

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015016
ARTICLE
Results of Fontan in Patients with Apicocaval Juxtaposition or/and Separated Hepatic Venous Drainage
Department of Pediatric Cardiac Surgery, National Center for Cardiovascular Disease and Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
*Corresponding Author: Jun Yan. Email: yanjun.1112@aliyun.com
Received: 16 November 2020; Accepted: 13 January 2021
Abstract: Objective: Modifications of the Fontan operation, which are also known as total cavopulmonary connection (TCPC), are widely applied for patients with functionally univentricular hearts (FUH). Herein, we summed up the different surgical pathways and clinical outcomes in FUH patients with apicocaval juxtaposition (ACJ) or/and separated hepatic venous (SHV) drainage. Methods: Between January 2009 and December 2019, 123 patients who undergone TCPC in our institute were included in this retrospective study. We have included 70 patients with ACJ (Group 1) and 53 patients with SHV (Group 2). Moreover, Group 2 included 17 cases combing with ACJ (32.1%). In Group 1, three different TCPC methods were conducted. While 45 cases were conducted with the extracardiac conduit-TCPC(EC-TCPC) method, 24 cases used the intracardiac conduit-TCPC(IC-TCPC) method, and only one case used the lateral tunnel-TCPC(LT-TCPC). In Group 2, four TCPC methods were conducted on patients. Forty cases used the EC-TCPC-common open technique, 6 cases with IC-TCPC technique, 4 cases with LT-TCPC, and 3 cases with intra-extracardiac conduit-TCPC(IEC-TCPC). Results: There were 7 patients in Group 1 and 14 patients in Group 2 who required early re-operation during hospitalization (p < 0.05). Postoperative mean pulmonary artery pressure (mPAP) greater than 15 mmHg emerged as a predictor for early re-operation (p < 0.01) and early death (p < 0.001) in univariate analysis. Conclusions: TCPC can be performed in these patients and shows beneficial results. Under the Fontan principle of connecting systemic venous to the pulmonary vasculature unimpededly, surgeons should carefully evaluate three components when choosing for the surgical technique: The distance between inferior vena cava (IVC) and the apex; the site of the vertebrae relative to the ACJ; the distance between ACJ and SHV if coexisting. However, the technique should be altered when the postoperative mPAP was greater than 15 mmHg.
Keywords: Modified Fontan operation; apicocaval juxtaposition; separated hepatic venous drainage; functionally univentricular hearts
Since the first successful repair of tricuspid atresia in 1971, Fontan operation has been continuously modified and received decreasing mortality over the recent years [1–3]. The modifications of Fontan procedure have been applied to a wide range of complex congenital heart defects with single-ventricular physiology. Two current modifications in common use are extracardiac conduit-total cavopulmonary connection (EC-TCPC) and lateral tunnel- total cavopulmonary connection (LT-TCPC) [4,5], especially EC-TCPC [6]. It has been well understood that apicocaval juxtaposition (ACJ) was characterized with the cardiac apex pointing toward the ipsilateral side of inferior vena cava (IVC) and separated hepatic venous (SHV) demonstrated with the IVC and the hepatic vein drained separately into the atrium. However, it was unclear on outcomes of the modified Fontan operations for ACJ or/and SHV, mainly for the relatively complex cardiac anatomy. Only limited case reports were found among published literature [7–10]. Therefore, we conducted a retrospect summed up the surgical pathways and the clinical results in these patients at the Fuwai Hospital.
We retrospectively reviewed all consecutive functionally univentricular hearts (FUH) patients with ACJ or/and SHV who underwent modified Fontan operation between January 2009 and December 2019 at Fuwai Hospital. In total, 123 patients were included in our study, with 70 patients who underwent ACJ (Group 1) and 53 patients who underwent SHV (Group 2). Patient characteristics of both groups were listed in Tab. 1 Preoperative mean pulmonary artery pressure (mPAP) was measured before CPB during the operations or the angiography. Postoperative mPAP was measured after sternal closure in the operating room. Group 1 included 36 males and 34 females with average age 7.39 ± 5.24 years (age ranging from 3 to 27 years), average weight 22.69 ± 11.79 kg (11 kg–57 kg), and preoperative average mPAP 9.30 ± 2.42 mmHg (5 mmHg–17 mmHg). In Group 1, forty-four patients (62.9%) were operated on by staged operation (forty cases of Glenn, two B-T shunt (Blalock-Taussig shunt), one Glenn with pulmonary trunk band, and one B-T with Glenn); the other 26 patients were operated on in one stage. There were 33 males and 20 females in Group 2. The average age for the group is 6.64 ± 3.25 years (2.67 years–15 years), the average weight was 20.13 ± 8.51 kg (12 kg–51 kg), and preoperative mPAP was 9.68 ± 2.29 mmHg (4 mmHg–15 mmHg). In Group 2, thirty-three patients (62.3%) had staged operations (thirty-two Glenn cases and one B-T). Another 20 patients were operated on in one stage. Group 2 also included 17 cases (32.1%) with ACJ. The mean follow-up period in this study was 5.5 years (0.42 years–11 years).
Table 1: Patient profiles [case/(¯x ± s)]

Modified Fontan operations were performed through a median sternotomy in all but one patient, for exception. Pulmonary artery, atrium, ventricle, IVC, and SHV were then dissected carefully and extensively with little tension. In Group 1, the arterial cannula was inserted into the ascending aorta in all but four patients (femoral arteries were used in three patients, and one patient did not use CPB), and then the venous cannula was inserted into SVC, innominate vein, femoral vein, or the atrium. Aortic cross-clamps and cardioplegic arrests were performed in 42 patients (60%) with an average cross-clamp time of 80.79 ± 33.45 min (21 min–155 min). In Group 2, 46 patients cannulated through the ascending aorta. Two patients cannulated through the femoral arteries, and both ascending aorta and femoral artery cannulation were conducted in three patients. Two patients did not use CPB during operations. As for the venous cannula, both the IVC and the SHV or the atrium were cannulated in 43 patients. Eight patients were cannulated only through the IVC, in which pump suction and simple intermittent clamp were used). Aortic cross-clamp and cardioplegic arrest were performed in 26 patients (49.1%) with the average clamping time of 90 ± 41.94 min (26 min–171 min).
The surgical techniques were selected under careful considerations of each patient’s preoperative angiography and echocardiography as well as during intraoperative explorations. Concomitant procedures were demonstrated in Tab. 2.
Table 2: Concomitant procedures (case)

In Group 1, three modified Fontan operation methods were performed. Forty-five cases performed EC-TCPC, in which an extracardiac conduit was anastomosed with the distal end of IVC and contralateral PA. That is, the conduit bridged the vertebra in consideration of the simplicity of this technique and thus avoided compression between conduit and ventricle, including one case, during which the Y-shaped conduit was anastomosed to LPA and RPA, respectively. No patient in Group 1 was operated on with the conduit running behind the ventricle, that the conduit was positioned straight between the IVC and ipsilateral PA. 24 cases were performed using IC-TCPC, and an intracardiac conduit was anastomosed with the PA and IVC. The remaining one case used LT-TCPC that intra-atrial Gore-Tex baffle was sutured inside the atrium and extended around both cavoatrial orifices, and then the route from IVC to PA was created. In total, 39 cases were performed with fenestration (3 mm–5 mm) on the conduit.
In Group 2, four different modified Fontan operation techniques were conducted. During the operation, when the IVC and SHV were ipsilateral or contralateral but not widely separated by the vertebra, we would perform the EC-TCPC-common open technique (en bloc resection of both IVC and SHV, and then anastomosed these veins to an extracardiac conduit). This surgical method was performed in 37 cases, including one case connecting with the two SHVs (en bloc resection) to the azygos vein through a lateral thoracotomy, which was performed with Kawashima operation in the initial palliation. IC-TCPC was performed in 6 cases. When the IVC and SHV were separated widely by the vertebrae, a modified EC-TCPC-common open technique was performed in 3 cases by connecting the IVC to SHV in a side-to-side fashion before anastomosing both of them to an extracardiac conduit. IEC-TCPC that the IVC was anastomosed to an extracardiac conduit and the SHV was anastomosed to the extracardiac conduit by an intracardiac conduit in 3 cases and the LT-TCPC in 4 cases. If an EC-TCPC-common open technique was used in patients with SHV and ACJ, conduits would bridge the vertebra to the contralateral PA. Thirty-five cases with fenestration (4 mm–7 mm) were performed on the conduit.
Python3.6 statistical software was used for data analysis. Data were expressed as mean±standard deviation (¯x ± s). Comparisons between the two groups were performed with a t-test. Risk factors were estimated by the chi-square (x2) test. Statistical significance was assigned based on a p-value of <0.05.
All patients were followed up with the last echocardiographic parameters, including ejection fraction, atrioventricular valve regurgitation (AVVR). Patients’ symptoms were also documented during follow-up, which included decreased activity, chest pain, dyspnea, cyanotic, etc. The general condition of the patients was evaluated using NYHA classification.
3.1 Early Postoperative Results
In Group 1, 7 cases required early re-operation, and one died. In Group 2, there were 14 cases that required early re-operations, and 4 cases died. The early re-operation incidence was higher in Group 2 (p < 0.05); other early postoperative results were not statistically significant. And there is no relationship between fenestration status and pleural effusions. The early survival rate was 98.6% in Group 1 and 92.5% in Group 2. Early postoperative results were listed in Tabs. 3 and 4, re-operations were listed in Tab. 5, and deaths were listed in Tab. 6.
Table 3: Early postoperative results
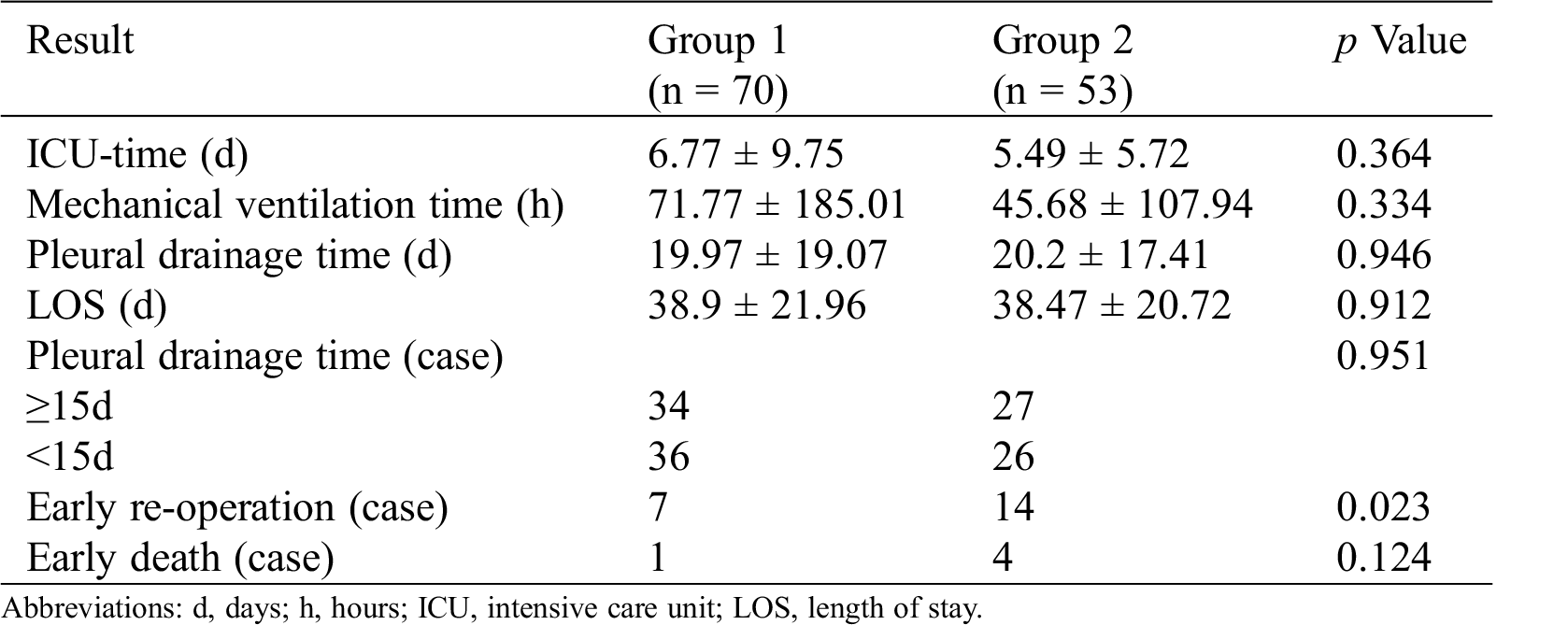
Table 4: Pleural drainage time and fenestration


Table 6: The causes of early deaths

3.2 Late Postoperative Results
The mean follow-up was 5.5 years (0.42 years–11 years). The late survival rate was 97.1% in Group 1 and 90.6% in Group 2 (p = 0.796).
In Group 1, one death occurred for renal failure one year after surgery, and 95.6% patients were categorized as NYHA I. Three cases were tested below NYHA I level, including two cases with AVVR of Grades III-IV and one with recurrent ascites.
In Group 2, one case died because of MODS 6 months after surgery. NYHA I was confirmed in 91.7% of patients. Four cases were tested below the NYHA I level, including two with AVVR of Grade IV, one with mild cyanosis, and one with protein-loss enteropathy.
Postoperative mPAP greater than 15 mmHg emerged as a predictor for early re-operation (p < 0.01) and early death (p < 0.001) in univariate analysis (Tab. 7).
Table 7: Risk factors analysis

In the wake of different modifications of Fontan operation and improvements of postoperative management for FUH patients with ACJ or/and SHV. Published studies have reported excellent clinical outcomes [11–14]. However, owing to the complex anatomical anomalies among these patients, the surgical techniques of modified Fontan operations are complicated and diverse.
For ACJ patients, many studies have shown no difference in the postoperative central venous pressure (CVP), cardiac function, and energy loss between the ipsilateral and the contralateral extracardiac conduit [15–17]. Yoshida M and colleagues have put forward that the IVC-index (IVC-index = IVC width overlapping the vertebra/width of the vertebra × 100%), and IVC-index of more than 40% is an indicator of contralateral EC-TCPC for optimal conduit routing [15]. What’s more, the contralateral EC-TCPC that bridges the vertebra may also be a better surgical technique in consideration of simplicity of the operation while shortening the CPB time and avoiding compression between the conduit and ventricle [16–18], especially for patients with poor cardiac function and enlarged ventricle [19]. The IC-TCPC should be chosen when EC-TCPC cannot be implemented for the conduit compression and severe intrapericardial adhesions [7]. LT-TCPC was hardly adopted on account of the difficulty of constructing, prolonged cross-clamp time, and the probability of obstruction to pulmonary veins orifices [17]. As for the selection of conduit materials, Kitayama H used autologous pedical pericardium to construct ipsilateral EC-TCPC. However, Kawahira et al. and colleagues reported their preference in Gore-Tex conduit for the reason that the pericardium was easily compressed [7,8].
For SHV patients, various surgical operation techniques were mainly obtained from case reports. The major technique used in current days was EC-TCPC-common open technique [20]. Nakata et al. and Schreiber et al. have also reported the modified common open technique by connecting the IVC to SHV in a side-to-side fashion before anastomosing both of them to an extracardiac conduit. However, the anastomosis may induce stenosis of IVC or SHV [10,20]. Lee et al. and colleagues used the IEC-TCPC just as we did, during which processes, careful anastomosis was needed to avoid hemorrhage [9]. Sughimoto K and colleagues have also described another kind of IEC-TCPC. While using atrial wall as the inner layer and the Gore-Tex baffle as the outer wall of the conduit, this technique has reduced the use of synthetic material [21]. For SHV patients with ACJ, Kawahira and colleagues supported using the second kind of modified common open technique (shoetree graft) to perform EC-TCPC [7]. For patients with first-stage Kawashima operation, Amodeo A and colleagues combined this technique with a direct anastomosis of the hepatic veins to azygos vein without conduit to minimize the risk of the thromboembolic accident [22]. Montesa and colleagues also connected the hepatic veins to PA by utilizing an extracardiac conduit with a conventional approach for facileness, especially under the concomitant intracardiac repairs [23]. But in view of incomplete regression of pulmonary arteriovenous malformations (PAVMs) after connecting hepatic veins to the pulmonary circulation, Alsoufi and colleagues thought either virtual surgical planning or computational flow dynamics might be helpful to select individualized optimal surgical procedure to balance the distribution of hepatic vein effluent to both lungs [24].
However, the published studies did not make clear recommendations as to which technique should be indicated by what anatomical presentation of each FUH patient with ACJ or/and SHV. Thus, under the precondition of constructing an unobstructed pathway for IVC and SHV to PA, we are trying to establish a coherent, anatomically based strategy for modified Fontan operation according to our surgical experience and successful case outcomes. The strategies are as follows.
Contralateral EC-TCPC is a preferable technique in view of avoiding compression between the conduit and ventricle. It also exhibits the simplicity of operations, especially among the patients with poor cardiac function, enlarged ventricle, and severe adhesion behind the ventricles. However, the ipsilateral EC-TCPC should be conducted by surgeons with an IVC-index of less than 40% to avoid conduit kinking.
IC-TCPC can also be selected when the heart could not be dissected adequately, and the conduit were not obstruct the orifices of pulmonary veins. However, according to a study done by Sakurai et al. and our own clinical experience, LT-TCPC ought not to be adopted because of the prolonged cross-clamp time, intricacy of the surgery, progressive atrial dilation, and arrhythmia [17].
1. Distance between IVC and SHV should be carefully considered before and during surgery.
2. When the IVC and SHV are not widely separated, EC-TCPC-common open technique is optimal. LT-TCPC can be chosen for low-body weight patients, although we do not recommend this technique at the expense of various complications.
When the IVC and SHV are widely separated, two different modified common open techniques (shoetree graft or connecting IVC to SHV in a side-to-side fashion) and IEC-TCPC can be chosen, especially among patients with TAPVC. If a patient has an enlarged heart without the criterion of EC-TCPC, IC-TCPC, and LT-TCPC could be the final choices.
For patients with SHV and ACJ, contralateral EC-TCPC and IEC-TCPC are preferable for the sake of avoiding obstruction of pulmonary veins and compression between the conduit and ventricle. Similarly, IC-TCPC and LT-TCPC can be chosen to avoid the above flaws.
For patients who underwent the first-stage Kawashima operation, direct anastomosis of the hepatic veins to the azygos vein with or without conduit can be performed for this technique is simple and can avoid complications of potential secondary median sternotomy. If the concomitant intracardiac repairs are required to perform, the aforementioned surgical pathways can be selected.
For the reason that the ipsilateral EC-TCPC was not performed in our hospital, the comparative analyses among ACJ patients between contralateral EC-TCPC and ipsilateral EC-TCPC could not be performed. This present study is retrospective, and the mean follow-up period was 5.5 years. On account of the complexity of the anatomy in the subjects, we recommend the surgical techniques according to our surgical experience and successful case outcomes at present. Therefore, longer follow-up is required to demonstrate the long-term outcomes among different surgical technique groups.
Modified Fontan operations can be performed in FUH patients with ACJ or/and SHV and have received good early and late results. Under the principle of Fontan that is connecting systemic venous to the pulmonary vasculature unimpededly, three important components should be considered when choosing surgical techniques: The distance between IVC and the apex; site of the vertebrae relative to the ACJ; distance between ACJ and SHV if both coexist. However, techniques should be altered if the postoperative mPAP is greater than 15 mmHg.
Data Availability Statement: Data will be available for a reasonable request.
Ethics Approval and Consent: The Research Ethics Board of the institution–Fuwai Hospital (Beijing, 2017, Approval No. 2017-898)–approved this retrospective study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: All authors declare no conflicts of interest to report regarding the present study.
1. Hirsch, J. C., Goldberg, C., Bove, E. L., Salehian, S., Lee, T. et al. (2008). Fontan operation in the current era: A 15-year single institution experience. Annals of Surgery, 248(3), 402–410. DOI 10.1097/SLA.0b013e3181858286. [Google Scholar] [CrossRef]
2. Ono, M., Kasnar-Samprec, J., Hager, A., Cleuziou, J., Burri, M. et al. (2016). Clinical outcome following total cavopulmonary connection: A 20-year single-centre experience. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 50(4), 632–641. DOI 10.1093/ejcts/ezw091. [Google Scholar] [CrossRef]
3. Fontan, F., Baudet, E. (1971). Surgical repair of tricuspid atresia. Thorax, 26(3), 240–248. DOI 10.1136/thx.26.3.240. [Google Scholar] [CrossRef]
4. Stamm, C., Friehs, I., Mayer, J. E.,Jr, Zurakowski, D., Triedman, J. K. et al. (2001). Long-term results of the lateral tunnel Fontan operation. Journal of Thoracic and Cardiovascular Surgery, 121(1), 28–41. DOI 10.1067/mtc.2001.111422. [Google Scholar] [CrossRef]
5. Nakano, T., Kado, H., Tachibana, T., Hinokiyama, K., Shiose, A. et al. (2007). Excellent midterm outcome of extracardiac conduit total cavopulmonary connection: Results of 126 cases. Annals of Thoracic Surgery, 84(5), 1619–1626. DOI 10.1016/j.athoracsur.2007.05.074. [Google Scholar] [CrossRef]
6. Tokunaga, S., Kado, H., Imoto, Y., Masuda, M., Shiokawa, Y. et al. (2002). Total cavopulmonary connection with an extracardiac conduit: Experience with 100 patients. Annals of Thoracic Surgery, 73(1), 76–80. DOI 10.1016/S0003-4975(01)03302-1. [Google Scholar] [CrossRef]
7. Kawahira, Y., Nishigaki, K., Ueno, T. (2006). Extracardiac fontan procedure bridging the vertebra for apico-caval juxtaposition. Annals of Thoracic Surgery, 82(1), 350–352. DOI 10.1016/j.athoracsur.2005.07.059. [Google Scholar] [CrossRef]
8. Kitayama, H., Oku, H., Matsumoto, T., Onoe, M. (2001). Total cavopulmonary connection using a pedicled pericardial conduit for a patient with apicocaval juxtaposition. Annals of Thoracic Surgery, 72(4), 1393–1394. DOI 10.1016/S0003-4975(00)02591-1. [Google Scholar] [CrossRef]
9. Lee, J. R., Lee, C., Chang, J. M., Bae, E. J., Noh, C. I. (2002). Modified extracardiac Fontan in a patient with separate hepatic venous drainage. Annals of Thoracic Surgery, 73(3), 992–993. DOI 10.1016/S0003-4975(01)03425-7. [Google Scholar] [CrossRef]
10. Schreiber, C., Hörer, J., Kostolny, M., Holper, K., Eicken, A. et al. (2005). Surgical management of an extracardiac total cavopulmonary connection in heterotaxy syndrome with isolated hepatic drainage. Herz, 30(2), 141–143. DOI 10.1007/s00059-005-2664-y. [Google Scholar] [CrossRef]
11. Azakie, A., Merklinger, S. L., Williams, W. G., van Arsdell, G. S., Coles, J. G. et al. (2001). Improving outcomes of the Fontan operation in children with atrial isomerism and heterotaxy syndromes. Annals of Thoracic Surgery, 72(5), 1636–1640. DOI 10.1016/S0003-4975(01)03039-9. [Google Scholar] [CrossRef]
12. Stamm, C., Friehs, I., Duebener, L. F., Zurakowski, D., Mayer, J. E. et al. (2002). Improving results of the modified Fontan operation in patients with heterotaxy syndrome. Annals of Thoracic Surgery, 74(6), 1967–1978. DOI 10.1016/S0003-4975(02)04124-3. [Google Scholar] [CrossRef]
13. Bartz, P. J., Driscoll, D. J., Dearani, J. A., Puga, F. J., Danielson, G. K. et al. (2006). Early and late results of the modified Fontan operation for heterotaxy syndrome 30 years of experience in 142 patients. Journal of the American College of Cardiology, 48(11), 2301–2305. DOI 10.1016/j.jacc.2006.07.053. [Google Scholar] [CrossRef]
14. Kim, S. J., Kim, W. H., Lim, H. G., Lee, C. H., Lee, J. Y. (2006). Improving results of the Fontan procedure in patients with heterotaxy syndrome. Annals of Thoracic Surgery, 82(4), 1245–1251. DOI 10.1016/j.athoracsur.2006.04.082. [Google Scholar] [CrossRef]
15. Yoshida, M., Menon, P. G., Chrysostomou, C., Pekkan, K., Wearden, P. D. et al. (2013). Total cavopulmonary connection in patients with apicocaval juxtaposition: Optimal conduit route using preoperative angiogram and flow simulation. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 44(1), e46–e52. DOI 10.1093/ejcts/ezt118. [Google Scholar] [CrossRef]
16. Menon, P. G., Yoshida, M., Pekkan, K. (2013). Presurgical evaluation of Fontan connection options for patients with apicocaval juxtaposition using computational fluid dynamics. Artificial Organs, 37(1), E1–E8. DOI 10.1111/j.1525-1594.2012.01555.x. [Google Scholar] [CrossRef]
17. Sakurai, T., Kado, H., Nakano, T., Hinokiyama, K., Oda, S. et al. (2010). The impact of extracardiac conduit-total cavopulmonary connection on apicocaval juxtaposition. European Journal of Cardio-Thoracic Surgery, 38(4), 439–444. DOI 10.1016/j.ejcts.2010.02.032. [Google Scholar] [CrossRef]
18. Gil-Jaurena, J. M., Pérez-Caballero, R., Pita, A., González-López, M. (2016). Extracardiac Fontan in apicocaval juxtaposition. Asian Cardiovascular and Thoracic Annals, 24(2), 178–180. DOI 10.1177/0218492314553613. [Google Scholar] [CrossRef]
19. Morizumi, S., Kato, H., Kanemoto, S., Noma, M., Abe, M. et al. (2012). Appropriate route selection for extracardiac total cavopulmonary connection in Apicocaval Juxtaposition. Annals of Thoracic Surgery, 94(1), 179–184. DOI 10.1016/j.athoracsur.2012.03.026. [Google Scholar] [CrossRef]
20. Nakata, T., Fujimoto, Y., Hirose, K., Osaki, M., Tosaka, Y. et al. (2010). Fontan completion in patients with atrial isomerism and separate hepatic venous drainage. European Journal of Cardio-Thoracic Surgery, 37(6), 1264–1270. DOI 10.1016/j.ejcts.2009.12.026. [Google Scholar] [CrossRef]
21. Sughimoto, K., Aoki, M., Naito, Y., Fujiwara, T. (2009). Novel modification of total cavopulmonary connection for isolated hepatic vein. Annals of Thoracic Surgery, 88(4), 1367–1370. DOI 10.1016/j.athoracsur.2009.02.005. [Google Scholar] [CrossRef]
22. Amodeo, A., di Carlo, D., Grigioni, M., de Santis, M., di Donato, R. M. (2005). Early primary Kawashima operation combined with direct hepatic vein-to-azygos vein connection: A new logical approach. Journal of Thoracic and Cardiovascular Surgery, 129(4), 949–950. DOI 10.1016/j.jtcvs.2004.08.032. [Google Scholar] [CrossRef]
23. Montesa, C., Karamlou, T., Ratnayaka, K., Pophal, S. G., Ryan, J. et al. (2019). Hepatic vein incorporation into the azygos system in Heterotaxy and interrupted inferior vena cava. World Journal for Pediatric and Congenital Heart Surgery, 10(3), 330–337. DOI 10.1177/2150135119842869. [Google Scholar] [CrossRef]
24. Alsoufi, B., Rosenblum, J., Travers, C., Kanter, K., Trusty, P. M. et al. (2019). Outcomes of single ventricle patients undergoing the kawashima procedure: Can we do better? World Journal for Pediatric and Congenital Heart Surgery, 10(1), 20–27. DOI 10.1177/2150135118809082. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |