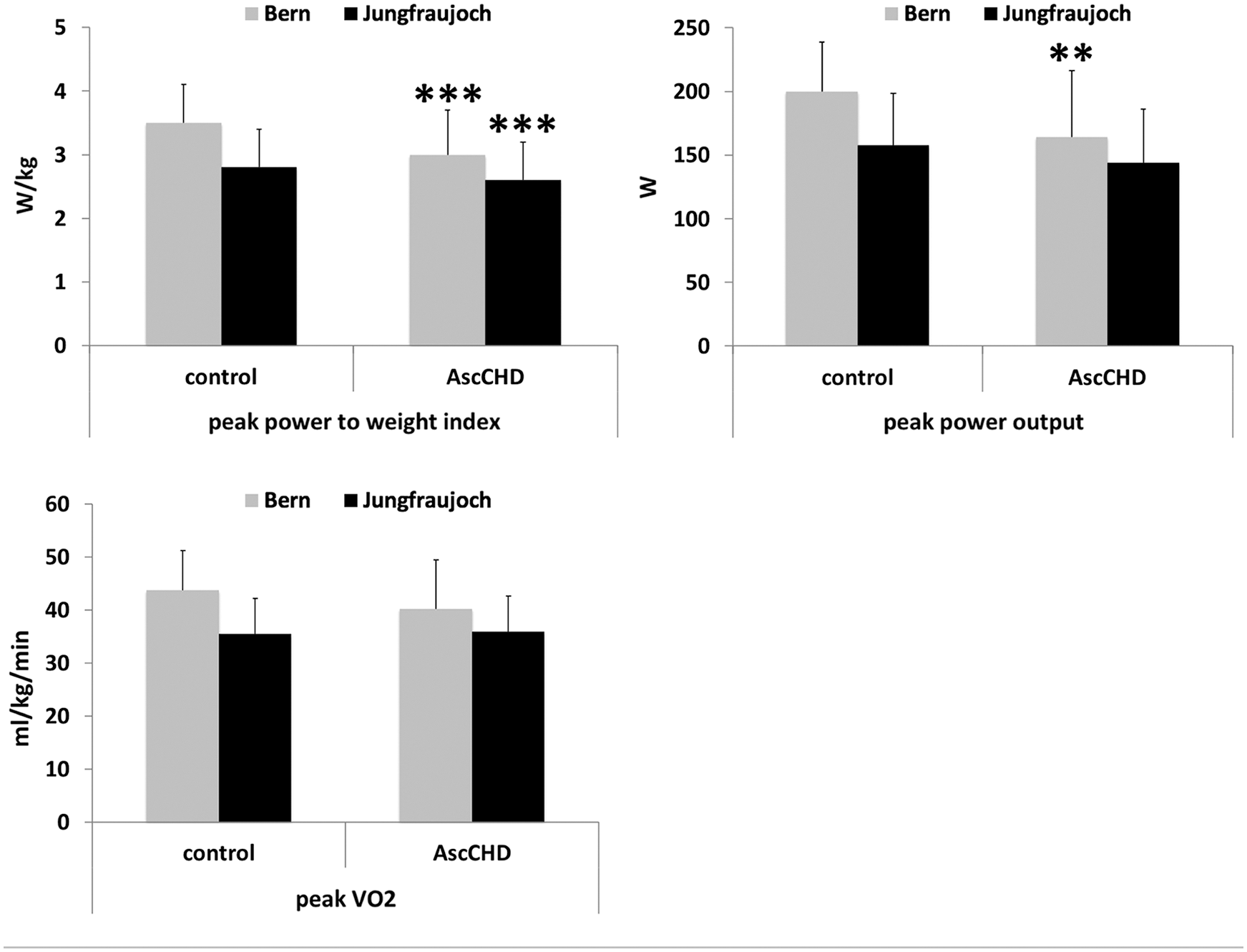
Figure 1: Exercise capacity parameters in control subjects and AscCHD patients in Bern (540 m) and at the Jungfraujoch (3450 m). Statistical differences control vs. AscCHD in Bern and on the Jungfraujoch, respectively: ** = p < 0.010; *** = p < 0.001
3.4 Non-Invasive Cardiac Output Measurements
At lowland, pulmonary blood flow (PBF) showed no difference between the control and the AscCHD group neither at rest (5.4 ± 0.8 l/min vs. 5.1 ± 0.9 l/min, p = 0.308), nor during the steady state test (10.6 ± 2.4 l/min vs. 10.5 ± 2.0 l/min, p = 0.825) (Fig. 2). During exercise it increased by 95% to 10.6 ± 2.4 l/min in the control group (p < 0.001) and by 105% to 10.5 ± 2.0 l/min in the AscCHD group (p = 0.001, inter-group difference: p = 0.825).
At high altitude, pulmonary blood flow at rest was 12% higher (6.1 ± 1.0 l/min) in the healthy group (p = 0.002) and 14% higher (5.9 ± 1.1 l/min) in the AscCHD group (p = 0.039) compared to lowland, without a significant difference in the change between the groups (p = 0.844). There was also no difference between groups during exercise, where pulmonary blood flow increased by 110% and 112%, respectively (12.8 ± 2.32 l/min vs. 12.5 ± 3.0 l/min; intergroup difference: p = 0.986) (Fig. 2).
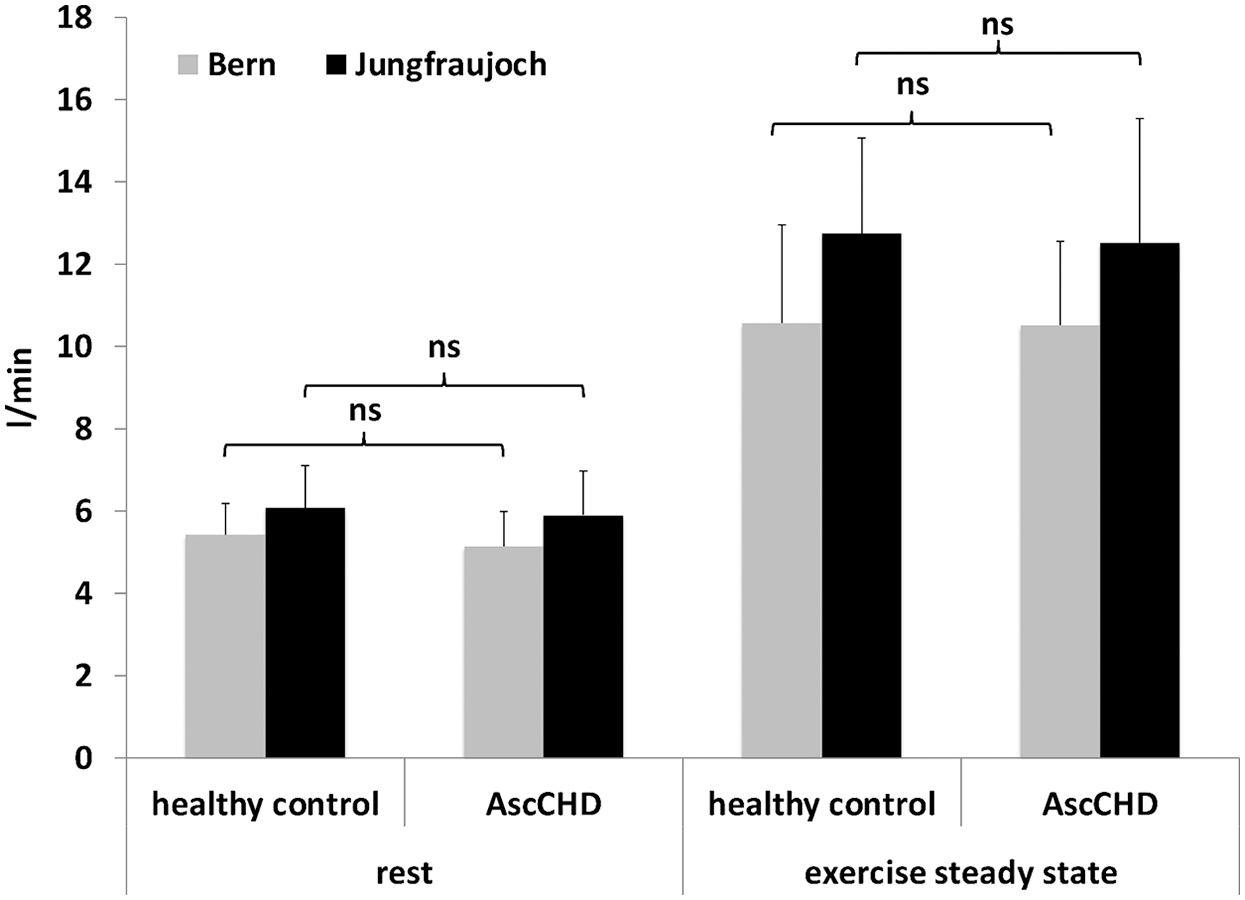
Figure 2: Pulmonary blood flow at rest and during submaximal exercise in healthy control subjects and AscCHD patients in Bern (540 m) and on the Jungfraujoch (3450 m)
Concerning heart rate, no difference was noticed between the two groups at rest at lowland (90 ± 13/min vs. 87 ± 12/min, p = 0.457) or at altitude (110 ± 17/min vs. 101 ± 15/min in the AscCHD group (p = 0.434). The same was true for heart rate during steady state exercise at lowland (133 ± 18/min vs. 134 ± 22/min, p = 0.825) or at high altitude (164 ± 13/min. vs. 164 ± 14/min, p = 0.138) (Fig. 3).
The rise in heart rate from rest to steady state exercise at lowland amounted to 49% in the control (p < 0.001) and to 54% in the AscCHD group (p = 0.003, ∆ intergroup: p = 0.825), at high altitude it rose by 49% (p < 0.001) and 62% (p = 0.001) in the two groups respectively (∆ intergroup: p = 0.138).
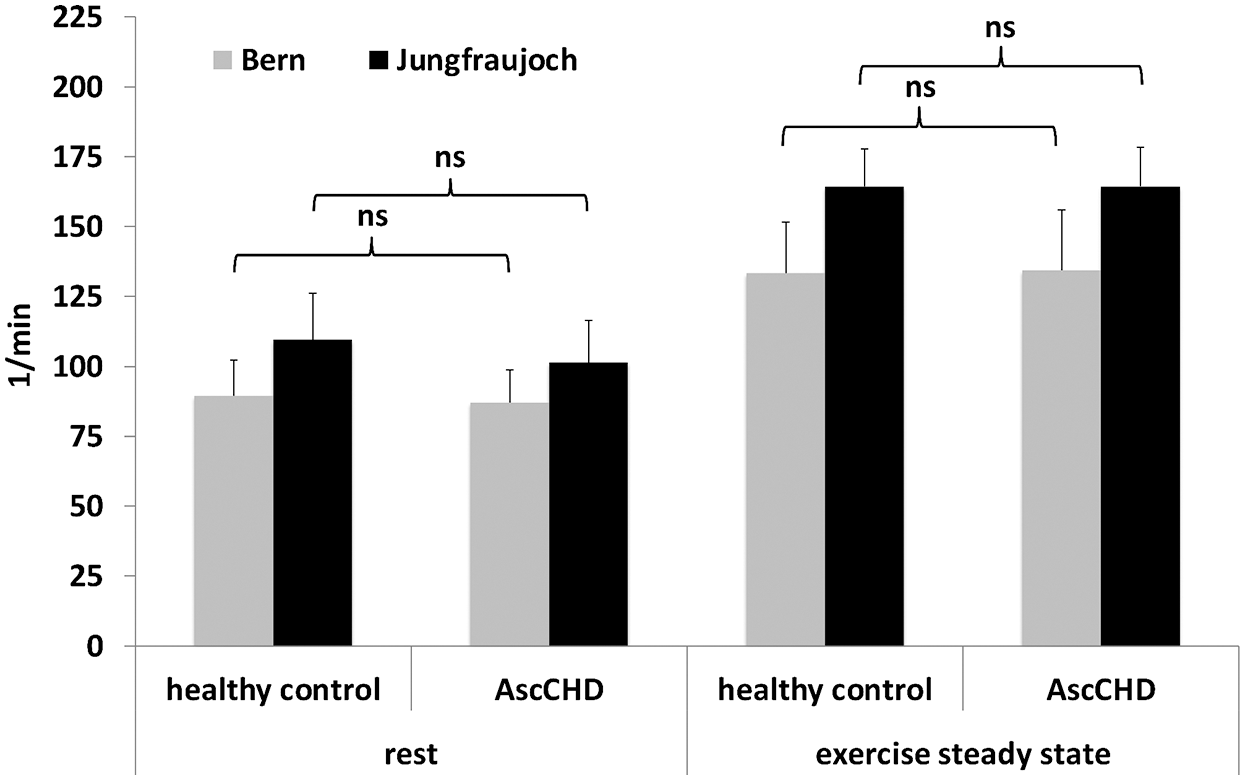
Figure 3: Heart rate at rest and during submaximal exercise in healthy control subjects and AscCHD patients in Bern (540 m) and on the Jungfraujoch (3450 m)
Stroke volume, as surrogate for cardiac contractility, was calculated by dividing pulmonary blood flow by heart rate.
At lowland stroke volume at rest was 62 ± 13 ml in the control group and 60 ± 12 ml in the AscCHD group (p = 0.660) (Fig. 4). During exercise it increased by 30% to 81 ± 19 ml (p = 0.002) in the healthy and by 34% to 80 ± 18 ml (p = 0.001) in the AscCHD group (∆ inter-group: p = 0.400).
At high altitude, stroke volume at rest was 12% lower compared to lowland in the control group (54 ± 14 ml, p = 0.205) and 1% lower (59 ± 12 ml, p = 0.959) in the AscCHD group. During exercise stroke volume increased by 45% to 79 ± 20 ml in the control group (p < 0.001) and by 29% to 77 ± 20 ml in the AscCHD group (p = 0.005, inter-group difference: p = 0.727).
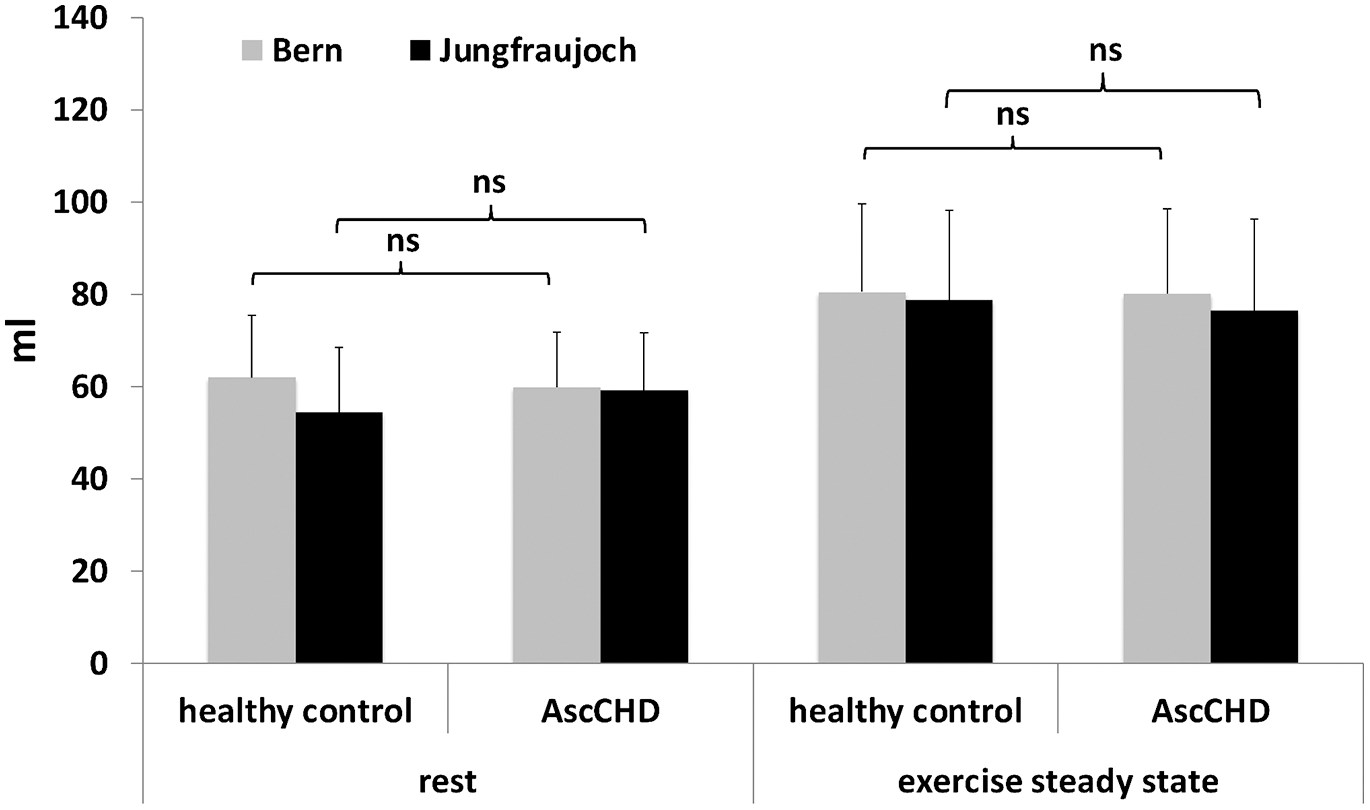
Figure 4: Stroke volume at rest and during submaximal exercise in healthy control subjects and AscCHD patients in Bern (540 m) and on the Jungfraujoch (3450 m)
At lowland systolic blood pressure at rest was 100 ± 9 mmHg in the control group and 107 ± 13 mmHg in the AscCHD group (p = 0.147). During exercise it increased to 121 ± 16 mmHg (p = 0.001) and 127 ± 22 mmHg (p = 0.004), respectively (inter-group difference p = 0.679).
At the Jungfraujoch resting blood pressure values were slightly higher with 107 ± 11 mmHg (p = 0.001) and 114 ± 17 mmHg (p = 0.001) for the healthy and AscCHD group, respectively. During exercise, systolic blood pressure rose to 125 ± 13 mmHg and to 132 ± 28 mmHg in the AscCHD patients (intragroup difference exercise systolic blood pressure: p = 0.285 and p = 0.307, respectively, intergroup difference: p = 0.536).
In this study, we included an unselected group of AscCHD patients without meaningful clinical or functional restriction. They tolerated high altitude exposure after a journey of 3 1/2 h and a stay at 3450 m a.s.l. for 3 to 4 h well, with a hemodynamic adaptation that was comparable to a population of healthy control subjects. Clinical symptoms in some isolated cases did not occur due to hemodynamic problems, but were related to mild mountain sickness, characterized by headache and dizziness. Acute mountain sickness can occur already at altitudes at or above 2000 m and may in susceptible persons develop as soon as 1 h after arrival, even though in general, an exposure of 6–12 h is needed. In severe cases, it can lead to changes in mental status, ataxia and pulmonary edema. No difference in the incidence is known between children and adults [15].
In another study on adolescents at the Jungfraujoch, Roach et al. [16] included 48 healthy non-acclimatized subjects aged 10 to 16 years. They were exposed to high altitude for 42 h and questioned about symptoms of acute mountain sickness with the Lake Louis questionnaire. Acute mountain sickness developed after 6 h and occurred in 25% of the subjects [17] compared with only 8% of our population. However, our subjects stayed only for 4 h. Nonetheless, an altitude exposure of 3450 m for a few hours was well tolerated in our adolescents with or without congenital heart disease and did not constitute a major problem. Symptoms were minor and did not necessitate an urgent descent. However, people should be aware of the symptoms of minor mountain sickness, which should not be mistaken for a hemodynamic problem related to the underlying heart disease.
The decrease in oxygen saturation (SpO2) due to the lower partial pressure of O2 at high altitude has to be compensated by an increase of cardiac output in order to maintain the adequate oxygen delivery to the tissues. In the acute phase, heart rate increases, as it was the case in our study population. Resting heart rate increased at altitude by 11 beats in the healthy subjects and by 15 beats in the AscCHD patients. For peak exercise, this marked heart rate difference between lowland and altitude disappeared (188/min vs. 189.6/min. in the healthy group and 179.4/min vs. 185.3/min in the AscCHD group). This might be explained by the fact, that heart rate reserve during maximal exercise was already exhausted at lowland and higher than maximal heart rate cannot be achieved during a symptom limited maximal exercise test. On the other hand, Lundby et al. [18] postulate a possible lowering of peak heart rate by high altitude, which however was not detected in our study.
The only significantly different exercise parameter at high altitude was peak power index (W/kg). Interestingly, the difference in peak VO2 was not significant, although power output is one of the main determinants of oxygen uptake. This would be in favor of only a minor, if ever, hemodynamic compromise of our AscCHD patients, as expected from the patient characteristics. However, it might reflect a slightly less developed peripheral muscle mass, potentially because of former avoidance of strenuous physical efforts. This fact could be an argument to encourage AscCHD patients to perform endurance activities at intensities limited by their symptoms of exhaustion and not by an overprotective attitude, including activities at high altitude.
An abnormal hemodynamic adaptation in AscCHD subjects would have discouraged us to recommend travel to and/ or exercise at high altitude in this patient group. However, in our study, both groups showed an adequate rise of cardiac output, measured as pulmonary blood flow, mainly achieved by a compensatory increase of heart rate. In this low risk AscCHD population with good exercise tolerance, NYHA functional class I & II, normal systolic left and right ventricular function and normal RV/RA gradient, the adaptation of exercise was the same as in the control group. As a consequence, no exercise restriction is required for these individuals, which is in line with the conclusions of the consensus paper of the Working Group of Grown Up Congenital Heart Disease and the Section of Sports Cardiology of the European Association of Preventive Cardiology (EAPC) (Tab. 4) [3]. Following their recommendations, in the absence of an aortic pathology or right-to-left shunt, if LV- and RV function and pulmonary artery pressure are normal and there is no evidence of arrhythmia, even sports with high static component and high intensity are possible.

The main limitation of the study is the small number of included patients, and the fact, that most of them corresponded to a case of simple or moderate complexity, reason why the findings cannot be generalized. Hemodynamic changes and thus adaptation to high altitude exposure can vary depending on the type of heart defect. This has to be taken into account when patients are advised. Based on our results, no heart defect specific recommendations can be given.
Validation of the inert gas rebreathing method showed that the method gives very accurate measurements of cardiac output, both at rest and during exercise [19]. However, its reliability at high altitude has not been validated.
Techniques based on gas exchange measurements are sensitive to changes in barometric pressure, which had to be taken into account for the CPET as well. Since the lowest possible pressure that could be entered into the software of the device accepted was 525 mmHg, while mean barometric pressure at 3450 m was 460 mmHg, we had to correct the measured values of pulmonary blood flow by a formula according to the ambient barometric pressure (formula see in the Appendix A). However, the correction factor was minor, amounting of a change in value of less than 1%.
A short stay up to four hours at an altitude of 3540 m in the setting of a single day excursion was well tolerated in an unselected group of AscCHD of simple or moderate complexity. This was true for light physical activity, but also for a symptom limited and an endurance exercise test. Symptoms of minor acute mountain sickness did occur, which should not be misinterpreted however as signs of hemodynamic maladaptation.
Data Statement: Parts of the data (non-invasive cardiac output measurement and data about acute mountain sickness) have been used for the non-published master thesis of the first-author at the University of Bern, Switzerland: Minder LM, Schmid JP. Höhenkrankheit und kardiopulmonale Anpassung an die Höhe bei Jugendlichen mit angeborenen Herzfehlern. University of Bern Master Thesis. 2015/04/01.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Giannakoulas, G., Dimopoulos, K. (2010). Exercise training in congenital heart disease: Should we follow the heart failure paradigm? International Journal of Cardiology, 138(2), 109–111. DOI 10.1016/j.ijcard.2009.06.024. [Google Scholar] [CrossRef]
2. Baumgartner, H., De Backer, J. (2020). The ESC clinical practice guidelines for the management of adult congenital heart disease 2020. European Heart Journal, 41(43), 4153–4154. DOI 10.1093/eurheartj/ehaa701. [Google Scholar] [CrossRef]
3. Budts, W., Börjesson, M., Chessa, M., van Buuren, F.,Trigo Trindade, P. et al. (2013). Physical activity in adolescents and adults with congenital heart defects: Individualized exercise prescription. European Heart Journal, 34(47), 3669–3674. DOI 10.1093/eurheartj/eht433. [Google Scholar] [CrossRef]
4. Parati, G., Agostoni, P., Basnyat, B., Bilo, G., Brugger, H. et al. (2018). Clinical recommendations for high altitude exposure of individuals with pre-existing cardiovascular conditions: A joint statement by the European Society of Cardiology, the Council on Hypertension of the European Society of Cardiology, the European Society of Hypertension, the International Society of Mountain Medicine, the Italian Society of Hypertension and the Italian Society of Mountain Medicine. European Heart Journal, 39(17), 1546–1554. DOI 10.1093/eurheartj/ehx720. [Google Scholar] [CrossRef]
5. Fredriksen, P. M., Veldtman, G., Hechter, S., Therrien, J., Chen, A. et al. (2001). Aerobic capacity in adults with various congenital heart diseases. American Journal of Cardiology, 87(3), 310–314. DOI 10.1016/S0002-9149(00)01364-3. [Google Scholar] [CrossRef]
6. Coats, A. J. (1996). The “Muscle Hypothesis” of chronic heart failure. Journal of Molecular and Cellular Cardiology, 28(11), 2255–2262. DOI 10.1006/jmcc.1996.0218. [Google Scholar] [CrossRef]
7. Reybrouck, T., Mertens, L. (2005). Physical performance and physical activity in grown-up congenital heart disease. European Journal of Cardiovascular Prevention and Rehabilitation: Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology, 12(5), 498–502. [Google Scholar]
8. Luks, A. M., Stout, K., Swenson, E. R. (2010). Evaluating the safety of high-altitude travel in patients with adult congenital heart disease. Congenital Heart Disease, 5(3), 220–232. DOI 10.1111/j.1747-0803.2010.00415.x. [Google Scholar] [CrossRef]
9. Staempfli, R., Schmid, J. P., Schenker, S., Eser, P., Trachsel, L. D. et al. (2016). Cardiopulmonary adaptation to short-term high altitude exposure in adult Fontan patients. Heart (British Cardiac Society), 102(16), 1296–1301. [Google Scholar]
10. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation, 139(14), e637–e697. [Google Scholar]
11. Schmid, J. P., Noveanu, M., Gaillet, R., Hellige, G., Wahl, A. et al. (2006). Safety and exercise tolerance of acute high altitude exposure (3454 m) among patients with coronary artery disease. Heart (British Cardiac Society), 92(7), 921–925. DOI 10.1136/hrt.2005.072520. [Google Scholar] [CrossRef]
12. Gabrielsen, A., Videbaek, R., Schou, M., Damgaard, M., Kastrup, J. et al. (2002). Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clinical Science, 102(2), 247–252. DOI 10.1042/cs1020247. [Google Scholar] [CrossRef]
13. Agostoni, P., Cattadori, G., Apostolo, A., Contini, M., Palermo, P. et al. (2005). Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: A new tool for heart failure evaluation. Journal of the American College of Cardiology, 46(9), 1779–1781. DOI 10.1016/j.jacc.2005.08.005. [Google Scholar] [CrossRef]
14. Binder, R. K., Wonisch, M., Corra, U., Cohen-Solal, A., Vanhees, L. et al. (2008). Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. European Journal of Cardiovascular Prevention and Rehabilitation: Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology, 15(6), 726–734. [Google Scholar]
15. Hackett, P. H., Roach, R. C. (2001). High-altitude illness. New England Journal of Medicine, 345(2), 107–114. DOI 10.1056/NEJM200107123450206. [Google Scholar] [CrossRef]
16. Roach, R., Bärtsch, P., Hackett, P., Oelz, O. (1993). The Lake Louise acute mountain sickness scoring system. In: Sutton, J. R., Houston, C. S., Coates, G. (eds.Hypoxia and molecular medicine, pp. 272–274. Burlington, VT: Queen City. [Google Scholar]
17. Bloch, J., Duplain, H., Rimoldi, S. F., Stuber, T., Kriemler, S. et al. (2009). Prevalence and time course of acute mountain sickness in older children and adolescents after rapid ascent to 3450 meters. Pediatrics, 123(1), 1–5. DOI 10.1542/peds.2008-0200. [Google Scholar] [CrossRef]
18. Lundby, C., Araoz, M., van Hall, G. (2001). Peak heart rate decreases with increasing severity of acute hypoxia. High Altitude Medicine & Biology, 2(3), 369–376. DOI 10.1089/15270290152608543. [Google Scholar] [CrossRef]
19. Agostoni, P. G., Wasserman, K., Perego, G. B., Guazzi, M., Cattadori, G. et al. (2000). Non-invasive measurement of stroke volume during exercise in heart failure patients. Clinical Science, 98(5), 545–551. DOI 10.1042/cs0980545. [Google Scholar] [CrossRef]
Appendix A
Correction of PBF at high altitude
Formula used by Innocor® (Innovision A/S, Odense, Denmark) software to determine
1:
2:
Conversion from ATP to STPD
3:
=
4:
Formula used by Innocor® (Innovision A/S, Odense, Denmark) software to determine PBF
Correction Factor (CF)
RH = Relative humidity
ATP = Ambient temperature and pressure
STPD = Standard temperature and pressure (273°K, 1 bar)
CF = Correction factor
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |