

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015588
ARTICLE
Relation or Influence of RVOTO in the Inflammatory Response to Reoxygenation in Patients with Tetralogy of Fallot
1Department of Cardiovascular Surgery, the First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, China
2Department of Cardiovascular Surgery, Graduate School, Tianjin Medical University, Tianjin, 300070, China
3Department of Cardiovascular Surgery, Graduate School, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, China
*Corresponding Authors: Hong Liu. Email: dr.hongliu@foxmail.com; Yongfeng Shao. Email: yfshaojph@sina.com
Received: 29 December 2020; Accepted: 18 March 2021
#These authors contributed equally to this work
Abstract: Background: This study evaluated differential inflammatory response to cardiopulmonary bypass reoxygenation in tetralogy of Fallot repair. Methods: We performed a retrospective study at a cardiovascular center from 2012 to 2018, including 500 patients aged 1 week–18 years who received complete repair of tetralogy of Fallot. Patients were grouped according to tertiles of preoperative RVOT gradient on echocardiography into mild, moderate, and severe stenosis. We measured the highest perfusate oxygenation (PpO2) during aortic occlusion as independent variable. Primary outcome was systemic inflammatory response syndrome (SIRS) within 7 days postoperatively or the time of death or discharge. Results: Overall, rate of SIRS was 24.2% without significant differences among three groups (P > 0.05). Older age, male, and smaller indexed left ventricular end-diastolic volume is independent risk factor of SIRS. There were significant interactions between RVOT stenosis and PpO2 on SIRS (P interaction = 0.011): higher PpO2 was associated with a greater SIRS risk among combined moderate and severe stenotic children (OR 1.463 95%CI [1.080, 1.981] per-SD increase, P = 0.014) but not among mild stenotic children (OR 0.900 [0.608, 1.333] per-SD increase; P = 0.600), independent of covariates. Conclusion: The association of PpO2 with SIRS was modified by RVOT obstruction severity in tetralogy of Fallot repair. (Clinical Trials gov: NCT03568357)
Keywords: Cardiopulmonary bypass; tetralogy of Fallot; hypoxia/reoxygenation injury; systemic inflammatory response syndrome
Abbreviations
| CPB: | cardiopulmonary bypass; |
| PpO2: | perfusate oxygenation; |
| RVOT: | right ventricular outflow tract; |
| SIRS: | systemic inflammatory response syndrome; |
| LVEDV: | left ventricular end-diastolic volume; |
| CI: | confidence interval. |
Cardiac surgery with cardiopulmonary bypass (CPB) is associated with ischemia-reperfusion injury and inflammatory response [1,2]. For patients with cyanotic heart diseases, sudden reoxygenation during CPB would lead to an extra hypoxia-reoxygenation injury induced by oxygen-derived free radicals and lipid peroxidation, thereby exacerbating the systemic inflammatory response to CPB [3–5]. Additionally, previous clinical and experimental studies indicate that hyperoxemic reoxygenation during CPB is associated with higher risk of critical organ injury and dysfunction by decreasing the adaptation and remodeling capacity compared to normoxemic reoxygenation [6–8].
However, there are no definitive reports regarding the effect of continuous perfusate oxygenation (PpO2) during CPB and its relationship with right ventricular outflow tract (RVOT) obstruction severity in pediatric patients with tetralogy of Fallot. Given the variety of pathological anatomy of tetralogy of Fallot [9,10], we investigate whether the differences in RVOT obstruction severity modifies the association of PpO2 with systemic inflammatory response syndrome (SIRS) in complete repair of tetralogy of Fallot, with a particular focus on the effect of interplay between obstruction severity and continuous PpO2.
2.1 Research Population and Design
This retrospective study focuses on quantitative evaluation of inflammatory responses of pediatric patients with different RVOT obstruction severities to CPB reoxygenation in complete repair of tetralogy of Fallot at a large cardiovascular tertiary academic medical center. The Institutional Review Board of Tianjin Economic Development Area Cardiovascular Hospital approved this study (2019-0826-3). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from parent and/or guardian of patient.
We screened pediatric patients aged 1 month–18 years who underwent complete repair of tetralogy of Fallot on bypass and were routinely admitted to intensive care unit (ICU) for inclusion between May 2012 and December 2018. Key criteria for exclusion included prematurity, previous palliation either with percutaneous or surgical procedures, severe renal dysfunction requiring dialysis, previous red-cell transfusion during the current admission, and use of extracorporeal membrane oxygenation or intra-aortic balloon pump for treatment of cardiogenic shock, as well as those with incomplete PpO2 records and missing outcome data, thereby leaving 500 infants and children in the final study population. This study was registered with Clinical Trials. gov No. NCT03568357.
Patients’ baseline clinical variables were collected and extracted from the specialized hospital information system (Java His, BlueCore, Tianjin), including age at surgery, sex, body mass index, body surface area, blood pressure, temperature, heart rate, respiratory rate, leukocyte count, hemoglobin, hematocrit, Tet spell history, percutaneous oxyhemoglobin saturation (SpO2), right bundle branch block (RBBB), NYHA class, ejection fraction, indexed left ventricular end-diastolic volume (LVEDV), McGoon index, CPB time, and aortic crossclamp time. In addition, procedural factors were also collected including cardioplegia solution, transannular patch, and tricuspid valve detachment.
Given this hypothesis that the differences in RVOT stenosis might have differential influences on inflammatory response to reoxygenation during CPB, we selected the highest value of PpO2 throughout aortic crossclamp as an independent variable for analysis. CPB is primed with Plasma-Lyte A, followed by the addition of packed red blood cells and fresh frozen plasma to achieve the desired hematocrit of 25% on CPB. At initiation of CPB, flow rates are maintained at approximately 150–200 mL/Kg/min and 100–150 mL/Kg/min for patients weighted <10 kg and >10 kg, respectively, subsequently adjusted according to the patient’s core temperature (usually a 20% decrease for core temperatures between 30°C and 34°C and an additional 10% decrease for core temperatures <30°C) following our institute protocol [11]. All patients received standardized a-stat CPB management and standardized critical care management according to our institute.
According to EAE/ASE Recommendations for Clinical Practice on Echocardiographic Assessment of Valve Stenosis [12,13], RVOT obstruction severity was evaluated by the peak instantaneous gradient of RVOT from transthoracic two-dimensional echocardiography [13]. Patients were grouped according to tertiles of the peak RVOT gradient into mild stenotic group (<45 mmHg), moderate stenotic group (45–95 mmHg), and severe stenotic group (>95 mmHg), regardless of the level of obstruction (RVOT, valvar or supravalvar).
Primary outcome was SIRS through postoperative day 7, hospital discharge, or death, whichever occurred first. SIRS was determined on the basis of International Pediatric Sepsis Consensus Conference: Definitions for Sepsis and Organ Dysfunction in Pediatrics [14], as the presence of at least two of the following four criteria appropriate for age-specific ranges for physiologic and laboratory variables, one of which must be abnormal temperature or leukocyte count: (i) Core temperature of >38.5°C or <36°C measured by rectal, oral, or central catheter probe; (ii) Tachycardia, defined as a mean heart rate >2 SD above normal for age in the absence of external stimulus, chronic drugs, or painful stimuli; or otherwise unexplained persistent elevation over a 0.5- to 4-hr time period, or for children <1 yr old: bradycardia, defined as a mean heart rate <10th percentile for age in the absence of external vagal stimulus; or otherwise unexplained persistent depression over a 0.5-hr time period; (iii) Mean respiratory rate >2 SD above normal for age, the prolonged mechanical ventilation defined as ventilation duration >48 hrs, or unplanned re-intubation for an acute process not related to underlying neuromuscular disease or the receipt of general anesthesia; (iv) Leukocyte count elevated or depressed for age or >10% immature neutrophils. All outcomes were adjudicated independently by an event collaborative team. Secondary outcomes were mechanical ventilation time, ICU duration, inotropic score [15], vasoactive-inotropic score [15], low cardiac output syndrome, and in-hospital mortality rate.
Patient characteristics were stratified by RVOT obstruction severity. Continuous data were presented as mean (SD) or median (IQRs) and compared using a t-test or Kruskal-Wallis testing depending on distributed characteristics, and categorical data were reported as percentages (%) and compared using χ2 testing. Because of covariates that were potentially missing not-completely-at-random, covariates were imputed for multivariable analysis by means of single imputation with 10 iterations using all covariates.
The variables affecting primary outcome that were significant (P < 0.10) in univariate analysis were included in multivariate logistic regression with backward stepwise analysis. We assessed the association of RVOT obstruction severity with the odd ratio (OR) of SIRS with 95% confidence interval (CI) using adjusted logistic regression model for potential risk factors. We test the interactions between RVOT obstruction severity and PpO2 with SIRS using interaction terms in a similar adjusted multivariable model. Restricted cubic splines were used to test the functional association of PpO2 with the probability of SIRS by RVOT obstruction severity. In patients of different RVOT obstruction severity, we also tested the 3-way interaction terms among procedural factors [16]. To investigate the cumulative incidence of SIRS by age at surgery, we used Kaplan-Meier estimates with age as the time scale by RVOT obstruction severity [17].
All statistical analyses were performed using Stata version 14 (Stata Corp, College Station, TX, USA) and R software (version 3.2.0). P values of less than 0.05 were considered statistically significant.
Among patients of different RVOT obstruction stenosis, there were similar distributions in terms of sex (P = 0.109), respiratory rate (P = 0.244), leukocyte count (P = 0.432), mean blood pressure (P = 0.052), CPB time (P = 0.740), crossclamp time (P = 0.698), NYHA class (P = 0.593), cardioplegia type (P = 0.999), the presence of RBBB (P = 0.133), McGoon index (P = 0.053), and tricuspid valve detachment (P = 0.125). However, there were different distributions in terms of age at surgery (P < 0.001), body mass index (P < 0.001), body surface area (P = 0.784), systolic blood pressure (P = 0.009), diastolic blood pressure (P = 0.031), temperature (P = 0.028), heart rate (P = 0.026), hemoglobin (P < 0.001), hematocrit (P < 0.001), Tet spell history (P = 0.006), SpO2 (P < 0.001), ejection fraction (P = 0.039), indexed LVEDV (P < 0.001), and transannular patch (P = 0.044) (Tab. 1).
Table 1: Baseline clinical, procedural, and outcome data of patients of different RVOT obstruction severity
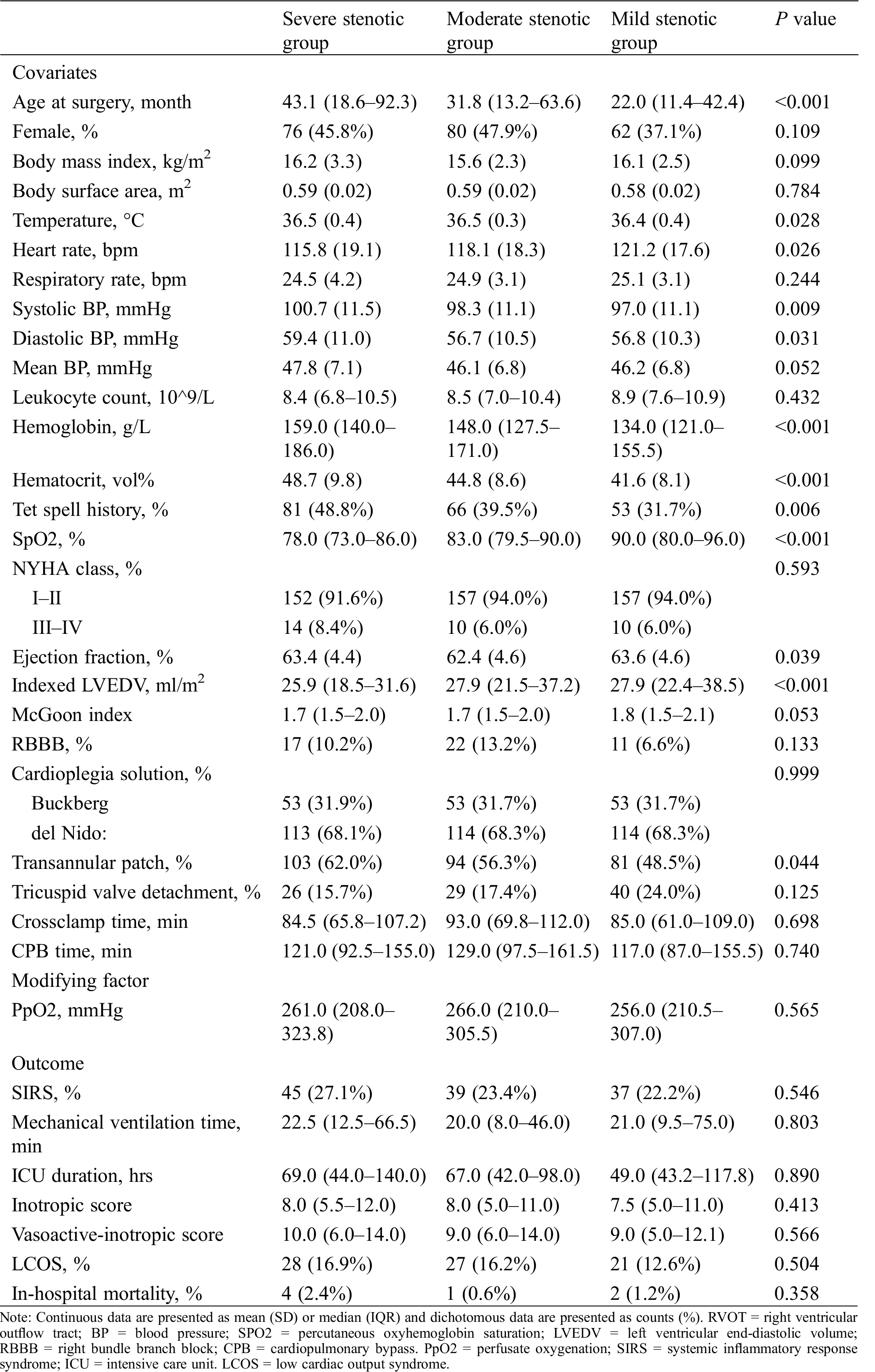
The hospital outcomes for all the study patients are listed in Tab. 1. Overall, the rate of SIRS was 24.2% without significant differences among the groups (22.2%, 23.4%, and 27.1% for mild, moderate and severe stenotic groups, respectively, P > 0.05). PpO2 were comparable among the groups (median 256.0 [IQR 210.5–307.0], 266.0 [210.0–305.5], and 261.0 [208.0–323.8] in mild, moderate and severe stenotic group; P > 0.05). Besides, there were no significant differences between groups in terms of mechanical ventilation time, ICU duration, inotropic score, vasoactive-inotropic score, low cardiac output syndrome, and in-hospital mortality (all P > 0.05).
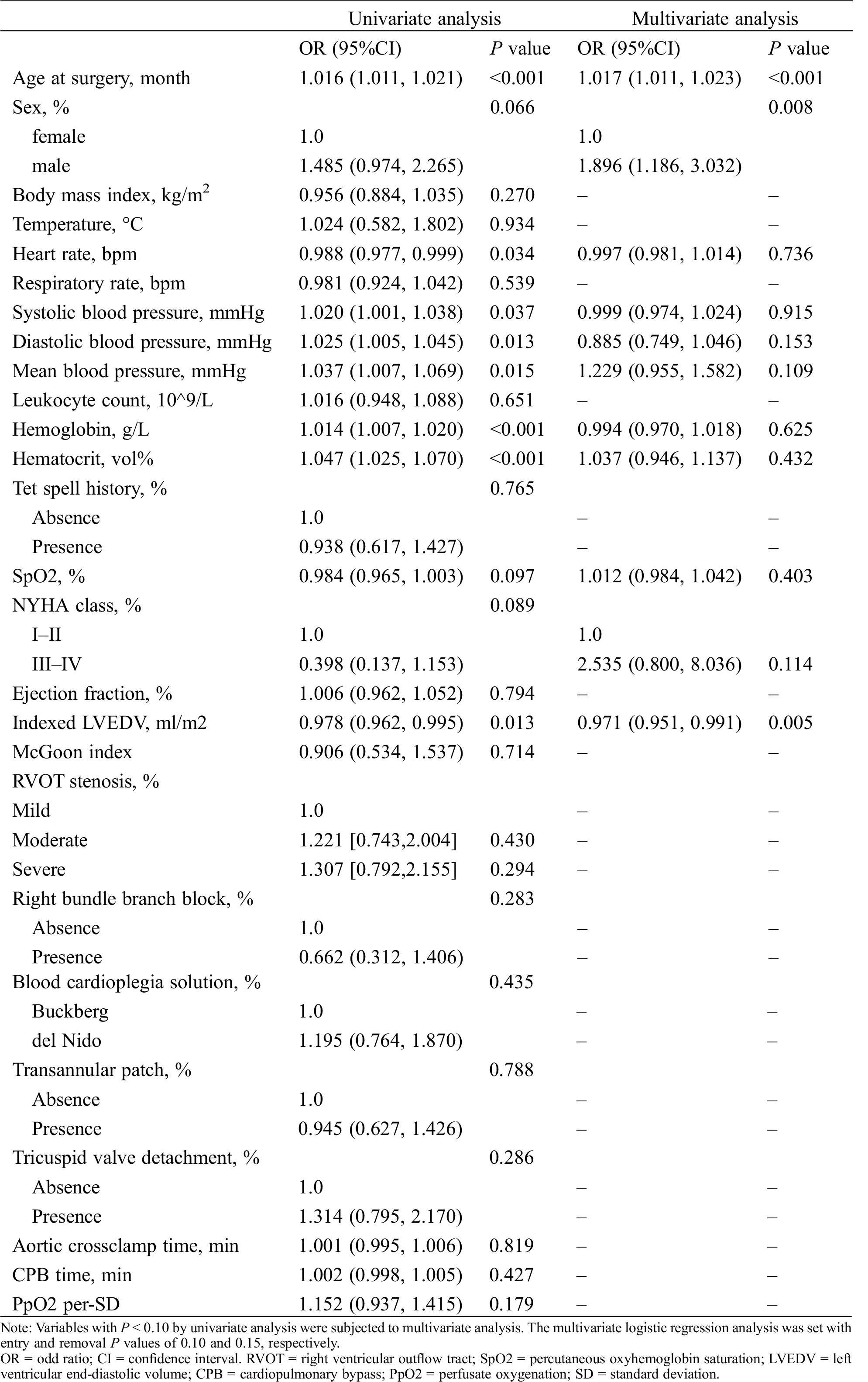
Risk factor analysis for SIRS with univariate logistic regression model revealed that older age, male, lower heart rate, lower SpO2, higher systolic, diastolic and mean blood pressure, higher hemoglobin, higher hematocrit, inferior NYHA classification, and lower indexed LVEDV were potential risk factors for SIRS (all with P < 0.10) (Tab. 2). Notably, neither RVOT obstruction stenosis (mild stenosis [reference 1.000]; moderate stenosis OR 1.221 95%CI [0.743,2.004], P = 0.430; severe stenosis OR 1.307 [0.792,2.155], P = 0.295) nor PpO2 as continuous variable (OR of 1.152 [0.937,1.415] per-SD increase, P = 0.179) was significant factor affecting the development of SIRS. Multivariate logistic regression analysis showed that only older age (OR of 1.017 [1.011,1.023] per-month increase, P < 0.001), male (OR 1.896 [1.186,3.032], P = 0.008), and smaller indexed LVEDV (OR of 0.971 [0.951,0.991] per-unit increase, P = 0.005) is independent risk factor for developing SIRS (Tab. 2).
Table 2: Logistic regression analysis for systemic inflammatory response syndrome
Overall, the association of PpO2 modelled as continuous variable with SIRS was assessed before and after adjusting for baseline and clinical covariates (Tab. 3). After adjustment for potential risk factors, there was a marginal significant interaction between different stenosis severity and continuous PpO2 on SIRS (Pinteraction = 0.061): higher PpO2 was associated with a trend toward a greater risk of SIRS among moderate stenotic children (adjusted OR 1.493 [0.946,2.354] per-SD increase, P = 0.061; Fig. 1B) and severe stenotic children (adjusted OR 1.438 [0.903,2.290] per-SD increase, P = 0.126; Fig. 1C) but not among mild stenotic children (adjusted OR 0.900 [0.608,1.333] per-SD increase; P = 0.600; Fig. 1A).
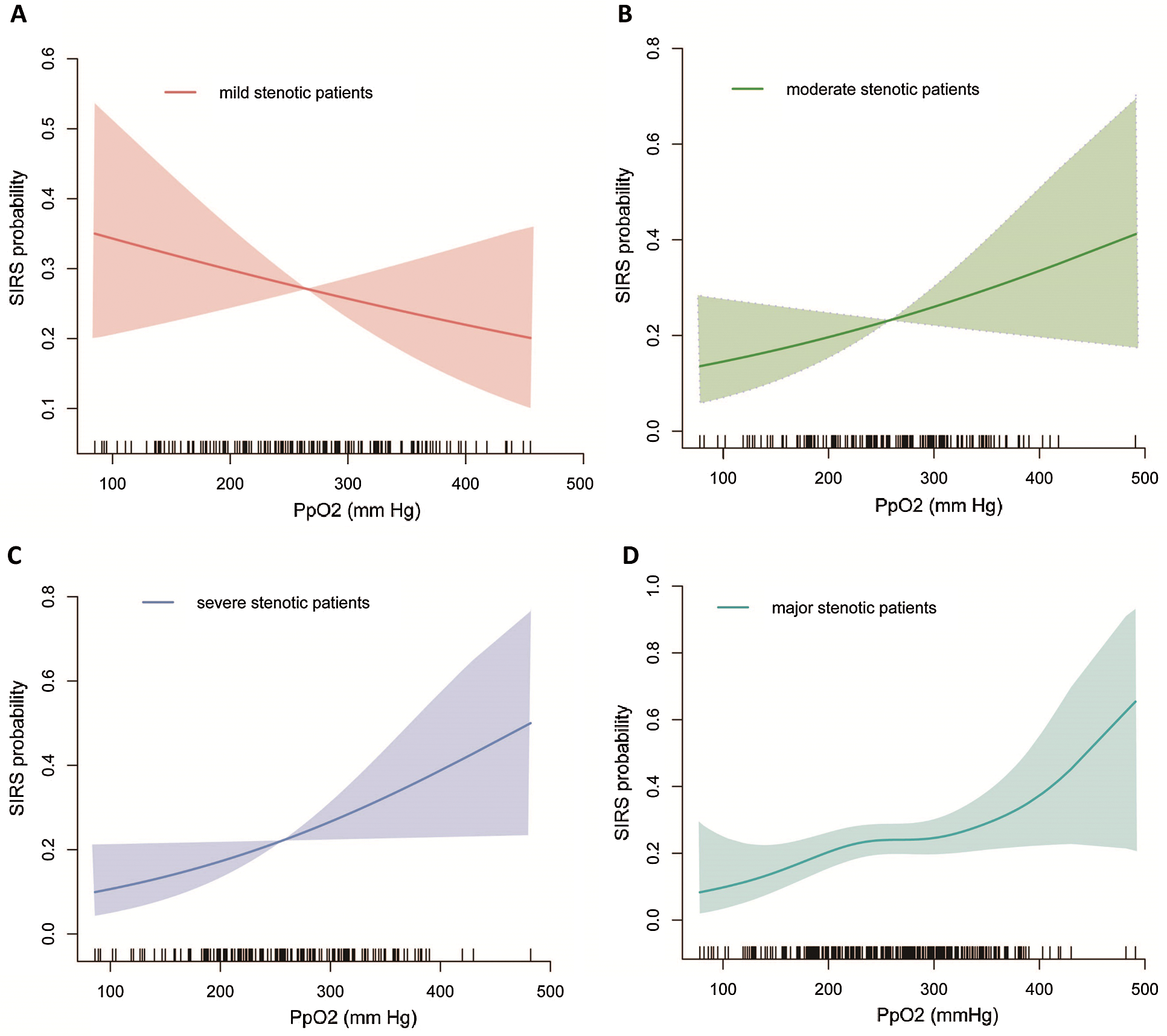
Figure 1: Functional association of PpO2 with SIRS probability by stenosis
Table 3: Association of PpO2 with systemic inflammatory response syndrome by overall and by RVOT obstruction severity
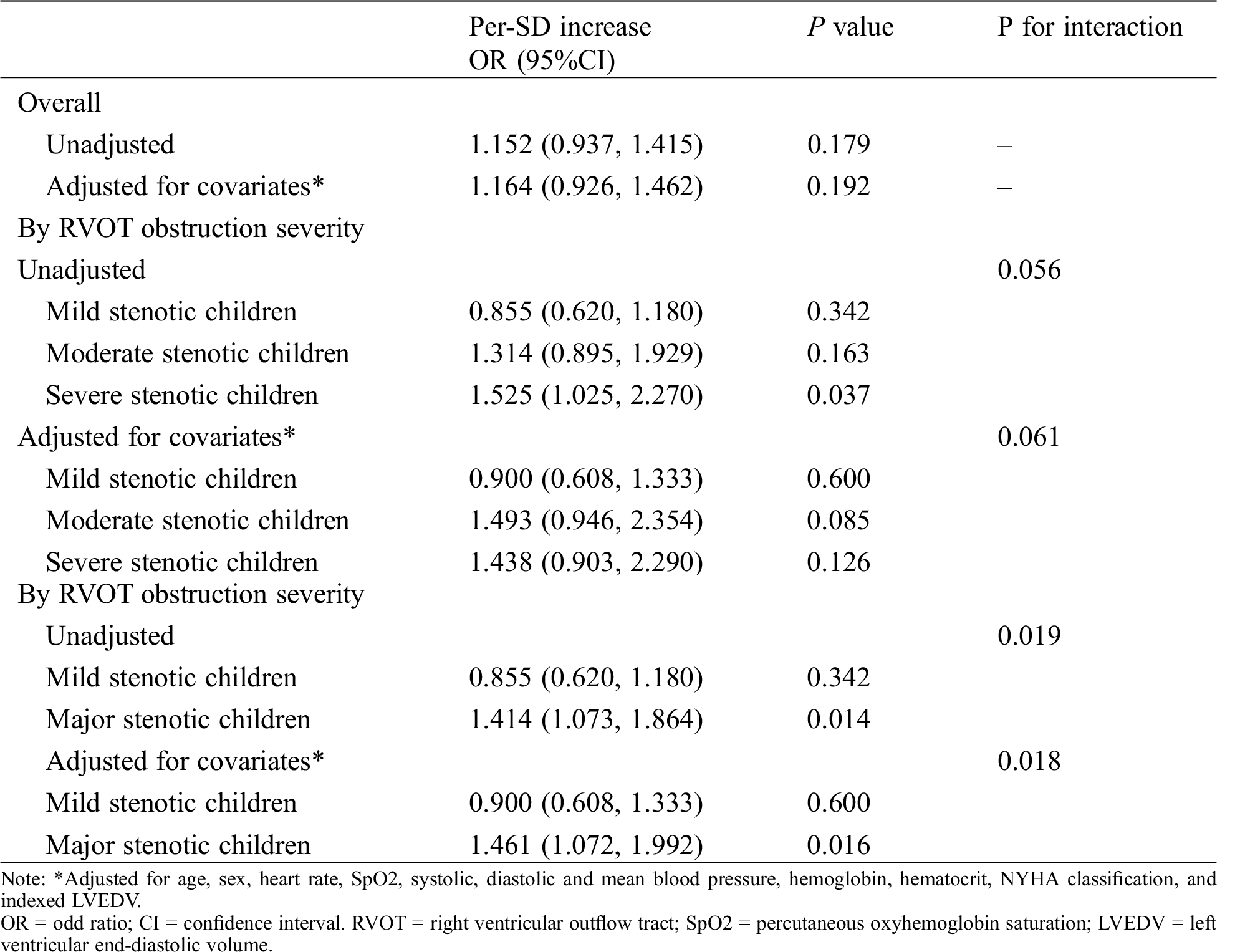
After a similar multivariable adjustment, however, a significant interactions between RVOT stenosis and PpO2 on SIRS was found when combining severe and moderate stenotic groups into an integral major stenotic group (Pinteraction = 0.0108): higher PpO2 was associated with a greater SIRS risk among major stenotic children (adjusted 1.463 [1.080,1.981] per-SD increase, P = 0.014; Fig. 1D) but not among mild stenotic children (Fig. 1A).
RVOT stenotic differences in the association between continuous PpO2 and SIRS were further accentuated in procedural differences after adjusting for baseline clinical covariates: cardioplegia type (Buckberg vs. del Nido: multivariable Pinteraction = 0.020 and 0.243, respectively), transannular patch (absence vs. presence: multivariable Pinteraction = 0.019 and 0.566, respectively), or tricuspid valve detachment (absence vs. presence: multivariable Pinteraction = 0.033 and 0.200, respectively) (Fig. 2).
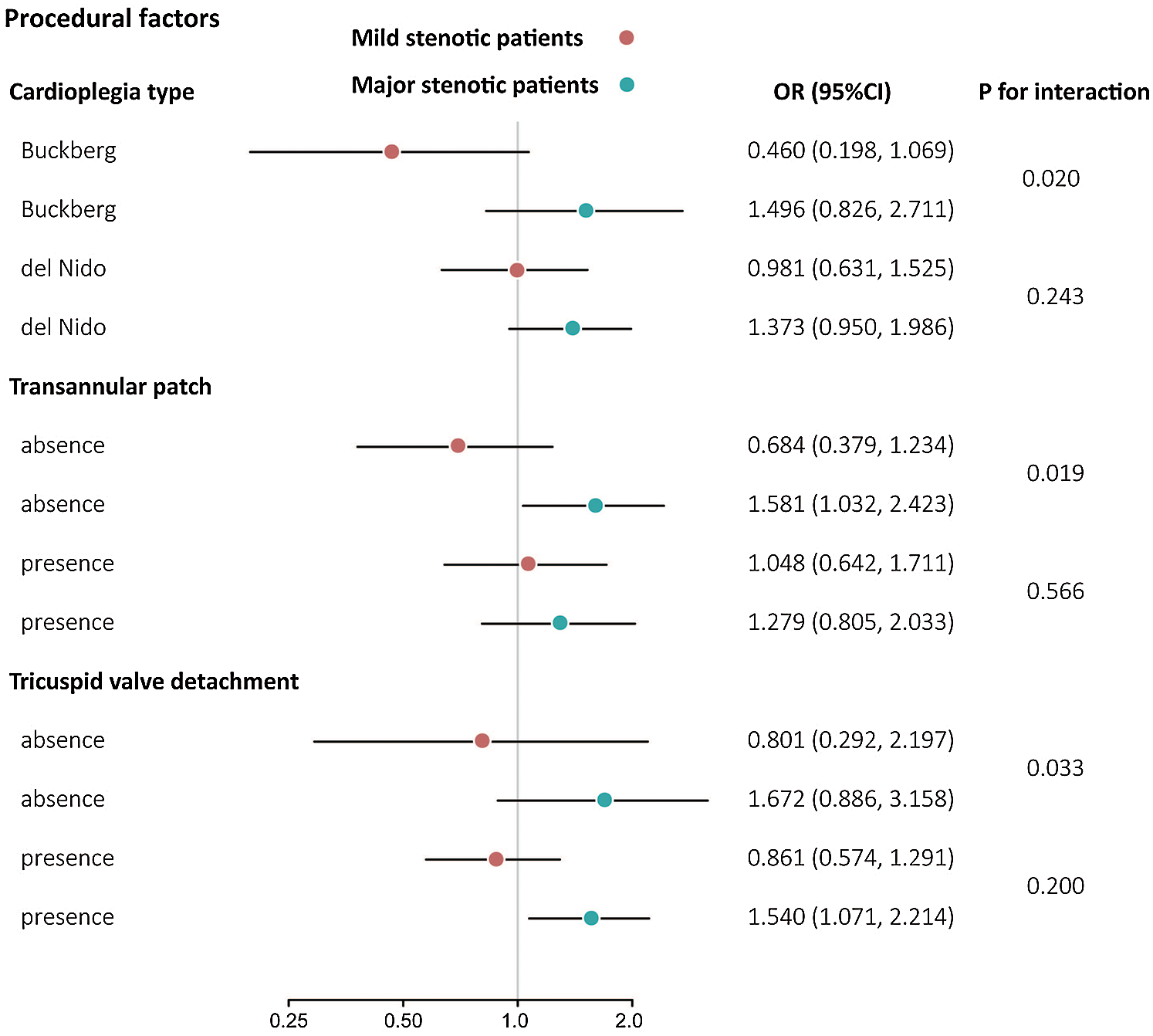
Figure 2: Three-way interaction analysis of PpO2 on SIRS by procedural factors
The cumulative incidence of SIRS by age at surgery and how it depends on triple-stenotic categorizations (Fig. 3A) and dual-stenotic categorizations (Fig. 3B) was diagrammatically presented. Patients with mild stenosis have the lowest risk probability compared to those with moderate and severe stenosis by triple-stenotic categorizations and those with major stenosis by dual-stenotic categorizations. By triple-stenotic categorizations, patients with moderate and severe stenosis have the lower SIRS-free probability than mild stenotic patients (P for log-rank < 0.001). By dual-stenotic categorizations, patients with major stenosis have the lower SIRS-free probability than mild stenotic patients (P for log-rank = 0.0005).
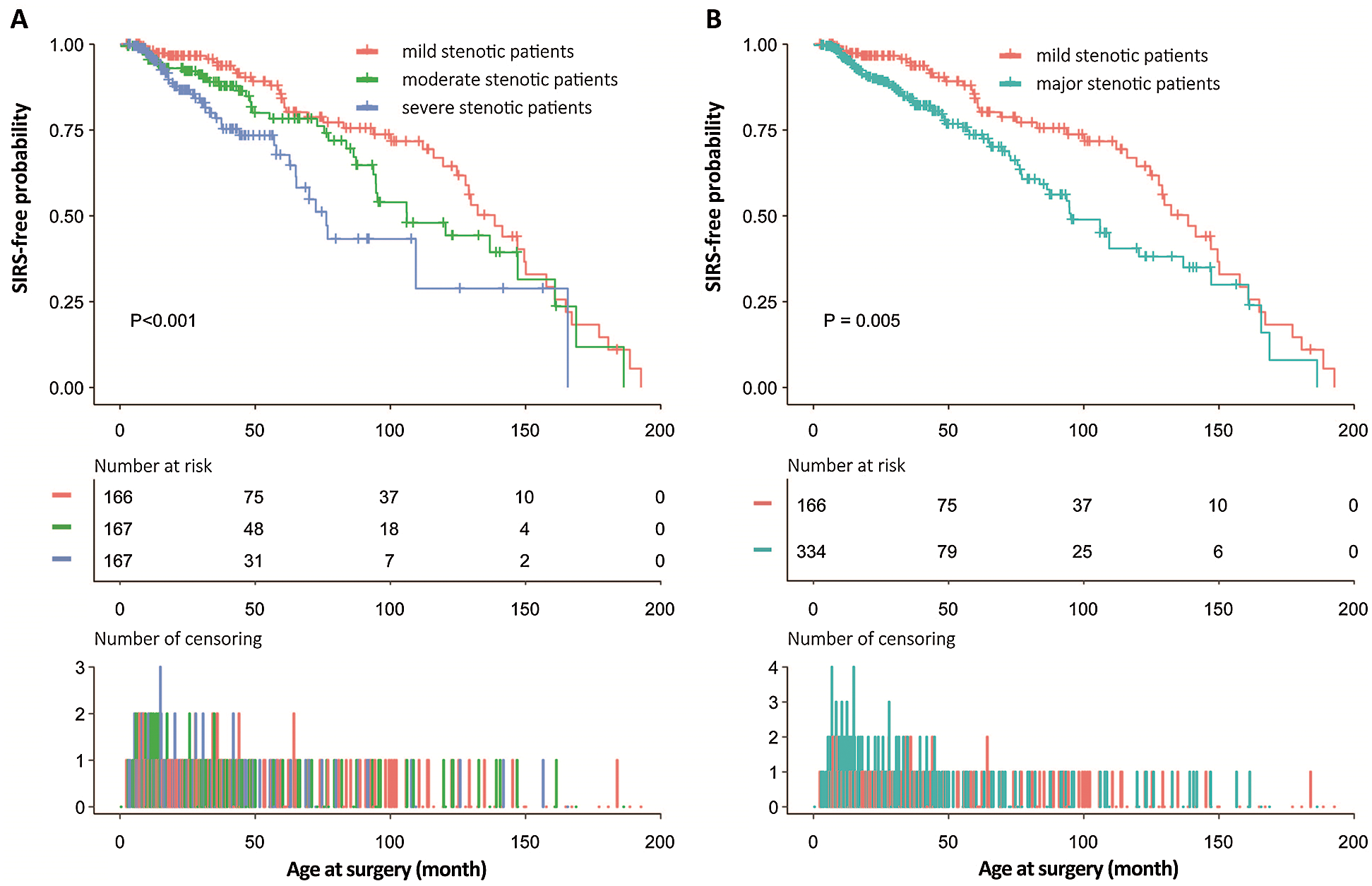
Figure 3: SIRS-free probabilities with increasing age at surgery
This study is the first report that compares PpO2 and it correlates with SIRS between different RVOT stenotic patients with tetralogy of Fallot using the real-world patient-based data. Findings suggest RVOT stenosis significantly modified the relationship between PpO2 and SIRS in complete repair of tetralogy of Fallot, with a steeper association between higher PpO2 and SIRS increase among moderate and severe stenotic children but not mild stenotic children.
Based on previous findings that hyperoxemic reoxygenation is associated with greater hypoxia-reoxygenation injury than did normoxemic reoxygenation in cyanotic and acyanotic individuals [5–8,18], we hypothesized that the differences in RVOT stenosis might have differential influences on inflammatory response to reoxygenation during CPB. While we indeed found higher PpO2 was associated with a trend towards increased risk of SIRS, we were surprised to find no significantly statistical differences between PpO2 and SIRS before and after adjusting potential confounding factors.
It has been well recognized that RVOT obstruction severity is a major determinant and indicator of hypoxic degree in patients with tetralogy of Fallot [10,19], so we extrapolated that the differences in RVOT stenosis might have differential influences on inflammatory response to reoxygenation. Our findings from interaction analysis showed that higher PpO2 was associated with a trend toward a greater SIRS risk for moderate and severe stenotic patients but not for mild stenotic patients with a marginal significant interaction. After we combined patients with severe and moderate stenosis into major stenotic integral, there was a significant interaction between stenosis and PpO2 on SIRS: higher PpO2 was associated with a greater SIRS risk among major stenotic patients but not among mild stenotic patients.
The mechanisms underlying the greater risk of SIRS with PpO2 increase in major stenotic children than mild stenotic children may relate to intrinsically severer impairment in autoregulatory capacity of systemic organs and systems among major stenotic patients than mild stenotic patients [20,21], thus rendering major organs and systems more susceptible to hypoxia-reoxygenation injury induced by oxygen-derived free radicals and lipid peroxidation when perfused with higher PpO2 during CPB.
On the contrary to the majority of prior conclusion that hyperoxemic reoxygenation is associated with poor outcome in cyanotic patients, we did not observe the association of higher PpO2 with greater risk of SIRS in mild stenotic children which is also consistent with Smith’s report [22]. The possible explanation is that PpO2 during CPB in our institute has not yet exceed the critical threshold for mild stenotic children, thereby not leading to significantly negative effect on inflammatory response.
Among mild stenotic group the likely hood of SIRS decreases with increased PpO2, but in moderate and severe group the likely hood of SIRS increases with increased PpO2. It highlights differential control of re-oxygenation for different stenosis-specific patients with tetralogy of Fallot in CPB management. Not all patients with tetralogy of Fallot should lower PpO2 levels on bypass. For the mild stenosis patients, a modest increase in PpO2 level is also beneficial.
While our findings are notable, we acknowledge that our observational data from this present study cannot provide conclusive evidence for different PpO2 cut-offs for mild, moderate, and severe stenotic children and cannot be extrapolated to response to treatment such as conventional oxygenation management [23]. Although we test for three-way interactions among RVOT stenosis, PpO2 and procedural factors such as repair pathway, transannular patch, and tricuspid valve detachment, the results should be interpreted with caution as we had limited statistical power to assess the stenosis-specific relationships of PpO2 with SIRS between subgroups due to relatively low patient numbers in those groups.
Based on our hypothesis, it is the highest value of PpO2 that was regarded as independent variable in the present study, which might overestimate the effect of PpO2 on SIRS. Results may therefore be considered hypothesis-generating and deserve further study using average value or lowest of PpO2 as independent variable. It is well known that different CPB strategies including prime constitutes and flow rates may have different effects on inflammatory response and organ functions [24]. In this study, our CPB strategies had little changes during the study period, leading to reducing the impact of prime constitutes and flow rates on the endpoint event. We carefully adjusted our analysis for the potential risk factors selected from available baseline clinical variables; however, residual confounding by unmeasured characteristics cannot be excluded. Taking into consideration the sample cohort age, the generalizability of our results must be interpreted in the context of demographic and clinical characteristics, especially for old age at surgery in our current study that is different from that of much of the rest of the world.
These first data that assess the association of PpO2 with SIRS between different severities of RVOT obstruction showed that RVOT stenosis significantly modified the relationship between PpO2 and SIRS in tetralogy of Fallot, with a steeper association between the increase in hyperoxemic reoxygenation and the increase in SISR probability among moderate and severe stenotic children but not among mild stenotic children. Further studies are needed to determine whether RVOT stenosis-specific PpO2 cut-offs during CPB for clinical decision-making regarding reoxygenation management in repair of tetralogy of Fallot should be considered.
Data Sharing: The data are available on request from the corresponding author.
Funding Statement: This work was supported by the National Natural Science Foundation of China (82000305, 81974033) and the National Natural Science Foundation of Jiangsu Province (BK20191069).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yellon, D. M., Hausenloy, D. J. (2007). Myocardial reperfusion injury. New England Journal of Medicine, 357(11), 1121–1135. DOI 10.1056/NEJMra071667. [Google Scholar] [CrossRef]
2. Kozik, D. J., Tweddell, J. S. (2006). Characterizing the inflammatory response to cardiopulmonary bypass in children. Annals of Thoracic Surgery, 81(6), S2347–S2354. DOI 10.1016/j.athoracsur.2006.02.073. [Google Scholar] [CrossRef]
3. Caputo, M., Mokhtari, A., Rogers, C. A., Panayiotou, N., Chen, Q. et al. (2009). The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: A randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery, 138(1), 206–214. DOI 10.1016/j.jtcvs.2008.12.028. [Google Scholar] [CrossRef]
4. Morita, K. (2012). Surgical reoxygenation injury of the myocardium in cyanotic patients: Clinical relevance and therapeutic strategies by normoxic management during cardiopulmonary bypass. General Thoracic and Cardiovascular Surgery, 60(9), 549–556. DOI 10.1007/s11748-012-0115-2. [Google Scholar] [CrossRef]
5. Allen, B. S., Rahman, S., Ilbawi, M. N., Kronon, M., Bolling, K. S. et al. (1997). Detrimental effects of cardiopulmonary bypass in cyanotic infants: Preventing the reoxygenation injury. Annals of Thoracic Surgery, 64(5), 1381–1388. DOI 10.1016/S0003-4975(97)00905-3. [Google Scholar] [CrossRef]
6. del Nido, P. J.,Mickle, D. A., Wilson, G. J., Benson, L. N., Coles, J. G. et al. (1987). Evidence of myocardial free radical injury during elective repair of tetralogy of Fallot. Circulation, 76(5 Pt 2), V174–V179. [Google Scholar]
7. Ghorbel, M. T., Mokhtari, A., Sheikh, M., Angelini, G. D., Caputo, M. (2012). Controlled reoxygenation cardiopulmonary bypass is associated with reduced transcriptomic changes in cyanotic tetralogy of Fallot patients undergoing surgery. Physiological Genomics, 44(22), 1098–1106. DOI 10.1152/physiolgenomics.00072.2012. [Google Scholar] [CrossRef]
8. Ihnken, K., Morita, K., Buckberg, G. D., Winkelmann, B., Beyersdorf, F. et al. (1996). Reduced oxygen tension during cardiopulmonary bypass limits myocardial damage in acute hypoxic immature piglet hearts. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 10(12), 1127–1135. DOI 10.1016/S1010-7940(96)80361-7. [Google Scholar] [CrossRef]
9. Restivo, A., Piacentini, G., Placidi, S., Saffirio, C., Marino, B. (2006). Cardiac outflow tract: A review of some embryogenetic aspects of the conotruncal region of the heart. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 288(9), 936–943. DOI 10.1002/ar.a.20367. [Google Scholar] [CrossRef]
10. Apitz, C., Webb, G. D., Redington, A. N. (2009). Tetralogy of fallot. The Lancet, 374(9699), 1462–1471. DOI 10.1016/S0140-6736(09)60657-7. [Google Scholar] [CrossRef]
11. Liu, H., Zheng, S. Q., Qian, S. C., He, H. H., Xue, J. R. (2020). Haematocrit differences modify the association of cardiopulmonary bypass reoxygenation with acute kidney injury after paediatric tetralogy of Fallot repair. Perfusion, 35(4), 284–289. DOI 10.1177/0267659119871777. [Google Scholar] [CrossRef]
12. Baumgartner, H., Hung, J., Bermejo, J., Chambers, J. B., Evangelista, A. et al. (2009). Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Journal of the American Society of Echocardiograph: Official Publication of the American Society of Echocardiography, 22(1), 1–102. DOI 10.1016/j.echo.2008.11.029. [Google Scholar] [CrossRef]
13. Valente, A. M., Cook, S., Festa, P., Ko, H. H., Krishnamurthy, R. et al. (2014). Multimodality imaging guidelines for patients with repaired tetralogy of fallot: A report from the American Society of Echocardiography. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography, 27(2), 111–141. DOI 10.1016/j.echo.2013.11.009. [Google Scholar] [CrossRef]
14. Goldstein, B., Giroir, B., Randolph, A., International Consensus Conference on Pediatric Sepsis (2005). International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 6(1), 2–8. [Google Scholar]
15. Gaies, M. G., Gurney, J. G., Yen, A. H., Napoli, M. L., Gajarski, R. J. et al. (2010). Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 11(2), 234–238. [Google Scholar]
16. Wielkopolan, B., Obrępalska-Stęplowska, A. (2016). Three-way interaction among plants, bacteria, and coleopteran insects. Planta, 244(2), 313–332. DOI 10.1007/s00425-016-2543-1. [Google Scholar] [CrossRef]
17. Qizilbash, N., Gregson, J., Johnson, M. E., Pearce, N., Douglas, I. et al. (2015). BMI and risk of dementia in two million people over two decades: A retrospective cohort study. The Lancet. Diabetes & Endocrinology, 3(6), 431–436. DOI 10.1016/S2213-8587(15)00033-9. [Google Scholar] [CrossRef]
18. Raddatz, E., Thomas, A. C., Sarre, A., Benathan, M. (2011). Differential contribution of mitochondria, NADPH oxidases, and glycolysis to region-specific oxidant stress in the anoxic-reoxygenated embryonic heart. American Journal of Physiology. Heart and Circulatory Physiology, 300(3), H820–H835. DOI 10.1152/ajpheart.00827.2010. [Google Scholar] [CrossRef]
19. Zabala, L. M., Guzzetta, N. A. (2015). Cyanotic congenital heart disease (CCHDFocus on hypoxemia, secondary erythrocytosis, and coagulation alterations. Paediatric Anaesthesia, 25(10), 981–989. DOI 10.1111/pan.12705. [Google Scholar] [CrossRef]
20. Rafiee, P., Shi, Y., Kong, X., Pritchard, K. A., Jr, Tweddell, J. S. et al. (2002). Activation of protein kinases in chronically hypoxic infant human and rabbit hearts: Role in cardioprotection. Circulation, 106(2), 239–245. DOI 10.1161/01.CIR.0000022018.68965.6D. [Google Scholar] [CrossRef]
21. Yuan, X., Lee, J. W., Bowser, J. L., Neudecker, V., Sridhar, S. et al. (2018). Targeting hypoxia signaling for perioperative organ injury. Anesthesia and Analgesia, 126(1), 308–321. DOI 10.1213/ANE.0000000000002288. [Google Scholar] [CrossRef]
22. Smith, J. M., Roberts, W. H., Miller, J. D., Hasselfeld, K. A., Hendy, M. P. (2006). Controlled cardiac reoxygenation does not improve myocardial function following global myocardial ischemia. International Journal of Surgery (London, England), 4(3), 153–159. DOI 10.1016/j.ijsu.2006.05.017. [Google Scholar] [CrossRef]
23. Medikonda, R., Ong, C. S., Wadia, R., Goswami, D., Schwartz, J. et al. (2019). Trends and updates on cardiopulmonary bypass setup in pediatric cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia, 33(10), 2804–2813. DOI 10.1053/j.jvca.2019.01.025. [Google Scholar] [CrossRef]
24. Tadphale, S. D., Ramakrishnan, K., Spentzas, T., Kumar, T., Allen, J. et al. (2020). Impact of different cardiopulmonary bypass strategies on renal injury after pediatric heart surgery. Annals of Thoracic Surgery, 111(4), 1374–1379. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |