DOI:10.32604/CHD.2021.013038

| Congenital Heart Disease DOI:10.32604/CHD.2021.013038 |  |
| Article |
Arrhythmic Risk in Paediatric Patients Undergoing Surgical Repair for Pulmonary Atresia with Intact Ventricular Septum
1Paediatric Cardiology and Cardiac Arrhythmia Unit, Department of Paediatric Cardiology and Cardiac Surgery, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
2European Reference Network for Rare and Low Prevalence Complex Disease of the Heart
3Paediatric Cardiac Surgery Unit, Department of Paediatric Cardiology and Cardiac Surgery, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
4Sport Medicine Unit, Department of Paediatric Cardiology and Cardiac Surgery, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
*Corresponding Author: Corrado Di Mambro. Email: corrado.dimambro@opbg.net
Received: 24 July 2020; Accepted: 10 November 2020
Abstract: Introduction: While previous studies only focused on the arrhythmic risk associated with specific correction strategies, this study evaluates this risk in a large cohort of paediatric patients with all phenotypes of PA-IVS after surgical repair. Methods: In this single centre observational cohort study, we retrospectively evaluated 165 patients with a diagnosis of PA-IVS and we excluded those with an exclusively percutaneous treatment, patients lost or with insufficient follow-up and those affected by other arrhythmic syndromes. Surgical history and clinical outcomes were reviewed. Results: 86 patients were included in the study (54 male [62.8%], mean age 16.4 ± 6.1 years), with median follow-up from definitive repair of 12.8 years (6.4–18.9 years). They underwent three different final repairs: 23 patients (26.7%) univentricular palliation, 43 (50%) biventricular correction, and 20 (23.3%) one and a half ventricle correction. Thirteen patients (15%) developed arrhythmia: 6 patients (all the subgroups) sinus node disfunction (SND); 2 (biventricular repair) premature ventricular complexes; 2 (one and a half ventricle repair) non-sustained ventricular tachycardia; 1 (biventricular repair) intra-atrial re-entrant tachycardia; 1 (one and a half ventricle repair) supraventricular tachyarrhythmia; 1 (biventricular repair) atrial fibrillation. Three patients with SND needed a pacemaker implantation. Only Fontan circulation showed an association with SND, while the other two groups heterogeneous types of arrhythmias. Conclusions: The low arrhythmic risk is related to surgical repair, it does not appear to be associated with native cardiomyopathy, and it appears to increase with length of follow up. Continuous follow-up in specialized centres is necessary to make an early diagnosis and to manage the potential haemodynamic impact at medium-long term.
Keywords: Pulmonary atresia with intact ventricular septum; arrhythmic risk; univentricular palliation; Fontan circulation; biventricular repair; one and a half repair
Pulmonary atresia with intact ventricular septum (PA-IVS) is a rare congenital heart malformation characterized by the complete obstruction of the right ventricular outflow tract (RVOT) in the absence of ventricular septal defect. Its estimated prevalence is 0.083 per 1000 live births [1], but more recently its incidence has been modified by advances in prenatal diagnosis and its phenotype is constantly changing [2].
Different phenotypes have been identified in the spectrum of PA-IVS: the main features of this pathology are different right ventricular (RV) morphologies, tricuspid valve (TV) defects and coronary artery abnormalities. Current management of PA-IVS is mainly based on surgery and percutaneous treatment. The therapeutic strategy consists in assessing RV morphology and its tripartite division, namely the inlet, apical trabecular, and outlet components, as described in Bull and De Leval’s classification [3]. In this regard, three phenotypes should be taken into consideration. First, a well-represented, tripartite RV in the presence of a regurgitant TV and a TV annulus Z-score > –2.5. Second, a bipartite RV, with a vestigial or absent infundibular septum and a TV annulus Z-score between –2.5 and –4.5. Lastly, the most extreme pathological form represented by a restrictive RV and a stenotic TV with a TV annulus Z-score < –4.5, often in the presence of coronary sinusoids, with or without RV dependence. The first phenotype usually goes to biventricular correction with opening of the obstructed outflow tract (infundibular patch, surgical valvulotomy) or placement of a RV to pulmonary artery conduit; the second one, having an intermediate ventricular volume, needs a partial volume unloading through a bidirectional cavo-pulmonary anastomosis (modified Glenn operation) and concomitant RVOT opening (one and a half ventricle correction); the third one, with the most reductive ventricles, also the most prone to abnormalities of the coronary circulation, are only amenable to univentricular palliation, starting with aorto-pulmonary shunt (BT Shunt or Laks Shunt), bidirectional Glenn and Fontan procedure [4,5] (Fig. 1).
The arrhythmic risk in all subsets of PA-IVS patients has not been evaluated to date. Previous studies only focused on the arrhythmic risk associated with a specific correction strategy without considering the different characteristics of the overall PA-IVS population [6,7]. The aim of this study was to evaluate the arrhythmic risk in a large cohort of paediatric patients with all phenotypes of PA-IVS after surgical repair.

Figure 1: Surgical management in patients with PA-IVS. RV, right ventricle; TV, tricuspid valve; RVOT, right ventricle outflow tract
This is a single centre observational cohort study. We retrospectively evaluated patients with a diagnosis of PA-IVS admitted to our Institution between 1 January 1990 and 31 December 2017. For all patients we collected data regarding demographics, right ventricular morphology (tripartite, bipartite, or monopartite), presence of associated abnormalities [Ebstein-like TV or RV-dependent coronary circulation (DCC)], Z-score of the TV, type of initial intervention, need for and type of subsequent additional interventions and cardiac catheterization.
All patients had undergone a cardiac examination once/twice a year with ECG and echocardiographic assessment. ECG data included standard 12-lead ECG, 24-hour ECG Holter monitoring and exercise stress test in collaborative children (>6 years). Electrophysiological studies (EPS), diagnostic and/or interventional studies and pacemaker (PM) interrogation were also considered if available. Echocardiographic data included right atrial (RA) and RV dilatation, tricuspid and pulmonary regurgitation in biventricular and in one and a half ventricle correction, and left ventricular ejection fraction (LVEF, %) for everyone. Echocardiographic assessment has been expressed in a qualitative way as mild, moderate or severe. Length of follow-up was calculated as the time from the first operation to the date of the last follow-up visit at our Institution.
Cardiac arrhythmia has been classified as supraventricular tachyarrhythmias (SVT), ventricular tachyarrhythmias and bradyarrhythmias. For patients with premature ventricular complexes (PVCs), only organized monomorphic/polymorphic couples or triplets were included, and for patients with atrio-ventricular block only the advanced/complete ones. Sinus node dysfunction (SND) was defined as a physiologically inappropriate atrial rate, with or without pathological pauses and/or junctional escape rhythm. The time of occurrence of arrhythmia was defined as the time elapsed between surgical repair and the first recorded arrhythmia (through diagnostic instruments with or without clinical findings). In addition, arrhythmia have been divided into early (within 30 days of surgical repair) and late (after 30 days from surgical repair). If available, the time between surgical repair and PM implantation was also evaluated. We then analysed the factors that may have caused arrhythmia: PA-IVS phenotype, the first and subsequent additional interventions, age and time from surgical repair. Exclusion criteria included an exclusively percutaneous treatment, a follow-up duration of <5 years, and the presence of other arrhythmic syndromes.
Continuous variables were first tested for Gaussian distribution with the one-sample Kolmogorov–Smirnov test. They were expressed as frequencies, median ± interquartile range (IQR) and mean ± standard deviation, as appropriate, and compared with unpaired Student’s t-test, if normally distributed, or with Mann-Whitney U test, if not normally distributed. Categorical variables were reported as frequencies and compared with Chi-square test. Freedom from arrhythmia was analysed with the Kaplan–Meier method and differences between groups were compared using log-rank test. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA).
A total of 76 patients were excluded from the overall population of 162 patients: 11 (6.8%) who received exclusively percutaneous treatment, 11 (6.8%) with insufficient follow-up (<5 years), 17 (10.5%) lost to follow-up, 1 (0.6%) affected by Wolff-Parkinson-White syndrome, and 38 (23.5%) who died. Among the latter, 35 died after the first palliative intervention (shunt ± outflow patch) during their recovery in intensive care unit (i.e., acute heart failure in BT Shunt). No further information about the cause of death was retrievable because of the lack of an electronic medical record system at that time. Only one child underwent heart transplantation for failing Fontan and died due to acute rejection. Another one died for heart failure after one and a half ventricle repair and another patient died for Fontan failure. A total population of 86 patients was included in the study (54 male [62.8%], mean age at last follow-up 16.4 ± 6.1 years); the median follow-up from definitive correction was 12.8 years (IQR 6.4–18.9 years). According to the heterogeneity of PA-IVS phenotypes, our cohort was characterized by different interventions and final corrections: 23 patients (26.7%) underwent univentricular palliation, 43 (50%) biventricular correction, and 20 patients (23.3%) one and a half ventricle repair.
The first group was composed by patients with a hypoplastic RV, associated with RV-DCC in 13 cases (56.5% of patients with univentricular palliation, 15.1% of the total population). Patients referred to univentricular palliation first underwent a BT Shunt and then a modified Glenn operation, before completion of the Fontan procedure with extracardiac conduit.
The second group included patients with a well-developed RV. Seven of these patients (35%, 8.1% of the total cohort) were managed with a percutaneous approach but surgical operation was performed in case of acute complications or treatment failure. The last group consisted of patients with a borderline RV, who underwent a modified Glenn operation after a BT Shunt and outflow patch. One of these patients presented an Ebstein like TV. The median age at surgical correction was 2.8 years (IQR 1.4–5 years).
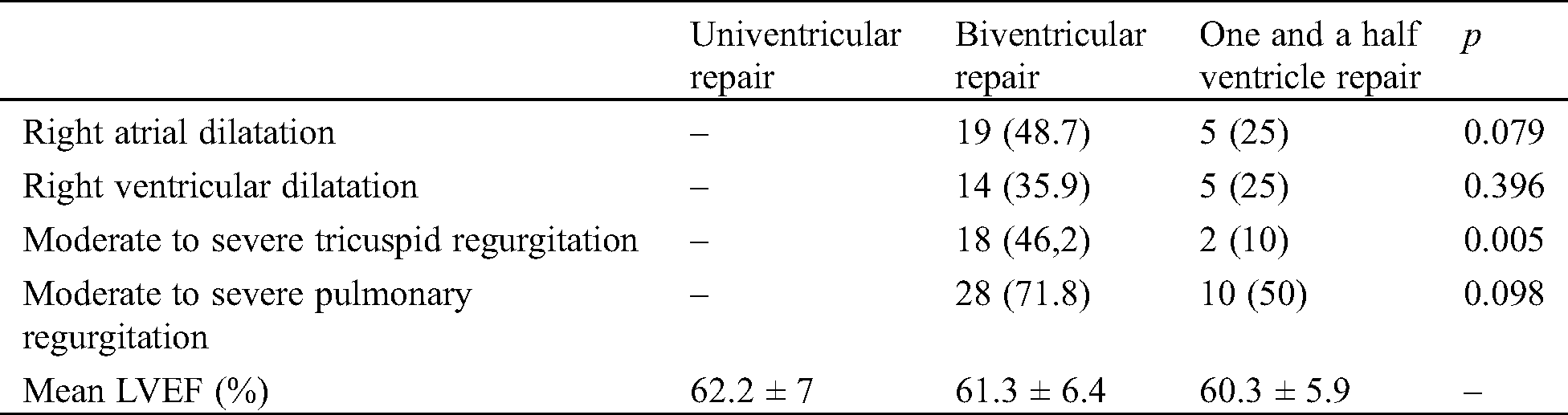
In the biventricular and one and a half ventricle correction groups, RA dilatation was observed in 24 patients (40.7%) (19 [48.7%] and 5 [25%] in the first and second group, respectively), while RV dilatation was observed in 19 patients (32.2%) (14 [35.9%] and 5 [25%] in the first and second group, respectively). The prevalence of atrial and ventricular dilatation (both p = NS) was slightly higher in the biventricular repair group compared with the one and a half ventricle correction group.
In the same groups, tricuspid regurgitation was detected in 20 patients (33.9%) (18 [46.2%] and 2 [10%] in the first and second group, respectively), while pulmonary regurgitation was detected in 38 patients (64.4%) (28 [71.8%] and 10 [50%] in the first and second group, respectively). The prevalence of tricuspid (p = 0.005) and pulmonary regurgitation (p = NS) was higher in the biventricular repair group than in the one and a half ventricle correction group.
No differences in LVEF were found among the three groups. Univentricular palliation showed a mean LVEF of 62.2 ± 7%, biventricular correction a mean LVEF of 61.3 ± 6.4%, and one and a half ventricle correction a mean LVEF of 60.3 ± 5.9% (Tab. 1).
Table 1: Echocardiographic data. Values are expressed as n (%) or mean ± standard deviation. LVEF, left ventricular ejection fraction
Thirteen patients (15%) developed arrhythmia during follow-up. The mean time (ΔT) from surgical correction was 7.9 years (minimum 0–maximum 20.3 years). In 6 of them (46.2% of arrhythmic patients, 7% of the total cohort), a bradyarrhythmias was observed, while 7 (53.8 % of arrhythmic patients, 8.1% of the total cohort) experienced a tachyarrhythmia (Tab. 2).
Table 2: Arrhythmia. AF, atrial fibrillation; IART, intra-atrial re-entrant tachycardia; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; SND, sinus node dysfunction; SVT, supraventricular tachycardia
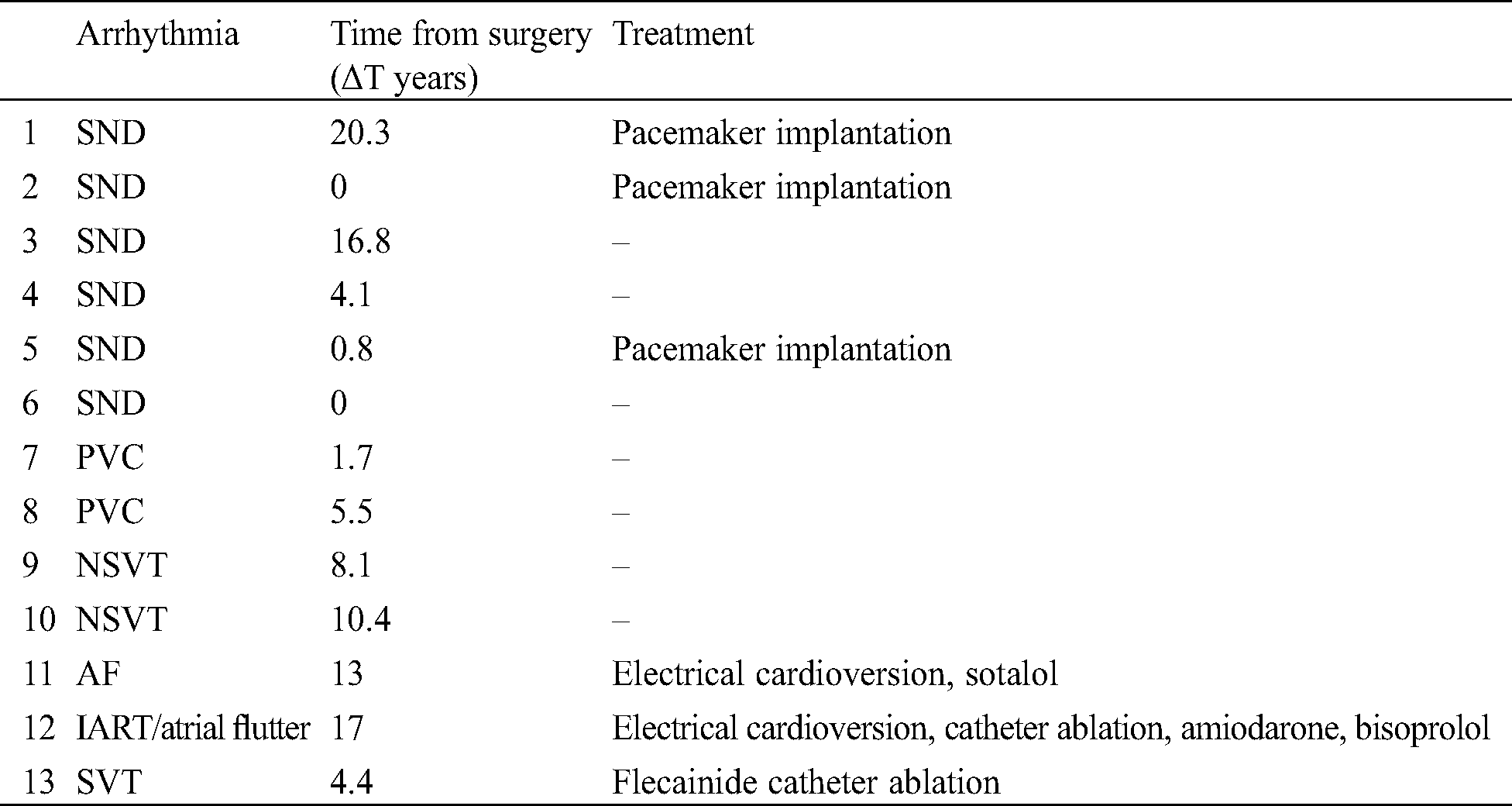
The first episode of bradyarrhythmia, represented by sinus node disfunction (SND), developed after a median of 7 years from correction, while tachyarrhythmia occurred after a mean of 8.6 years. However, 2 patients developed bradyarrhythmia immediately after the surgical procedure.
Only 3 symptomatic patients (50% of patients with SND, 3.5% of the total cohort) needed a PM implantation after a median of 1.7 years, according to pacing guidelines [8]. One of them underwent PM implantation immediately after correction due to acute complications related to the surgical procedure (Fontan procedure). In 2 patients an epicardial PM was implanted, due to young age and low weight. All PM were in DDD modality. During PM interrogation during their routine FU, only atrial pacing was detected.
Two patients who underwent biventricular repair showed monomorphic PVCs, in couples or triplets, during ECG Holter monitoring after 1.7 and 5.5 years from surgical correction. In both patients, at 12-lead ECG, PVCs exhibited a left bundle branch block (LBBB) morphology with an inferior axis and QRS precordial transition in V3, typical characteristics of PVCs originating from the RVOT.
Two patients who underwent one and a half ventricle repair showed non-sustained ventricular tachycardia (NSVT) after 8.1 and 10.4 years from surgical correction. One of these developed two episodes of NSVT (LBBB morphology, heart rate 187 bpm, duration 21 and 15 s) during ECG Holter monitoring and an EPS was therefore performed, which was negative for inducible ventricular tachycardia. The other patient experienced only one episode of NSVT (LBBB morphology, 130 bpm, duration 17 s) in the ECG Holter monitoring made during his routine FU.
One patient with severe RA dilatation who received biventricular repair developed atrial fibrillation (AF) after 13 years from surgical correction. He underwent successful electrical cardioversion, and maintenance therapy with sotalol was initiated.
One patient of the biventricular repair group developed an intra-atrial re-entrant tachycardia (IART) with an atrio-ventricular conduction 3:1/4:1 (atrial cycle length 270–280 ms) after 17 years from surgical correction. Echocardiography showed severe RA and right ventricular dilatation and severe tricuspid regurgitation. He underwent an EPS who showed RA low-voltage areas during IART/atrial flutter and was treated successfully with catheter ablation. After 1 year, he experienced recurrent IART/atrial flutter of different morphology and P-wave axis and underwent various attempts of electrical cardioversion. At the moment, he is on amiodarone and bisoprolol.
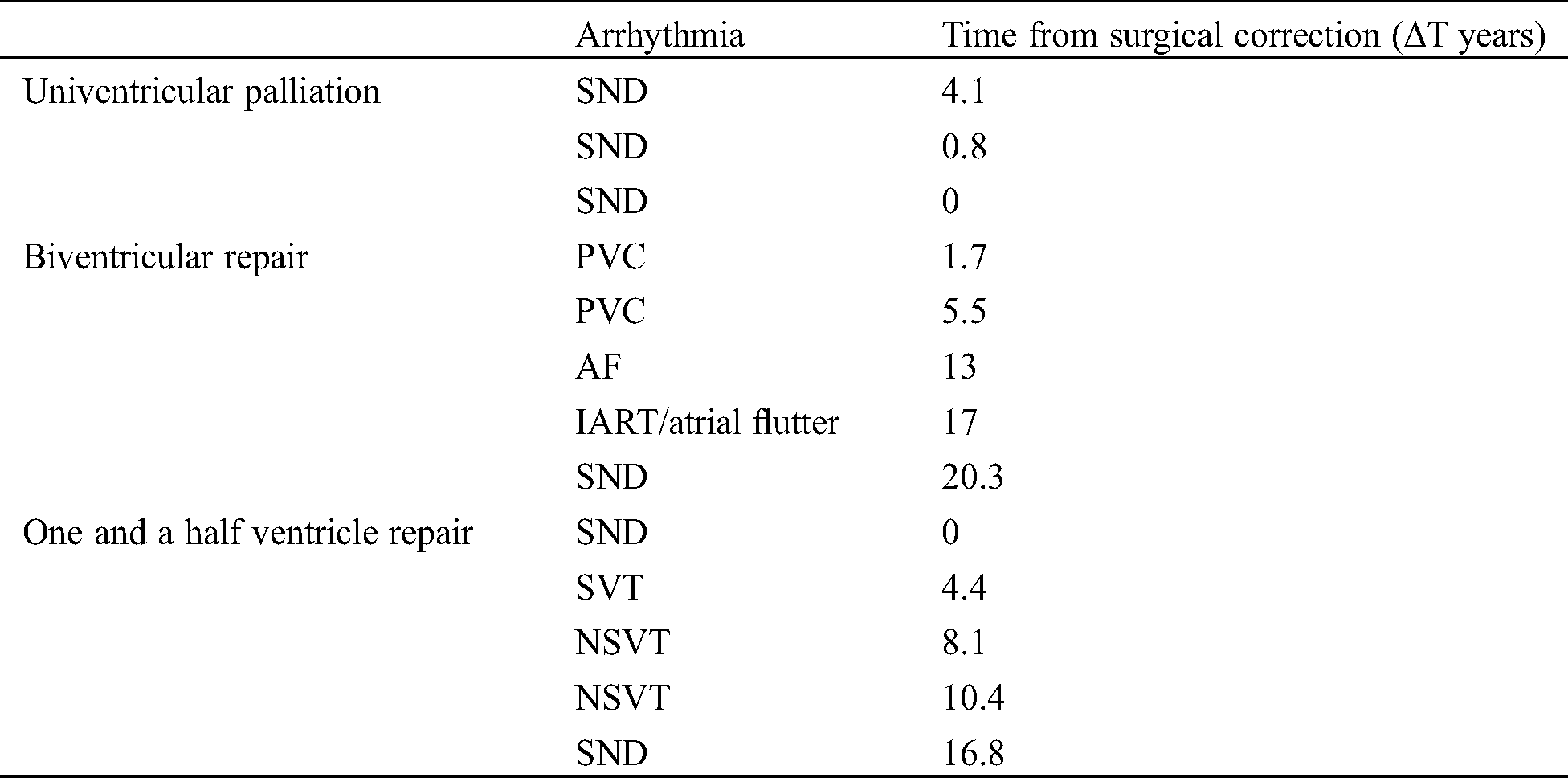
One patient of the one and a half ventricle repair group showed an SVT (an ectopic atrial tachycardia originating from the right atrium) after 4.4 years from surgical repair. After a transoesophageal EPS negative for inducible IART/atrial flutter, he was treated with flecainide and 2 years later he underwent a successful catheter ablation. No direct correlation was found between the development of arrhythmia, PA-IVS phenotypes and age at surgical correction (p = NS). Stratification by arrhythmia among the three groups of correction is showed in Tab. 3.
Table 3: Arrhythmia in the three groups of surgical correction. AF, atrial fibrillation; IART, intra-atrial re-entrant tachycardia; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; SND, sinus node dysfunction
Kaplan-Meier analysis showed a significant difference in arrhythmia-free survival with a higher arrhythmic risk in patients who received one and a half ventricle repair (log-rank p = 0.45) (Fig. 2).

Figure 2: Kaplan-Meier curves showing arrhythmia-free survival in the three groups of surgical correction, over the first 26 years after surgical correction
Only Fontan circulation showed a particular association with SND, though without reaching statistical significance, while the other two groups seemed more likely to present heterogeneous types of arrhythmias. Moreover, in our cohort, arrhythmia after Fontan procedure developed earlier compared with the other groups.
When comparing echocardiographic parameters (right atrial and ventricular dilatation, pulmonary and tricuspid regurgitation) in biventricular and one and a half ventricle repair, only RA dilatation was found to be significantly related to the presence of arrhythmia (p = 0.05). When analysing LVEF in patients with Fontan palliation, a significant correlation was observed between reduced LVEF and the occurrence of arrhythmia (p = 0.042).
The PA-IVS is a rare and complex congenital heart disease. In line with the heterogeneous phenotype of this pathology, different treatments and therapeutic approaches have been adopted to tailor its management. As a result, many studies have focused on outcomes after a particular surgical repair strategy, overshadowing the arrhythmic stratification related to PA-IVS. In contrast, we aimed at evaluating arrhythmia in a cohort of affected patients who underwent different types of surgical repair.
In our study, a heterogeneous management reflected the composite nature of the population: 23 patients with a hypoplastic RV underwent univentricular Fontan palliation, 43 patients with a well-developed RV received biventricular repair, and 20, presenting a borderline morphology, were managed with one and a half ventricle repair.
Although the analysis of mortality data falls outside the scope of this study, the mortality rate we observed was comparable to that reported in other studies. In the vast majority of cases, an infant death occurred for hemodynamic failure (i.e., heart failure after BT Shunt), mainly in patients showing a hypoplastic RV, a small TV and RV-DCC [9–11]. Consistently with literature data [12], none of the patients died from arrhythmic complications.
In our study, arrhythmia have been reported in 13 patients, accounting for 15% of our cohort. These complications seem to be only correlated to the type of surgical repair and probably due to the overload of RV with different grade of hypoplasia. Currently, no microscopic or macroscopic abnormalities have been identified that may represent an arrhythmogenic substrate in PA-IVS patients: the conduction system is similar to that in normal hearts [13], and only coronary abnormalities could explain the occurrence of atrio-ventricular block or ventricular tachyarrhythmias [14]. There was a slightly higher prevalence of tachyarrhythmias compared to bradyarrhythmias (ratio 7:6), and, within tachyarrhythmias, a slightly higher prevalence of ventricular arrhythmias compared to atrial arrhythmias (ratio 4:3). Among patients undergoing Fontan palliation, 13% developed an arrhythmia (i.e., SND), occurring immediately after surgery in one patient and after 0.8 and 4.1 years from surgery in the other 2 patients. These complications were mainly observed in patients with reduced LVEF, confirming previous data about arrhythmic risk after Fontan procedure: even considering the evolution of the original technique, which prevents excessive atrial enlargement and use of long suture lines, no reduction of arrhythmia has been reported [15,16]. The high incidence of SND could be explained by the surgical dissection near the sinoatrial node or its blood supply to separate the right pulmonary artery from the right atrial roof during extra-cardiac conduit Fontan completion after a modified Glenn operation. This may reduce blood supply to the sinoatrial node, which arises from the circumflex coronary artery in 40% of cases [17]. Stephenson et al. reported an increased prevalence of arrhythmias, especially atrial arrhythmias due to atrial fibrosis [18].
Patients who had undergone biventricular repair showed the lowest prevalence of arrhythmia (11.7%). The mean time of arrhythmia development was 11.8 years after surgical repair. SVT, represented by atrial flutter and AF, may be secondary to atrial dilatation and tricuspid regurgitation, as observed in our population. Similarly, ventricular arrhythmias, manifested as PVCs in 2 patients, may be caused by either ventricular dilatation or pulmonary regurgitation—though no statistically significance was found in our cohort—as well as by ventricular or pulmonary artery scarring (valvulotomy or placement of a RV to pulmonary artery conduit). Moreover, with increasing length of follow-up, marked enlargement and severe regurgitation may increase the risk of arrhythmic events [19].
Among patients who received one and a half ventricle repair, 25% showed arrhythmia. This cohort had the highest prevalence of arrhythmias but not the earliest manifestation. Numata et al. supposed that the increased occurrence of atrial arrhythmias could be related to relatively high RA pressure, due to the development of collateral communications through veno-venous shunts. As a treatment of elevated RA pressure and/or atrial arrhythmias, conversion to total cavo-pulmonary connection may be performed in these patients [20,21]. Therefore, it is of utmost importance to choose the best correction in patients with a borderline RV, such as patients with a bipartite RV [22].
In contrast to other studies [20], we analysed a large cohort of patients with PA-IVS without including patients with pulmonary stenosis. Additionally, the single centre nature of this study avoided differences in surgical strategies, which are seen when more centres are involved; as an example, Fontan palliation was performed with the extracardiac technique in all cases.
The major limitation of our study is its retrospective nature. In addition, the lack of data before the introduction of an electronic medical record system has also limited our sample size. The unavailability of remote monitoring data, in many cases due to the age of our patients, did not allow an accurate analysis free from operator-dependent variability, which characterizes echocardiographic assessment.
Our findings are not representative of all arrhythmia that may occur after surgical repair in PA-IVS patients because of the relatively short follow-up time period (12.8 years). Some arrhythmia may develop late after surgical repair: The early occurrence of postoperative arrhythmias, particularly SND, may be secondary to surgical procedures performed near the conduction system, whereas a late onset may be due to progressive fibrosis in the surgical site with slow sclerosis [23].
In patients with PA-IVS undergoing surgical repair, there is generally a relatively low risk of arrhythmia. The arrhythmic risk is related to surgical repair and does not appear to be associated with native cardiomyopathy.
Although our study cohort may not be representative of all patients with PA-IVS, several interesting findings were obtained. Patients with univentricular heart have an intermediate risk of developing SND and this risk seems to increase during follow-up. Patients with biventricular repair show a low risk of developing different types of arrhythmia that may increase with chamber dilatation and valve regurgitation. Ultimately, one and a half ventricle repair is associated with a high risk for various arrhythmia during follow-up, which mandates accurate evaluation of the best correction in patients with a borderline RV.
In conclusion, considering the increased risk of arrhythmia with any type of surgical repair, continuous follow-up in specialized centres is necessary to make an early diagnosis and to manage the potential haemodynamic impact at medium-long term.
Acknowledgement: The authors would like to thank Cardioenglish for English language services, and Dr. Elisa Del Vecchio for editorial revision.
Data Sharing: Data will be available on request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Freedom, R. M. (1989). Pumonary Atresia with Intact Ventricular Septum. Mount Kisco, NY: Futura Publishing. ISBN: 0-87993-339-9. [Google Scholar]
2. Daubeney, P. E., Sharland, G. K., Cook, A. C., Keeton, B. R., Anderson, R. H. et al. (1998). Pulmonary atresia with intact ventricular septum: Impact of fetal echocardiography on incidence at birth and postnatal outcome UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation, 98(6), 562–566. [Google Scholar]
3. Bull, C., de Leval, M. R., Mercanti, C., Macartney, F. J., Anderson, R. H. (1982). Pulmonary atresia and intact ventricular septum: a revised classification. Circulation, 66(2), 266–272. DOI 10.1161/01.CIR.66.2.266. [Google Scholar] [CrossRef]
4. Alwi, M. (2006). Management algorithm in pulmonary atresia with intact ventricular septum. Catheterization and Cardiovascular Interventions, 67(5), 679–686. DOI 10.1002/ccd.20672. [Google Scholar] [CrossRef]
5. Burkholder, H., Balaguru, D. (2012). Pulmonary atresia with intact ventricular septum: management options and decision-making. Pediatrics & Therapeutics, S5, 007. [Google Scholar]
6. John, A. S., Warnes, C. A. (2012). Clinical outcomes of adult survivors of pulmonary atresia with intact ventricular septum. International Journal of Cardiology, 161(1), 13–17. DOI 10.1016/j.ijcard.2011.04.026. [Google Scholar] [CrossRef]
7. Shi, J. Z., Chow, P. C., Li, W., Kwok, S. Y., Wong, W. H. et al. (2019). Fifty-five years follow-up of 111 adult survivors after biventricular repair of PAIVS and PS. Pediatric Cardiology, 40(2), 374–383. DOI 10.1007/s00246-018-2041-2. [Google Scholar] [CrossRef]
8. Brignole, M., Auricchio, A., Baron-Esquivias, G., Bordachar, P., Boriani, G. et al. (2013). 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. European heart journal, 34(29), 2281–2329. DOI 10.1093/eurheartj/eht150. [Google Scholar] [CrossRef]
9. Rychik, J., Goldberg, D., Dodds, K. (2010). Long-term results and consequences of single ventricle palliation. Progress in Pediatric Cardiology, 29(1), 19–23. DOI 10.1016/j.ppedcard.2010.02.001. [Google Scholar] [CrossRef]
10. Dyamenahalli, U., McCrindle, B. W., McDonald, C., Trivedi, K. R., Smallhorn, J. F. et al. (2004). Pulmonary atresia with intact ventricular septum: Management of, and outcomes for, a cohort of 210 consecutive patients. Cardiology in the Young, 14(3), 299–308. DOI 10.1017/S1047951104003087.
11. Ashburn, D. A., Blackstone, E. H., Wells, W. J., Jonas, R. A., Pigula, F. A. et al. (2004). Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. Journal of Thoracic and Cardiovascular Surgery, 127(4), 1000–1008. DOI 10.1016/j.jtcvs.2003.11.057. [Google Scholar] [CrossRef]
12. Wright, L. K., Knight, J. H., Thomas, A. S., Oster, M. E., St Louis, J. D. et al. (2019). Long-term outcomes after intervention for pulmonary atresia with intact ventricular septum. Heart (British Cardiac Society), 105(13), 1007–1013. [Google Scholar]
13. Ansari, A., Goltz, D., McCarthy, K. P., Cook, A., Ho, S. Y. (2003). The conduction system in hearts with pulmonary atresia and intact ventricular septum. Annals of Thoracic Surgery, 75(5), 1502–1505. DOI 10.1016/S0003-4975(02)04896-8. [Google Scholar] [CrossRef]
14. Patel, A. R., Goldberg, D., Shah, M. (2011). Paroxysmal complete atrioventricular block in a patient with pulmonary atresia and intact ventricular septum. Cardiology in the Young, 21(1), 94–96. DOI 10.1017/S1047951110001368. [Google Scholar] [CrossRef]
15. Egbe, A. C., Connolly, H. M., Khan, A. R., Niaz, T., Said, S. S. et al. (2017). Outcomes in adult Fontan patients with atrial tachyarrhythmias. American Heart Journal, 186, 12–20. DOI 10.1016/j.ahj.2016.12.015. [Google Scholar] [CrossRef]
16. de Groot, N. M. S., Bogers, A. J. J. C. (2017). Development of tachyarrhythmias late after the fontan procedure: The role of ablative therapy. Cardiac Electrophysiology Clinics, 9(2), 273–284. DOI 10.1016/j.ccep.2017.02.009. [Google Scholar] [CrossRef]
17. Rajanbabu, B. B., Gangopadhyay, D. (2016). Sinus node dysfunction after extracardiac conduit and lateral tunnel fontan operation: the importance of the type of prior superior cavopulmonary anastomosis. World Journal for Pediatric & Congenital Heart Surgery, 7(2), 210–215. DOI 10.1177/2150135115616370. [Google Scholar] [CrossRef]
18. Stephenson, E. A., Lu, M., Berul, C. I., Etheridge, S. P., Idriss, S. F. et al. (2010). Arrhythmias in a contemporary fontan cohort: Prevalence and clinical associations in a multicenter cross-sectional study. Journal of the American College of Cardiology, 56(11), 890–896. DOI 10.1016/j.jacc.2010.03.079. [Google Scholar] [CrossRef]
19. Li, F. F., Du, X. L., Chen, S. (2015). Biventricular repair versus uni-ventricular repair for pulmonary atresia with intact ventrical septum: A systematic review. Journal of Huazhong University of Science and Technology, 35(5), 656–661. [Google Scholar]
20. Numata, S., Uemura, H., Yagihara, T., Kagisaki, K., Takahashi, M. (2003). Long-term functional results of the one and one half ventricular repair for the spectrum of patients with pulmonary atresia/stenosis with intact ventricular septum. European Journal of Cardio-Thoracic Surgery, 24(4), 516–520. DOI 10.1016/S1010-7940(03)00378-6. [Google Scholar] [CrossRef]
21. Chowdhury, U. K., Airan, B., Talwar, S., Kothari, S. S., Saxena, A. et al. (2005). One and one-half ventricle repair: Results and concerns. Annals of Thoracic Surgery, 80(6), 2293–2300. DOI 10.1016/j.athoracsur.2005.05.052. [Google Scholar] [CrossRef]
22. Mavroudis C., Backer C. L., Kohr L. M., Deal B. J., Stinios J., Muster A. J., Wax D. F. (1999). Bidirectional Glenn shunt in association with congenital heart repairs: The 112 ventricular repair. The Annals of Thoracic Surgery, 68(3), 976–981. DOI 10.1016/S0003-4975(99)00562-7. [Google Scholar] [CrossRef]
23. Di Mambro, C., Calvieri, C., Silvetti, M. S., Tamburri, I., Giannico, S. et al. (2018). Bradyarrhythmias in repaired atrioventricular septal defects: Single-center experience based on 34 years of follow-up of 522 patients. Pediatric Cardiology, 39(8), 1590–1597. DOI 10.1007/s00246-018-1934-4. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |