DOI:10.32604/CHD.2021.013233

| Congenital Heart Disease DOI:10.32604/CHD.2021.013233 |  |
| Article |
Contemporary Patterns of Management of Tetralogy of Fallot: Data from a Single Center in China
1Center for Pediatric Cardiac Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
2Department of Cardiac Surgery, Yunnan Fuwai Cardiovascular Hospital, Kunming, China
*Corresponding Author: Qiang Wang. Email: wangqiang_cory@163.com
Received: 31 August 2020; Accepted: 22 October 2020
Abstract: Background: There is scarce research on large cohorts with tetralogy of Fallot (TOF) from China. The database in Fuwai Hospital was reviewed to ascertain current trends in the management of TOF and to determine the prevalence of various surgical techniques and the optimal early outcome. Methods: This cross-sectional study included 1861 patients who underwent surgery between 2012 and 2017 and were aged 0-18 years old with a primary diagnosis of TOF. A total of 1760 eligible patients were included in the analyses. Results: A total of 1683 patients underwent repair of TOF as a one-stage operation (primary repair). Sixty-one patients underwent repair of TOF after prior palliation. Of patients who underwent one-stage repair (n = 1683): 858 were 6 months to 1 year old, 421 were 1 to 2 years old, 251 were 2-18 years old, and 145 were 3 to 6 months old. Of patients who underwent repair following prior palliation (n = 61), 58 (95.1%) were older than 1 year of age. Of 1744 complete repairs, 986 (56.0%) had annulus-sparing (AS) repair. Total in-hospital mortality was 15 of 1744 (0.9%) for complete repair (including one-stage and staged repairs). The total incidence of the optimal early outcome was 78.2% in terms of a composite of the absence of death in the first year, significant right ventricular outflow tract obstruction, significant pulmonary valve insufficiency, or catheter or surgical reintervention. Conclusions: Surgical correction in patients with TOF can achieve an acceptable outcome in terms of death and reintervention. Primary repair at 6 months to 1 year of age is the most prevalent strategy in our centre. However, the relatively high incidence of early undesirable surgical adequacy of the pulmonary valve (PV) represents a wake-up call.
Keywords: Tetralogy of Fallot; chinese contemporary patterns of management; cross-sectional study
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease, with a large body of literature having described the diagnosis, treatment, and prognosis since it was first defined in 1888 [1] and first surgically treated in 1944 [2]. As the negative effects of long-standing pulmonary valve insufficiency, in terms of ventricular dimensions and function, exercise capacity, arrhythmias, heart failure, and sudden cardiac death, became clear, focus has shifted towards preserving pulmonary valve function [3,4]. However, after nearly 76 years, despite all of the accumulated knowledge and experience, the optimal timing and strategy of repair remain controversial [5]. The persistent uncertainty reflects ambiguity regarding the concept of treatment and divergent opinions when discussing the roles of annular preservation versus transannular patch enlargement and how best to accomplish pulmonary valve function preservation, as well as lack of consensus about end points. The recent report of Jeon and colleagues [6] introduced more reasonable approaches, including the redefinition of confusing concepts and the proposal of new end points, termed the optimal early outcome in TOF repair for neonates.
Against this backdrop, this study aims to describe the prevalence of various types of operative strategies used in the contemporary management of TOF in one of the largest heart centres in China. Although reports from individual institutions reflect several schools of thought about optimal timing, technique of repair, and the role of palliation, data from a large cohort reveal overall trends. Consideration of these data in relation to previously reported patterns of practice could answer questions of whether contemporary awareness of the late sequelae of chronic pulmonary regurgitation (PR) has affected the relative prevalence of various surgical techniques and whether contemporary treatment strategies yield similar early results in China.
This study was a cross-sectional study. The protocol for this study was reviewed and approved by the Medical Ethics Review Committee of Fuwai Hospital (2020–1318). Informed consent was waived. The study was supported by the National Key R&D Programme of China (2017YFC1308100) and Central Public-interest Scientific Institution Basal Research Fund (2019XK320050). This study was registered at www.chictr.org.cn (ChiCTR2000033234).
Clinical data were collected from the hospital database and extracted from the medical records and from the outpatient records at the available follow-up, including basic demographic information, preoperative factors, intraoperative details, surgical procedure performed, postoperative complications, in-hospital mortality and post-discharge surgical outcomes. The postoperative information of some patients was followed up by telephone call.
Patients who met all of the following criteria were included in the analysis.
1. The operation occurred from 2012 to 2017 inclusive.
2. The primary diagnosis was TOF.
3. The primary procedure was any of the choices, including one-stage repair, staged repair or palliation, listed in Tab. 1.
The analysis excluded patients who met any of the following criteria.
1. The patient had a primary diagnosis or secondary diagnosis that was any of the complicated cardiac malformations demonstrated in Fig. 1.
2. Critical information for patients was missing.
3. The patient was not involved in any surgical repair.

Figure 1: Patient flowchart
For this analysis, age groups were defined as follows:
1. Age in days 0 to 30 0 to 30 Days
2. Age in days 31 to 90 >30 Days to 3 Months
3. Age in days 91 to 180 >3 Months to 6 Months
4. Age in days 181 to 365 >6 Months to 1 Year
5. Age in days 366 to 730 >1 Year to 2 Years
6. Age in days 731 to 6574 >2 Years to 18 Years
Echocardiographic reports were obtained using standard views recommended by the published guidelines [7] and were reviewed for the degree of pulmonary regurgitation (PR) and peak annular gradient (APG). The pulmonary valve annulus (PVA) Z-score was calculated according to the previous equation [7]. Morphological features of the preoperative pulmonary valve (PV), which was investigated with preoperative echocardiography and intraoperative assessment, included unicuspid, bicuspid and tricuspid. One-stage repair was defined as patients who underwent primary repair of TOF as the initial operation. Double channel repair (DCR) was defined as complete repair followed by RV-PA connection with a conduit. Staged repair was defined as patients who underwent primary repair of TOF followed by prior palliation. Palliative surgery included modified Blalock-Taussig shunt (MBTS), central shunt (from aorta or to main pulmonary artery), RV-PA connection, main pulmonary artery stent implantation or right ventricular outflow tract (RVOT) muscle resection. Annulus-sparing (AS) repair was defined as uncut PVA [6]. We adopted a PV-specific technical performance score (TPS) as reported [8] to evaluate surgical adequacy by echo reports. We defined PV TPS as class 1 (peak annular gradient <20 mm Hg or none/trivial PR), class 2 (peak annular gradient 20–40 mm Hg or mild/mild to moderate PR), or class 3 (peak annular gradient >40 mm Hg or moderate or greater PR) [8]. The optimal early outcome was defined as a composite of the absence in the first year of death, significant right ventricular outflow tract obstruction (> 40 mmHg, TPS for peak annular gradient as class 3), significant pulmonary valve insufficiency (moderate or greater PR, TPS for PR as class 3), or catheter or surgical reintervention, as previously reported [6].
Categorical variables were presented as frequencies and percentages, continuous variables as the means and standard deviations or medians and ranges, where appropriate. The clinical characteristics of the patients and control subjects were compared with the use of the Mann-Whitney test for continuous variables and the chi-square test for categorical variables. The Kaplan-Meier method was used to determine cumulative probabilities of the early primary outcome defined as a composite of significant right ventricular outflow tract obstruction (> 40 mmHg, TPS for peak annular gradient as class 3) and significant pulmonary valve insufficiency (moderate or greater PR, TPS for PR as class 3). To explore the relationship between risk factors and the early primary outcome, variables at admission that had a P value < 0.05 in univariate Cox proportional-hazards analyses were introduced as covariates in a multivariate model with a stepwise forward method. All of the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solution, Inc., Boston, MA, USA).
3.1 Operation Types and Age at Operation
From January 2012 to December 2017, 1760 included patents (1064 male, 60.5%) underwent surgical repair of TOF at a median age of 318 days old (range: 18-6497 days) and a median weight of 8.9 kg (range: 2.1–80.0 kg). Detailed patient characteristics are depicted in Tab. 1. The inclusion and exclusion of patients are shown by a flow chart in Figs. 1 and 2 depicts the relationship between the type of procedure and age at operation. Beyond the neonatal period, one-stage repair (primary repair) was by far the most prevalent approach. The largest number of repairs with one-stage repair (primary repair) was within the 6-month to 1-year age category followed by the 1-year to 2-year category. The largest number of complete repairs after previous palliative repair was in the 2-year to 18-year age category, with 27.9% of complete repairs performed within 1 year after a previous palliative repair. Tab. 2 depicts the relationship between the specific type of procedure and the age at surgery for all instances of TOF palliation. Palliative RVOT muscle resection was the most prevalent palliative procedure in all age categories. Tab. 3 depicts the relationship between the type of procedure and PVA Z-score in complete repairs. TAPE was the most prevalent procedure in all age categories with PVA Z-score < −2, while AS was often used for PVA Z-score ≥ −2.
Table 1: Main characteristics of the study population
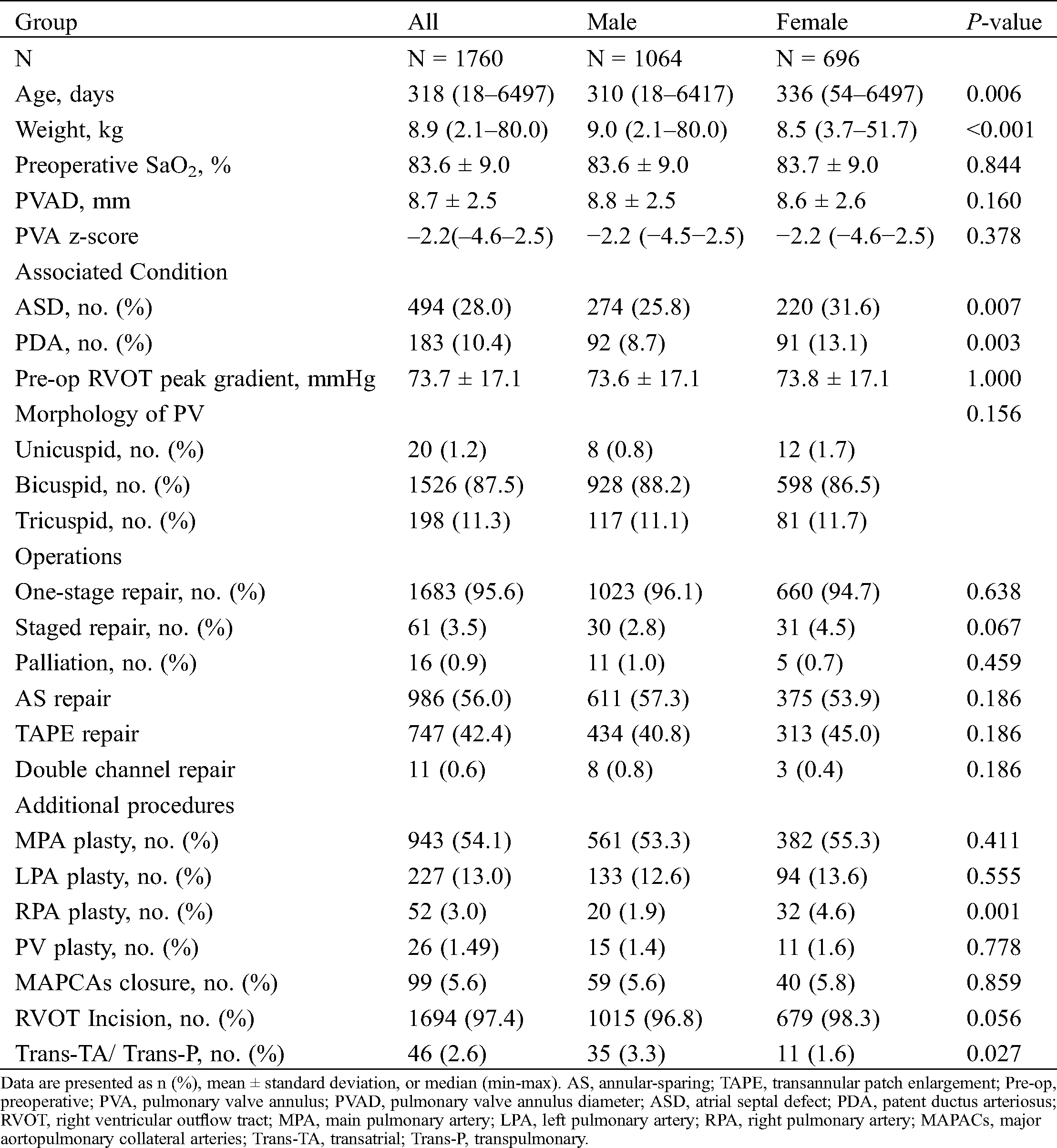

Table 3: The relationship between type of procedure and PVA z-score in complete repairs


Figure 2: The relationship between type of procedure and age at operation
3.2 Selection of Intraoperative Patch Materials
Fig. 3 demonstrates the selection of patch materials to relieve any part of the obstruction or stenosis in patients with AS repair and to enlarge the RVOT-MPA connection in TAPE repair. Glutaraldehyde-treated autologous pericardium was often used for PA enlargement, and autologous pericardium lined with a Dacron patch was often used for RVOT enlargement in AS repair. In the TAPE cohort, the Valved Bovine Jugular Vein patch was often used for reconstruction.

Figure 3: Selection of patch materials in AS and TAPE repair
Tab. 4 displays clinical outcomes for complete repair. The in-hospital mortality rate was 0.9%, and there were no deaths or reinterventions in the first year after the operations. The total incidence of adverse composite outcomes was 21.8% in terms of severe valvular dysfunction at an early stage, due to peak annular gradient (APG) > 40 mm Hg, moderate or greater PR, or both. In unadjusted Kaplan-Meier estimates, the early primary outcome was similar between female and male (see the Appendix A in the Appendix). In univariate Cox proportional-hazards analyses for factors at admission, patients with lower saturation, smaller PVA, smaller PVA Z-score, TAPE, secondary procedure, RVOT incision, transatrial approach, LPA enlargement and PV palsty had a higher risk of the early primary outcome. Detailed results of univariate analyses are shown in (Appendix B). According to the multivariate Cox model, TAPE was associated with the higher risk for the early primary outcome.
Table 4: Clinical outcomes for complete repair

Our current review of available data describing contemporary practice at a single centre in China suggests that primary repair in infancy remains the most prevalent therapeutic approach. More than half of the included patients received AS repair, while TAPE was the most prevalent procedure in all age categories with PVA Z-score < −2. These findings demonstrated that repair by means of right ventriculostomy with patched reconstruction of RVOT was the most prevalent surgical technique. Repair without ventriculostomy accounted for only 2.6% of complete repair. Surgical correction in patients with TOF can achieve an acceptable outcome with an in-hospital mortality of 0.9%. However, there are still some early potential adverse outcomes that must be treated with caution.
In palliative repair for TOF, systemic-to-pulmonary artery shunt and muscle resection and patch enlargement of RVOT have been commonly used worldwide since they were first introduced by Blalock in 1944 and Lillehei in 1954 [9]. For at least 2 decades, initial palliation followed by reparative operation later in childhood was the most prevalent strategy. By the early 1980s, primary repair in early infancy had been performed by Castaneda [10] and had gained wide acceptance. Thereafter, the concept of early primary repair was extended to symptomatic neonates, and low operative mortality was achieved [11]. Over time, mortality, progressive RV dilation, biventricular dysfunction, ventricular arrhythmias, and sudden cardiac death have been well recognized as late sequelae of chronic PR in patients with repaired TOF, leading some surgeons to advocate for approaches aimed at the preservation of pulmonary valve integrity to the extent possible. These recent surgical efforts have focused on PV structural integrity preservation, including valvoplasty with autologous pericardium after TAPE [12], pulmonary arterioplasty and valvular sinus plasty with an autologous pantaloon pericardial patch [13], pulmonary valve cusp plasty [14], pulmonary root enlargement [15], and AS repairs with intraoperative balloon dilation and delamination plasty [16–19], emphasizing valve-sparing or annulus-sparing repair. Other techniques include transatrial/transpulmonary artery approaches, avoidance of disrupting the pulmonary annulus to the extent possible, and palliative shunting (rather than primary repair) for symptomatic neonates and some infants in the hope that growth of the pulmonary annulus and maturation of the valve will lead to the reduced likelihood of transannular incision and patching at the time of eventual repair. These latter approaches also emphasize minimizing the length of transannular ventriculostomy incisions in those instances when they are unavoidable [13,20].
In our cohort, early complete repair could be carried out safely, and early mortality of the total cohort in our centre was lower than the pooled estimate of early mortality, which is as high as 2.84% [5]. However, despite all of the previously accumulated knowledge and experience, the timing of repair and these strategies adopted varied from our centre to others. The included patients who were completely repaired were slightly older than those typically encountered in North America [21], with the largest proportion of patients at 6 months to 1 year old as high as 49.2%, similar to a recent pooled estimate [5]. In case TAPE was used, monocusp reconstruction was applied in 79.7%, with the Valved Bovine Jugular Vein patch. There was no significant correlation between repair of all age categories and TAPE. In early smaller TOF cohorts, safe execution with sufficient reduction in RV to left ventricle pressure ratios has been described in patients with AS repair with a PVA Z-score < –5 [15], but our minimum value was −4.19. Additionally, we rarely adopted PV plasty as previously reported during this period [12,13,17,18]. Furthermore, despite many studies suggesting that the “classic” transventricular repair, with its large ventricular incision, should give way to newer transatrial/transpulmonary techniques focusing on preserving PV competency whenever possible and limiting the insult to the ventricle [20,22], we adopted RVOT incision for most RVOT muscle resections.
We introduced the early optimal outcome, as previously reported by Jeon and colleagues [6], to comprehensively estimate surgical effects, and it demonstrated early potential PV dysfunction, which could remind us of paying more attention to these types of patients earlier.
We presented the first largest, single-centre TOF cohort in China, to the best of our knowledge, on contemporary patterns of management and the early optimal outcome. The results presented here could help members of other congenital heart teams understand the current strategies.
Our study has several limitations. First, the study was retrospective and descriptive, with treatment decided by surgical judgement. The study design did not enable a determination of the extent to which choices of treatment strategies were determined by surgeon philosophies and preferences or were ordained by specific features of the cardiac morphology and the clinical status of the individual patient. A prospective, protocolized approach based on PVA Z-score would have perhaps avoided ‘‘inappropriate’’ treatment assignments. Second, the study was limited by long-term follow-up, so the observational incidence of death and reintervention was low. Therefore, we must follow up the cohort further. Third, our data only reflect the treatment patterns of congenital cardiac centres with relatively high medical levels in China but not the patterns of hospitals at different levels. Accordingly, multicentre TOF cohorts must be incorporated. Fourth, during this period, we lacked data on neonatal surgery, which will be made up for in the future.
This study was only a small part of the ambispective cohort study (ChiCTR2000033234). Under contemporary treatment patterns, more questions will be answered in future research.
A review of data pertaining to 1862 instances of palliation or repair of TOF from 2012 to 2017 suggested that primary repair at 6 months to 1 year old is the most prevalent strategy in our centre. AS repair has become the most prevalent technique due to contemporary awareness of the late consequences of pulmonary sufficiency. TAPE repair was more likely to be used in patients with pulmonary valve annulus (PVA) Z-scores < −2, both for primary repair and for repair following palliation. Surgical correction in patients with TOF could achieve an acceptable outcome in terms of death and reintervention. However, the data highlight the shortcomings of early repair, and the relatively high incidence of early undesirable adequacy of the pulmonary valve (PV) requires a wake-up call.
This study was intended to be descriptive of current practice in our centre. In this context, we are not advocating for any of the specific treatment strategies for the management of patients with TOF. To answer important questions about the effects of various initial treatment strategies on long-term adverse events, such as deterioration of ventricular function, decreasing exercise capacity, arrhythmias, heart failure, and sudden cardiac death, will likely require prospective investigation by further follow-up.
Acknowledgement: We appreciate Dr. Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for statistics, study deign consultations and editing the manuscript.
Ethical Statement: This study was reviewed and approved by the Medical Ethics Review Committee of Fuwai Hospital (2020-1318). Informed consent was waived.
Data Sharing: No more data will be shared.
Funding Statement: The study was supported by the National Key R&D Program of China (2017YFC1308100) and Central Public-interest Scientific Institution Basal Research Fund (2019XK320050).
Conflicts of Interest: The authors have no conflicts of interest to declare.
1. Fallot, A. (1888). Contribution à l'anatomie pathologique de la maladie bleue (cyanose cardiaquepar le Dr. A. Fallot. Marseille: Impr de Barlatier-Feissat, http://refhub.elsevier.com/s0022-5223(19)31954-3/sref1. [Google Scholar]
2. Blalock, A., Taussig, H. B. (1945). The surgical treatment of malformations of the heart: In which there is pulmonary stenosis or pulmonary atresia. Journal of the American Medical Association, 128(3), 189–202. DOI 10.1001/jama.1945.02860200029009. [Google Scholar] [CrossRef]
3. Geva, T., Sandweiss, B. M., Gauvreau, K., Lock, J. E., Powell, A. J. (2004). Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. Journal of the American College of Cardiology, 43(6), 1068–1074. DOI 10.1016/j.jacc.2003.10.045. [Google Scholar] [CrossRef]
4. Apitz, C., Webb, G. D., Redington, A. N. (2009). Tetralogy of Fallot. Lancet (London, England), 374(9699), 1462–1471. DOI 10.1016/S0140-6736(09)60657-7. [Google Scholar] [CrossRef]
5. Romeo, J., Etnel, J., Takkenberg, J., Roos-Hesselink, J., Helbing, W. A. et al. (2020). Outcome after surgical repair of tetralogy of Fallot: A systematic review and meta-analysis. Journal of Thoracic and Cardiovascular Surgery, 159(1), 220–236.e8. DOI 10.1016/j.jtcvs.2019.08.127. [Google Scholar] [CrossRef]
6. Jeon, B., Kim, D. H., Kwon, B. S., Choi, E. S., Park, C. S. et al. (2020). Surgical treatment of tetralogy of Fallot in symptomatic neonates and young infants. Journal of Thoracic and Cardiovascular Surgery, 159(4), 1466–1476.e2. DOI 10.1016/j.jtcvs.2019.10.172. [Google Scholar] [CrossRef]
7. Pettersen, M. D., Du, W., Skeens, M. E., Humes, R. A. (2008). Regression equations for calculation of Z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents, an echocardiographic study. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography, 21(8), 922–934. DOI 10.1016/j.echo.2008.02.006. [Google Scholar] [CrossRef]
8. Nathan, M., Marshall, A. C., Kerstein, J., Liu, H., Fynn-Thompson, F. et al. (2014). Technical performance score as predictor for post-discharge reintervention in valve-sparing tetralogy of Fallot repair. Seminars in Thoracic and Cardiovascular Surgery, 26(4), 297–303. DOI 10.1053/j.semtcvs.2014.12.001. [Google Scholar] [CrossRef]
9. Jacobs, M. L., Jacobs, J. P. (2008). The early history of surgery for patients with tetralogy of Fallot. Cardiology in the Young, 18(S3), 8–11. DOI 10.1017/S1047951108003247. [Google Scholar] [CrossRef]
10. Castaneda, A. R., Freed, M. D., Williams, R. G., Norwood, W. I. (1977). Repair of tetralogy of Fallot in infancy early and late results. Journal of Thoracic and Cardiovascular Surgery, 74(3), 372–381. DOI 10.1016/S0022-5223(19)41351-2. [Google Scholar] [CrossRef]
11. Hirsch, J. C., Mosca, R. S., Bove, E. L. (2000). Complete repair of tetralogy of Fallot in the neonate, results in the modern era. Annals of Surgery, 232(4), 508–514. DOI 10.1097/00000658-200010000-00006. [Google Scholar] [CrossRef]
12. Sung, S. C., Kim, S., Woo, J. S., Lee, Y. S. (2003). Pulmonic valve annular enlargement with valve repair in tetralogy of Fallot. Annals of Thoracic Surgery, 75(1), 303–305. DOI 10.1016/S0003-4975(02)03926-7. [Google Scholar] [CrossRef]
13. Stewart, R. D., Backer, C. L., Young, L., Mavroudis, C. (2005). Tetralogy of Fallot: Results of a pulmonary valve-sparing strategy. Annals of Thoracic Surgery, 80(4), 1431–1439. DOI 10.1016/j.athoracsur.2005.04.016. [Google Scholar] [CrossRef]
14. Hua, Z., Li, S., Wang, L., Hu, S., Wang, D. (2011). A new pulmonary valve cusp plasty technique markedly decreases transannular patch rate and improves midterm outcomes of tetralogy of Fallot repair. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 40(5), 1221–1226. [Google Scholar]
15. Ito, H., Ota, N., Murata, M., Tosaka, Y., Ide, Y. et al. (2013). Technical modification enabling pulmonary valve-sparing repair of a severely hypoplastic pulmonary annulus in patients with tetralogy of Fallot. Interactive CardioVascular and Thoracic Surgery, 16(6), 802–807. DOI 10.1093/icvts/ivt095. [Google Scholar] [CrossRef]
16. Bautista-Hernandez, V., Cardenas, I., Martinez-Bendayan, I., Loyola, H., Rueda, F. et al. (2013). Valve-sparing tetralogy of Fallot repair with intraoperative dilation of the pulmonary valve. Pediatric Cardiology, 34(4), 918–923. DOI 10.1007/s00246-012-0574-3. [Google Scholar] [CrossRef]
17. Vida, V. L., Guariento, A., Castaldi, B., Sambugaro, M., Padalino, M. A. et al. (2014). Evolving strategies for preserving the pulmonary valve during early repair of tetralogy of Fallot: Mid-term results. Journal of Thoracic and Cardiovascular Surgery, 147(2), 687–696. DOI 10.1016/j.jtcvs.2013.10.029. [Google Scholar] [CrossRef]
18. Vida, V. L., Angelini, A., Guariento, A., Frescura, C., Fedrigo, M. et al. (2015). Preserving the pulmonary valve during early repair of tetralogy of Fallot: Anatomic substrates and surgical strategies. Journal of Thoracic and Cardiovascular Surgery, 149(5), 1358–63.e1. DOI 10.1016/j.jtcvs.2015.01.030. [Google Scholar] [CrossRef]
19. Vida, V. L., Guariento, A., Zucchetta, F., Padalino, M., Castaldi, B. et al. (2016). Preservation of the pulmonary valve during early repair of tetralogy of Fallot: Surgical techniques. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 19(1), 75–81. DOI 10.1053/j.pcsu.2015.12.008. [Google Scholar] [CrossRef]
20. Morales, D. L., Zafar, F., Fraser, C. D.Jr. (2009). Tetralogy of Fallot repair: The right ventricle infundibulum sparing (RVIS) strategy. In Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 54–58. DOI 10.1053/j.pcsu.2009.02.001. [Google Scholar] [CrossRef]
21. Al Habib, H. F., Jacobs, J. P., Mavroudis, C., Tchervenkov, C. I., O’Brien, S. M. et al. (2010). Contemporary patterns of management of tetralogy of Fallot, data from the Society of Thoracic Surgeons Database. Annals of Thoracic Surgery, 90(3), 813–820. DOI 10.1016/j.athoracsur.2010.03.110. [Google Scholar] [CrossRef]
22. Wald, R. M., Haber, I., Wald, R., Valente, A. M., Powell, A. J. et al. (2009). Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation, 119(10), 1370–1377. DOI 10.1161/CIRCULATIONAHA.108.816546. [Google Scholar] [CrossRef]
Appendix:

Appendix A: Kaplan-Meier curves for the early primary outcome. Unadjusted Kaplan-Meier estimates for the probability of freedom from the early primary outcome
Appendix B: The early optimal outcome for TOF patients undergoing complete repair with RVOT incision, analyzed by Cox regression

 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |