

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015527
ARTICLE
Morphology and Function of the Aortic Valve after Transcatheter Closure of Ventricular Septal Defect with Aortic Valve Prolapse
1Graduate School, The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
2Department of Cardiac Pediatrics, Guangdong Provincial Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
3Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
4School of Medicine, South China University of Technology, Guangzhou, China
*Corresponding Author: Zhiwei Zhang. Email: drzhangzw@sohu.com
Received: 25 December 2020; Accepted: 10 March 2021
#All three authors contributed equally to this article
Abstract: Objective: This study aims to evaluate the morphology and function of the aortic valve after transcatheter closure of ventricular septal defect (VSD) with aortic valve prolapse (AVP) abased on clinical and radiological outcomes. Methods: From January 2013 to November 2014, 164 consecutive patients (97 males, 59.1%) with VSD and AVP were treated by transcatheter closure. The patients were divided into the mild AVP group (n = 63), moderate AVP group (n = 89) and severe AVP group (n = 12). The clinical and radiological outcomes of these patients were analyzed retrospectively. Results: In total, 146 (89.0%) patients were successfully treated with VSD occluders, including 59/63 (93.7%) with mild AVP, 80/89 (89.9%) with moderate AVP and 7/12 (58.3%) with severe AVP. The degree of AVP was ameliorated or disappeared in 39 (26.7%) patients, and remained unchanged in 103 (70.5%) patients after the intervention. In the 35 patients who initially had trivial-to-moderate aortic regurgitation (AR), the degree of AR was ameliorated or disappeared in 25 (71.4%) patients, aggravated from trivial to mild AR in 1 (2.9%) patient, and remained unchanged in 9 (25.7%) patients. In 111 patients without AR, 1 (0.9%) patient had mild AR and 24 (21.6%) patients had trivial AR after intervention. The depth and width of the prolapsed aortic valve decreased after transcatheter closure of VSD in all three groups. During the 70-month (range, 54–77) follow-up period, no patients with AVP and AR needed an aortic valve intervention. Conclusions: Transcatheter closure of VSD with AVP is feasible. The morphology and function of the prolapsed aortic valve improved and remained stable for a long period after intervention.
Keywords: Ventricular septal defect; aortic valve prolapse; aortic regurgitation
Perimembranous ventricular septal defect (PmVSD) is the most common type of ventricular septal defect (VSD), which is prone to aortic valve prolapse (AVP) and even aortic regurgitation (AR). Transcatheter closure has achieved good results in the treatment of VSD with AVP [1,2]. However, due to the proximity of the PmVSD to the prolapsed aortic valve, AR easily occurs. Therefore, transcatheter closure of PmVSD with AVP is still controversial.
For patients with PmVSD and AVP, transcatheter closure is constantly being explored, and the short-term outcome is satisfactory [3,4]. Nevertheless, the long-term morphology and function of the aortic valve are rarely reported.
In this study, we retrospectively evaluated the morphology and function of the aortic valve after transcatheter closure of the VSD with AVP based on clinical and radiological outcomes.
From January 2013 to November 2014, 164 consecutive patients (97 males, 59.1%) with VSD and AVP were treated with transcatheter closure at our institution. The clinical and imaging outcomes of these patients were retrospectively reviewed. The degree of AVP was evaluated by aortic root angiography and the degree of AR was evaluated by transthoracic echocardiography (TTE).
The morphology and function of the aortic valve were investigated in all subjects who met the following inclusion criteria: (i) diagnosis of PmVSD with AVP by cardiac catheterization; and (ii) age ≥ 24 months. The exclusion criteria were as follows: (i) PmVSD with AVP combined with other congenital heart diseases; (ii) VSD with a right to left shunt; (iii) bicuspid aortic valve; and (iv) other heart malformations requiring surgical treatment or residual shunt after VSD surgical repair.
This retrospective study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2016427A). Individual written informed consent was obtained from each patients’ guardian before intervention.
The technique was performed as described previously [5,6]. The procedure was under the monitoring of TTE and fluoroscopy. After vascular access was established through the right femoral artery and vein, right and left cardiac catheterization and aortic root angiography were performed at the left anterior oblique 60° and cranial 20°. The location, shape, and size of the VSD, the degree and morphology of AVP and the relationship between the VSD and AVP were confirmed. A 5-F cut pigtail angiographic catheter was sent into the left ventricle and passed through the VSD. A 0.032-inch guide wire was fed along the 5-F catheter into the superior vena cava, inferior vena cava or pulmonary artery. A snare pulled the guide wire from the femoral vein, forming a femoral artery-VSD-femoral vein loop. An appropriate delivery sheath was introduced into the femoral vein along the guide wire, through the VSD into the ascending aorta, and then back to the left ventricle. An occluder size 1–3 mm larger than the size of VSD measured by left ventriculography was selected. An occluder was advanced along the delivery sheath, then the left disc and the waist were deployed in the left ventricle, the right disc was deployed in the right ventricle. Finally, left cardiac catheterization, aortic root angiography and TTE were performed again to evaluate the position, shape of occluder, the presence of residual shunts and AR before releasing the device.
Symmetrical VSD occluders (Lifetech Scientific Corporation, Shenzhen, China), eccentric VSD occluders (Lifetech Scientific Corporation, Shenzhen, China), and the Amplatzer Duct Occluder II (ADO-II; AGA Medical Corporation, Minnesota, USA) were used in our study.
2.4 Morphology and Degree of AVP
Aortic root angiography was performed at the left anterior oblique 60° and cranial 20° in all patients before and after transcatheter closure of the VSD.
The degree of AVP was evaluated according to aortic root angiography at the end of ventricular diastole. AVP was divided into three grades [7,8]: mild (buckling of the aortic cusp down the left ventricular outflow tract without right coronary sinus prolapse into the VSD), moderate (buckling of the cusp with obvious herniation and right coronary sinus prolapse into the VSD) and severe (prolapse of the cusp and right coronary sinus prolapse into the right ventricle through the VSD).
The shape of the AVP was measured by aortic root angiography at the end of ventricular diastole. A straight line was drawn horizontally through the tip of the left coronary valve, parallel to the junction of the aortic sinus and ascending aorta. The depth and width of the AVP were measured. The data were corrected according to the diameter of the pigtail catheter used during radiography (Fig. 1).

Figure 1: Method for measuring the morphology of AVP
2.5 Data Collection and Follow-Up
Clinical data were obtained from databases, outpatient or telephone follow-up. All patients were required to undergo TTE, electrocardiogram (ECG) and clinical evaluation before intervention, and then 1 month, 3 months, 6 months, and 1 year after intervention and annually thereafter. The aborted procedure included death, more than moderate AR, new arrhythmias requiring pacemaker treatment, residual shunts or other complications requiring surgical or interventional treatment during follow-up.
The grading of AR was evaluated by TTE using the ratio of jet width and left ventricular outflow tract diameter during ventricular systole. AR was divided into four grades [8,9]: trivial (ratio < 10%), mild (ratio = 10%–24%), moderate (ratio = 25%–49%) and severe (ratio > 50%).
Aortic root angiography was performed at the left anterior oblique 60°and cranial 20°.The shape of the AVP was measured by aortic root angiography at the end of ventricular diastole. A straight line was drawn horizontally through the tip of the left coronary valve, parallel to the junction of the aortic sinus and ascending aorta. The data were corrected according to the diameter of the pigtail catheter used during radiography. A is the depth of AVP and b is the width of AVP.
The results are shown as n (%), mean ± standard deviation or median and IQRs. Statistical analysis was performed with SPSS 25.0 software (IBM Corporation, Chicago, USA). The Shapiro–Wilk test was used to verify normal distribution. The Student’s t-test was used to compare the groups of continuous variables with normal distribution, and the Mann–Whitney U-test was used to compare the groups of continuous variables without normal distribution or rank variable. Differences were considered significant when two-tailed P values were <0.05.
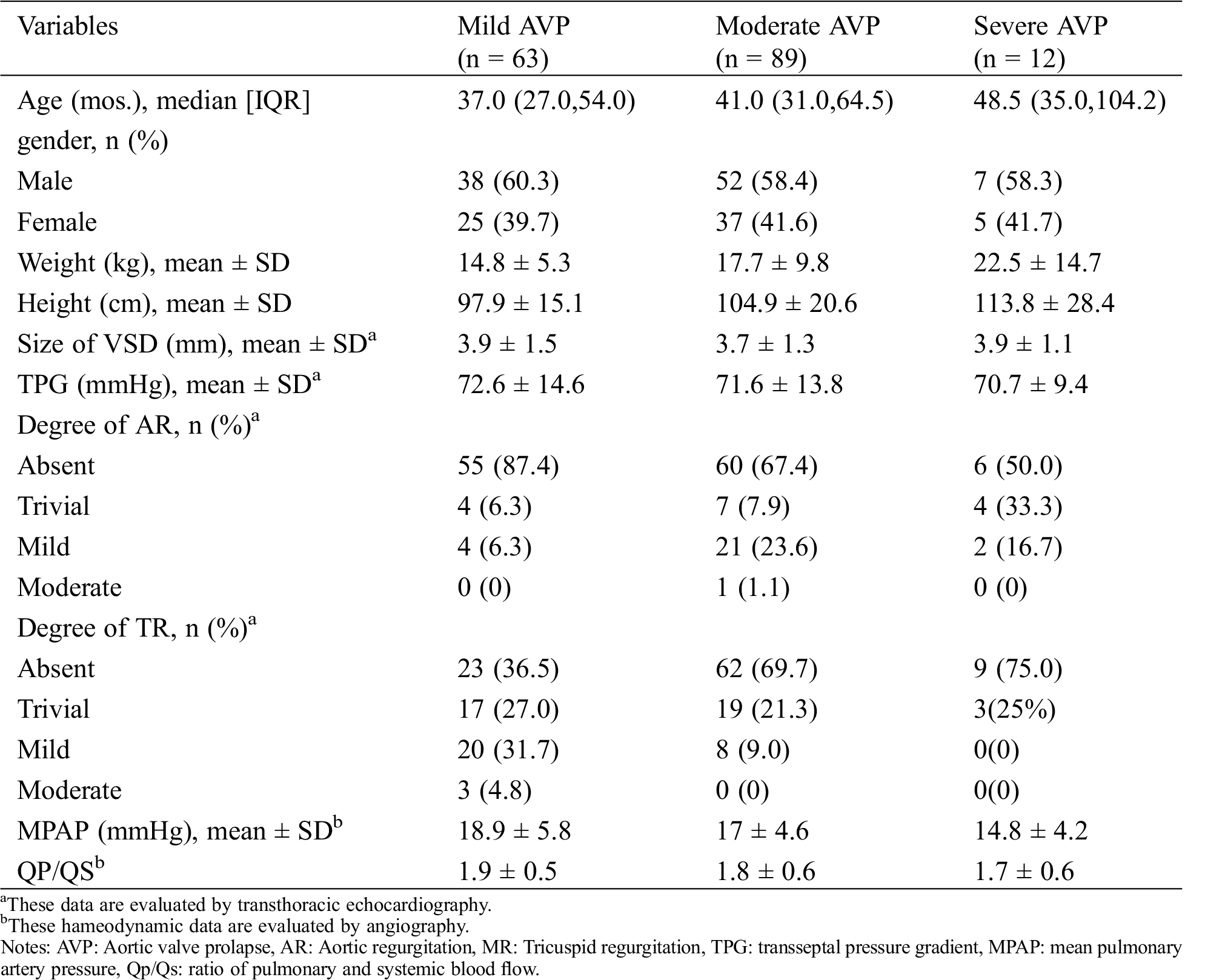
Patients’ characteristics are shown in Tab. 1. The mean age was 55.0 months (range 24–330 months), and mean weight was 16.94 ± 9.02 kg. All patients were divided into the mild AVP group (n = 63), moderate AVP group (n = 89) and severe AVP group (n = 12) according to the morphology of the aortic valve examined by cardiac catheterization before transcatheter closure.
Table 1: Patient characteristics
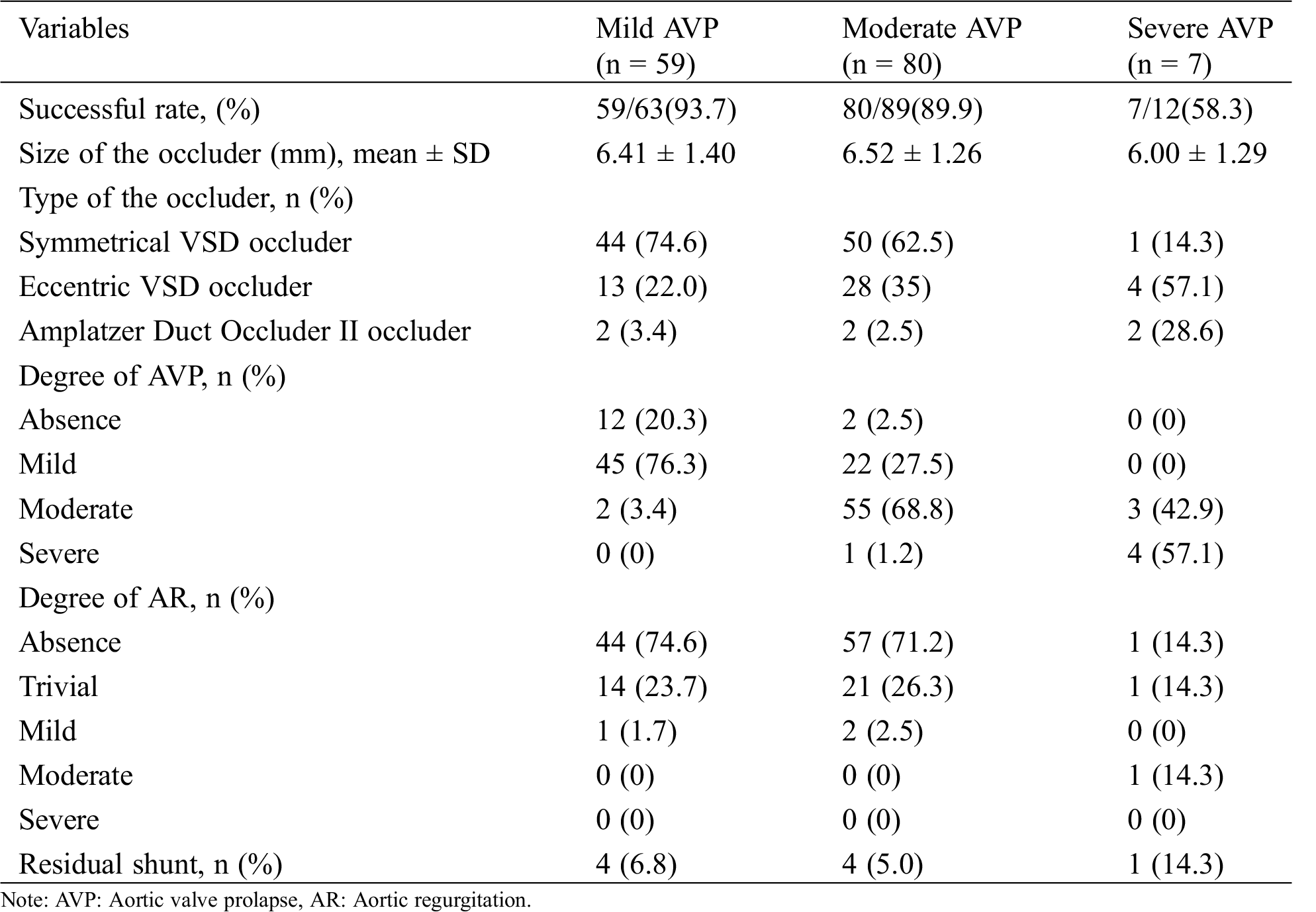
3.2 Success Rate of Transcatheter Closure
Of the 164 patients, 146 (89.0%) were successfully treated with VSD occluders, including 59/63 (93.7%) with mild AVP, 80/89 (89.9%) with moderate AVP and 7/12 (58.3%) with severe AVP. A trivial to small residual shunt was found in 4 patients (6.8%) with mild AVP, in 4 patients (5.0%) with moderate AVP and in 1 patient (14.3%) with severe VSD. No deaths, hemolysis, or pericardial tamponade occurred during the perioperative period (Tab. 2). All patients with unsuccessful interventional therapy were admitted to pediatric cardiac surgery for surgical treatment (Fig. 2).

Figure 2: Aortic root angiography of VSD with different degrees of AVP before and after transcatheter closure. A, B, C: Preoperative aortic root angiography of mild, moderate and severe AVP. D, E, F: Postoperative aortic root angiography of mild, moderate and severe AVP
Table 2: Postoperative clinical outcome
Of the 146 patients with VSD and AVP, the degree of AVP was ameliorated or even disappeared in 39 (26.7%) patients, and remained unchanged in 103 (70.5%) patients after the intervention. Two patients (3.4%) with mild AVP increased to moderate AVP, and one (1.2%) patient with moderate AVP increased to severe AVP after the intervention.
In patients with VSD and mild AVP (n = 59), the depth of the AVP was reduced from 1.27 ± 0.33 mm to 0.94 ± 0.53 mm (P < 0.0001), and the width of the AVP was reduced from 3.84 ± 1.32 mm to 2.82 ± 1.63 mm (P < 0.0001). In patients with VSD and moderate AVP (n = 87), the depth of the AVP was reduced from 2.24 ± 0.27 mm to 1.88 ± 0.56 mm (P < 0.0001), and the width of the AVP was decreased from 6.74 ± 1.66 mm to 5.56 ± 1.86 mm (P < 0.0001). In patients with VSD and severe AVP (n = 7), the depth of the AVP was reduced from 4.03 ± 0.68 mm to 2.92 ± 0.75 mm (P = 0.0015), and the width of the AVP was changed from 10.02 ± 1.74 mm to 9.20 ± 2.35 mm (P = 0.0503) (Fig. 3).

Figure 3: Morphological changes of AVP before and after transcatheter closure of VSD in different degrees of AVP. *** P < 0.001, ** P < 0.01, ns: no significance
3.4 Functional Valvular Outcome
Of the patients with AR, 8 patients with mild AVP, 25 patients with moderate AVP and 2 patients with severe AVP successfully completed transcatheter closure of VSD. Two patients with VSD and AVP, accompanied by moderate AR were not successfully treated with transcatheter closure of VSD (Fig. 4).

Figure 4: Results of postprocedural AR
Of the 35 patients who initially had trivial-to-moderate AR, the degree of AR was ameliorated or even disappeared in 25 (71.4%) patients, aggravated from trivial to mild AR in 1 (2.9%) patient, and remained unchanged in 9 (25.7%) patients after the intervention. In 111 patients without preprocedural AR, 1 (0.9%) patient had mild AR and 24 (21.6%) patients had trivial AR after transcatheter closure of VSD.
Of the 35 patients who initially had trivial-to-moderate AR, the degree of AR was ameliorated or even disappeared in 25 patients, aggravated from trivial to mild AR in 1 patient, and remained unchanged in 9 patients after the intervention. In 111 patients without preprocedural AR, 1 patient had mild AR and 24 patients had trivial AR after transcatheter closure of VSD.
Clinical follow-up ended in October 2020 and was complete in 137/146 (93.8%) of patients. The median (1st–3rd quartile) follow-up time was 70 months (54–77 months). During the follow-up period, no patients with AVP and AR required aortic valve intervention. Only one patient (0.7%) with moderate AVP increased from mild AR to moderate AR at one year after the intervention, and one patient (0.7%) with mild AVP increased from mild AR to moderate AR at two years after the intervention. The degree of AR in other patients remained stable at trivial-to-mild AVP or even disappeared during the follow-up period. No cases of valve perforation or aggravated regurgitation due to long-term attrition of the occluder occurred.
Due to the lack of sufficient tissue support in the aortic valve ring and the Venturi effect under the valve [10], AVP and AR develop in patients with VSD [11]. Approximately 7.8%–24.8% of patients with VSD also have associated AVP [12–15], and AR gradually appears as the disease develops. Traditionally, surgery is generally recommended if AVP or AR occurs in patients with VSD [11]. However, during surgical thoracotomy, for patients with VSD combined with AVP or mild AR, simply repairing VSD can be corrected, without treating the prolapsed aortic valve [12]. Based on this principle, transcatheter closure was attempted and has achieved good results in patients with VSD and AVP in recent years [3,4,8,16]. Considering that transcatheter closure can prevent the progression of aortic valve disease and further hemodynamic disorder and is minimally invasive, we also treated patients with intervention for those with VSD and AVP or even AR, and achieved satisfactory results.
Chen et al. [3] reported a success rate of 96.9% for VSD combined with AVP and mild AR, with few complications. In the present study, 146 (89.0%) patients were successfully treated with VSD occluders, including 59/63 (93.7%) with mild AVP, 80/89 (89.9%) with moderate AVP and 7/12 (58.3%) with severe AVP. In patients with VSD and mild AVP, the success rate of transcatheter closure in our center was similar to that of Chen and colleagues. Satisfactory outcomes were been obtained for patients with moderate AVP. No complications such as residual shunt and severe arrhythmia requiring interventional or surgical therapy occurred in our cohort.
AVP and AR have been shown to be associated with VSD [11,17]. However, few data are available regarding postoperative morphology and function of the aortic valve in patients who have undergone transcatheter closure of VSD alone. In the present study, in 97% of patients with successful occlusion, the degree of AVP remained stable, was ameliorated or even disappeared. For those with AVP of different grades, the depth and width of the prolapsed aortic valve decreased after transcatheter closure of VSD, which meant that abnormal morphology of the aortic valve was improved. In addition, no AVP requiring intervention was found during the long-term follow-up period. This proves the feasibility of interventional therapy in patients with VSD and AVP.
AR is a criterion for evaluating aortic valve function, which is associated with the progression of AVP [18]. AVP can gradually develop into AR, which usually takes 3 to 5 years [15]. Chen et al. [3] demonstrated that the degree of AR was stable after intervention in most patients and only 3.6% of patients exhibited aggravated AR. Similarly, in a small series of VSD with mild AR, the degree of AR decreased or remained unchanged in most patients, and only one patient developed severe AR [4]. In the present study, the degree of AR in 93.8% of patients remained stable, was ameliorated or even disappeared after transcatheter closure of VSD, while 6.2% of patients developed trivial-to-mild AR. During the postoperative follow-up period, only two patients progressed to moderate AR and did not need surgical therapy. These results suggest that transcatheter closure of VSD is feasible and safe in patients with AVP and AR.
In order to prevent the progression of AVP or AR, transcatheter closure of VSD should be carried out as soon as possible in patients with VSD and AVP. When the VSD is occluded successfully, the subaortic Venturi effect will no longer exist and the morphology and function of the aortic valve will be improved. As a minimally invasive, safe and effective treatment, transcatheter closure of VSD could be an alternative treatment choice for patients with VSD and AVP.
Among the 18 patients who were unsuccessful with interventional treatment, 2 patients failed due to transient arrhythmia during procedure, 9 patients failed because of more than moderate aortic regurgitation, 6 patients failed because of obvious residual shunt and 1 patient failed due to severe tricuspid regurgitation after closure. In our constitution, we consider that patients with larger VSD, larger QP/QS, and more severe degree of AVP are more inclined to fail. Many patients with severe AVP tried interventional closure, but the results were not satisfactory, with a success rate of only 58.3%. We suggest that patients with severe AVP are not suitable for interventional closure. Therefore, since 2015, almost all patients with severe AVP have treated with surgery.
Transcatheter closure of VSD is feasible in patients with VSD and AVP. The morphology and function of the prolapsed aortic valve were improved and remained stable for a long period after intervention.
Ethical Statement: This study was reviewed and approved by the Institutional Review Board of Guangdong Province People’s Hospital (Guangzhou, Guangdong, China) (No. GDREC2020213H). Individual written informed consent was obtained from each patients’ guardian before intervention.
Funding Statement: This study was supported by National Key R&D Program of China (Grant No. 2016YFC1100305); and Sanming Medical Project of China (Grant No. SZSM201612057).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yang, J., Yang, L. F., Yu, S. Q., Liu, J. C., Zuo, J. et al. (2014). Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: A randomized controlled trial. Journal of the American College of Cardiology, 63(12), 1159–1168. DOI 10.1016/j.jacc.2014.01.008. [Google Scholar] [CrossRef]
2. Yang, J., Yang, L. F., Wan, Y., Zuo, J., Zhang, J. et al. (2010). Transcatheter device closure of perimembranous ventricular septal defects: Mid-term outcomes. European Heart Journal, 31(18), 2238–2245. DOI 10.1093/eurheartj/ehq240. [Google Scholar] [CrossRef]
3. Chen, G. L., Li, H. T., Li, H. R., Zhang, Z. W. (2015). Transcatheter closure of ventricular septal defect in patients with aortic valve prolapse and mild aortic regurgitation: Feasibility and preliminary outcome. Asian Pacific Journal of Tropical Medicine, 8(4), 315–318. DOI 10.1016/S1995-7645(14)60337-0. [Google Scholar] [CrossRef]
4. Chen, F., Li, P., Liu, S. X., Du, H., Zhang, B. L. et al. (2015). Transcatheter closure of intracristal ventricular septal defect with mild aortic cusp prolapse using zero eccentricity ventricular septal defect occluder. Circulation Journal: Official Journal of the Japanese Circulation Society, 79(10), 2162–2168. DOI 10.1253/circj.CJ-15-0301. [Google Scholar] [CrossRef]
5. Kanaan, M., Ewert, P., Berger, F., Assa, S., Schubert, S. (2015). Follow-up of patients with interventional closure of ventricular septal defects with Amplatzer Duct Occluder II. Pediatric Cardiology, 36(2), 379–385. DOI 10.1007/s00246-014-1017-0. [Google Scholar] [CrossRef]
6. Li, H., Shi, Y. Y., Zhang, S. Y., Ren, Y., Rong, X. et al. (2019). Short- and medium-term follow-up of transcatheter closure of perimembranous ventricular septal defects. BMC Cardiovascular Disorders, 19(1), 222. DOI 10.1186/s12872-019-1188-y. [Google Scholar] [CrossRef]
7. Leung, M. P., Chau, K. T., Chiu, C., Yung, T. C., Mok, C. K. (1996). Intraoperative TEE assessment of ventricular septal defect with aortic regurgitation. Annals of Thoracic Surgery, 61(3), 854–860. DOI 10.1016/0003-4975(95)01133-1. [Google Scholar] [CrossRef]
8. Lin, H. C., Lin, M. T., Chen, C. A., Hsu, J. Y., Lin, S. M. et al. (2021). Safety and efficacy of transcatheter closure of outlet-type ventricular septal defects in children and adults with Amplatzer Duct Occluder II. Journal of the Formosan Medical Association, 120(1), 180–188. [Google Scholar]
9. Singh, J. P., Evans, J. C., Levy, D., Larson, M. G., Freed, L. A. et al. (1999). Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). American Journal of Cardiology, 83(6), 897–902. DOI 10.1016/S0002-9149(98)01064-9. [Google Scholar] [CrossRef]
10. Chiu, S. N., Wang, J. K., Lin, M. T., Wu, E. T., Lu, F. L. et al. (2005). Aortic valve prolapse associated with outlet-type ventricular septal defect. Annals of Thoracic Surgery, 79(4), 1366–1371. DOI 10.1016/j.athoracsur.2004.10.012. [Google Scholar] [CrossRef]
11. Monsefi, N., Zierer, A., Risteski, P., Primbs, P., Miskovic, A. et al. (2014). Long-term results of aortic valve resuspension in patients with aortic valve insufficiency and aortic root aneurysm. Interactive Cardiovascular and Thoracic Surgery, 18(4), 432–437. DOI 10.1093/icvts/ivt530. [Google Scholar] [CrossRef]
12. Ugan, A. S., Guler, E. A. (2017). Aortic valve prolapse and aortic regurgitation during long-term follow up in children with ventricular septal defect. Journal of Heart Valve Disease, 26(6), 616–623. [Google Scholar]
13. Eroglu, A. G., Atik, S. U., Sengenc, E., Cig, G., Saltik, I. L. et al. (2017). Evaluation of ventricular septal defect with special reference to the spontaneous closure rate, subaortic ridge, and aortic valve prolapse II. Pediatric Cardiology, 38(5), 915–921. DOI 10.1007/s00246-017-1597-6. [Google Scholar] [CrossRef]
14. Kazmi, U., Sadiq, M., Hyder, S. N. (2009). Pattern of ventricular septal defects and associated complications. Journal of the College of Physicians and Surgeons, 19(6), 342–345. [Google Scholar]
15. Layangool, T., Kirawittaya, T., Sangtawesin, C., Kojaranjit, V., Makarapong, P. et al. (2008). Natural aortic valve complications of ventricular septal defect: A prospective cohort study. Journal of the Medical Association of Thailand, 91(3), S53–S59. [Google Scholar]
16. Li, J. J., Zhang, Z. W., Qian, M. Y., Li, Y. F., Wang, S. S. (2009). Transcatbeter closure of perimembranous ventricular septal defects: A comparative study between the asymmetric device and the symmetric device. Chinese Journal of Interventional Cardiology, 17(6), 301–304. [Google Scholar]
17. Lun, K., Li, H., Leung, M. P., Chau, A. K., Yung, T. et al. (2001). Analysis of indications for surgical closure of subarterial ventricular septal defect without associated aortic cusp prolapse and aortic regurgitation. American Journal of Cardiology, 87(11), 1266–1270. DOI 10.1016/S0002-9149(01)01517-X. [Google Scholar] [CrossRef]
18. Tomita, H., Arakaki, Y., Ono, Y., Yamada, O., Yagihara, T. et al. (2005). Impact of noncoronary cusp prolapse in addition to right coronary cusp prolapse in patients with a perimembranous ventricular septal defect. International Journal of Cardiology, 101(2), 279–283. DOI 10.1016/j.ijcard.2004.03.023. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |