

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016054
ARTICLE
Clinical Effect of an Improved Post-Operative Feeding Protocol “in Transition” Infants of Congenital Heart Disease with Pulmonary Hypertension
1The Children’s Department of Cardiovascular and Thoracic Surgery, Children’s Heart Center, The Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, 325027, China
2Institute of Cardiovascular Development And Translational Medicine, the Second School of Medicine, Wenzhou Medical University, Wenzhou, 325027, China
*Corresponding Author: Qifeng Zhao. Email: zhaoqf1862@163.com
Received: 02 April 2021; Accepted: 04 June 2021
Abstract: Background: To achieve successful management of infants with congenital heart disease (CHD) together with pulmonary hypertension (PH), postoperative care, especially feeding care is vital in addition to surgery. Postoperative feeding is comprised of three stages: feeding in the intensive care unit, feeding in the general ward and family feeding, in which the general ward is considered as the “transitional stage”. At present, there is little research on the optimal mode of feeding care for the transitional stage, and there is no universally recognized and accepted protocol. Methods: We retrospectively analyzed 114 CHD infants with PH who underwent family-centered (FC) feeding care from July 2017 to December 2018, and prospectively studied 122 CHD infants with the same baseline level who adopted the improved mode, nurse-parent-driven (NPD) feeding mode from January 2019 to June 2020. The feasibility and efficacy of NPD as a “transitional” feeding nursing mode in CHD infants with PH were compared with the FC cohort by observing and analyzing the stress of family caregivers, feeding-related complications, the proportion of breastfeeding, improvement of nutritional status, acquisition of knowledge and skills of feeding care, inpatient’s satisfaction rating and prognosis. Results: When compared with the FC feeding care, the NPD mode significantly reduced the burden of family caregivers, improved the rate of feeding care knowledge and skills and inpatient’s satisfaction rating, reduced the incidence of improper feeding-related complications, and enhanced the proportion of breastfeeding and nutritional status of infants at the “transitional stage” (all P < 0.05). The self-assessment score of care ability of family caregivers and weight gain of children in the NPD group were significantly higher than those in the FC group (all P < 0.05) during the follow-up. Conclusions: As a transitional mode of feeding in CHD infants with PH, NPD feeding care is superior to the conventional FC mode, which therefore can be adopted as a standard protocol in clinical practice.
Keywords: Congenital heart disease; pulmonary hypertension; postoperative feeding care; infant
Abbreviations
| SD: | Standard deviation |
| IQR: | Interquartile range |
| CICU: | Cardiac intensive care unit |
| NEC: | Necrotizing enterocolitis |
| LOS: | Length of stay |
| VSD: | Ventricular septal defect |
| ASD: | Atrial septal defect |
| PDA: | Patent ductus arteriosus |
| PFO: | Patent foramen ovale |
| APSD: | Aorticopulmonary septal defect |
| PAPVC: | Partial anomalous pulmonary venous connect |
| AVSD: | Atrioventricular septal defect |
| DORV: | Double- outlet right ventricle |
| IAA: | Interruption of aortic arch |
| CoA: | Coarctation of the aorta |
| TGA: | Transposition of the great arteries |
| TAPVC: | Total anomalous pulmonary venous connect |
| MI: | Mitral insufficiency |
| CHD: | Congenital heart disease |
| PH: | Pulmonary hypertension |
| ND: | Nursing-driven |
| FC: | Family-centered |
| NPD: | Nurse-parent-driven |
| NPDFC: | Nurse-parent-driven feeding care |
| mPAP: | mean pulmonary artery pressure |
| RASCHDS: | Risk adjustment for surgery for congenital heart disease score |
| EN: | Enteral nutrition |
Congenital heart disease (CHD) with pulmonary hypertension (PH) is common and the proportion of CHD infants with PH under 1-year-old undergoing surgical treatment has increased in recent years, demonstrating a trend of low age, low weight and complexity of cases. Studies have shown that the preoperative nutritional status of these infants is significantly poorer than that of healthy children of the same age [1], with more than half of the children with CHD suffer from severe malnutrition [2] and the incidence of growth retardation is approximately 45.4% [3]. Surgery remains the mainstay of treatment for infants with CHD and PH that carries significant risks. Thus, postoperative nursing care is critical in the course of the treatment [4]. Despite recent advances in surgical and postoperative management, the nutritional support for infants with CHD is often suboptimal [5]. Recently, perioperative nutritional support has drawn significant attention and has become a hot topic of discussion in the clinical management of CHD [6]. In particular, among infants with CHD, perioperative nutritional management requires a multidisciplinary team approach, with evidence showing that nutritional intervention improves prognosis by adopting the nurse-directed care on these children [7] during postoperative feeding.
CHD infants with PH, even newborns, have different degrees of abnormality in the cardiopulmonary function. In the perioperative phase, patients may suffer from consequences of endotracheal intubation, extracorporeal circulation and surgery, which therefore requires timely management and care to prevent potential complications or even death [8]. Furthermore, infants with CHD and PH are often complicated by having other congenital malformations, including tracheal stenosis, cleft lip and palate, esophageal atresia, and anal atresia. Moreover, postoperative feeding may be compromised by several other factors, such as recurrent laryngeal nerve injury from surgery leading to sucking or swallowing dysfunction, nausea or vomiting as a result of medications, etc.
Postoperative feeding is comprised of three stages: feeding in the intensive care unit, feeding in the general ward and family feeding following discharge from the hospital. Nurse-driven feeding care (ND) is the main mode of feeding in the ICU [9,10], while family-centered feeding care (FC) is adopted after discharge from the hospital. Feeding in the general ward is considered as the transitional stage, bridging between the care provided by the intensive care unit and the family. To date, clinical research is scarce on the feeding care mode during this transitional period with no universally accepted standardized protocols or guidelines. Although the family-centered nursing mode has many advantages [11], family caregivers often feel anxious in caring for postoperative infants, leading to poor sleep, and insufficient feeding-related knowledge and skills increase the risks of adverse events. Thus, nursing intervention on family caregivers optimizes the nursing effect and plays a key role in preventing the occurrence of adverse events among infants with CHD [12]. This study aimed to investigate the feasibility and efficacy of NPD as a transitional feeding mode in comparison with the conventional FC mode by observing and analyzing several outcomes, including feeding difficulties, improvement of nutritional status, feeding-related complications, prognosis, the stress of family caregivers, sleep disturbances, acquisition of knowledge and skills of feeding care, and satisfaction of caregivers.
2.1 Study Design and Patient Selection
This was a cohort study exploring the effects on nutritional status and prognosis of CHD infants with PH “in transition” following the implementation of an improved post-operative feeding mode. A retrospective analysis was performed on 114 CHD infants with PH who were fed using the conventional mode, FC from July 2017 to December 2018. Then, a prospective study was conducted on 122 CHD infants with similar conditions who were fed using the improved protocol, NPD from January 2019 to June 2020. CHD children older than 1 year, who did not receive cardiopulmonary bypass or did not suffer from PH were excluded. This study was approved by the Institutional Review Board of the Yuying Children’s Hospital of Wenzhou Medical University and informed consent was obtained from the parents of all the infants. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
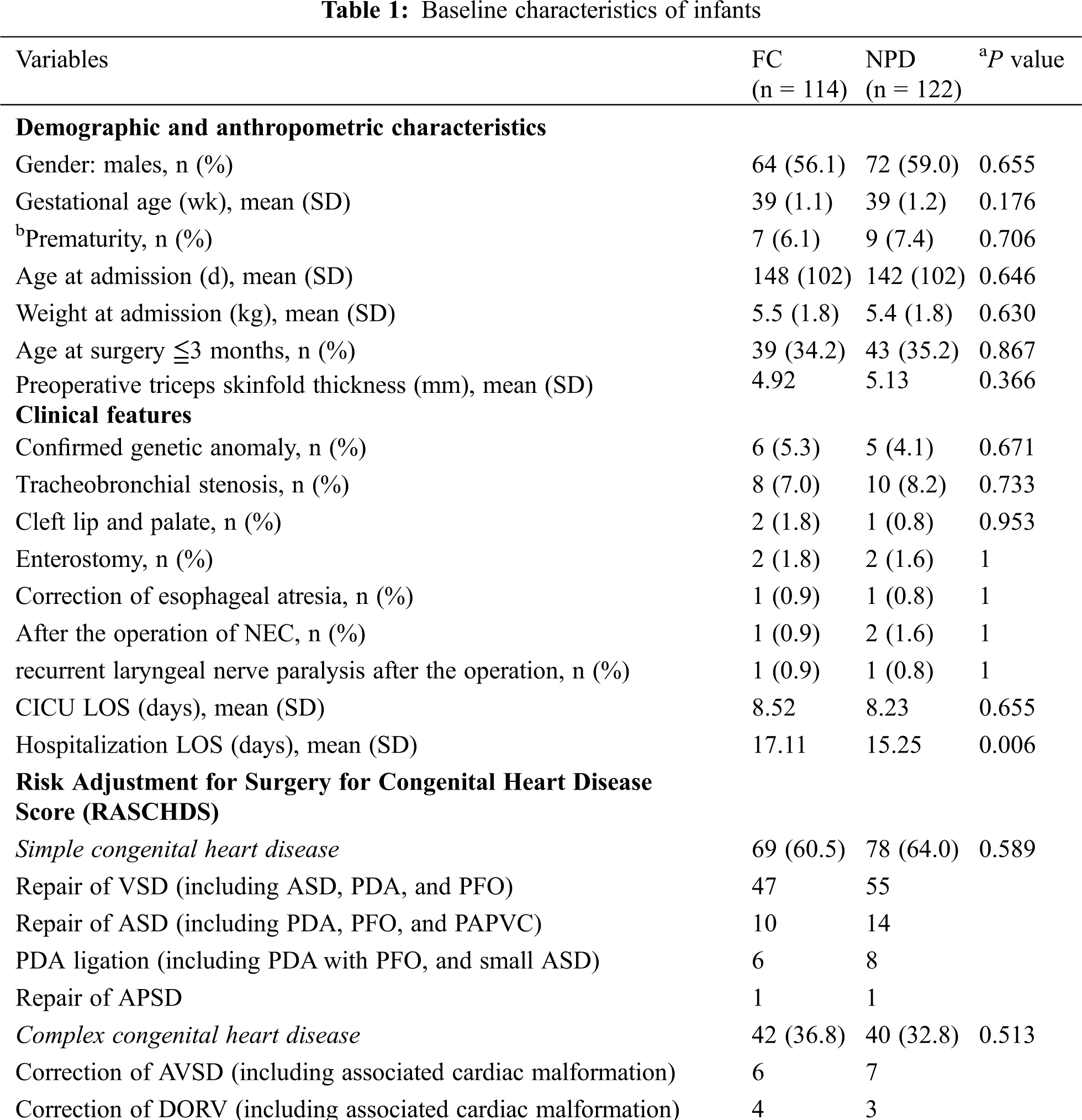
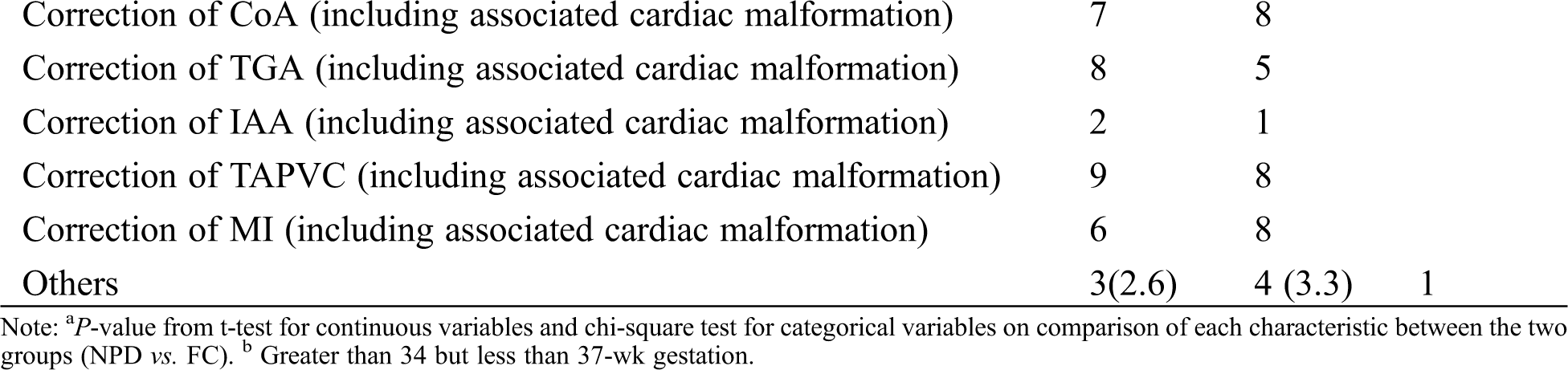
All patients with CHD were diagnosed by echocardiography, computed tomography angiography, chest X-ray and ECG, while PH was defined as mean pulmonary arterial pressure of over 26 mmHg as evaluated by Ultrasonography. A standardized data extraction tool was employed to collect demographic and anthropometric characteristics (gender, gestational age, prematurity, age and weight on admission, age at surgery, preoperative triceps skinfold thickness), clinical features (confirmed genetic anomaly, tracheobronchial stenosis, cleft lip and palate, enterostomy, following an operation for necrotizing enterocolitis, recurrent laryngeal nerve paralysis after the operation, length of stay in CICU, length of stay in the hospital) and risk adjustment for surgery for CHD. The baseline characteristics of infants of the FC and NPD groups were outlined in Tab. 1.
2.2 Feeding Risk Factor Scale for Infants in the Transition Period
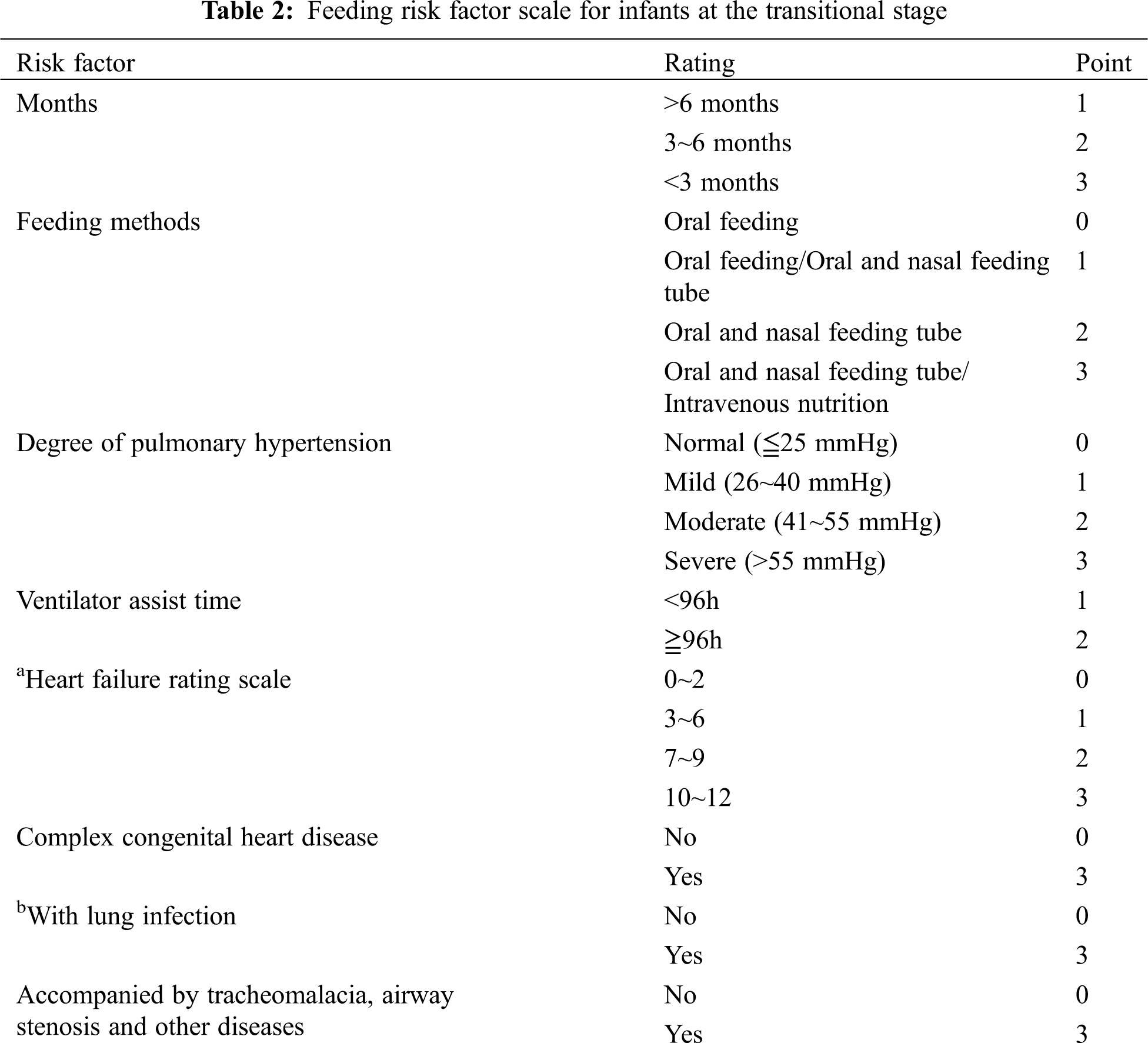
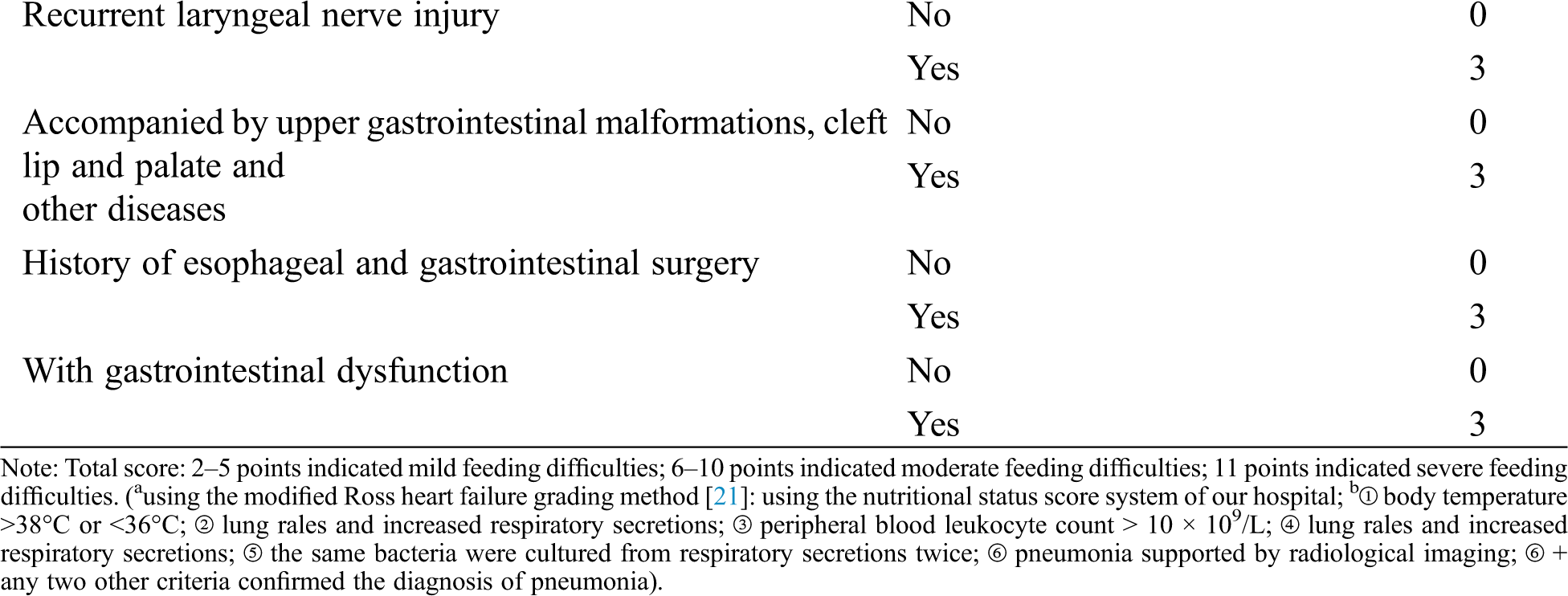
Studies have shown that optimal nutritional status shortens the time of mechanical ventilation, reduces the incidence of infection, improves wound healing, promotes the rehabilitation of children, and plays an important role in reducing complications and mortality [13,14]. The guidelines for clinical practice of pediatric enteral and parenteral nutrition support at home and abroad have indicated that the nutritional risk screening should be carried out for hospitalized infants, and the nutritional assessment and nutritional intervention should be conducted for children with nutritional risk to improve the nutritional status of children and promote the recovery of disease [15,16]. At present, several screening tools for nutritional risk assessment in children with CHD have been described [17–20]. However, in China, the mature pediatric feeding and nutritional risk screening scales have been used widely but it lacks pertinence. Based on our clinical practice, the “transitional stage” infant feeding risk factor score system was developed (see Tab. 2). From the score, an individualized and refined intervention was instituted to form a suitable postoperative feeding protocol for Chinese infants with CHD in order to improve the nutritional level and resistance of infants, reduce the occurrence of postoperative complications, and shorten the postoperative recovery time.
2.3 Feeding Ability Score of Family Caregivers at “Transitional Stage”
In addition to abnormal hemodynamics, insufficient energy intake, impaired nutrient absorption capacity, excessive nutrient consumption, various treatments and the subjective behavior of feeders can also lead to malnutrition in children with CHD [14,22,23]. The adverse effects of malnutrition on the disease and the prognosis of infants with CHD have been overlooked by clinical workers. Studies have shown that increasing postoperative energy supply improves malnutrition and clinical outcomes in infants with CHD [24]. Therefore, family caregivers play an integral role in improving the nutritional status of children with CHD. In the transitional stage, we also designed a feeding ability score system concerning family caregivers (see Tab. 3). Combined with the infant feeding risk score described above, targeted management could be formulated and instituted by nurses

2.4 Description of NPD Feeding Care (NPDFC) Protocol
Feeding risk factor scale for infants at the transitional stage
The feeding difficulty of CHD infants with PH after the operation was statistically analyzed, and a feasible risk factor scoring system was formulated with reference to relevant literature and analysis results.
Feeding ability scale of family caregivers at the transitional stage
The data of the main caregivers of CHD infants with PH were collected, specific situations of family caregivers and their executive ability were recorded, and a scoring system was developed.
Establishment of NPDFC intervention team
A standardized nursing intervention management team for postoperative feeding and care of CHD infants with PH at “transitional stage” was established. The head nurse of the Department was the supervisor for the quality of the whole process. There were 2–3 lead nurses managing CHD infants in the transitional stage. These nurses were N3 level nurses or above (bachelor degree or above) or nurse-in-charge. They had tremendous clinical experience and knowledge and skills of infant feeding, good communication and education management ability, and received professional training in CHD postoperative nutrition and feeding knowledge. The team also consisted of attending doctors and nutritionists, who were responsible for the nutritional assessment and monitoring of children.
NPDFC intervention
Based on both the feeding risk factor score for infants and the feeding ability score of family caregivers, various individualized and standardized feeding programs and practice processes were developed in advance.
Interview of the family caregiver, evaluation of feeding ability and provision of early guidance
1–2 days before transferring the infant to the ward, the lead nurse determined the most suitable caregivers, interviewed them and evaluated their feeding ability. According to the degree of acceptance of feeding care knowledge and grading results, a series of evidence-based intervention methods were employed as early guidance to appreciate the general situation, nutritional status, feeding stage, and feeding difficulties of children. Then, a standardized, individualized feeding program and feeding intervention were introduced. Audio, manual and other materials were also used to facilitate preliminary understanding of various corresponding methods among family caregivers. The early guidance training of caregivers was as important as standardized training of medical staff.
Assessment of infants at “transitional stage”
The lead nurses conducted a comprehensive handover with the ICU staff to appreciate the current general situation of the children, including vital signs, tracheal intubation time, mode of nutritional intake, feeding ability, and nutritional status of the infants. In addition, with the assistance from the ICU nurses and attending doctors, risk factors associated with infant feeding were evaluated to obtain the score and grading results.
Joint participation of lead nurses and family caregivers in the feeding program
On the day of discharging from the ICU, the lead nurse and family caregivers received the infants together. The feeding scheme that was individualized and standardized, was determined based on the grading results. The lead nurses played a vital role in guiding, emphasizing the optimal time and way of feeding, and assisting in the implementation of the feeding scheme.
Highlighting the importance of breastfeeding
Lead nurses advocated and encouraged breastfeeding, addressed caregivers’ concerns regarding breastfeeding and guided the correct implementation. During the infants’ ICU stay, the lead nurse could provide breast milk to the ICU nurses through acceptable ways as a form of indirect breastfeeding support, which was particularly important for premature infants, newborns and infants with gastrointestinal diseases.
Roles of lead nurses at several key points
Lead nurses played an active role in every turning point of feeding or transitional stage during the change of the feeding mode. They evaluated the condition and feeding situation of the infants, guided the correct feeding skills, ensured that the feeding measures were in place, and facilitated the family caregivers feeding the infants correctly, competently and confidently.
Implementation of NPDFC mode at different levels
According to the grading results of infant feeding risk factor score and family caregiver feeding ability score at the transitional stage, different levels of targeted nursing intervention were taken, including the number of intervention personnel, implementation requirements, intensity and frequency of implementation, tools of intervention implementation, the content of the nursing plan, supervision and evaluation of personnel. These aimed to reflect the individualization, refinement, efficiency and effectiveness of standardized nursing requirements. At the transitional stage, the nurses in the general ward were different from those in the ICU, the proportion of nursing staff for children and the nursing grade were not comparable to those in ICU. With targeted nursing intervention, the limited nursing resources could be more effectively distributed while ensuring the quality of nursing care.
2.4.3 Continuous Improvement and Achievement of Goals
Continuous quality improvement
During the implementation of NPDFC, feeding difficulties and care ability were dynamically evaluated, difficulties identified were evaluated and resolved timely, adverse factors of feeding were removed, and the safe implementation of feeding care measures was ensured.
Achievement of feeding goals
Measures were taken to effectively assist caregivers in transitioning to different feeding methods, relieving their stress, establishing a more confident state of feeding care, achieving the goal of oral feeding, providing support for caregivers after discharge and eventually ensuring full competency.
2.5 Comparisons between the Improved Protocol (NPD) and the Conventional Protocol (FC)
The advantages and disadvantages of NPD and FC were outlined in Tab. 4.

2.6 Data Presentation and Statistical Analysis
Continuous variables were presented as means with standard deviations or standard errors. Categorical variables were presented as frequencies and proportions. Descriptive comparisons included t-test were used for continuous variables, while the chi-square test was used for categorical variables. Differences between the groups (for instance, care burden and satisfaction of family caregivers “in transition”) were compared using the Mann-Whitney U test for nonparametric data.
All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
3.1 The Impacts of the FC and NPD Modes on the Care Burden of Family Caregivers at Transitional Stage
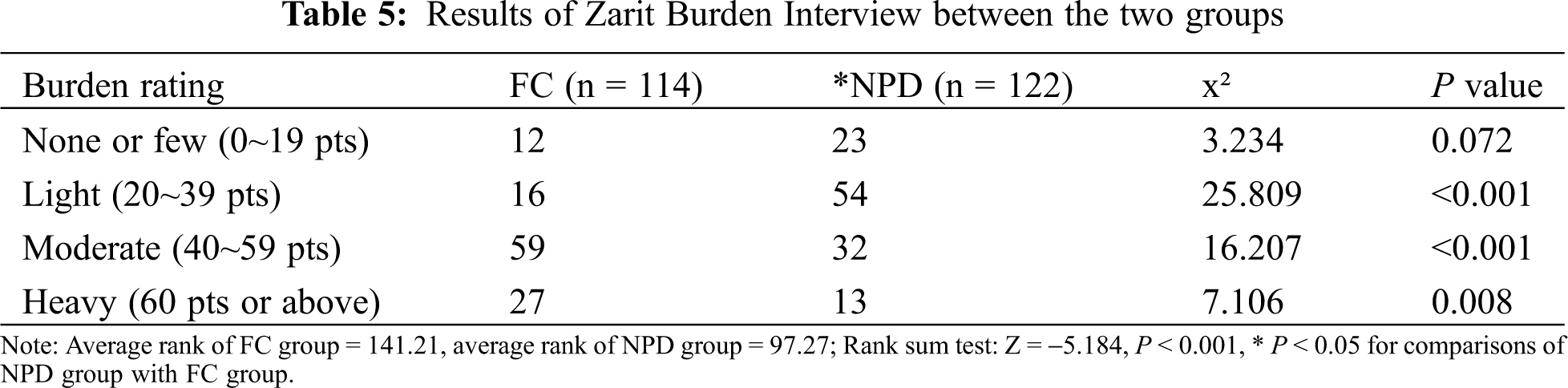
Zarit Burden Interview (ZBI) [25] was used to assess the care burden of family caregivers, including psychological stress, anxiety, stress and sleep disturbance caused by lack of feeding care knowledge, which was divided into four grades (see Tab. 5). The average rank of the FC group was 141.21 and that of the NPD group was 97.27 (rank-sum test z = −5.184). When compared with the FC mode, the NPD mode significantly reduced the care burden of family caregivers (P < 0.05).
3.2 Comparison of Acquisition of Knowledge and Skills of Feeding Care among Family Caregivers at the Transitional Stage
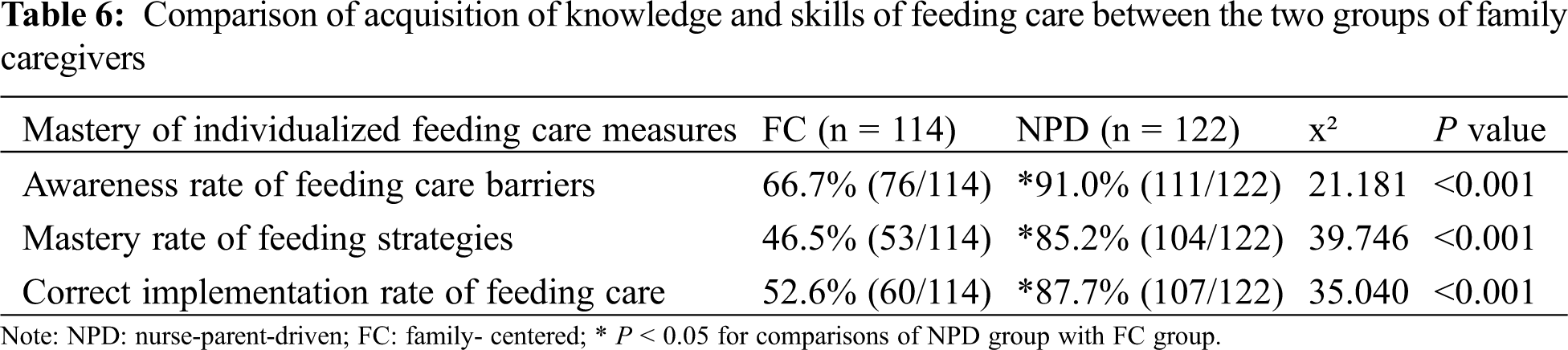
When comparing the comprehension of health education and individualized feeding care measures between the two groups, the NPD group demonstrated significantly higher rates in the awareness of adverse factors for individualized feeding care (FC/NPD = 66.7%/91.0%), comprehension of individualized feeding strategy of CHD infants with pulmonary hypertension (FC/NPD = 46.5%/85.2%) and correct implementation of feeding care (FC/NPD = 52.6%/87.7%) than the FC group (all P < 0.05, see Tab. 6).
3.3 Incidence of Complications and ICU Readmission Rate of Infants at the Transitional Stage due to Improper Feeding Care
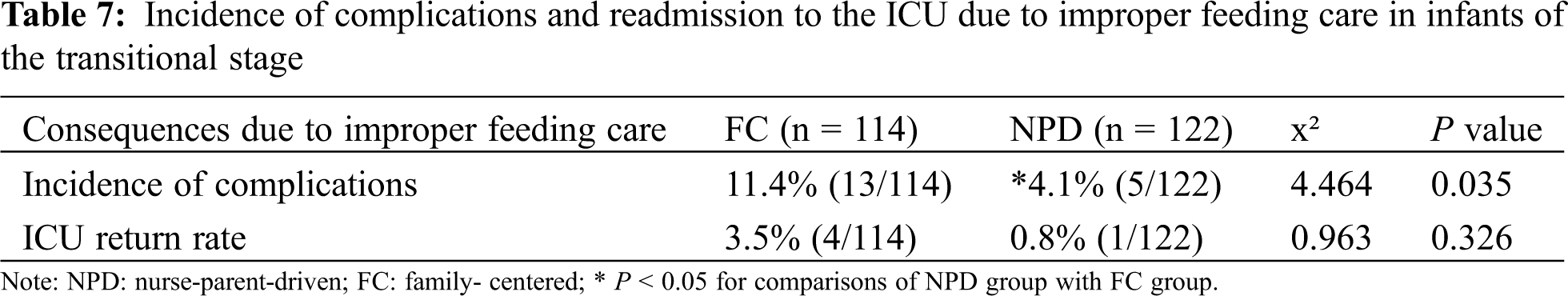
Several direct complications were associated with improper feeding and care in CHD infants with PH, including aspiration pneumonia, aspiration and asphyxia caused by milk spilling, vomiting, etc, leading to respiratory and cardiac arrest in serious cases. Indirect consequences of improper feeding and care included frequent vomiting (>3 times/day), diarrhea (>5 times/day), and even dysfunction aggravation, as a result of overfeeding.
A total of 13 cases of complications related to improper feeding care were identified in the FC group, with an incidence rate of 11.4%. Of these, 4 were re-admitted to the ICU, with an ICU readmission rate of 3.5%. There were two infants who suffered from aspiration pneumonia and respiratory insufficiency due to improper feeding that required mechanical ventilation; one infant who had undergone surgery for ventricular septal defect, atrial septal defect and PH developed cardiac insufficiency as a result of improper feeding and was re-admitted to the ICU for further management; one infant of Tetralogy of Fallot was re-admitted to the ICU following cardiopulmonary resuscitation as a result of improper nursing position by caregivers, but suffered hypoxic encephalopathy despite successful resuscitation.
In the NPD group, 5 cases of complications related to improper were identified, with an incidence rate of 4.1%. Only one infant required readmission to the ICU (0.8%). This infant initially underwent surgery for tracheobronchial stenosis and tracheomalacia due to double outlet right ventricle, ventricular septal defect, atrial septal defect and PH. The family caregivers had insufficient feeding skills and feeding obstacles, leading to the infant repeatedly developing aspiration pneumonia and hypoxic episodes requiring re-admission to the ICU.
The incidence of direct and indirect complications related to improper feeding care in the NPD group was significantly lower than that in the FC group (P < 0.05). Although the ICU readmission rate was lower in the NPD group, the difference was not statistically significant when compared with the FC group (see Tab. 7).
3.4 Comparison of Breastfeeding between the Two Groups at the Transitional Stage
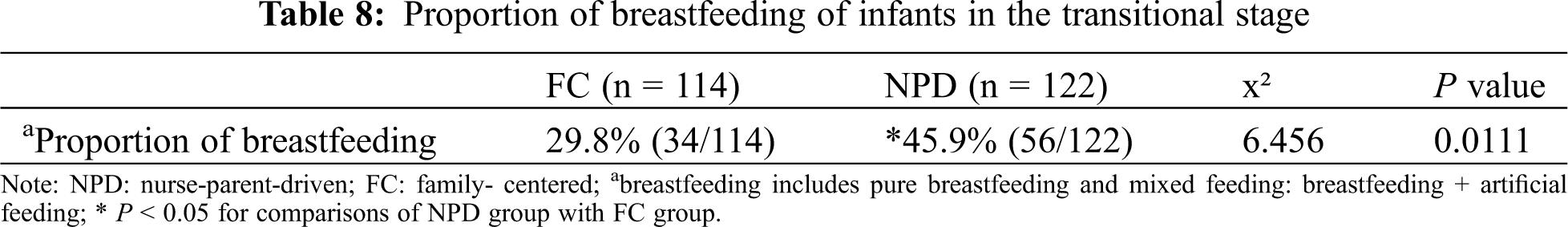
The NPD group demonstrated a significantly higher proportion of breastfeeding at the transitional stage when compared with the FC group (see Tab. 8).
3.5 Comparison of Nutritional Status between the Two Groups at Transitional Stage
The nutritional status was evaluated by measuring the plasma prealbumin, albumin and body weight gain (measurement taken at the point of hospital discharge subtracted the measurement taken when the infant was transferred to the general ward from the ICU). After excluding infants who had albumin infusion in the general ward, there were 105 cases in the FC group and 116 in the NPD group for analysis. The plasma prealbumin and body weight gain in the NPD group were significantly higher than those in the FC group (all P < 0.05, see Fig. 1), suggesting that the NPD mode was superior to the FC mode in improving the nutritional status of infants in “transition period”.
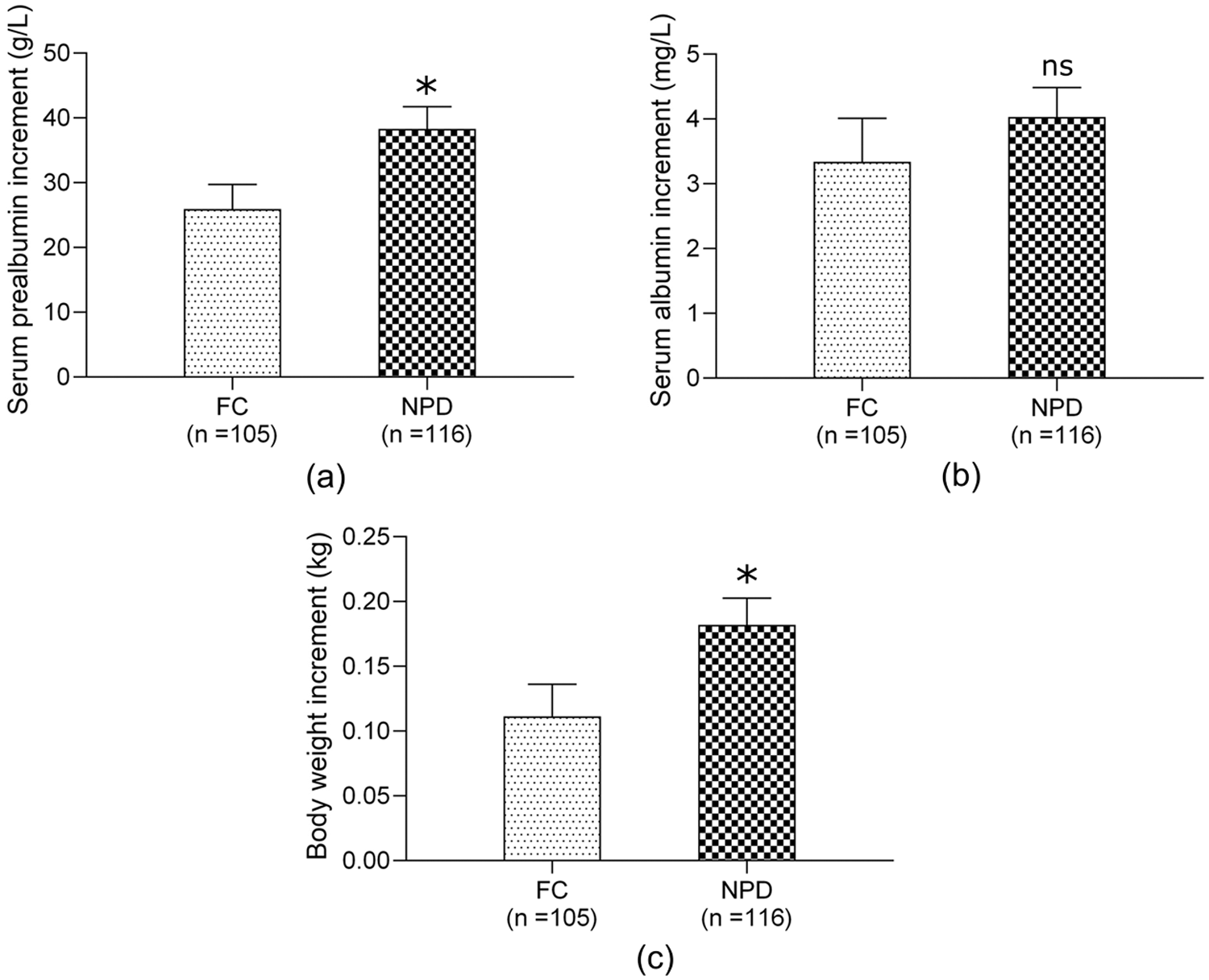
Figure 1: Comparison of nutritional status between FC group and NPD group “in transition”. (a) plasma prealbumin increment; (b) plasma albumin increment; (c) body weight increment; the increment indicates the level at discharge point minus the level at the point when infant was transferred to the general ward from ICU; Abbreviations: FC, family- centered; NPD, nurse-parent-driven; *P < 0.05 for comparisons of NPD group with FC group
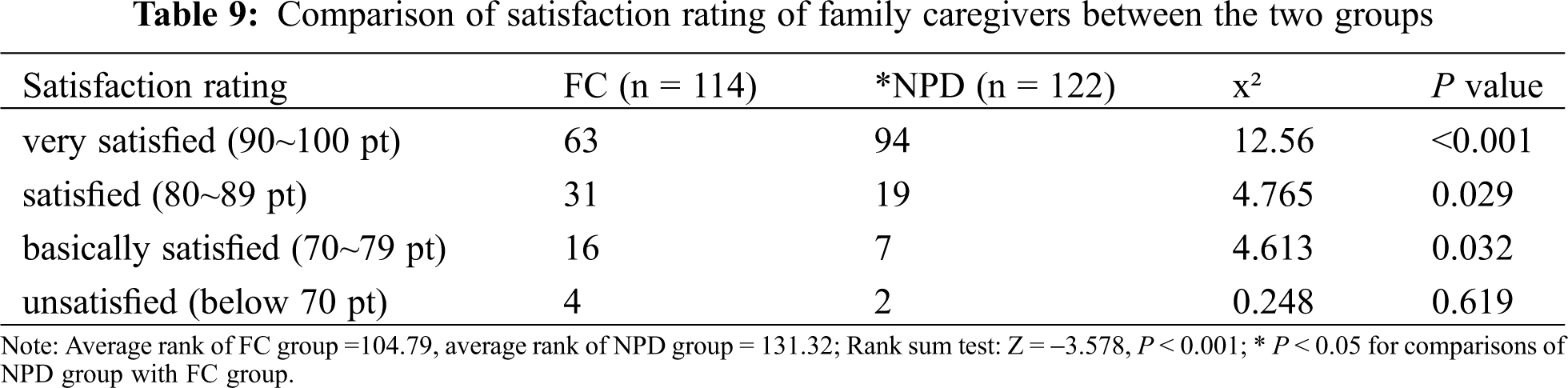
3.6 Satisfaction Rating of Family Caregivers at the Transitional Stage
Satisfaction rating was divided into four grades: very satisfied, satisfied, basically satisfied, unsatisfied (Tab. 9). The proportions of very satisfied, satisfied, basically satisfied and unsatisfied in the FC group were 64.0%, 22.8%, 9.6%, 3.5%, respectively, as compared with 77.0%, 15.6%, 5.7% and 1.6%, respectively in the NPD group. The average ranks were 104.79 and 131.32 in the FC group and NPD group (Z = −3.578, P < 0.05), respectively, indicating a significantly higher level of satisfaction of family caregivers following the intervention with the NPD feeding mode.
3.7 Follow-Up in 1 Month after Discharge from the Hospital
At one month after discharge, a self-assessment of family caregivers’ care ability (on a scale of 1–10) was conducted, and the plasma prealbumin, albumin and body weight gain (the measurement taken at one month after hospital discharge subtracted the measurement at hospital discharge) were measured. There were 82 and 120 cases in the FC and NPD groups, respectively. The score of self-assessment of care ability and body weight gain in the NPD group were significantly higher than those in the FC group (all P < 0.05, see Fig. 2).
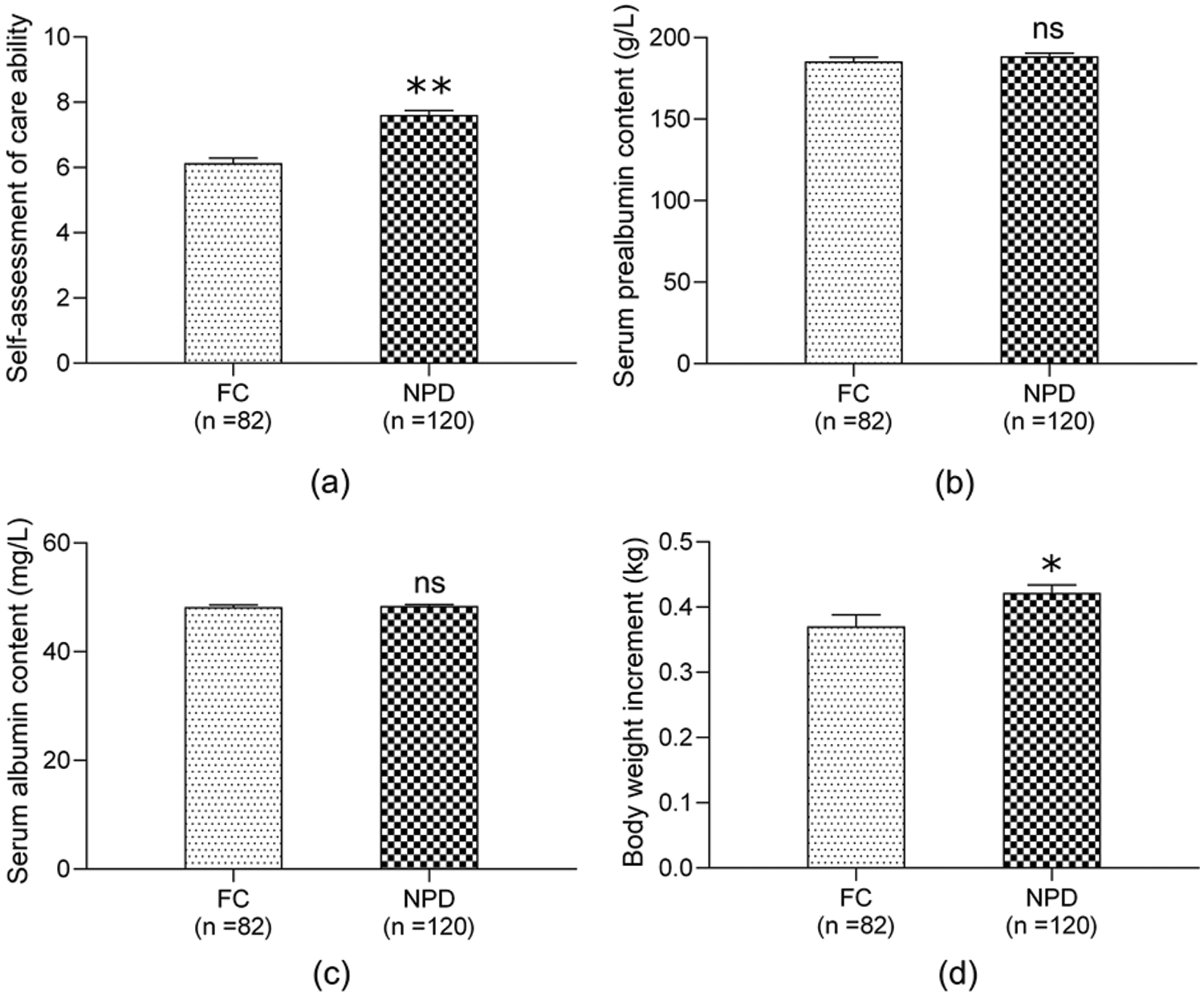
Figure 2: Follow up of 1 month after discharge between FC group and NPD group. (a) self-assessment of care ability; (b) plasma prealbumin increment; (c) plasma albumin increment; (d) body weight increment; the increment indicates the level at one month after discharge minus the level at discharge; Abbreviations: FC, family-centered; NPD, nurse-parent-driven; *P < 0.05 for comparisons of NPD group with FC group; **P < 0.01 for comparisons of NPD group with FC group
Although great progress has been made in surgery and postoperative management of CHD infants with PH, nutritional support for these patients is often suboptimal. The importance of postoperative feeding in particular, has frequently been neglected. During the early phase of ICU stay after operation [11], family caregivers do participate in the care of infants but ND remains the main feeding care mode [9,10], while FC mode is mostly adopted after discharge. The children are transferred to the general ward from the ICU when they have recovered from the critical period with stable condition. The care on the general ward following the transfer is considered as a “transitional stage”, which plays an important role in bridging the care delivered by the ICU and the family caregivers. Even though the ventilatory support is removed and the condition of infants is stable, their body tolerance remains poor. Furthermore, the lack of support and education from nurses to the caregivers, in addition to insufficient evaluation of the feeding care of the family caregivers may lead to the family members who lack knowledge about feeding and related diseases performing feeding improperly. Consequently, some infants may deteriorate further as a result of aspiration pneumonia and even asphyxia, requiring readmission to the ICU. This not only prolongs the hospitalization of children and increases the medical costs but also incurs potential medical disputes due to adverse consequences.
During the postoperative period following the surgery for CHD, the infants are prone to malnutrition due to hypermetabolism and high catabolism. Most centers utilize “internal guidelines” or “informal practice” to determine the feeding mode [26]. At present, studies on the feeding care mode of the transitional stage are scarce and there is no universally accepted, standardized protocol on the best-practice feeding mode for infants undergoing surgery. At the transitional stage, both the FC and ND have respective advantages and disadvantages. Combining the advantages of both modes and compensating for the disadvantages can achieve the best feeding care mode. On the one hand, the convenience of feeding provided by family caregivers can be brought into play, especially that the parent-child contact is crucial for the development of the infants. On the other hand, nurse-led delivery of targeted health education, psychological counseling and nursing intervention can improve the acquisition of feeding knowledge, skills and processes among family caregivers, leading to successful completion of the feeding tasks and goals at this stage. Such feeding care mode is scientific and effective, with studies showing that standardized nutrition care together with the active participation of nurses is valuable in improving nutritional outcomes and shortening hospital stay [27]. In addition, mothers of infants after CHD surgery also have to handle other affairs besides providing medical care, such as employment, family chores, and parenting other school-age children [28]. This “transitional period” feeding mode can strengthen further the mother’s care at home after hospital discharge through nursing intervention.
CHD infants after surgery suffer from varying degrees of dysphagia. Moreover, postoperative cardiopulmonary support or the inflammatory response to cardiopulmonary bypass may alter the intestinal epithelial barrier function. Prolonged intubation and duration of withholding enteral feeds, and the presence of gastroesophageal reflux disease are risk factors for poor oral feeding [29]. Others have also postulated that an increased RASCHDS (Risk Adjustment for Surgery for Congenital Heart Disease Score) and prolonged postoperative intubation [30] appear to be the most important risk factors for feeding difficulties. Unsurprisingly, studies have demonstrated that postoperative feeding difficulties significantly prolonged the hospital stay [31] and affected the rehabilitation of infants [32,33].
The feeding methods of infants after the operation are complex and variable. Upon review of the literature and our clinical practice, we defined several risk factors and characteristics of caregivers, and graded the feeding risk and caregiver’s feeding ability at the “transitional stage”, in order to identify those children who might have feeding difficulties and at risk of malnutrition, which can be avoided by systematic assessment and standardization of feeding care [34].
The key to NPDFC is to set up a nurse-led intervention team to formulate various individualized, systematic and standardized feeding plans and practices according to the grading system. After complex heart surgery, the medical teams and family caregivers often struggle with the problems of infant feeding. Compelling evidence exists that this multi-factorial problem must be addressed with both physiological and behavioral strategies [35]. Feeding disorders are present in infants who are on full oral feeds and also those dependent on supplemental feeding tubes. Caregivers’ feeding management, acceptance of nasogastric tube, psychological pressure, feeding behavior and caloric intake of infants are all issues that need to be addressed by nursing staff during the “transitional stage”. Among these, parental stress over feeding including sleep deprivation and weight gain have been identified as key areas to be addressed during hospitalization [36]. CHD surgery impacts majorly on the emotions and daily lives of children and their caregivers. In addition, caregivers have to constantly adapt to caring activities with self-fulfillment. Therefore, nurses should evaluate and obtain valuable information in order to appreciate the needs of caregivers, which helps to formulate effective future nursing plans [37]. In our study, when compared with the FC mode, the NPD feeding mode significantly reduced the care burden of family caregivers and improved inpatient satisfaction. Our dual-driven feeding care mode of nurses and family caregivers ensures the parents receiving sufficient support from the health care team [38]. Concurrently, the nursing team also requires cooperation from the caregivers. At the transitional stage, the management team developed a standardized and individualized feeding plan, which was improved continuously to guide caregivers in achieving an effective and competent role in feeding. The NPD mode was significantly superior to the FC mode with regards to the rates of awareness of obstacles in individual feeding care, acquisition of individual feeding strategies and correct implementation of feeding care for infants with CHD and pulmonary hypertension. These contributed to a significantly lower incidence of direct and indirect complications related to improper feeding care than the FC mode.
To date, there is a lack of standardized feeding protocols with variable caloric goals and practices among centers. The latest American Society for Parenteral and Enteral Nutrition guidelines underlie the importance of proteins and recommend early introduction of enteral nutrition. Newborns and infants can tolerate enteral nutrition in the first 24 h after CHD surgery [39]. Postoperative enteral nutrition (EN) has many advantages, including the protection of intestinal mucosa, reduction in the risk of infection and clinical costs. However, EN disruption may adversely affect nutritional support and clinical outcomes. Qi et al. [6] have reported that gastrointestinal factors accounted for 32.2% of all postoperative enteral nutrition interruptions, which however were the main factors in infants less than 12 months old. EN interruption due to gastrointestinal factors can be reduced by tube feeding and the use of gastrointestinal peristaltic drugs. Necrotizing enterocolitis (NEC) and gastrointestinal surgery are often encountered in infants after CHD surgery, which adversely affects gastrointestinal absorption. Davis et al. have postulated that infants with CHD are at increased risk of NEC, feeding difficulties and growth disorders. Breast milk is an ideal source of nutrition for infants with CHD and should be encouraged by the nursing team. Furthermore, breastfeeding may reduce the incidence of NEC, especially among newborns and premature infants [40]. Occasionally, the uncertainty of breastfeeding intake hinders the implementation of this feeding mode. Thus, increased education on breastfeeding for the healthcare provider is essential, especially after surgery. Also, the provision of evidence-based lactation education and care to family caregivers is paramount [41,42], given that many mothers give up breastfeeding at the transitional stage because they are concerned regarding the inadequacy and immeasurable amount of breast milk consumed by their babies, or they want to take this opportunity to wean in order to maintain their body shape. They do not maintain artificial breast suckling during the period when their babies are in the ICU, resulting in the reduction of milk production and natural weaning. The medical factors are the important factors that affect the proportion of breastfeeding. In the NPD feeding mode, the lead nurse can address the caregivers’ concerns regarding breastfeeding and guide the correct implementation by correctly advocating and encouraging breastfeeding. Moreover, during the ICU stay, the lead nurse can provide breast milk to nurses in the ICU through acceptable methods as indirect breastfeeding support. This invariably increases significantly the proportion of breastfeeding of infants in the transitional period.
Conforming to evidence-based guidelines decreases variability in clinical practice. Standardized practice, education and counseling to caregivers and hospital healthcare providers would improve the understanding and selection of appropriate feeding methods that positively impact patient outcomes [43]. We aimed to transition infants to oral feeding, dynamically assess the feeding ability of caregivers, and determine the related factors affecting feeding. For instance, the feeding characteristics of cyanotic and non-cyanotic infants with CHD are different. The feeding of cyanotic infants with CHD is significantly delayed in four aspects: (a) feeding readability, (b) successful gastric feeding, (c) oromotor readability and (d) successful oromotor skills [44]. Thus, nursing staff should make individualized feeding plans according to the actual feeding situation. In our study, the plasma prealbumin and body weight gain of infants in the NPD group were significantly higher than those in the FC group (all P < 0.05), indicating the superiority of NPD feeding mode in improving the nutritional status of infants at the “transitional stage”. Oral feeding before hospital discharge is a difficult task. Future prospective studies are warranted to identify multimodal strategies to optimize the early introduction of oral feeding [45]. In addition, the NPD feeding mode represents a test to control the caregivers, to establish evidence-based family-centered interventions [46]. Our findings revealed a significantly higher score of self-assessment of care ability and body weight gain in the NPD group than the FC group (all P < 0.05) at one month after hospital discharge, suggesting the continued effectiveness of the NPD feeding mode following discharge.
To date, there is no nationally or internationally accepted assessment tool for risk factors for infant feeding during the transition period, nor there is any validated tool for assessing the feeding competency of family caregivers. Therefore, with the help from the nursing staff, dietitians, physicians in the care unit and medical support staff, we developed the “feeding risk factor scale for infants at transitional stage” based on the age of the child with CHD, postoperative feeding status, nutritional status, the impact of disease, condition on feeding and experience from previous feeding-related adverse events, with reference to relevant literature. Concurrently, we also developed the feeding ability scale of family caregivers at the transitional stage based on their age, education level, knowledge of feeding and feeding skills. These two scales were clinically validated in a single center by our preliminary investigation. The individualized and refined targeted interventions based on the scoring systems improved the postoperative nutrition of the infant, reduced complications and shortened the postoperative recovery time. Such interventions not only allow caregivers to target their work and ensure that each family caregiver plays a defined role in the "transition period" that is beneficial to the infant’s postoperative recovery but also reduce the workload of caregivers overall, reflecting the significance of different care measures for different scoring levels. These two scoring systems were developed internally and had not been widely recognized by our peers, which therefore demand further studies for validation.
In this study, through a systematic and comprehensive evaluation of CHD infants with PH, determining causes of feeding disorders in infants with different types of diseases, followed by scientifically formulating the corresponding feeding process and methods, the nurses are much more competent in providing effective and safe support and delivering comprehensive intervention measures with anticipation. Through objective evaluation of family caregivers’ ability, a series of nursing intervention can be instituted to improve all aspects of health education, so that caregivers appreciate the causes of feeding disorders, feeding performance, feeding process, effective feeding methods and other key links, all of which would lead to better care ability for infants and avoiding blind obedience and randomness of feeding care. Through continuous active and effective doctor-patient communication and interaction, caregivers will be familiar with each stage of the treatment and rehabilitation process, and participate in the whole treatment and nursing of children. In the process of implementation, dynamic assessment and continuous quality improvement are carried out to ensure oral feeding is established the soonest, which will provide security for family care after discharge.
This study demonstrates that the NPD feeding mode is significantly superior to the FC mode in reducing the burden of family caregivers and the incidence of complications related to improper feeding care, improving the acquisition of caregivers’ knowledge and skills of feeding care and inpatient’s satisfaction. In addition to increasing the proportion of breastfeeding, NPD feeding mode significantly improves the nutritional status of infants at the transitional stage and in one month after hospital discharge. All in all, our findings shed light on the feasibility, effectiveness and many advantages of NPDFC as a “transitional stage” nurse-led feeding mode, which provides a theoretical and practical basis for clinical implementation.
Author Contributions: Concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, data collection, statistics: Qifeng Zhao; Concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, data collection, implementation of clinical nursing: Huaying He; Concept/design, data analysis/interpretation, statistics, approval of article: Zhiyong Lin; Concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, implementation of clinical nursing: Yuelan Weng, Jianjie Zhou; Concept/design, data analysis/interpretation, approval of article, data collection, implementation of clinical nursing: Man Ye, Xiaowei Luo.
Funding Statement: This work was supported by the Science and Technology Project of Science and Technology Bureau of Wenzhou (Y20170467), the Science and Technology Project of Medical and Health of Zhejiang Province (2017RC021), Key Discipline Program of Pediatric Surgery of Health Bureau of Zhejiang Province (No. 11-ZC27), Research Center for diagnosis and treatment of cardiac and vascular disease of Zhejiang, China (JBZX-202001).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Radman, M., Mack, R., Barnoya, J., Castañeda, A., Rosales, M. et al. (2014). The effect of preoperative nutritional status on postoperative outcomes in children undergoing surgery for congenital heart defects in San Francisco (UCSF) and Guatemala City (UNICAR). Journal of Thoracic and Cardiovascular Surgery, 147(1), 442–450. [Google Scholar]
2. Vaidyanathan, B., Radhakrishnan, R., Sarala, D. A., Sundaram, K. R., Kumar, R. K. et al. (2009). What determines nutritional recovery in malnourished children after correction of congenital heart defects? Pediatrics, 124(2), e294–299. [Google Scholar]
3. Batte, A., Lwabi, P., Lubega, S., Kiguli, S., Karamagi, C. et al. (2017). Wasting, underweight and stunting among children with congenital heart disease presenting at Mulago hospital. Uganda BMC Pediatrics, 17(1), 10. [Google Scholar]
4. Ni, Z., Chao, Y., Xue, X. (2016). An empowerment health education program for children undergoing surgery for congenital heart diseases. Journal of Child Health Care, 20(3), 354–364. [Google Scholar]
5. Ju, R. M., Yong, A. C., Sun, I. M., Yang, J. H., Jung, Y. Y. et al. (2009). Development and application of a feeding program for infants postoperatively following cardiac surgery. Journal of Korean Academy of Nursing, 39(4), 508–517. [Google Scholar]
6. Qi, J., Zhuo, L., Cun, Y., Li, X. (2017). Causes of interruptions in postoperative enteral nutrition in children with congenital heart disease. Asia Pacific Journal of Clinical Nutrition, 26(3), 402–405. [Google Scholar]
7. Magalhäes, M., De, A., Oliveira, L., Resende, C., Amorim, B. B. et al. (2012). Nutritional status of children with congenital heart disease. Revista Latino-Americana de Enfermagem, 20(6), 1024–1032. [Google Scholar]
8. Alrddadi, S. M., Morsy, M. M., Albakri, J. K., Mohammed, M. A., Ghassan, A. A. et al. (2019). Risk factors for prolonged mechanical ventilation after surgical repair of congenital heart disease. experience from a single cardiac center. Saudi Medical Journal, 40(4), 367–371. [Google Scholar]
9. Tume, L. N., Balmaks, R., Cruz, E. D., Latten, L., Verbruggen, S. et al. (2018). Enteral feeding practices in infants with congenital heart disease across European PICUs: A European society of pediatric and neonatal intensive care survey. Pediatric Critical Care Medicine, 19(2), 137–144. [Google Scholar]
10. Lisanti, A. J., Cribben, J., Connock, M. M., Lessen, R., Medoff-Cooper, B. (2016). Developmental care rounds: An interdisciplinary approach to support developmentally appropriate care of infants born with complex congenital heart disease. Clinics in Perinatology, 43(1), 147–156. [Google Scholar]
11. Lisanti, A. J., Vittner, D., Medoff-Cooper, B., Fogel, J., Wernovsky, G. et al. (2019). Individualized family-centered developmental care: An essential model to address the unique needs of infants with congenital heart disease. Journal of Cardiovascular Nursing, 34(1), 85–93. [Google Scholar]
12. Nieves, J. A., Kohr, L. (2010). Nursing considerations in the care of patients with pulmonary hypertension. Pediatric Critical Care Medicine, 11(2 Suppl), S74–78. [Google Scholar]
13. Owens, J. L., Musa, N. (2009). Nutrition support after neonatal cardiac surgery. Nutriton in Clinical Practice, 24(2), 242–249. [Google Scholar]
14. Medoff-Cooper, B., Ravishankar, C. (2013). Nutrition and growth in congenital heart disease: A challenge in children. Current Opinion in Cardiology, 28(2), 122–129. [Google Scholar]
15. Society of Parenteral and Enteral Nutrition Pediatric Collaborative Group (2010). Guidelines for pediatric clinical application of enteral and parenteral nutritional support in China. Chinese Journal of Pediatrics, 48(6), 436–441. [Google Scholar]
16. Corkins, M. R., Griggs, K. C., Groh-Wargo, S., Han-Markey, T. L., Helms, R. A. (2013). Standards for nutrition support: Pediatric hospitalized patients. Nutriton in Clinical Practice, 28(2), 263–276. [Google Scholar]
17. Mccarthy, H., Mcnulty, H., Dixon, M., Eaton-Evans, M. J. (2008). Screening for nutrition risk in children: The validation of a new tool. Journal of Human Nutrition and Dietetics, 21(4), 395–396. [Google Scholar]
18. Wong, S., Graham, A., Hirani, S. P., Grimble, G., Forbes, A. (2013). Validation of the screening tool for the assessment of malnutrition in paediatrics (STAMP) in patients with spinal cord injuries (SCIs). Spinal Cord, 51(5), 424–429. [Google Scholar]
19. Hulst, J. M., Zwart, H., Hop, W. C., Joosten, K. (2010). Dutch national survey to test the STRONG kids nutritional risk screening tool in hospitalized children. Clinical Nutrition, 29(1), 106–111. [Google Scholar]
20. Pierre, A. S., Khattra, P., Johnson, M., Cender, L., Manzano, S. et al. (2010). Content validation of the infant malnutrition and feeding checklist for congenital heart disease: A tool to identify risk of malnutrition and feeding difficulties in infants with congenital heart disease. Journal of Pediatric Nursing–Nursing Care of Children & Families, 25(5), 367–374. [Google Scholar]
21. Läer, S., Mir, T. S., Behn, F., Eiselt, M., Scholz, H. et al. (2002). Carvedilol therapy in pediatric patients with congestive heart failure: A study investigating clinical and pharmacokinetic parameters. American Heart Journal, 143(5), 916–922. [Google Scholar]
22. Okoromah, C., Ekure, E. N., Lesi, F., Okunowo, W. O., Tijani, B. O. et al. (2011). Prevalence, profile and predictors of malnutrition in children with congenital heart defects: A case-control observational study. Archives of Disease in Childhood, 96(4), 354–360. [Google Scholar]
23. Dalili, M., Meraji, S. M., Davari, P., Moghaddam, M., Abkenar, H. B. et al. (2011). Growth status of Iranian children with hemodynamically important congenital heart disease. Acta Medica Iranica, 9(2), 103–108. [Google Scholar]
24. Huang, J. T., Lu, X. L., Xiao, Z. H., Zang, P., Gong, L. et al. (2019). Clinical effect of feeding with calorie-enriched formula in children with ventricular septal defect and severe pneumonia. Chinese Journal of Contemporary Pediatrics, 21(10), 998–1004. [Google Scholar]
25. Wang, L., Yang, X., Hou, Z. (2006). Application and evaluation of Chinese version of Zarit caregiver burden interview. Chinese Journal of Public Health, 22(8), 970–972. [Google Scholar]
26. Slicker, J., Sables-Baus, S., Lambert, L. M., Peterson, L. E., Woodard, F. K. et al. (2016). Perioperative feeding approaches in single ventricle infants: A survey of 46 centers. Congenital Heart Disease, 11(6), 707–715. [Google Scholar]
27. Newcombe, J., Fry-Bowers, E. (2017). A post-operative feeding protocol to improve outcomes for neonates with critical congenital heart disease. Journal of Pediatric Nursing-nursing Care of Children & Families, 35, 139–143. [Google Scholar]
28. Imperial-Perez, F., Heilemann, M. (2019). Having to be the one: Mothers providing home care to infants with complex cardiac needs. American Journal of Critical Care, 28(5), 354–360. [Google Scholar]
29. Indramohan, G., Pedigo, T. P., Rostoker, N., Cambare, M., Grogan, T. et al. (2017). Identification of risk factors for poor feeding in infants with congenital heart disease and a novel approach to improve oral feeding. Journal of Pediatric Nursing-Nursing Care of Children & Families, 35, 149–154. [Google Scholar]
30. Kogon, B. E., Ramaswamy, V., Todd, K., Plattner, C., Kirshbom, P. M. et al. (2007). Feeding difficulty in newborns following congenital heart surgery. Congenital Heart Disease, 2(5), 332–337. [Google Scholar]
31. Desena, H. C., Nelson, D. P., Cooper, D. S. (2015). Cardiac intensive care for the neonate and child after cardiac surgery. Current Opinion in Cardiology, 30(1), 81–88. [Google Scholar]
32. Souza, P., Gigoski, V. S., Etges, C. L., Barbosa, L. (2018). Findings of postoperative clinical assessment of swallowing in infants with congenital heart defect. CoDAS, 30(1), e20170024. [Google Scholar]
33. Typpo, K. V., Larmonier, C. B., Deschenes, J., Redford, D., Kiela, P. R. et al. (2015). Clinical characteristics associated with postoperative intestinal epithelial barrier dysfunction in children with congenital heart disease. Pediatric Critical Care Medicine, 16(1), 37–44. [Google Scholar]
34. Ehrmann, D. E., Mulvahill, M., Harendt, S., Church, J., Stimmler, A. et al. (2018). Toward standardization of care: The feeding readiness assessment after congenital cardiac surgery. Congenital Heart Disease, 13(1), 31–37. [Google Scholar]
35. Medoff-Cooper, B., Naim, M., Torowicz, D., Mott, A. (2010). Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiology in the Young, 20(S3), 149–153. [Google Scholar]
36. Hartman, D. M., Medoff-Cooper, B. (2012). Transition to home after neonatal surgery for congenital heart disease. MCN–The American Journal of Maternal Child Nursing, 37(2), 95–100. [Google Scholar]
37. Ni, Z. H., Lv, H. T., Ding, S., Yao, W. Y. (2019). Home care experience and nursing needs of caregivers of children undergoing congenital heart disease operations: A qualitative descriptive study. PLoS One, 14(3), e0213154. [Google Scholar]
38. Sjostrom-Strand, A., Terp, K. (2019). Parents’ experiences of having a baby with a congenital heart defect and the child’s heart surgery. Comprehensive Child and Adolescent Nursing–Buildng Evidence for Practice, 42(1), 10–23. [Google Scholar]
39. Kalra, R., Vohra, R., Negi, M., Joshi, R., Aggarwal, N. et al. (2018). Feasibility of initiating early enteral nutrition after congenital heart surgery in neonates and infants. Clinical Nutrition ESPEN, 25, 100–102. [Google Scholar]
40. Mangili, G., Garzoli, E., Sadou, Y. (2018). Feeding dysfunctions and failure to thrive in neonates with congenital heart diseases. Medical and Surgical Pediatrics, 40(1), 196. [Google Scholar]
41. Gregory, C. (2018). Use of test weights for breastfeeding infants with congenital heart disease in a cardiac transitional care unit: A best practice implementation project. JBI Database of Systematic Reviews and Implementation Reports, 16(11), 2224–2245. [Google Scholar]
42. Davis, J. A., Spatz, D. L. (2019). Human milk and infants with congenital heart disease: A summary of current literature supporting the provision of human milk and breastfeeding. Advances in Neonatal Care, 19(3), 212–218. [Google Scholar]
43. Hoch, J. M., Fatusin, O., Yenokyan, G., Thompson, W. R., Lefton-Greif, M. A. (2019). Feeding methods for infants with single ventricle physiology are associated with length of stay during stage 2 surgery hospitalization. Congenital Heart Disease, 14(3), 438–445. [Google Scholar]
44. Jadcherla, S. R., Vijayapal, A. S., Leuthner, S. (2009). Feeding abilities in neonates with congenital heart disease: A retrospective study. Journal of Perinatology, 29(2), 112–118. [Google Scholar]
45. Sables-Baus, S., Kaufman, J., Cook, P., Cruz, E. M. D. (2012). Oral feeding outcomes in neonates with congenital cardiac disease undergoing cardiac surgery. Cardiology in the Young, 22(1), 42–48. [Google Scholar]
46. Harvey, K. A., Kovalesky, A., Woods, R. K., Loan, L. A. (2013). Experiences of mothers of infants with congenital heart disease before, during, and after complex cardiac surgery. Heart & Lung, 42(6), 399–406. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |