

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015808
ARTICLE
Arrhythmias in Common Arterial Trunk (CAT): Uncommon Atrial Tachycardia in CAT with Anomalous Pulmonary Venous Connection and Re-entry Atrial Tachycardia in CAT with HIV Seropositive Mother
1Mediterranean Pediatric Cardiology Center “Bambino Gesù”, San Vincenzo Hospital, Taormina, Italy
2Cardiac Electrophysiology Research Center, Rajaie, University of Medical Sciences, Tehran, Iran
*Corresponding Author: Silvia Farruggio. Email: silvifar@msn.com
Received: 15 January 2021; Accepted: 22 February 2021
Abstract: We show a brief report of two common arterial trunk cases (CAT) with different arrhythmias and discuss anatomy, clinical and diagnostic management. The burden of volume and pressure overload of this cardiac malformation may predispose to different types of arrhythmia before and after surgical repair. Because of labile hemodynamic state in this group of patients, prompt diagnosis of any arrhythmia is mandatory as the devastating factor on prognosis. The first patient with a diagnosis of CAT Type II Collett and Edwards (CE) had a particular history with HIV seropositive mother assuming antiretroviral therapy during pregnancy, who presented hyperbilirubinemia and liver dysfunction at birth, and re-entry atrial tachycardia after repair. The second patient had CAT Type I CE with a partial anomalous venous connection of left superior pulmonary vein and uncommon type of atrial tachycardia with dual AV nodal physiology.
Keywords: Common arterial trunk; arrhythmia; anomalous pulmonary venous connection; HIV
Common arterial trunk (CAT) is a rare congenital heart disease (CHD) presenting in 0.034 to 0.56 per 1,000 live births and in 1.4% to 2.8% of all cases of CHD [1].
The association of CAT and other cardiac anomalies as anomalous pulmonary venous connections (APVC), aortic arch interruption, truncal valve regurgitation, and coronary artery anomalies negatively influence the outcome. CAT and APVC is extremely rare and few cases have been described until now [2–5]; APVC can complicate the surgical management of these patients and represents a risk factors for death after the repair of CAT [6].
Arrhythmias in CAT are very rare and it is crucial to identify any lesions that may be haemodynamically significant and therefore precipitate arrhythmias, such as valvar insufficiency and chamber dilation, as well as the presence of ventricular pre-excitation on a baseline electrocardiogram, atrioventricular node dysfunction, the presence of heterotaxy syndrome.
Pre-operative arrhythmia assessment may be useful for surgeons and anaesthesiologists during cardiac surgery and increase awareness for similar post-operative arrhythmias, which may be less well tolerated [7].
We describe two types of supraventricular arrhythmia: A re-entry atrial tachycardia after repair in a patient with CAT Type II Collett and Edwards (CE) who had a particular history with HIV seropositive mother and hyperbilirubinemia, and a second one uncommon type of atrial tachycardia with dual AV nodal physiology presented before surgery in a patient affected of CAT Type I CE with partial anomalous venous connection of a left superior pulmonary vein.
A full-term male newborn with prenatal diagnosis of CAT, born to HIV seropositive mother, a cesarean delivery was necessary to reduce the risk of vertical transmission, even if antiretroviral therapy was performed during pregnancy. At birth his body weight was 2.9 kg and peripheral oxygen saturation was 89%.
Newborn received antiretroviral treatment (zidovudine and nevirapine) and underwent to serial blood samples to check viral PCR at 7th, 15th and 30th day after birth.
Echocardiogram showed CAT Type II CE (Fig. 1), a quadricuspid truncal valve (Figs. 2A and 2B) with moderate stenosis (Gmax 40 mmHg), reversal flow in descending aorta due to significant truncal valve regurgitation, and ventricular septal defect of 8 mm shunting left-to-right. Single coronary ostium was detected at 10 o’ clock in short axis view.
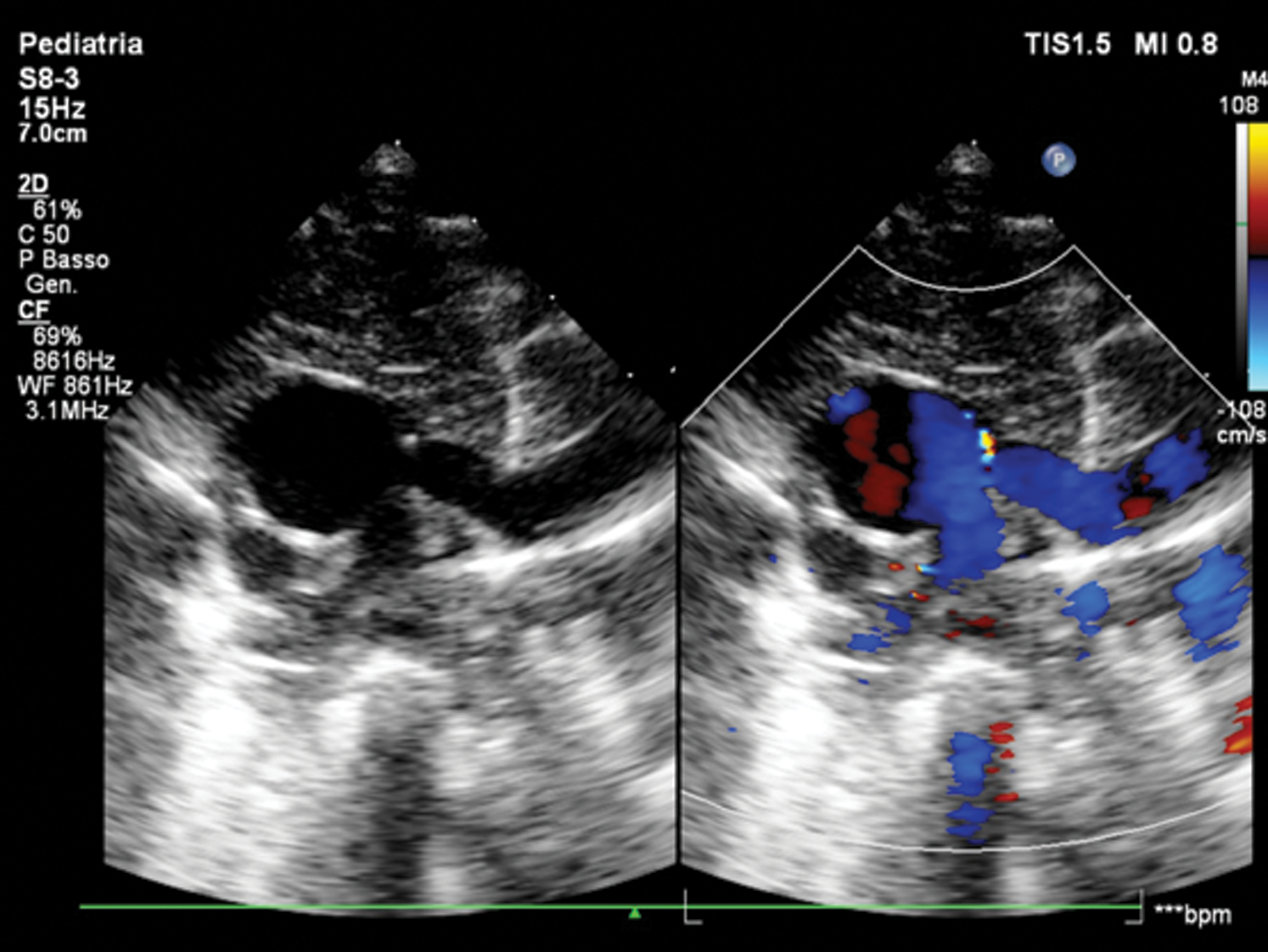
Figure 1: Short-axis view of common arterial trunk Type 2 CE
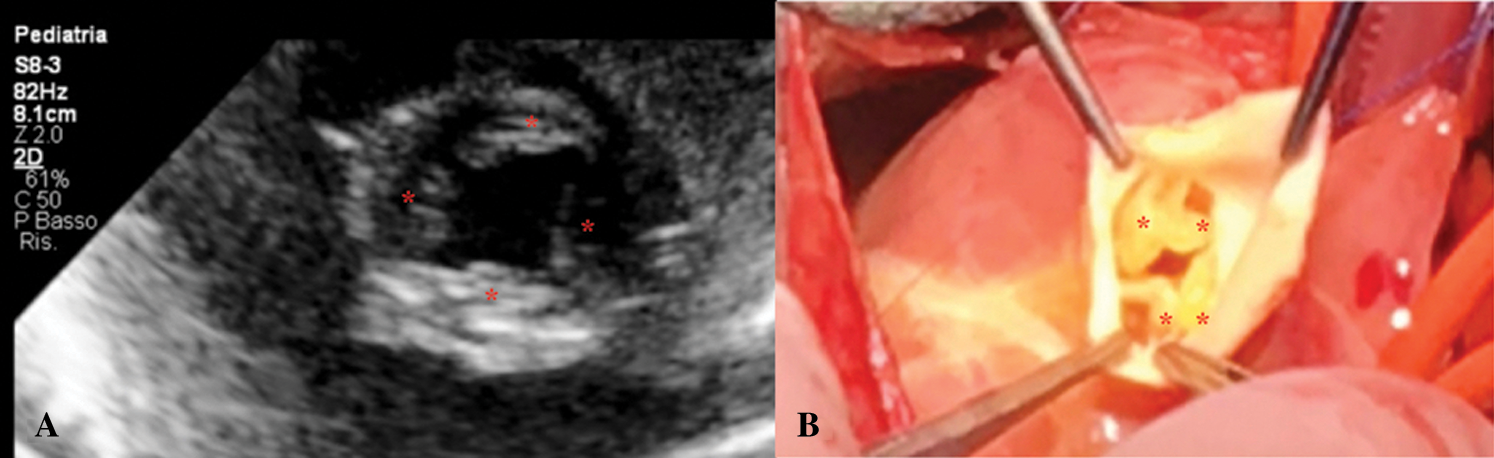
Figure 2: A. Short-axis view of quadricuspid (asterisk) common arterial trunk valve. B. Surgical view of quadricuspid (asterisk) common arterial trunk valve
In the first week the patient was affected by severe hyperbilirubinemia and elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, then surgery was delayed on 10th after birth in order to operate with proper liver function. A 12 mm size Contegra valve conduit was placed from the right ventricle to the pulmonary arteries confluence, ventricular septal defect closure and truncal valvuloplasty was performed. After sternal closure, the patient needed inotropic drugs because of transient right ventricle dysfunction.
In the first day after surgery, the newborn presented a supraventricular arrhythmia (Fig. 3) in intensive care unit (ICU), presenting narrow QRS as a re-entry atrial tachycardia 2:1 conduction (atrial HR 300 bpm, ventricular HR 150 bpm), interrupted with overdrive pacing via temporary epicardial wires placed after surgery. The post-surgical course was uneventful. Patient was discharged on amiodarone therapy and underwent to serial echocardiographic exams, Holter ECG and blood samples during follow-up without presenting hemodynamic or arrhythmic complications.
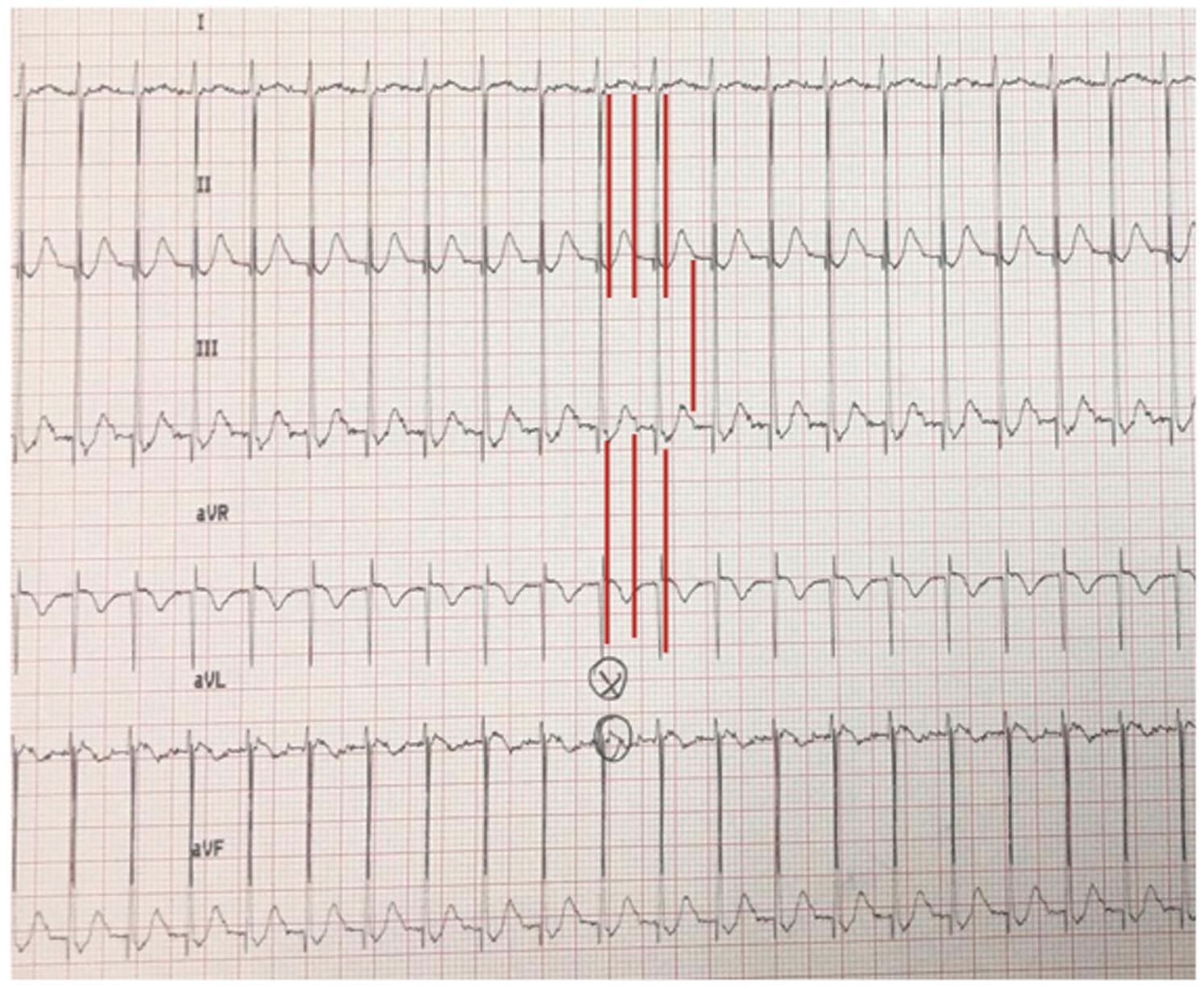
Figure 3: Limb leads ECG shows re-entry atrial tachycardia 2:1 (red lines) conduction with atrial HR of 300 bpm and ventricular HR of 150 bpm
After 12 months underwent to transoesophageal electrophysiological study without evidence of arrhythmias.
A female patient with prenatal diagnosis of CAT, born at term from eutocic delivery with 3.4 kg body weight and peripheral oxygen saturation was 85%. There were no associated extracardiac malformations. A sequential segmental echocardiographic analysis confirmed CAT Type I CE (Fig. 4B) with normal coronary artery anatomy, bicuspid semilunar truncal valve with mild insufficiency, and the presence of a small patent ductus arteriosus (PDA) (Fig. 4A) with left-sided unobstructed aortic arch. Additionally, echo detected a moderate dilatation of the vertical vein, without hemodynamic consequences (Fig. 4A).
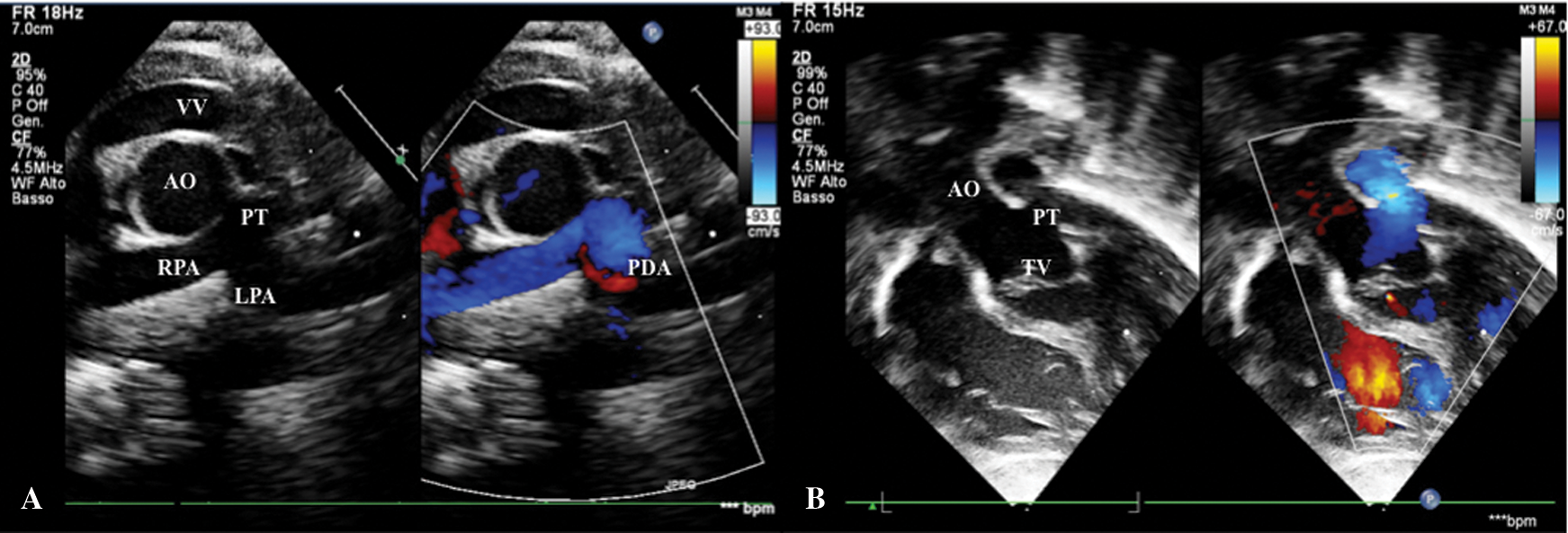
Figure 4: A. Short-axis view shows CAT Type I CE, small patent ductus arteriosus in left pulmonary artery and dilated vertical vein. B. Subcostal view shows mild truncal valve insufficiency. AO: Aorta, LPA: Left Pulmonary Artery, PDA: Patent Ductus Arteriosus, PT: Pulmonary Trunk, RPA: Right Pulmonary Artery, TV: Truncal Valve, VV: Vertical Vein
On 7th day of life, newborn presented a non-sustained (NS) narrow QRS tachycardia (Fig. 5) HR 280 bpm, with a first diagnosis of uncommon atrial tachycardia and dual AV physiology. The ECG showed a premature atrial contraction (PAC) inside the T wave and that comes before a prolonged PQ interval (black line); the PQ triggered the tachycardia, with a retroconducted P waves (asterisks) on a slow conducting pathway and followed by a QRS with a 1:1 VA. Eventually, a fusion beat (F) interrupted tachycardia. Some characteristic findings are the positive P wave in limb lead as the previous sinus P wave, the P wave with prolonged PQ conducted with antegrade P-QRS suggests a dual AV node physiology and PACs triggered arrhythmia.
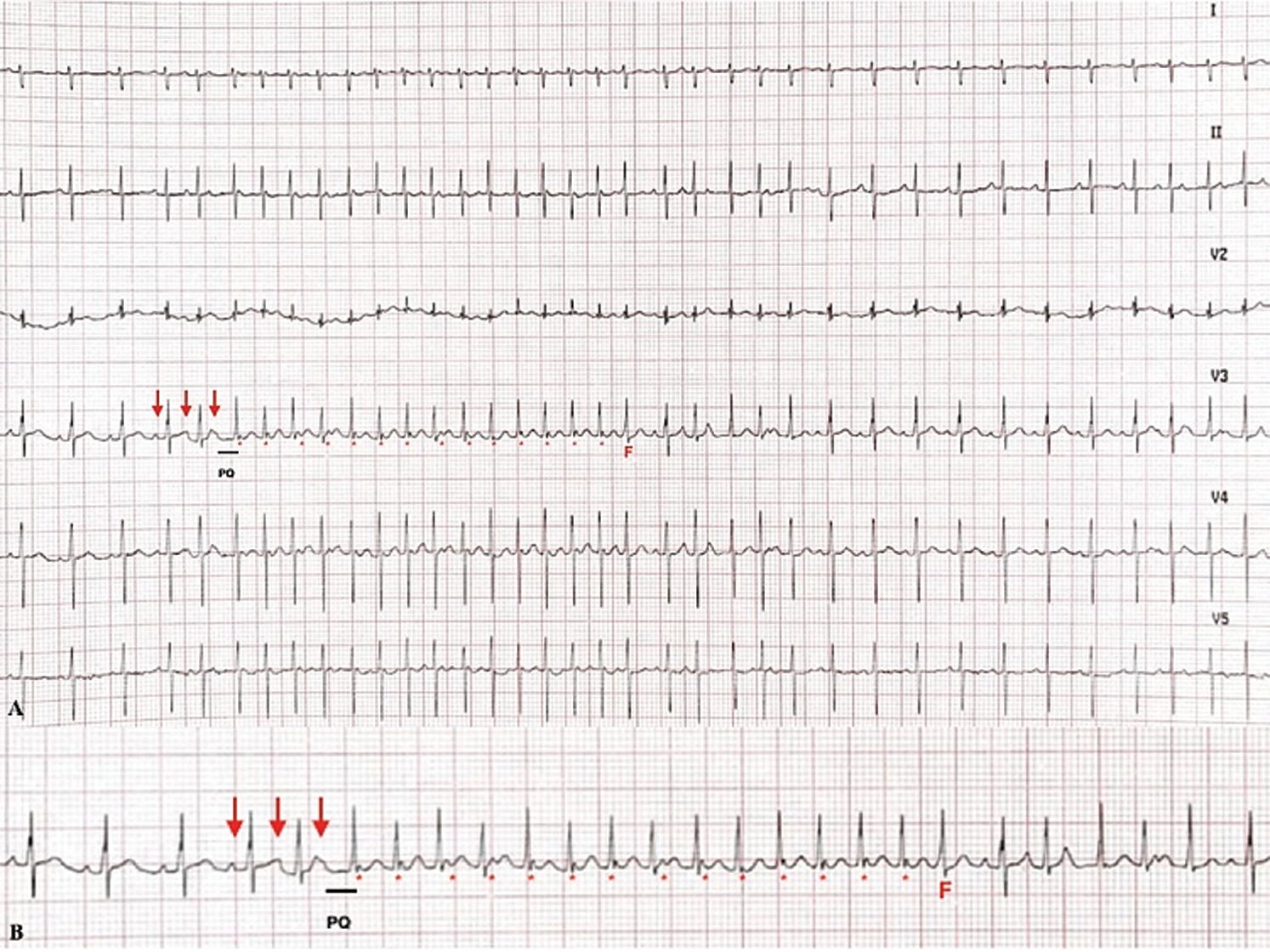
Figure 5: A. 6-lead ECG. B. A longer ECG strip shows the beginning of arrhythmia: The first P wave (red arrow) is a sinus beat, the second one inside T wave is a PAC and the third arrow indicate the last P wave with prolonged PQ interval after that begin tachycardia with P wave (asterisk) retroconducted; a fusion beat (F) signs the spontaneous termination of tachycardia
Treatment with propranolol 1 mg/kg per os every 8 h was started and then increased to 1,5 mg/kg per os every 8 h.
Heart team decided to avoid transesophageal electrophysiological study because it was NS tachycardia treated with propranolol and to plan it at 12 months of follow up.
The patient underwent surgery repair at 8th day of life; cardiopulmonary bypass was initiated and truncus arteriosus anatomy was assessed through ventricular septal defect closure, a 12 mm size Contegra valve conduit was placed from the right ventricle to the pulmonary arteries confluence.
On the sixth day post-surgery, due to inability for ventilatory and inotropic support weaning, added to presence of abnormal blood flow at retrocardiac level seen in echocardiography images, it was decided to perform CT scan that demonstrated APVC: A left upper pulmonary vein draining into a retrocardiac vertical vein (Fig. 6). A new surgical procedure needed to establish correct anatomy left upper pulmonary vein draining into a retrocardiac vertical vein was identified and then anastomosed to the left atrial posterior wall. Patient returned to ICU and was discharged after complete recovery and without any complications.
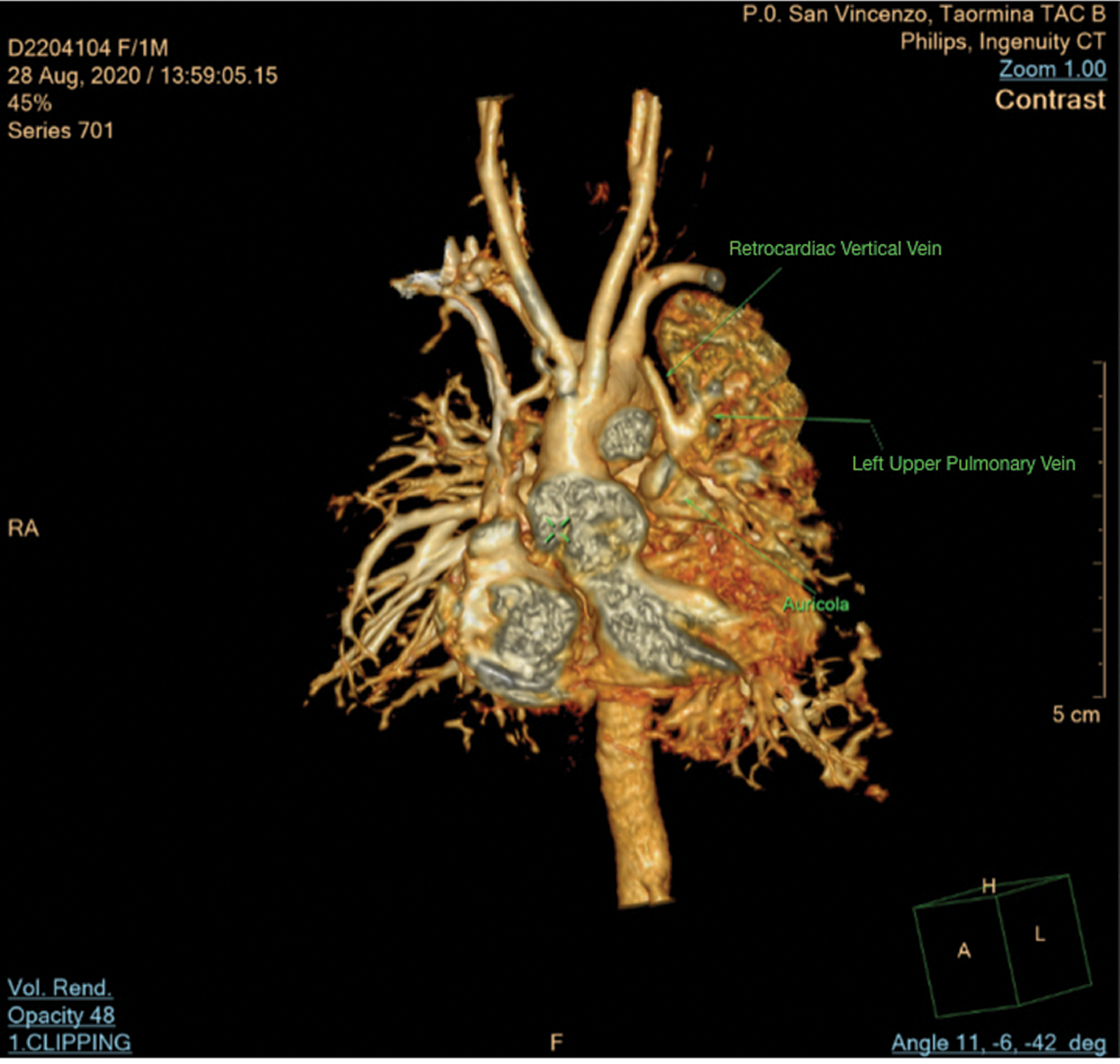
Figure 6: Computed tomography scan shows left upper pulmonary vein draining in retrocardiac vertical vein
The impact and prognosis of arrhythmia specifically in CAT have been poorly described. Several long-term surgical studies are available from literature, but they do not characterise arrhythmia burden [7].
In 1984 Toussaint et al. [8] described four cases of multifocal atrial tachycardia of which one case was associated to CAT.
The identification of the underlying arrhythmia mechanism and hemodynamic impact is critical because the management and prognosis differ among the various disorders [9].
Anatomical cardiac lesions may be haemodynamically significant and induce arrhythmias, as well as the presence of ventricular pre-excitation, atrioventricular node dysfunction, heterotaxy syndrome. The arrhythmia risk should be minimized by correcting electrolyte imbalances and acidosis, similarly, an arrhythmia may significantly worse haemodynamic balance and pulmonary/systemic blood flow ratio or overall systemic output [7].
With burden of volume and pressure overload this group of patients may be predispose to different types of tachyarrhythmia before and after surgical repair. Because of labile hemodynamic state in this group of patients, prompt diagnosis of any tachyarrhythmia is mandatory as it could be a devastating factor on prognosis.
Usually, the most common post-surgery arrhythmia in CAT and transposition of the great arteries with a complex ventricular septal defect is junctional ectopic tachycardia, and its origin is the result of irritation to the atrioventricular node, His bundle and its associated conduction tissue due to ventricular septal defect repair or when there is traction applied close to the atrioventricular node. Conotruncal pathology requiring prolonged bypass times, are at risk for junctional tachycardia [7].
Propranolol, digoxin, amiodarone, and sotalol are all commonly used as antiarrhythmic therapy in the newborn period for peri-operative arrhythmias.
It is important to have a detailed knowledge of age-dependent changes on pediatric electrocardiogram to avoid misinterpretation as sinus arrhythmia, ectopic atrial rhythm, wandering pacemaker, and functional rhythm can be normal characteristics in children. Paroxysmal supraventricular re-entry tachycardia is the most common arrhythmia in pediatric population, accounting about 82% of arrhythmias. In neonatal period, supraventricular tachycardia (SVT) is frequent and triggered by PACs or rapid acceleration of sinus rhythm during feeding or crying, but after the first year of life its recurrence tends to decrease because of a better sympathovagal balance and it have been demonstrated that transesophageal electrophysiological study performed at 1 year of age show a disappearance of the re-entry circuit in 50% of cases [10].
A paroxysmal AV nodal re-entry tachycardia (AVNRT) is rare in childhood (13%–16% of all SVTs), caused by a re-entry through the slow and the fast pathways. Only 30% to 40% of children AVNRT show a double nodal conduction at the electrophysiologic study. Ectopic atrial tachycardia represents 14% of all SVTs, uncommon in children, due to an increased automaticity of myocardial cells or, less frequent, to triggered activity. The most common ectopic foci are placed in the right appendage, in the crista terminalis, around the pulmonary veins, but a focus can exist at any place in the right or left atrium, also scar regions due to surgical repair, or rarely in the limbus fossa ovalis [10,11].
Our cases are extremely uncommon either to anatomical association of cardiac anomalies either to type of arrhythmias presented.
The rarity of anatomical cardiac association in our second patient is the coexistence of APVC and PDA in CAT, both uncommon in this CHD. Ductus arteriosus is usually persistent in CAT and aortic arch interruption. Van Praagh in his previous report of 100 patients with CAT, the persistence of PDA was observed only in 2% of patients without aortic arch interruption or anomalous ductal origin of the pulmonary artery [12]. Similarly, few cases of APVC associated with CAT have been described until now [2–5].
Slow pathway is very rare as atrial tachycardia. On the ECG showed in Fig. 5, the P wave can be visible and with different morphology from the sinus rhythm with conduction from slow pathway the RP is shorten as opposed focal AT usually present as long RP tachycardia. Atrial rate during tachycardia ranges from 130 to 280 bpm and it is influenced by autonomic tone variations. First- and second-degree AV blocks can be present [10].
For proper approach to neonatal tachyarrhythmia, awareness of arrhythmia mechanism is essential.
Dual atrioventricular nodal physiology has been reported in 35% children with uncorrected congenital heart disease [13,14].
Differential diagnosis was made with other arrhythmias. Multifocal atrial tachycardia is usually idiopathic and more common in neonatal period, characterized by 3 or more different P wave morphologies and incessant trend. In our case multifocal atrial tachycardia was ruled out as ECG showed no more than one different P wave and this was too close to QRS with a VA interval less than 70 msec.
Junctional ectopic tachycardia frequently occurs after surgery for CHD involving the AV junction (tetralogy of Fallot, ventricular septal defect, AV defect, CTA) as mentioned above.
It is caused by automatic foci inside the AV junction and it is usually incessant [15]. In our patient arrhythmia occurred before surgery and ECG shows a NS tachycardia and positive P wave as a high atrial focus as opposed to JET.
In cases of mothers suffering from pathologies such as HIV, fetal echocardiography is crucial for improving the clinical management of mother and child at birth. The use of antiretroviral drugs in pregnancy can cause congenital abnormalities and complex CHD. In a retrospective study the rate of congenital abnormalities reported was only 3% in HIV pregnant women who undertaken antiretroviral treatment [16].
There may be a relationship between in utero antiretroviral therapy and heart defects as reported by Brogly et al. Specifically exposure of zidovudine in the first trimester and association of conotruncal and obstructive defects were marginally significant [17].
However, even this case required a thorough genetic investigation, the fundamental point is that all HIV seropositive mothers with fetuses affected from CHD must undergo to a careful infectious disease evaluation because of antiretroviral fetal toxicity. Fetal echocardiography must always be included in HIV-pregnancy screening because some CHD need to be repaired in the first 2 weeks from birth and each possible cause of delay have been avoided. Specifically, in CAT, a delay in surgical repair could lead to coronary steal and a fatal outcome.
In fact, at birth, newborn from HIV seropositive mother, who needs cardiac surgery repair in the first weeks of life, requires the use of antiretroviral drugs immediately after delivery, knowing the infectious causes certainly improves management in order to promptly treat any side adverse effects and not delay surgery.
If CAT is associated with other very important extracardiac diseases such as hyperbilirubinemia and liver dysfunction, the surgery could be postponed. In our case, as guidelines recommended, antiretroviral treatment was performed for postnatal prophylaxis of HIV-exposed infant, but combination of antiretrovirals, as zidovudine and nevirapine, could determine severe adverse events as hyperbilirubinemia, anemia, neutropenia, thrombocytopenia, elevated AST and ALT [18].
In addition, the onset of post-operative atrial tachycardia, very rare in CAT according to literature, could worsen the postoperative course. The arrhythmia was interrupted with overdrive but recurred in the post-operative period and therefore it was necessary to start amiodarone.
The combination of CAT Type II CE, quadricuspid truncal valve, that has an incidence of 28% [19], with only single coronary ostium, reported in 13,6% of quadricuspid truncal valve by Suzuki et al. [20], the use of antiretroviral drugs, hyperbilurubinemia and liver dysfunction at birth during follow-up makes the case truly clinically unique and complex.
CAT and arrhythmias in new-borns are very rarely associated. We described a brief report of two singular cases: One CAT Type II CE with single coronary ostium in quadricuspid truncal valve, born to HIV mother presenting a rare neonatal arrhythmia, a re-entry atrial tachycardia after cardiac surgery, and one CAT Type I CE and APVC associated to pre-operative uncommon type of atrial tachycardia with dual AV nodal physiology. Both cases presented rare arrhythmias, unusual clinical findings and complex-anatomical cardiac anomalies. Preoperative and post operative management of complex CHD is extremely difficult and needs detailed diagnostic monitoring and clinical accuracy.
Tachyarrhythmia in complex CHD needs to prompt management especially in neonatal period and in complex cardiac malformations which have to be repaired within the first weeks of life. In CHD as cases above described, the burden of volume and pressure overload may predispose to different types of arrhythmia before and after surgical repair. Considering of etiologic mechanism, appropriate diagnostic tools and correct selection of therapy allow a timely control haemodynamic and arrhythmic complication.
Acknowledgement: The authors are grateful to Dr. Placido Romeo for his contribution.
Ethics Approval and Consent:Written informed consent have been obtained from all patients’ parents.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kalavrouziotis, G., Purohit, M., Ciotti, G., Corno, A. F., Pozzi, M. (2006). Truncus arteriosus communis: Early and midterm results of early primary repair. Annals of Thoracic Surgery, 82(6), 2200–2206. DOI 10.1016/j.athoracsur.2006.07.017. [Google Scholar] [CrossRef]
2. Ayyildiz, P., Kasar, T., Güzeltas, A. (2015). A rare type of common arterial trunk with interrupted aortic arch, partial anomalous pulmonary venous connection, and phenylketonuria. Cardiology in the Young, 25(5), 996–998. DOI 10.1017/S1047951115000128. [Google Scholar] [CrossRef]
3. Konstantinov, I. E., Karamlou, T., Blackstone, E. H., Mosca, R. S., Lofland, G. K. et al. (2006). Truncus arteriosus associated with interrupted aortic arch in 50 neonates: A congenital heart surgeons society study. Annals of Thoracic Surgery, 81(1), 214–222. DOI 10.1016/j.athoracsur.2005.06.072. [Google Scholar] [CrossRef]
4. Mair, D. D., Ritter, D. G., Davis, G. D., Wallace, R. B., Danielson, G. K. et al. (1974). Selection of patients with truncus arteriosus for surgical correction; anatomic and hemodynamic considerations. Circulation, 49(1), 144–151. DOI 10.1161/01.CIR.49.1.144. [Google Scholar] [CrossRef]
5. Litovsky, S. H., Ostfeld, I., Bjornstad, P. G., van Praagh, R., Geva, T. (1999). Truncus arteriosus with anomalous pulmonary venous connection. American Journal of Cardiology, 83(5), 801–A10. DOI 10.1016/S0002-9149(98)00999-0. [Google Scholar] [CrossRef]
6. Brown, J. W., Ruzmetov, M., Okada, Y., Vijay, P., Turrentine, M. W. (2001). Truncus arteriosus repair: Outcomes, risk factors, reoperation and management. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 20(2), 221–227. DOI 10.1016/S1010-7940(01)00816-8. [Google Scholar] [CrossRef]
7. Decker, J. A., McCormack, J., Cohen, M. I. (2012). Arrhythmia management in patients with a common arterial trunk and d-transposition of the great arteries. Cardiology in the Young, 22(6), 748–754. DOI 10.1017/S1047951112001576. [Google Scholar] [CrossRef]
8. Toussaint, R., Hofstetter, R., von Bernuth, G. (1984). Multifokale atriale Tachykardie im Säuglingsalter [Multifocal atrial tachycardia in infancy]. Klinische Padiatrie, 196(2), 118–120. DOI 10.1055/s-2007-1025591. [Google Scholar] [CrossRef]
9. Jaeggi, E., Öhman, A. (2016). Fetal and neonatal arrhythmias. Clinics in Perinatology, 43(1), 99–112. DOI 10.1016/j.clp.2015.11.007. [Google Scholar] [CrossRef]
10. Drago, F., Battipaglia, I., di Mambro, C. (2018). Neonatal and pediatric arrhythmias: Clinical and electrocardiographic aspects. Cardiac Electrophysiology Clinics, 10(2), 397–412. DOI 10.1016/j.ccep.2018.02.008. [Google Scholar] [CrossRef]
11. di Pino, A., Caruso, E., Gitto, P. (2016). The limbus of the fossa ovalis: An unusual location for incessant focal atrial tachycardia in children. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 18(8), 1251. DOI 10.1093/europace/euw047. [Google Scholar] [CrossRef]
12. Calder, L., van Praagh, R., van Praagh, S., Sears, W. P., Corwin, R. et al. (1976). Truncus arteriosus communis. Clinical, angiocardiographic, and pathologic findings in 100 patients. American Heart Journal, 92(1), 23–38. DOI 10.1016/S0002-8703(76)80400-0. [Google Scholar] [CrossRef]
13. Casta, A., Wolff, G. S., Mehta, A. V., Tamer, D., Garcia, O. L. et al. (1980). Dual atrioventricular nodal pathways: A benign finding in arrhythmia-free children with heart disease. American Journal of Cardiology, 46(6), 1013–1018. DOI 10.1016/0002-9149(80)90360-4. [Google Scholar] [CrossRef]
14. Blaufox, A. D., Rhodes, J. F., Fishberger, S. B. (2000). Age related changes in dual AV nodal physiology. Pacing and Clinical Electrophysiology: PACE, 23(4), 477–480. DOI 10.1111/j.1540-8159.2000.tb00830.x. [Google Scholar] [CrossRef]
15. Kylat, R. I., Samson, R. A. (2019). Junctional ectopic tachycardia in infants and children. Journal of Arrhythmia, 36(1), 59–66. DOI 10.1002/joa3.12282. [Google Scholar] [CrossRef]
16. Montgomery-Taylor, S., Hemelaar, J. (2015). Management and outcomes of pregnancies among women with HIV in Oxford, UK, in 2008-2012. International Journal of Gynecology & Obstetrics, 130(1), 59–63. DOI 10.1016/j.ijgo.2015.02.019. [Google Scholar] [CrossRef]
17. Brogly, S. B., Abzug, M. J., Watts, D. H., Cunningham, C. K., Williams, P. L. et al. (2010). Birth defects among children born to human immunodeficiency virus-infected women: Pediatric AIDS clinical trials protocols 219 and 219C. Pediatric Infectious Disease Journal, 29(8), 721–727. DOI 10.1097/INF.0b013e3181e74a2f. [Google Scholar] [CrossRef]
18. Smith, C., Forster, J. E., Levin, M. J., Davies, J., Pappas, J. et al. (2015). Serious adverse events are uncommon with combination neonatal antiretroviral prophylaxis: A retrospective case review. PLoS One, 10(5), e0127062. DOI 10.1371/journal.pone.0127062. [Google Scholar] [CrossRef]
19. Sun, L. C., Wang, J. K., Lin, M. T., Wu, E. T., Lu, F. L. et al. (2005). Persistent truncus arteriosus: Twenty years experience in a tertiary care center in Taiwan. Acta Paediatrica Taiwanica = Taiwan er Ke Yi Xue Hui Za Zhi, 46(1), 6–10. [Google Scholar]
20. Suzuki, A., Ho, S. Y., Anderson, R. H., Deanfield, J. E. (1989). Coronary arterial and sinusal anatomy in hearts with a common arterial trunk. Annals of Thoracic Surgery, 48(6), 792–797. DOI 10.1016/0003-4975(89)90672-3. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |