

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015260
ARTICLE
Plasma HGF and OPN as Potential Biomarkers of Pulmonary Arterial Hypertension in Congenital Heart Disease
1Department of Cardiovascular Internal Medicine, Affiliated Hospital of Nantong University, Nantong, 226001, China
2Department of Cardiovascular Internal Medicine, Tongzhou People’s Hospital Affiliated to Nantong University, Nantong, 226300, China
*Corresponding Author: Xiaofei Li. Email: lixiaofeint1991@163.com
Received: 04 December 2020; Accepted: 23 February 2021
#These authors contributed equally to this work
Abstract: Objectives: Pulmonary arterial hypertension in congenital heart disease (PAH-CHD) is the most common type of PAH and increases morbidity and mortality in patients with CHD. Right heart catheterization (RHC) is the standard method to diagnose PAH. However, RHC is an invasive and complicated method with relatively high cost. Noninvasive, feasible, and cost-efficient methods are urgently needed. The objective of this study was to evaluate three potential biomarkers of PAH-CHD: Hepatocyte growth factor (HGF), osteopontin (OPN), and suppression of tumorigenicity 2 (ST2). Methods: Plasma samples were collected from patients with CHD (n = 46) and healthy individuals (n = 22) and divided into four groups according to the severity of PAH. The levels of HGF, OPN, and ST2 were then analyzed using an enzyme-linked immunosorbent assay. Correlations between HGF, OPN, ST2, and clinical parameters of PAH-CHD were analyzed. Results: The plasma HGF levels in the moderate to the severe group were significantly higher than those in the mild group, nonPAH group, and healthy control group (p < 0.05). Derived from a receiver operating characteristic (ROC) curve analysis, a cut-off value of 356.75 ng/ml for the HGF concentration was able to predict PAH-CHD with 53% sensitivity and 89% specificity. The HGF level was positively related to pulmonary arterial systolic pressure (PASP) (r = 0.36, p < 0.05) and pulmonary vascular resistance (PVR) (r = 0.36, p < 0.05). Plasma OPN levels in the mild group were significantly higher than other groups and positively correlated with the pulmonary-systemic shunt ratio (Qp/Qs) (r = 0.33, p < 0.05). There was no statistically significant between-group difference in plasma soluble ST2 (sST2) levels. Conclusion: The plasma HGF level was elevated in PAH-CHD patients and can be used as a potential biomarker. The plasma OPN level was positively correlated with the Qp/Qs.
Keywords: Congenital heart disease; pulmonary hypertension; hepatocyte growth factor; osteopontin; suppression of tumorigenicity 2; biomarker
Pulmonary arterial hypertension (PAH) has various causes. The most common etiological agent of PAH in the South-East Asian population is congenital heart disease (CHD) (hereafter, PAH-CHD) [1]. Common defects observed in CHD include ventricular septal defects, atrial septal defects, and persistent ductus arteriosus. A European study showed that 5%–10% of adults with CHD eventually developed PAH [2]. The same study showed that high pulmonary artery pressure (PAP) was associated with right heart dysfunction, decreased exercise endurance, and an increased risk of death [2]. Therefore, for patients with CHD, early diagnosis of PAH and appropriate treatments are particularly important. Right heart catheterization (RHC) is the gold standard for the diagnosis of PAH. However, RHC is invasive and costly, which limits its range of applications. Transthoracic echocardiography is an alternative to RHC that can be used to evaluate pulmonary arterial systolic pressure (PASP). However, transthoracic echocardiography is not sufficient to diagnose PAH when considering the operation. For convenient and early diagnosis of PAH-CHD, efficient and accurate circulating biomarkers are urgently needed in clinical practice.
Hepatocyte growth factor (HGF) is a cytokine secreted by mesenchymal cells and plays a major role in myogenesis, adult organ regeneration, and wound healing through its receptor c-MET [3]. Left-to-right shunts in CHD can cause pulmonary vascular endothelial cell injury and right ventricular remodeling [4]. The HGF/MET pathway plays a prominent role in promoting proliferation, angiogenesis, anti-inflammatory, and antifibrosis after cardiovascular injury [4]. HGF can slow the progression of PAH by promoting vascular endothelial cell proliferation and, promoting angiogenesis [5] and, inhibiting the apoptosis of vascular endothelial cells through multiple pathways, such as extracellular signal-regulated kinase (ERK)/signal transducer and activator of transcription 3 (STAT3)/protein kinase B (AKT) [6]. It can also, activate nitric oxide synthase (NOS) through the phosphatidylinositol 3-kinase (PI3K) -AKT pathway [7], inhibit the generation of Endothelin 1 in the lungs, inhibit pulmonary interstitial remodeling and pulmonary fibrosis [8], and play a role of an anti-inflammatory role by reducing inflammatory factor IL-6 and meanwhile increasing anti-inflammatory factor IL-10 through the HGF/c-MET pathway [9]. A study showed the therapeutical effect of exogenous HGF on PAH in rats [10]. A previous study showed that plasma HGF levels in PAH patients were higher than those in healthy individuals and that the HGF level was positively related to the mean PAP (PAPm) and pulmonary vascular resistance (PVR) [11]. However, the mechanism of HGF elevation in PAH patients is not clear, and the relationship between HGF and PAH-CHD has not been studied.
Osteopontin (OPN) is a glycoprotein biosynthesized by a variety of tissue types, including cardiac fibroblasts [12]. Previous studies revealed that OPN played a role in ventricular remodeling in animal models of cardiac hypertrophy and heart failure and that it can be used as a prognostic marker of chronic heart failure [13,14]. Studies also showed that senescent pulmonary artery smooth muscle cells contributed to the pathogenesis of pulmonary hypertension by releasing OPN [15]. In addition, research demonstrated that PAH may develop in CHD patients with left-to-right (systemic-to-pulmonary) shunts due to persistent exposure of the pulmonary vasculature to increased blood flow and pressure [16]. Furthermore, a previous study reported an increased OPN level in the lungs and plasma of patients with Eisenmenger syndrome and in the rat model which lungs exposed to systemic-to-pulmonary shunts [17]. In previous research, plasma OPN levels were significantly associated with arterial hypertension [18]. Research also demonstrated that elevated pressure and pulsatile flow may cause the release of OPN, in part, by increasing aortic strain and reactive oxygen species production [19]. In the case of CHD, continuous left-to-right shunting leads to increased pulmonary circulation volume and pressure and blood vessel damage, which can lead to an increase in OPN released by endothelial cells. However, the relationship between OPN and PAP, PVR, and the pulmonary-systemic shunt ratio (Qp/Qs) remains to be studied.
Suppression of tumorigenicity 2 (ST2) is a member of the interleukin-1 (IL-1) receptor family. Interleukin-33 (IL-33) is its functional ligand. There are four subtypes of ST2: Soluble ST2 (sST2), transmembrane type ST2 (ST2L), ST2V, and ST2LV, with the latter being two alternative splicing products of ST2 [20]. Previous research demonstrated the cardiovascular protective effect of the ST2/IL-33 pathway against myocardial fibrosis and myocardial hypertrophy when exposed to biological stress overload [21]. Several recent studies showed that sST2 served as a circulating biomarker of heart failure and that it had prognostic value [22–24]. A prospective cohort study indicated that sST2 was associated with CHD, specifically in females, but that the specificity of the diagnosis needed further research [25].
In this study, we aimed to explore the association of HGF, OPN, and ST2 levels with PAH-CHD and the possibility of their usage as plasma biomarkers of PAH-CHD.
2.1 Study Subjects and Clinical Data
Ethical approval for the study was granted by the ethics committee of the Affiliated Hospital of Nantong University Hospital (No. 2016-052), and written informed consent was obtained from all the participants. Forty-six patients with CHD and symptoms (i.e., atrial septal defects, n = 34; ventricular septal defects, n = 2; persistent ductus arteriosus, n = 10) indicative of the need for interventional CHD treatment were enrolled between June 2018 and October 2019. Clinical data were recorded.
The exclusion criteria were as follows: children, infants, and other patients who required general anesthesia for interventional surgery; heart failure (New York Heart Association (NYHA) functional classification III and above); coronary heart disease; severe liver or kidney dysfunction; malignant tumors; idiopathic pulmonary hypertension; obstructive pulmonary disease; restrictive lung disease; active infectious diseases; Eisenmenger syndrome; bleeding or a bleeding tendency; pulmonary hypertension caused by factors other than CHD; or receiving medications to control PAP. The enrolled patients were divided into three groups based on the average PAPm measured by RHC: A moderate to severe group (Group H, PAPm ≥ 35 mmHg, n = 7), a mild group (Group M, 25 mmHg ≤ PAPm < 35 mmHg, n = 16), and no pulmonary hypertension group (Group W, PAPm < 25 mmHg, n = 23). The Qp/Qs value was measured through the right heart catheter and calculated based on the Fick principle.
Twenty-two healthy controls were also recruited during physical check-ups in the physical examination center of our hospital as Group C. All the controls had normal pulmonary artery systolic blood pressure, as estimated by color Doppler echocardiography, and they were free of the following conditions: history of obstructive pulmonary disease or restrictive lung disease, history of coronary heart disease, heart failure (NYHA Class II and above), severe liver or kidney dysfunction, active infectious diseases, diseases other than CHD that could cause pulmonary hypertension, or taking medications that could cause increased pulmonary pressure.
2.2 Sample Collection and Measurement
Venous blood (2–4 ml) was collected early morning after an overnight fast. The blood samples were allowed to stand for 10–20 min at room temperature and then centrifuged at 3,000 g for 10 min. The serum samples were stored at –80°C before being analyzed. All the samples were tested for HGF, OPN, and sST2 levels using commercially available enzyme-linked immunosorbent assay kits.
Data are shown as mean ± standard deviations. The t-test was used for comparisons between two groups, and one-way analysis of variance was used for analysis among three or more groups. The χ2 test was used for categorical variables, and correlations between two variables were assessed by Spearman’s correlation analysis. Receiver operating characteristic (ROC) curves were plotted to evaluate the diagnostic value of the biomarkers, and the area under the curve (AUC) was calculated. A value of p < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 23 (IBM Corp., Armonk, NY).
Seven CHD patients with a high PAPm, 16 CHD patients with mild pulmonary hypertension, 23 CHD patients without pulmonary hypertension, and 22 healthy individuals were enrolled in the study. Their clinical information is summarized in Tab. 1.
Table 1: Summary of clinical information of the study population
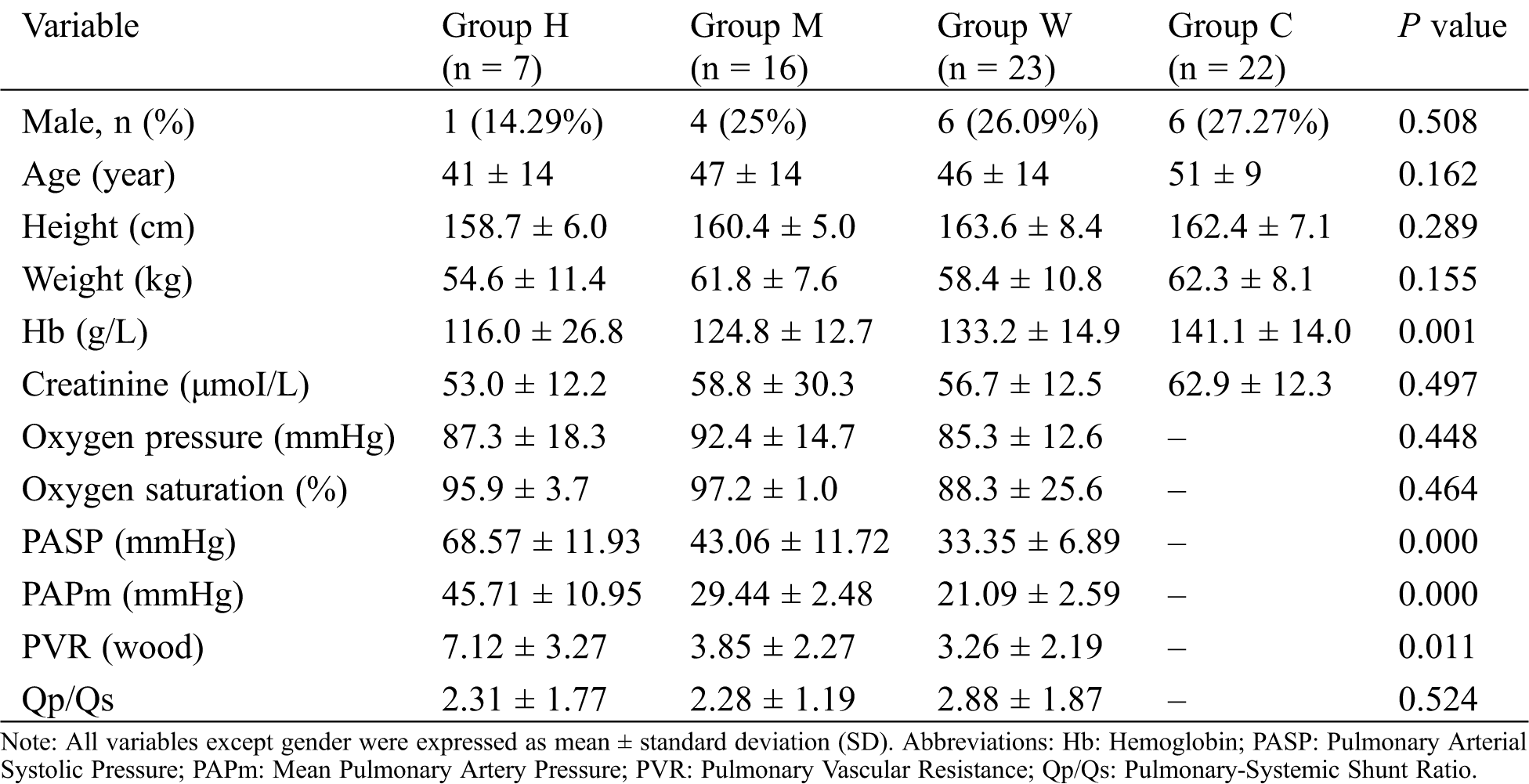
There were no significant differences among the groups in terms of sex, age, height, weight, creatinine, oxygen pressure, and oxygen saturation. However, there was a significant difference among the groups in the concentrate of hemoglobin, which was negatively related to PASP and PAPm.
3.2 Association of Plasma HGF, OPN, and ST2 Concentrations with PAH-CHD
The plasma levels of HGF in Group H were significantly higher than those in Group M, Group W, and Group C (p < 0.05). There was no significant difference in the HGF level between any two of Group M, Group W, and Group C (p ≥ 0.05) (Fig. 1a).

Figure 1: Concentration of HGF (A), OPN (B), and ST2 (C) in each group. Group H: PAPm ≥ 35 mmHg, n = 7; Group M: 25 mmHg ≤ PAPm < 35 mmHg, n = 16; Group W, PAPm < 25 mmHg, n = 23; Group C: Healthy controls. ***: p < 0.001; **: p < 0.01; *: p < 0.05; ns: not significant. HGF: Hepatocyte Growth Factor; OPN: Osteopontin; ST2: Suppression of Tumorigenicity 2; PAPm: Mean Pulmonary Artery Pressure
The plasma OPN level in Group W and Group M was significantly elevated versus that in Group C. There was no significant difference in the OPN levels of Group W and Group M. Interestingly, there was no difference in the OPN level in Group H as compared with that in the control group (Fig. 1b).
The results revealed no evidence of a statistically significant difference in the plasma ST2 level after multiple comparisons between Group H, Group M, and Group W (Fig. 1c).
3.3 Correlation between Plasma HGF and OPN Concentrations and Clinical Parameters
To further investigate the potential biomarkers and PAH-CHD, we performed a correlation analysis between the plasma biomarker level and clinical parameters. The results showed that the plasma HGF level was significantly and positively correlated with PASP and PVR (r = 0.36, p = 0.027) (Figs. 2a and 2b). Furthermore, the plasma OPN level was significantly and positively correlated with the Qp/Qs (r = 0.33, p = 0.035) (Fig. 2c). No correlation was found between HGF and PAPm, hemoglobin, the Qp/Qs, creatinine, oxygen pressure, oxygen saturation. OPN was also correlated with PASP, PAPm, PVR, creatinine, oxygen pressure, and oxygen saturation. When the PAH-CHD patients were regrouped according to the Qp/Qs, there was a significant difference in the plasma OPN level in those with a Qp/Qs of ≥ 1.5 versus those with a Qp/Qs of < 1.5 (Fig. 3).

Figure 2: Plasma HGF concentrations were positively correlated with PASP (A). Plasma HGF concentrations were positively correlated with PVR (B). Plasma OPN concentrations were positively correlated with Qp/Qs (C). HGF: Hepatocyte Growth Factor; PASP: Pulmonary Arterial Systolic Pressure; PVR: Pulmonary Vascular Resistance; Qp/Qs: Pulmonary-Systemic Shunt Ratio

Figure 3: Plasma OPN concentrations were significantly higher in patients with a Qp/Qs of ≥ 1.5. OPN: Osteopontin; Qp/Qs: Pulmonary-Systemic Shunt Ratio. PAH: Pulmonary Arterial Hypertension; Dynamic PAH: Qp/Qs ≥ 1.5; Resistance PAH: Qp/Qs of < 1.5
3.4 Prognostic Value of HGF for PAH-CHD
The results of the ROC curve plotted to analyze the diagnostic efficacy of HGF for PAH-CHD revealed an acceptable diagnostic ability (AUC = 0.692) (Fig. 4). The cut-off value derived from the ROC curve analysis was 356.75 ng/ml, with sensitivity of 53% and specificity of 89% in predicting PAH-CHD.

Figure 4: ROC analysis of the sensitivity and specificity of HGF (AUC = 0.692) in differentiating PAH from non-PAH. ROC: Receiver Operating Characteristic; AUC: Area Under Curve; HGF: Hepatocyte Growth Factor; PAH: Pulmonary Arterial Hypertension
HGF is a mesenchymal-derived cytokine and has been found elevated in PAH patients in the previous study [11]. In this study, we analyzed the plasma HGF concentration in CHD patients with different severities of PAPm and healthy individuals. We found that the plasma HGF level in the group with moderate to severe pulmonary hypertension (Group H) was significantly higher than that in the other groups. We detected no significant difference in the HGF level in the mild group versus that in the healthy control group. Moreover, the plasma HGF concentration was positively correlated with PASP and PVR. Notably, the correlation between HGF and PAPm was not statistically significant (p = 0.33). This may have been due to the small sample size. Using PVR of ≥ 3 Wood units as the standard for PAH, the ROC curve showed that plasma HGF exhibited acceptable diagnostic efficiency as a biomarker (p < 0.05). The results indicate that elevated plasma HGF is linked to the severity of PAH and that HGF can be used as a potential biomarker for early diagnosis of PAH-CHD.
Interestingly, in the present study, the OPN level in the mild group was higher than that in both the non-PAH group and moderate and severe group. One explanation is that in the early stage of PAH-CHD, the systemic pressure is higher than the pulmonary pressure, as the shunt direction is left to right, with a high shunt load. As the disease progresses, load and pressure on the right heart and pulmonary increases, and pulmonary vascular remodeling leads to increased pulmonary pressure, bi-directional shunting, and even reverse shunting, with a reduction or disappearance in left-to-right shunting. In accordance with this hypothesis, the results of Spearman’s analysis in this study showed that the plasma OPN concentration was positively correlated with the Qp/Qs. We regrouped the experimental group by a threshold of Qp/Qs of ≥1.5 and found the plasma OPN level in patients with Qp/Qs of ≥1.5 was significantly higher than that in patients with a Qp/Qs of <1.5. The Qp/Qs is one of a number of indicators that reflect the magnitude of the shunt. Further research with different methods, such as echocardiography, and a larger cohort is needed.
In recent years, sST2, as a circulating biomarker of heart failure, has been widely studied. In combination with other indicators, such as brain natriuretic peptide (BNP) and N-terminal pro BNP, it has been used to predict the prognosis in chronic heart failure. A previous study reported that higher sST2 levels were associated with more severe pulmonary hypertension [26]. Given the important role of sST2 in the inflammatory response, fibrosis, and ventricular remodeling, it may be associated with the development of PAH-CHD. However, the results of this study did not reveal any significant differences in the plasma sST2 levels of the various groups. Furthermore, they did not reveal any correlation between the sSS2 level and hemodynamic parameters of PAH-CHD.
Our study has some limitations. First, the small cohort size, especially the insufficient number of CHD patients with moderate to severe pulmonary hypertension (Group H), may compromise the validity of the statistical findings. Second, although the study pointed to the relevance of plasma levels of OPN and HGF in PAH-CHD, the specific pathways through which OPN and HGF may exert their actions remain to be elucidated. Moreover, we did not detect BNP in this study, the diagnostic efficiency of HGF may increase in combination with BNP.
In conclusion, we found that the plasma level of HGF was elevated in CHD patients with moderate to severe PAH and that the HGF level was an acceptable marker in diagnosing CHD-PAH. The plasma OPN level was higher in CHD patients with mild PAH, which may be related to the load of the left-to-right shunt. The sST2 level was not associated with hemodynamic parameters of PAH-CHD.
Data Sharing: The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Funding Statement: This work was supported by the Nantong Municipal Science and Technology Plan (JCZ19107).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Lim, Y., Low, T. T., Chan, S. P., Teo, T. W., Jang, J. J. et al. (2019). Pulmonary arterial hypertension in a multi-ethnic Asian population: Characteristics, survival and mortality predictors from a 14-year follow-up study. Respirology, 24(2), 162–170. DOI 10.1111/resp.13392. [Google Scholar] [CrossRef]
2. Kaemmerer, H., Apitz, C., Brockmeier, K., Eicken, A., Gorenflo, M. et al. (2018). Pulmonary hypertension in adults with congenital heart disease: Updated recommendations from the Cologne Consensus Conference 2018. International Journal of Cardiology, 272(18), 79–88. DOI 10.1016/j.ijcard.2018.08.078. [Google Scholar] [CrossRef]
3. Madonna, R., Rokosh, G., de Caterina, R., Bolli, R. (2010). Hepatocyte growth factor/Met gene transfer in cardiac stem cells–potential for cardiac repair. Basic Research in Cardiology, 105(4), 443–452. DOI 10.1007/s00395-010-0102-7. [Google Scholar] [CrossRef]
4. Gallo, S., Sala, V., Gatti, S., Crepaldi, T. (2015). Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clinical Science, 129(12), 1173–1193. DOI 10.1042/CS20150502. [Google Scholar] [CrossRef]
5. Nakamura, Y., Morishita, R., Higaki, J., Kida, I., Aoki, M. et al. (1996). Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: Additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. Journal of Hypertension, 14(9), 1067–1072. DOI 10.1097/00004872-199609000-00004. [Google Scholar] [CrossRef]
6. Nakagami, H., Morishita, R., Yamamoto, K., Taniyama, Y., Aoki, M. et al. (2001). Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension, 37(2), 581–586. DOI 10.1161/01.HYP.37.2.581. [Google Scholar] [CrossRef]
7. Purdie, K. J., Whitley, G. S., Johnstone, A. P., Cartwright, J. E. (2002). Hepatocyte growth factor-induced endothelial cell motility is mediated by the upregulation of inducible nitric oxide synthase expression. Cardiovascular Research, 54(3), 659–668. DOI 10.1016/S0008-6363(02)00255-9. [Google Scholar] [CrossRef]
8. Ono, M., Sawa, Y., Mizuno, S., Fukushima, N., Ichikawa, H. et al. (2004). Hepatocyte growth factor suppresses vascular medial hyperplasia and matrix accumulation in advanced pulmonary hypertension of rats. Circulation, 110(18), 2896–2902. DOI 10.1161/01.CIR.0000146342.30470.30. [Google Scholar] [CrossRef]
9. Chen, J., Zhang, H., Zhang, R., Liu, Z., Wang, J. et al. (2014). Transfer of human hepatocyte growth factor reduces inflammation and prevents pulmonary arterial remodeling in monocrotaline-induced. International Journal of Clinical and Experimental Pathology, 7(12), 8763–8769. [Google Scholar]
10. Pang, Y., Liang, M. T., Gong, Y., Yang, Y., Bu, P. L. et al. (2018). HGF reduces disease severity and inflammation by attenuating the NF-κB signaling in a rat model of pulmonary artery hypertension. Inflammation, 41(3), 924–931. DOI 10.1007/s10753-018-0747-1. [Google Scholar] [CrossRef]
11. Liang, M., Pang, Y., Zhang, S., Zhang, M. (2016). Utility of hepatocyte growth factor as a biomarker for early diagnosis of pulmonary artery hypertension. Molecular Diagnosis & Therapy, 20(5), 463–468. DOI 10.1007/s40291-016-0214-3. [Google Scholar] [CrossRef]
12. Ashizawa, N., Graf, K., Do, Y. S., Nunohiro, T., Giachelli, C. M. et al. (1996). Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. Journal of Clinical Investigation, 98(10), 2218–2227. DOI 10.1172/JCI119031. [Google Scholar] [CrossRef]
13. Singh, K., Sirokman, G., Communal, C., Robinson, K. G., Conrad, C. H. et al. (1999). Myocardial osteopontin expression coincides with the development of heart failure. Hypertension, 33(2), 663–670. DOI 10.1161/01.HYP.33.2.663. [Google Scholar] [CrossRef]
14. Rosenberg, M., Zugck, C., Nelles, M., Juenger, C., Frank, D. et al. (2008). Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circulation: Heart Failure, 1(1), 43–49. DOI 10.1161/CIRCHEARTFAILURE.107.746172. [Google Scholar] [CrossRef]
15. Saker, M., Lipskaia, L., Marcos, E., Abid, S., Parpaleix, A. et al. (2016). Osteopontin, a key mediator expressed by senescent pulmonary vascular cells in pulmonary hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(9), 1879–1890. DOI 10.1161/ATVBAHA.116.307839. [Google Scholar] [CrossRef]
16. Brida, M., Gatzoulis, M. A. (2018). Pulmonary arterial hypertension in adult congenital heart disease. Heart, 104(19), 1568–1574. DOI 10.1136/heartjnl-2017-312106. [Google Scholar] [CrossRef]
17. Meng, L., Liu, X., Teng, X., Gu, H., Yuan, W. et al. (2019). Osteopontin plays important roles in pulmonary arterial hypertension induced by systemic-to-pulmonary shunt. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 33(6), 7236–7251. DOI 10.1096/fj.201802121RR. [Google Scholar] [CrossRef]
18. Stępień, E., Wypasek, E., Stopyra, K., Konieczyńska, M., Przybyło, M. et al. (2011). Increased levels of bone remodeling biomarkers (osteoprotegerin and osteopontin) in hypertensive individuals. Clinical Biochemistry, 44(10–11), 826–831. DOI 10.1016/j.clinbiochem.2011.04.016. [Google Scholar] [CrossRef]
19. Caesar, C., Lyle, A. N., Joseph, G., Weiss, D., Alameddine, F. M. F. et al. (2017). Cyclic strain and hypertension increase osteopontin expression in the aorta. Cellular and Molecular Bioengineering, 10(2), 144–152. DOI 10.1007/s12195-016-0475-2. [Google Scholar] [CrossRef]
20. Iwahana, H., Yanagisawa, K., Ito-Kosaka, A., Kuroiwa, K., Tago, K. et al. (1999). Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. European Journal of Biochemistry, 264(2), 397–406. DOI 10.1046/j.1432-1327.1999.00615.x. [Google Scholar] [CrossRef]
21. Sanada, S., Hakuno, D., Higgins, L. J., Schreiter, E. R., McKenzie, A. N. et al. (2007). IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. Journal of Clinical Investigation, 117(6), 1538–1549. DOI 10.1172/JCI30634. [Google Scholar] [CrossRef]
22. Aimo, A., Januzzi, J. L.,Jr., Bayes-Genis, A., Vergaro, G., Sciarrone, P. et al. (2019). Clinical and prognostic significance of sST2 in heart failure: JACC review topic of the week. Journal of the American College of Cardiology, 74(17), 2193–2203. DOI 10.1016/j.jacc.2019.08.1039. [Google Scholar] [CrossRef]
23. Lichtenauer, M., Jirak, P., Wernly, B., Paar, V., Rohm, I. et al. (2017). A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. European Journal of Internal Medicine, 44, 31–38. DOI 10.1016/j.ejim.2017.05.027. [Google Scholar] [CrossRef]
24. Lotierzo, M., Dupuy, A. M., Kalmanovich, E., Roubille, F., Cristol, J. P. (2020). sST2 as a value-added biomarker in heart failure. Clinica Chimica Acta, 501(2017), 120–130. DOI 10.1016/j.cca.2019.10.029. [Google Scholar] [CrossRef]
25. Geenen, L. W., Baggen, V. J. M., van den Bosch, A. E., Eindhoven, J. A., Cuypers, J. et al. (2019). Prognostic value of soluble ST2 in adults with congenital heart disease. Heart, 105(13), 999–1006. DOI 10.1136/heartjnl-2018-314168. [Google Scholar] [CrossRef]
26. Geenen, L. W., Baggen, V. J. M., Kauling, R. M., Koudstaal, T., Boomars, K. A. et al. (2019). The prognostic value of soluble ST2 in adults with pulmonary hypertension. Journal of Clinical Medicine, 8(10), 1517. DOI 10.3390/jcm8101517. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |