

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012599
ARTICLE
Comprehensive Long-Term Follow up of Adults with Arterial Switch Operation–European Collaboration for Prospective Outcome Research in Congenital Heart Disease (EPOCH-ASO)–Study Design and Protocols
1Center for Congenital Heart Disease, Cardiology, University Hospital Inselspital, University of Bern, Bern, Switzerland
2Université de Paris, Hôpital Européen Georges Pompidou, AP-HP, Adult Congenital Heart Disease Unit, Centre de référence des Malformations Cardiaques Congénitales Complexes, M3C. Inserm U970, Paris Centre de Recherche Cardiovasculaire, Paris, France
3Adult Congenital Heart Disease Unit, Department of Cardiology, Hospital Universitario Virgen del Rocio, Instituto de BioMedicina de Sevilla and CIBERCV, Sevilla, Spain
4Unitat de Cardiopaties Congènites de l’Adolescent i l’Adult Vall d’Hebron-Sant Pau, Hospital Universitari Vall d’Hebron, Barcelona, Spain
5Adult Congenital Heart Disease Unit, Department of Cardiology, Hospital Universitari i Politècnic La Fe and CIBERCV, València, Spain
6Department of Cardiology, Academic Medical Center, Amsterdam, The Netherlands
7Adult Congenital Heart Disease Program, Department of Cardiology, Medical University of Vienna, Vienna, Austria
8Department of Cardiology and Cardiac Surgery, University Hospital Lausanne, Lausanne, Switzerland
9Division of Cardiology, University Hospital Geneva, Geneva, Switzerland
10Division of Cardiology, University Hospital of Basel, University of Basel, Basel, Switzerland
11University Heart Center, Department of Cardiology, University of Zurich, Zurich, Switzerland
# These authors contributed equally to the study design, data interpretation, and manuscript preparation
*Corresponding Author: Francisco Javier Ruperti-Repilado. Email: javier.rupertirepilado@insel.ch
Received: 06 July 2020; Accepted: 24 August 2020
Abstract: Background: Long-term outcomes in adults with prior arterial switch operation (ASO) have not yet been well defined. The aim of this study is to elucidate incidence and predictors of adverse cardiac outcomes in a prospectively followed cohort of adults after their ASO. Methods: The comprehensive long-term follow up of adults with ASO is a project within the European collaboration for prospective outcome research in congenital heart disease (EPOCH). It is designed as a prospective, international multicenter cohort study. Consecutive patients (age ≥ 16 years) with prior ASO will be included at 11 European tertiary care centers. Participants will be followed according to a standardized protocol following international recommendations, including standardized protocols for imaging and for exercise testing. Results: Main outcome measures are all-cause and cardiac-related mortality, rate of cardiac re-intervention, neo-aortic dissection, myocardial infarction, stroke, infective endocarditis, sustained atrial and ventricular arrhythmias, new-onset or worsening pulmonary hypertension or heart failure. Secondary endpoints are frequency and progression of right ventricular outflow stenosis, neo-aortic root dilatation, neo-aortic valve regurgitation and ventricular dysfunction. The impact of demographic, anatomic (e.g., coronary artery anatomy) and functional variables on the above-mentioned outcomes, as well as quality of life and incidence of pregnancy related complications will also be assessed. Conclusion: The prospective, international, multicenter EPOCH-ASO study will provide a better understanding of adverse outcomes and their predictors in adults after ASO. The results of the EPOCH-ASO study may help to optimize future care of this novel patient cohort in adult cardiology.
Keywords: Transposition of the great arteries; arterial switch operation; coronary artery anomaly; outcome
Complete transposition of the great arteries (TGA) accounts for 3 percent of all congenital heart lesions and for 20 percent of all cyanotic heart defects [1–3]. Without intervention, survival beyond the neonatal period is unlikely [4]. With the advent of open-heart surgery, the atrial switch operation has become the standard repair operation for many decades before it was superseded by the arterial switch operation (ASO) [5–10]. The ASO, first successfully performed by Dr. Adip Jatene in 1975, has become the standard operation at most centers since the late 1980s with very low operative mortality and excellent childhood survival [11–15]. Therefore, adult patient cohorts after ASO are rapidly evolving. Although good long-term outcomes are expected, preliminary studies in young adults have shown several areas of concern, including dilatation of the neo-aortic root and malfunction of the neo-aortic valve, obstruction of the right ventricular outflow (RVO) and concerns about long-term patency and dysfunction of the re-implanted coronary arteries [15–26].
In this manuscript, we describe the setting and organization of our prospective, international, multicenter cohort study in adults after childhood ASO. Our aim is to define frequency of and risk factors for adverse events in a large cohort of adults after ASO. The study will eventually provide better identification of patients at risk, improve follow-up protocols and may aid to improve long-term outcomes within this novel patient cohort in adult congenital heart disease.
2.1 Setting and Study Population
EPOCH (European collaboration for Prospective Outcome research in Congenital Heart disease) is a research initiative among several European tertiary care centers for adults with congenital heart disease. Its aim is to design and execute multicenter outcome studies in the field of adult congenital heart disease. A complete list of participating centers and investigators is shown in Appendix 1. EPOCH-ASO is a research project of the EPOCH-consortium, designed as a prospective, multicenter, international cohort study of adults after the ASO. The study is registered at ClinicalTrials.gov (Identifier: NCT04335448).
All adults (≥16 years) with TGA or a Taussig-Bing anomaly who underwent repair by an ASO, and who are actively followed at one of the participating centers will be enrolled. Exclusion criteria are incapability of giving informed consent and previous heart transplant. Enrollment started in October 2019. The cohort will be prospectively followed according to a standardized follow-up protocol, detailed below, based on recommendations in current guidelines for the management of adults with congenital heart disease [27,28].
The EPOCH-ASO platform is a web-based data management system and electronic database (secuTrial®). The required application software is implemented on a central server at the University Hospital Zurich and maintained by the Clinical Trial Unit of the University Hospital Zurich, Switzerland. Access can be obtained from any personal computer using some of the most popular internet browsers (e.g., Microsoft Internet Explorer, Mozilla Firefox, and Apple Safari). The secuTrial® system complies with all regulatory requirements regarding data safety. Each participant will be pseudonymized (depersonalized) and participants’ data will be entered by center representatives or trained study nurses.
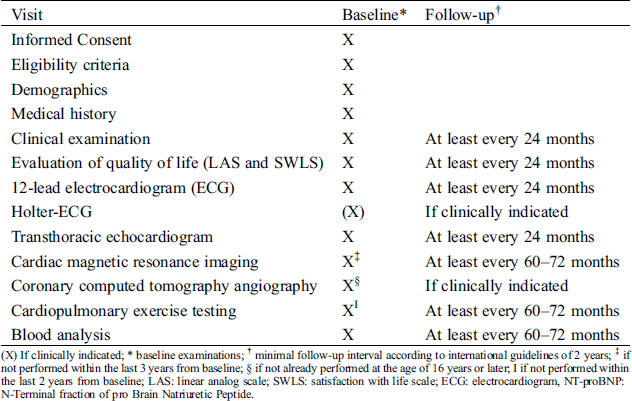
Enrollment into the study will occur at routine clinic visits. Follow-up visits will occur at least every 24 months or more frequently if indicated in an individual patient. A framework of minimal requirements of follow-up is outlined in Tab. 1.
Table 1: Framework and timing of follow-up visits and examinations
3.1.1 Data Collection and eCRF-Forms
There are three different types of electronic case reports forms (eCRF): a baseline form, a visit form and an outcome form. The information contained in these forms will be collected at routine clinic visits and entered electronically (eCRF) into the electronic databased platform (secuTrial®). Adverse events are recorded and entered into the eCRF continuously as they occur.
Inclusion into EPOCH-ASO will occur at the time of routine clinic visits at the participating centers. All patients will undergo physical examination, basic blood work testing (including N-terminal fraction of pro-B-type natriuretic peptide (NT-proBNP) levels and measurement of blood lipids), 12-lead electrocardiogram (ECG), comprehensive transthoracic echocardiography and cardiopulmonary exercise testing. As part of these routine clinic visits, quality of life will be assessed by a linear analog scale (LAS) [29,30] and the Satisfaction with Life Scale (SWL) [31]. If no contraindications are present and unless already available within the last 3 years prior to study inclusion, cardiac magnetic resonance imaging (CMR) will be performed for evaluation of RVO obstruction, neo-aortic root dilatation and neo-aortic valve regurgitation [27]. In order to evaluate the anatomy and integrity of the coronary arteries, all patients will undergo coronary computed tomography angiography (CCTA). If CCTA was already performed prior to inclusion at ≥ 16 years of age, this test will be analyzed as baseline CCTA. In case of any cardiac intervention between baseline CMR/CCTA and the time of inclusion into EPOCH-ASO, these investigations will be repeated.
Baseline characteristics include the original cardiac anatomy at birth, including all associated congenital cardiac defects. When TGA is accompanied by other heart defects such as a ventricular septal defect (VSD) or aortic coarctation, the condition is described as complex TGA. If TGA occurs without associated defects it is denominated simple TGA. Detailed coronary artery anatomy and pattern will be recorded [32].
Details and timing of prior surgical and interventional procedures will be recorded after careful review of interventional and operative notes. All cardiac complications until inclusion into EPOCH-ASO will be extracted from chart review.
All study investigations will be standardized among participating centers as detailed below.
3.1.3 Timing of Routine Follow-Up Visits and Follow-Up Examinations
Frequency of routine follow up will be performed according to the clinical status of the individual patient, following general recommendations in published guidelines [27,28]. A framework of minimal requirements of follow-up examinations is outlined in Tab. 1.
The main outcome measures of the study are the incidence of major adverse cardiac events. Secondary endpoints are deterioration of cardiac function and functional capacity. Type and definitions of primary and secondary outcomes are outlined in Tabs. 2 and 3, respectively. All major adverse cardiac events will be reviewed periodically within the steering committee by means of videoconferences.
Table 2: Definition of primary outcomes
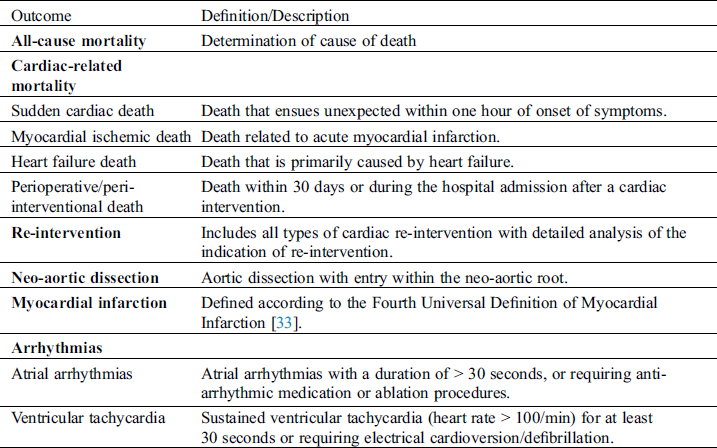
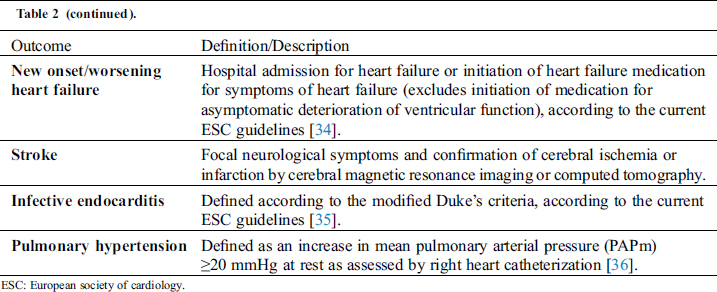
Table 3: Secondary outcomes and potential predictors of mortality and morbidity outcomes
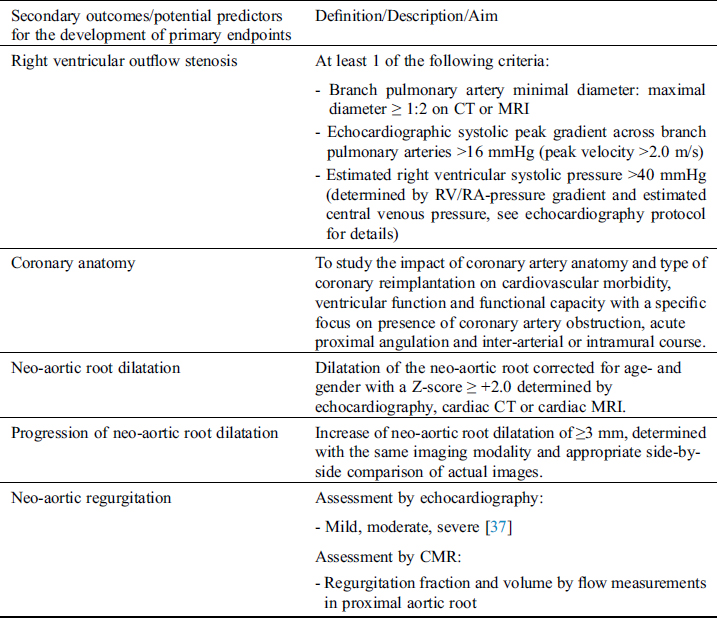
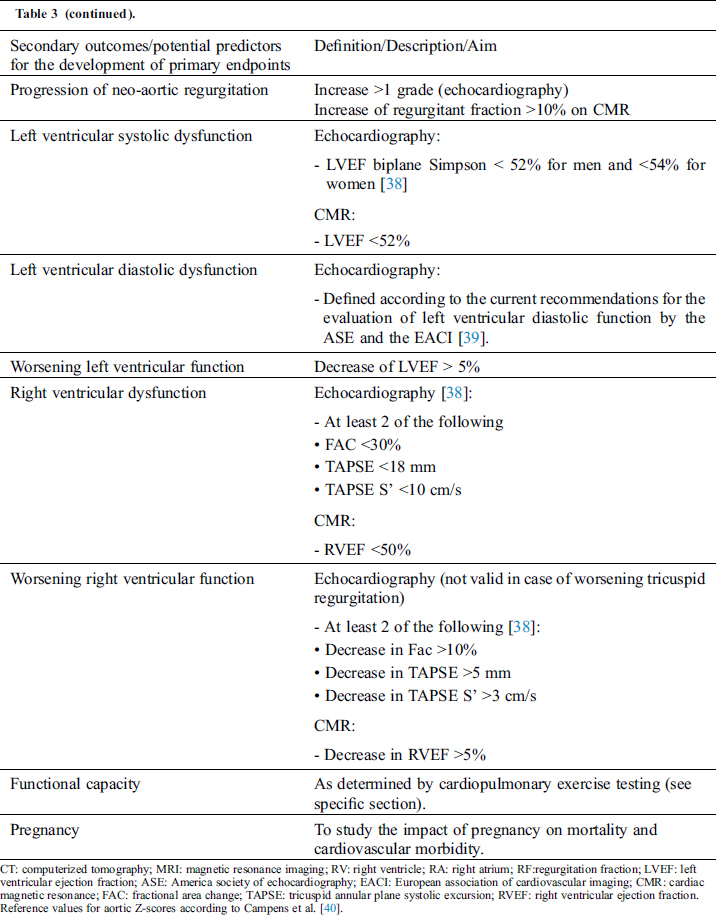
A predefined list of potential predictors for the development of primary endpoints is presented in Tab. 3.
3.3 Standard Operating Procedure (SOP) for Study Procedures
In order to standardize and harmonize cardiac imaging (echocardiography, CMR and CCTA) and cardio-pulmonary exercise testing, standardized operating procedures (SOP) were developed for these investigations. These SOPs, as outlined below, contain minimum requirements for image acquisition and reporting of results. All SOPs are based on recommendations of national and international guidelines and imply measurements commonly used in clinical practice. The essence of the SOPs is outlined below, while the complete SOPs are available as supplement tables (Appendix 2) or under: http://www.sacher-registry.com/epoch/.
3.3.1 SOP for Transthoracic Echocardiography
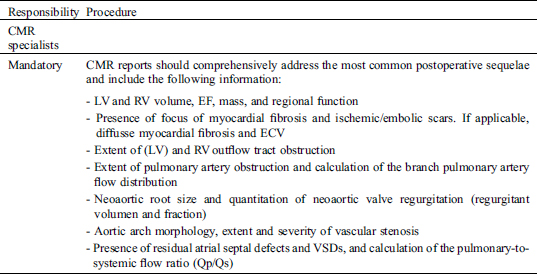
All patients will undergo a comprehensive standard transthoracic echocardiographic examination, according to international recommendations [38]. The echocardiographic examination will specifically focus on qualitative and quantitative assessment of the left and right ventricular dimensions, systolic and diastolic function, neo-aortic root dimensions and neo-aortic valve function as well as obstruction within the right ventricular outflow (including assessment of branch pulmonary artery stenosis, if technically feasible).
3.3.2 SOP for Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing will be performed preferably on a cycle ergometer but a treadmill can be used instead, if clinically indicated (e.g., patients with pacemakers requiring pacemaker accelerometer sensor function to maintain chronotropic competence). Follow-up examinations for individual patients must use the same test modality as with the initial investigation. Before exercise, respiratory flow loops will be acquired and maximal breathing capacity determined. A ramp protocol will be used, individualized to the patient’s expected exercise capacity with the aim of exercise duration of 8–12 minutes. Details on specifics of recording and protocols are outlined in the supplements. For follow-studies, identical ramp protocols must be used for the individual patient. Measurements and reporting of data from exercise testing will follow the recommendations of the European Association for Cardiovascular Prevention and Rehabilitation [41].
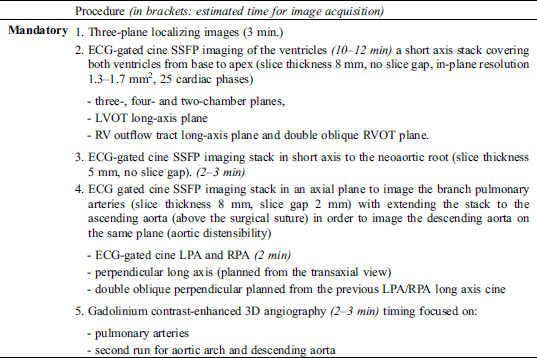
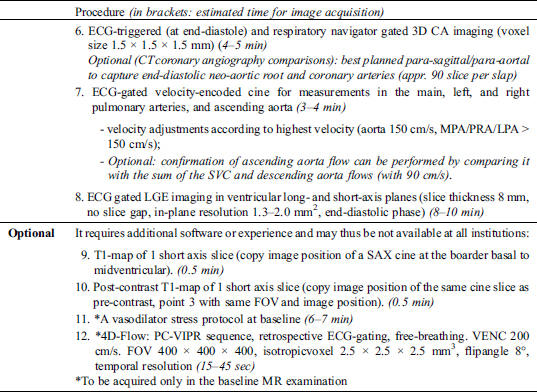
3.3.3 SOP for Cardiac Magnetic Resonance Imaging
Among patients without contraindication a CMR will be performed. The CMR protocol will specifically focus on biventricular volumes and function (including late gadolinium enhancement sequences to detect myocardial scars), neo-aortic root dilatation and quantification of neo-aortic valve regurgitation. In addition, the protocol includes assessment of branch pulmonary artery stenosis and pulmonary artery flow distribution. A semi quantitative evaluation of myocardial perfusion will be performed by the analysis of myocardial perfusion first-pass perfusion images as previously described [42,43].
3.3.4 SOP for Coronary Computed Tomography Angiography
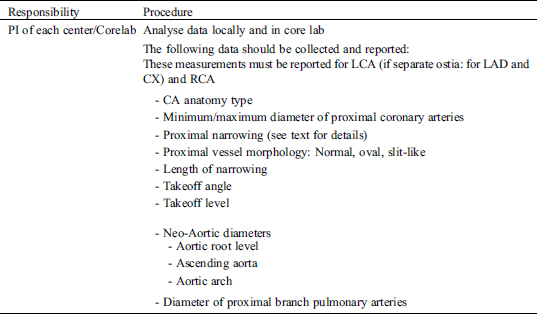
Before the CCTA examinations, beta-blockers will be given if heart rate >65/min unless contraindicated, to optimize acquisition. Data acquisition will be performed prospectively with ECG-triggered sequential scan or retrospectively with ECG-gated spiral scan. Acquisition includes the entire heart and proximal pulmonary artery branches. Inclusion of the thoracic aorta and aortic arch is optional, if good quality images from cardiac magnetic resonance imaging are available within the last 3 years prior to CCTA. The specific focus of analysis of the CCTA will be the origin and the proximal course of the re-implanted coronary arteries. This includes detailed analysis of coronary ostial dimensions and geometry as well as angles of coronary artery takeoff and potential length of inter-arterial or intramural course. To minimize bias in image analysis, the analysis of proximal coronary artery anatomy will be performed by experienced observers in a core lab.
3.4 Analysis Plan and Statistical Considerations
Given that enrollment started by the end of 2019 and all patients after the ASO are seen at least every 24 months in outpatient clinic, we expect that the majority of eligible adults followed at the participating centers will have been enrolled until the end of 2021. It is thus planned to perform a descriptive cross-sectional characterization of the study cohort by the end of 2021. Analyses of primary and secondary outcomes are planned at least every five years. Dedicated biostatisticians at the Clinical Trial Unit of the University Hospital Zurich Switzerland, or the other corresponding units of the other participating centers will support statistical analysis of data. Investigator initiated sub-studies are encouraged and research proposals with a specific research aim and a study plan can be submitted to the steering committee at any time. The steering committee will decide mutually upon acceptance of the research proposal. Publication of sub-studies within EPOCH-ASO is regulated by the steering committee of EPOCH by means of a publication strategy.
Cohorts of adult survivors after the ASO are rapidly evolving. Although we generally expect a good long-term outcome for these patients, experience taught us that there is no cure for congenital heart disease [44]. Indeed, preliminary analyses of outcomes of young adults after the ASO have shown several areas of concern [45–47]. These include particularly dilatation of the neo-aortic root, regurgitation of the neo-aortic valve and obstruction of the RVO [15,17–19,21–23,25,26]. These may affect ventricular function and have an impact on long-term outcomes. Furthermore, complications of the re-implanted coronary arteries due to geometric distortion or altered vasomotion require our attention [16]. In adulthood, the frequency of cardiac structural and functional deterioration, their predictors and their impact on adverse outcomes are largely unknown.
Only a better knowledge of all of these factors will allow early detection of patients at risk and will potentially allow appropriate indication and timing of preventive interventions to reduce the risk of complications and hence improve long-term outcomes. EPOCH-ASO has the potential to fill some of these gaps in our knowledge. Given the multicenter nature of the study, we expect to enroll up to 700 adults after ASO within 2 years, which will create a large cohort that will allow valid estimates of the frequency and predictors of these adverse outcomes.
Several studies have documented impaired cardiopulmonary exercise capacity in patients after the ASO [47]. This has mainly been attributed to right ventricular outflowright ventricular outflow obstruction and neo-aortic valve dysfunction [48–50]. However, the extent of impaired exercise capacity of adults after the ASO, their course over time and its impact on long-term outcomes remains to be elucidated. A better understanding of factors contributing to limited exercise capacity may allow tailored treatment strategies and have the potential to improve quality of life.
The prospective nature of the project with clearly defined follow-up protocols will help to identify predictors of adverse outcomes and will improve the validity of the collected data. As learnt from other multicenter registries, larger patient cohorts provide a better base for data analysis and allow a greater granularity of data analysis [51,52]. Only such precise data will inform us on optimal strategies for conduction of prospective-ideally randomized-studies comparing different treatment strategies, which will finally improve patient outcomes.
The nature of this prospective registry will only allow defining associations between potential predictors and adverse outcomes. We hope that the results of the EPOCH-ASO study will alleviate the planning of prospective, ideally randomized trials to test preventive strategies for improved outcomes.
Although ideally all investigations were analyzed within a core-laboratory, funding of our project currently does not allow core-laboratory analyses of all imaging and exercise data. Currently only CCTA is planned to be analyzed in a core-lab.
The prospective, international multicenter EPOCH-ASO study will provide a better understanding of adverse outcomes and their predictors in a large cohort of adults after the arterial switch operation. The results of the EPOCH-ASO study may help to optimize the future care of this novel patient cohort in adult cardiology and may inform us about the optimal targets for planning prospective trials to compare treatment strategies.
Acknowledgement: JR and DT contributed in drafting of the manuscript, in the conception of the research, critical revision of the manuscript for important intellectual content and supervision. ML, MG, PG, LD, JRS, BB, HG, MS, and JB contributed in the conception and design of the research, critical revision of the manuscript for important intellectual content and supervision.
Availability of Data and Materials: Not applicable for this manuscript.
Ethical Considerations: The study will be carried out according to the ICH GCP Guidelines. It has been submitted to the responsible ethics committee and to the competent authorities. The clinical study can only begin once approval from all required authorities has been received. Any additional requirements imposed by the authorities will be implemented. So far, ethical approval for the participating centers located in Amsterdam, Barcelona, Basel, Bern, Geneva, Lausanne, Valencia, Vienna and Zurich has been obtained. The available ethical approvals can be downloaded from http://www.sacher-registry.com/epoch/.
Funding Statement: EPOCH-ASO is funded by internal grants without support from the pharmaceutical industry.
Conflicts of Interest: There are no conflicts of interest to disclose.
1. Centers for Disease Control and Prevention. (2006). Improved national prevalence estimates for 18 selected major birth defect-United States, 1999-2001. Morbidity and Mortality Weekly Report, 54(51), 1301–1305.
2. Ferencz, C., Rubin, J. D., McCarter, R. J., Brenner, J. I., Neill, C. A. et al. (1985). Congenital heart disease, prevalence at livebirth. The Baltimore-Washington infant study. American Journal of Epidemiology, 121(1), 31–36. DOI 10.1093/oxfordjournals.aje.a113979.
3. Grabitz, R. G., Joffres, M. R., Collins-Nakai, R. L. (1988). Congenital heart disease, incidence in the first year of life. The Alberta Heritage Pediatric Cardiology Program. American Journal of Epidemiology, 128(2), 381–388.
4. Liebman, J., Cullum, L., Belloc, N. B. (1969). Natural history of transpositon of the great arteries. Anatomy and birth and death characteristics. Circulation, 40(2), 237–262.
5. Borromee, L., Lecompte, Y., Batisse, A., Lemoine, G., Vouhe, P. et al. (1988). Anatomic repair of anomalies of ventriculoarterial connection associated with ventricular septal defect. II. Clinical results in 50 patients with pulmonary outflow tract obstruction. Journal of Thoracic and Cardiovascular Surgery, 95(1), 96–102. DOI 10.1016/S0022-5223(19)35392-9.
6. Metras, D., Kreitmann, B. (2000). Modified Rastelli using an autograft, A new concept for correction of transposition of the great arteries with ventricular septal defect and left ventricular outflow tract obstruction (with an extension to other congenital heart defects). Seminars in Thoracic and Cardiovascular Surgery Annual, Pediatric Cardiac Surgery Annual, 3(1), 117–124. DOI 10.1053/tc.2000.6035.
7. Rastelli, G. C. (1969). A new approach to “anatomic” repair of transposition of the great arteries. Mayo Clinic Proceedings, 44(1), 1–12.
8. Senning, A. (1959). Surgical correction of transposition of the great vessels. Surgery, 45(6), 966–980.
9. Mustard, W. T. (1964). Successful two–stage correction of transposition of the great vessels. Surgery, 55, 469–472.
10. Nikaidoh, H. (1984). Aortic translocation and biventricular outflow tract reconstruction. A new surgical repair for transposition of the great arteries associated with ventricular septal defect and pulmonary stenosis. Journal of Thoracic and Cardiovascular Surgery, 88(3), 365–372. DOI 10.1016/S0022-5223(19)38323-0.
11. Jatene, A. D., Fontes, V. F., Paulista, P. P., Souza, L. C., Neger, F. et al. (1976). Anatomic correction of transposition of the great vessels. Journal of Thoracic and Cardiovascular Surgery, 72(3), 364–370. DOI 10.1016/S0022-5223(19)40063-9.
12. Bautista-Hernandez, V., Marx, G. R., Bacha, E. A., del Nido, P. J. (2007). Aortic root translocation plus arterial switch for transposition of the great arteries with left ventricular outflow tract obstruction, intermediate–term results. Journal of the American College of Cardiology, 49(4), 485–490. DOI 10.1016/j.jacc.2006.09.031.
13. de Koning, W. B.,van Osch-Gevers, M.,Ten Harkel, A. D., van Domburg, R. T.,Spijkerboer, A. W. et al. (2008). Follow-up outcomes 10 years after arterial switch operation for transposition of the great arteries, comparison of cardiological health status and health-related quality of life to those of the a normal reference population. European Journal of Pediatrics, 167(9), 995–1004. DOI 10.1007/s00431-007-0626-5.
14. Hazekamp, M. G., Gomez, A. A., Koolbergen, D. R., Hraska, V., Metras, D. R. et al. (2010). Surgery for transposition of the great arteries, ventricular septal defect and left ventricular outflow tract obstruction, European Congenital Heart Surgeons Association multicentre study. European Journal of Cardio-Thoracic Surgery, 38(6), 699–706. DOI 10.1016/j.ejcts.2010.03.030.
15. Lo Rito, M., Fittipaldi, M., Haththotuwa, R., Jones, T. J., Khan, N. et al. (2015). Long-term fate of the aortic valve after an arterial switch operation. Journal of Thoracic and Cardiovascular Surgery, 149(4), 1089–1094. DOI 10.1016/j.jtcvs.2014.11.075.
16. Possner, M., Buechel, R. R., Vontobel, J., Mikulicic, F., Grani, C. et al. (2020). Myocardial blood flow and cardiac sympathetic innervation in young adults late after arterial switch operation for transposition of the great arteries. International Journal of Cardiology, 299, 110–115. DOI 10.1016/j.ijcard.2019.07.041.
17. Bonhoeffer, P., Bonnet, D., Piechaud, J. F., Stumper, O., Aggoun, Y. et al. (1997). Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. Journal of the American College of Cardiology, 29(1), 202–206. DOI 10.1016/S0735-1097(96)00433-0.
18. Bonnet, D., Bonhoeffer, P., Piechaud, J. F., Aggoun, Y., Sidi, D. et al. (1996). Long-term fate of the coronary arteries after the arterial switch operation in newborns with transposition of the great arteries. Heart, 76(3), 274–279. DOI 10.1136/hrt.76.3.274.
19. Co-Vu, J. G., Ginde, S., Bartz, P. J., Frommelt, P. C., Tweddell, J. S. et al. (2013). Long-term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries. Annals of Thoracic Surgery, 95(5), 1654–1659. DOI 10.1016/j.athoracsur.2012.10.081.
20. Delmo Walter, E. M., Miera, O., Nasseri, B., Huebler, M., Alexi-Meskishvili, V. et al. (2011). Onset of pulmonary stenosis after arterial switch operation for transposition of great arteries with intact ventricular septum. HSR Proceedings in Intensive Care & Cardiovascular Anesthesia, 3(3), 177–187.
21. Koolbergen, D. R., Manshanden, J. S., Yazdanbakhsh, A. P., Bouma, B. J., Blom, N. A. et al. (2014). Reoperation for neoaortic root pathology after the arterial switch operation. European Journal of Cardio-Thoracic Surgery, 46(3), 474–479, discussion 479. DOI 10.1093/ejcts/ezu026.
22. Legendre, A., Losay, J., Touchot-Kone, A., Serraf, A., Belli, E. et al. (2003). Coronary events after arterial switch operation for transposition of the great arteries. Circulation, 108(Suppl 1), II186–II190.
23. McMahon, C. J., Ravekes, W. J., Smith, E. O., Denfield, S. W., Pignatelli, R. H. et al. (2004). Risk factors for neo-aortic root enlargement and aortic regurgitation following arterial switch operation. Pediatric Cardiology, 25(4), 329–335. DOI 10.1007/s00246-003-0483-6.
24. Nellis, J. R., Turek, J. W., Aldoss, O. T., Atkins, D. L., Ng, B. Y. (2016). Intervention for supravalvar pulmonary stenosis after the arterial switch operation. Annals of Thoracic Surgery, 102(1), 154–162. DOI 10.1016/j.athoracsur.2016.01.068.
25. van der Bom, T.,van der Palen, R. L.,Bouma, B. J., van Veldhuisen, S. L.,Vliegen, H. W. et al. (2014). Persistent neo-aortic growth during adulthood in patients after an arterial switch operation. Heart, 100(17), 1360–1365. DOI 10.1136/heartjnl-2014-305702.
26. van der Palen, R. L. F.,van der Bom, T.,Dekker, A., Tsonaka, R., van Geloven, N. et al. (2019). Progression of aortic root dilatation and aortic valve regurgitation after the arterial switch operation. Heart, 105(22), 1732–1740. DOI 10.1136/heartjnl-2019-315157.
27. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease, executive summary, a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation, 139(14), e637–e697.
28. Baumgartner, H., Bonhoeffer, P., De Groot, N. M., de Haan, F.,Deanfield, J. E. et al. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249.
29. Hyland, M. E., Sodergren, S. C. (1996). Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Quality of Life Research, 5(5), 469–480. DOI 10.1007/BF00540019.
30. Gudex, C., Dolan, P., Kind, P., Williams, A. (1996). Health state valuations from the general public using the visual analogue scale. Quality of Life Research, 5(6), 521–531. DOI 10.1007/BF00439226.
31. Diener, E., Emmons, R. A., Larsen, R. J., Griffin, S. (1985). The satisfaction with life scale. Journal of Personality Assessment, 49(1), 71–75. DOI 10.1207/s15327752jpa4901_13.
32. Gittenberger-de Groot, A. C., Koenraadt, W. M. C., Bartelings, M. M., Bokenkamp, R., DeRuiter, M. C. et al. (2018). Coding of coronary arterial origin and branching in congenital heart disease, The modified Leiden Convention. Journal of Thoracic and Cardiovascular Surgery, 156(6), 2260–2269. DOI 10.1016/j.jtcvs.2018.08.009.
33. Thygesen, K., Alpert, J. S., Jaffe, A. S., Chaitman, B. R., Bax, J. J. et al. (2018). Fourth universal definition of myocardial infarction. Journal of the American College of Cardiology, 72(18), 2231–2264. DOI 10.1016/j.jacc.2018.08.1038.
34. Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure, The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure, 18(8), 891–975. DOI 10.1002/ejhf.592.
35. Habib, G., Lancellotti, P., Antunes, M. J., Bongiorni, M. G., Casalta, J. P. et al. (2015). 2015 ESC guidelines for the management of infective endocarditis, The task force for the management of infective endocarditis of the european society of cardiology (ESC). Endorsed by, European association for cardio-thoracic surgery (EACTS), the European association of nuclear medicine (EANM). European Heart Journal, 36(44), 3075–3128. DOI 10.1093/eurheartj/ehv319.
36. Galie, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I. et al. (2016). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension, The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European respiratory society (ERS), Endorsed by, Association for European paediatric and congenital cardiology (AEPC), International society for heart and lung transplantation (ISHLT). European Heart Journal, 37(1), 67–119. DOI 10.1093/eurheartj/ehv317.
37. Zoghbi, W. A., Adams, D., Bonow, R. O., Enriquez-Sarano, M., Foster, E. et al. (2017). Recommendations for noninvasive evaluation of native valvular regurgitation, a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. Journal of the American Society of Echocardiography, 30(4), 303–371. DOI 10.1016/j.echo.2017.01.007.
38. Lang, R. M., Badano, L. P., Mor-Avi, V., Afilalo, J., Armstrong, A. et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults, an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28(1), 1–39.e14. DOI 10.1016/j.echo.2014.10.003.
39. Nagueh, S. F., Smiseth, O. A., Appleton, C. P., Byrd, B. F.3rd, Dokainish, H. et al. (2016). Recommendations for the evaluation of left ventricular diastolic function by echocardiography, an update from the American society of echocardiography and the European association of cardiovascular imaging. European Heart Journal Cardiovascular Imaging, 17(12), 1321–1360. DOI 10.1093/ehjci/jew082.
40. Campens, L., Demulier, L., De Groote, K., Vandekerckhove, K., De Wolf, D. et al. (2014). Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. American Journal of Cardiology, 114(6), 914–920. DOI 10.1016/j.amjcard.2014.06.024.
41. Guazzi, M., Adams, V., Conraads, V., Halle, M., Mezzani, A. et al. (2012). EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation, 126(18), 2261–2274. DOI 10.1161/CIR.0b013e31826fb946.
42. Pingitore, A., Lombardi, M., Scattini, B., De Marchi, D., Aquaro, G. D. et al. (2008). Head to head comparison between perfusion and function during accelerated high-dose dipyridamole magnetic resonance stress for the detection of coronary artery disease. American Journal of Cardiology, 101(1), 8–14. DOI 10.1016/j.amjcard.2007.07.076.
43. Rutz, T., Wustmann, K., Prsa, M., Vallée, J., Donner, B. et al. (2016). Cardiac magnetic resonance imaging in congenital heart disease. Cardiovascular Medicine, 19(6), 176–184. DOI 10.4414/cvm.2016.00411.
44. Stark, J. (1989). Do we really correct congenital heart defects?. Journal of Thoracic and Cardiovascular Surgery, 97(1), 1–9. DOI 10.1016/S0022-5223(19)35118-9.
45. Khairy, P., Clair, M., Fernandes, S. M., Blume, E. D., Powell, A. J. et al. (2013). Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation, 127(3), 331–339. DOI 10.1161/CIRCULATIONAHA.112.135046.
46. Shim, M. S., Jun, T. G., Yang, J. H., Park, P. W., Kang, I. S. et al. (2016). Current expectations of the arterial switch operation in a small volume center, a 20-year, single-center experience. Journal of Cardiothoracic Surgery, 11(1), 34. DOI 10.1186/s13019-016-0428-9.
47. Tobler, D., Williams, W. G., Jegatheeswaran, A., Van Arsdell, G. S., McCrindle, B. W. et al. (2010). Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. Journal of the American College of Cardiology, 56(1), 58–64. DOI 10.1016/j.jacc.2010.03.031.
48. Baggen, V. J., Driessen, M. M., Meijboom, F. J., Sieswerda, G. T., Jansen, N. J. et al. (2015). Main pulmonary artery area limits exercise capacity in patients long-term after arterial switch operation. Journal of Thoracic and Cardiovascular Surgery, 150(4), 918–925. DOI 10.1016/j.jtcvs.2015.07.101.
49. Giardini, A., Khambadkone, S., Taylor, A., Derrick, G. (2010). Effect of abnormal pulmonary flow distribution on ventilatory efficiency and exercise capacity after arterial switch operation for transposition of great arteries. American Journal of Cardiology, 106(7), 1023–1028. DOI 10.1016/j.amjcard.2010.05.035.
50. Morgan, C. T., Mertens, L., Grotenhuis, H., Yoo, S. J., Seed, M. et al. (2017). Understanding the mechanism for branch pulmonary artery stenosis after the arterial switch operation for transposition of the great arteries. European Heart Journal Cardiovascular Imaging, 18(2), 180–185. DOI 10.1093/ehjci/jew046.
51. Ruys, T. P., Roos-Hesselink, J. W., Hall, R., Subirana-Domenech, M. T., Grando Ting, J. et al. (2014). Heart failure in pregnant women with cardiac disease, data from the ROPAC. Heart, 100(3), 231–238. DOI 10.1136/heartjnl-2013-304888.
52. Iyengar, A. J., Winlaw, D. S., Galati, J. C., Gentles, T. L., Weintraub, R. G. et al. (2014). The Australia and New Zealand Fontan Registry, description and initial results from the first population-based Fontan registry. Internal Medicine Journal, 44(2), 148–155. DOI 10.1111/imj.12318.
Infrastructure of EPOCH
Steering Committee (in alphabetical order)
J. Bouchardy, BJ. Bouma, L. Dos, H. Gabriel, P. Gallego, M. Greutmann, M. Ladouceur, M. Schwerzmann, D. Tobler
Participating Centers (in alphabetical order)
Austria: University Hospital Vienna; France: CHU Paris IdF Ouest - HEGP Hôpital Européen Georges Pompidou; Netherlands: Netherlands: Amsterdam University Medical Center, location AMC; Spain: Hospital Universitario y Politécnico La Fe Valencia, Hospital Universitari Vall d'Hebron Barcelona, Hospital Universitario Virgen del Rocio, Sevilla; Switzerland: University Hospital Basel, University Hospital Inselspital Bern, University Hospital Geneva, University Hospital Lausanne, University Hospital Zurich.
Electronic Data Capture and Statistical Analyses (CTU University hospital Zurich).
Contributors of the EPOCH (mentioned as 'on behalf of the EPOCH):
Amsterdam University Medical Center, location AMC: BJ. Bouma, D. Robbers-Visser, M. Groenink, SM. Boekholdt, L. Engele, T. Konings.
Barcelona: L. Dos, A. Pijuan Doménech, B. M. Barrio, MT. Subirana Doménech, B. Gordon Ramírez, V. González Fernández, H. Cuéllar Calabria, G. Burcet Rodríguez.
University Hospital Basel: D. Tobler, Ph. Haaf.
University Hospital Inselspital Bern: M. Schwerzmann, F. Schwitz, E. Goulouti, M. Wilhelm, K. Wustmann, FJ. Ruperti-Repilado, C. Gräni.
University Hospital Geneva: J. Bouchardy, C. Blanche.
University Hospital Lausanne: J. Bouchardy, T. Rutz.
Hôpital Européen Georges Pompidou, Paris: M. Ladouceur, E. Mousseaux, G. Soulat, L. Iserin, P. Vouhé, L. Du Puy Montbrun, R. Ly, A-S. Chaussade, A. Legendre, V. Wadmann, A. Boubrit.
University Hospital Virgen del Rocio, Sevilla: P. Gallego, M. J. Rodriguez-Puras, N. Romero, P. Serrano, A. Ramiro, B. Manso, R. Camacho, J. M. Cubero, R. Hosseinpour, A. Gonzalez-Calle, A. Adsuar.
University Hospital La Fe, Valencia: J. Rueda, Francisco Buendía, A. Osa, C. Fonfría.
University Hospital Vienna: H. Gabriel; IM. Lang, J. Mascherbauer, M. Schneider, R. Pokan.
University Hospital Zurich: M Greutmann, Ronny R. Buechel, C. H. Attenhofer Jost, L. Meier, H. Schneider, F. Bonassin Tempesta, E. Valsangiacomo Büchel.
Standard Operating Procedures
Study Specific SOP
The purpose of this SOP is to describe the standards for performing a cardiopulmonary exercise test as part of the EPOCH studies.
The SOP is valid for all clinical research functions participating at EPOCH ASO STUDY and aims to improve the comparability of CPET exams performed at different investigating sites. The SOP shall set a common standard for the performing a cardiopulmonary exercise test in compliance with GCP, other SOP and regulatory requirement(s).
3 Abbreviations

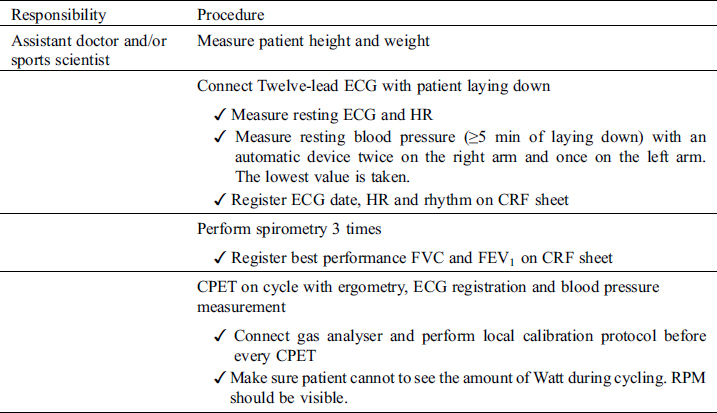
4.3 Performing Study
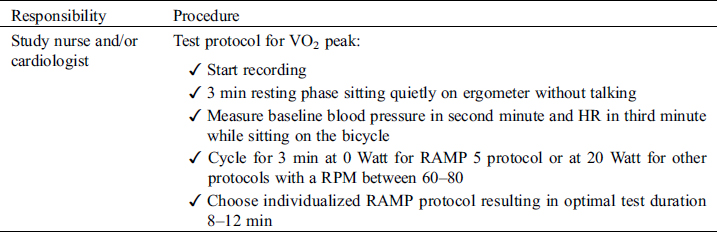
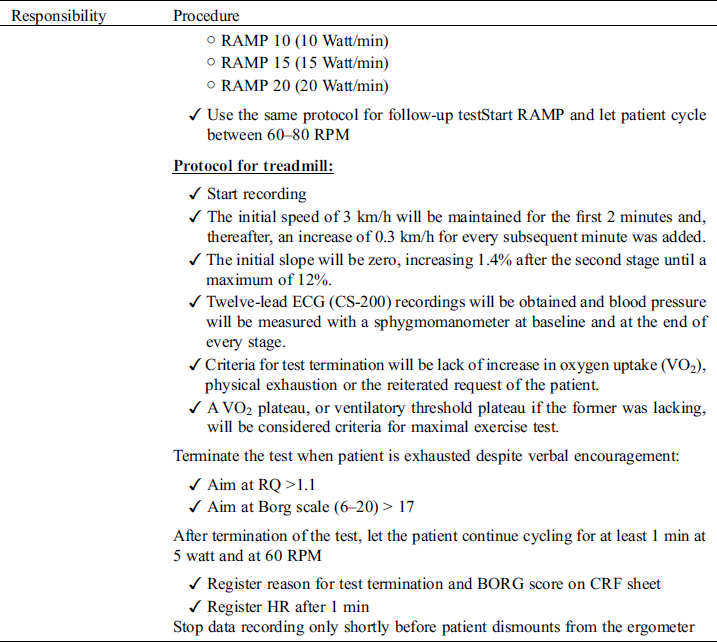
4.4 Results/Report
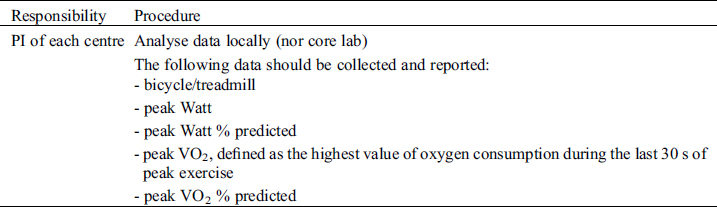
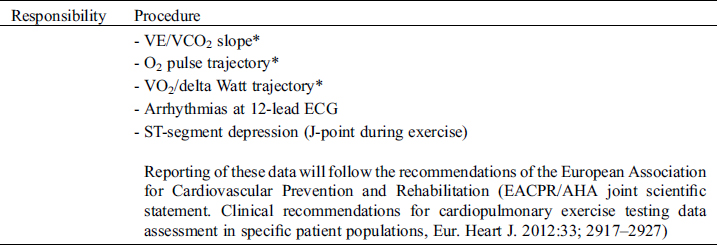
Study Specific SOP
The purpose of this standard operating procedure (SOP) is to describe the standards for performing a transthoracic echocardiography (TTE) as part of the EPOCH-ASO study. The SOP is valid for all clinical research functions participating at EPOCH-ASO and aims to improve the comparability of echocardiography exams performed at different investigating sites. The SOP shall set a common standard for the performing of an echocardiographic study in compliance with GCP, other SOP and regulatory requirement(s).
• University Hospital Basel
PI: Daniel Tobler (daniel.tobler@usb.ch)
• University Hospital Zurich
PI: Matthias Greutmann (matthias.greutmann@usz.ch)
• University Hospital Lausanne (CHUV)
PI: Judith Bouchardy (judith.bouchardy@chuv.ch)
• University Hospital Geneva (HUG)
PI: Judith Bouchardy (judith.bouchardy@chuv.ch)
• Inselspital Bern
PI: Markus Schwerzmann (markus.schwerzmann@insel.ch)
• Medical Unversity Vienna
PI: Harald Gabriel (harald.gabriel@meduniwien.ac.at)
• Amsterdam University Medical Center, location AMC, Amsterdam
PI: Berto Bouma (b.j.bouma@amc.uva.nl)
• Hospital Universitario Virgen Macarena, Sevilla
PI: Pastora Gallego (pgallegogv@gmail.com)
• Service de Cardiologie-Hôpital européen Georges-Pompidou (HEGP)-AP-HP, Paris
PI: Magalie Ladouceur (magalie.ladouceur@gmail.com)
• Hospital Universitario Virgen del Rocío, Sevilla
PI: Begoña Manso (begogui@gmail.com)
• University Hospital Vall d'Hebron, Barcelona
PI: Laura dos Subira (laura_dos_subira@hotmail.com)
Transthoracic echocardiography
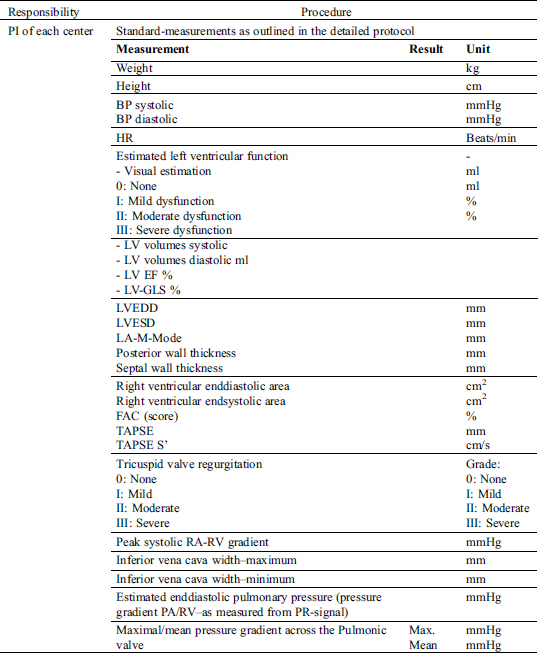
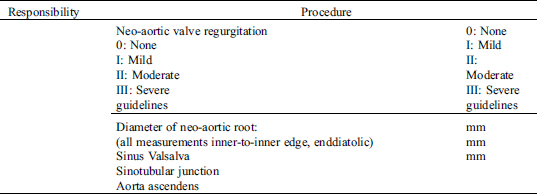
4.1 Requirements/Responsibilities

4.2 Detailed Echocardiography Protocol


4.3 Results/Report
Study Specific SOP

The purpose of this SOP is to describe the standards for image acquisition, image analysis and reporting of cardiac mangnetic resonance tomography for assessment of cardiac anatomy and function as part of the EPOCH-ASO study.
The SOP is valid for all clinical research functions participating at EPOCH-ASO study and aims to improve the comparability of CMR exams performed at different investigating sites. The SOP shall set a common standard for image acquisition, image analysis and reporting of results. The CMR protocol is in compliance with GCP, other SOP and regulatory requirement(s).
The general objectives of the standardized CMR-protocols part of the EPOCH-ASO study are:
(1) To describe the standards for CMR examinations in adults with TGA after ASO as part of the EPOCH-ASO study
(2) To improve comparability among examinations performed at different centers by different operators.
(3) To detect and quantify aortic dilatation and regurgitation
(4) To detect, localize and quantify right ventricular outflow tract obstructions and to evaluate pulmonary branches anatomy.
(5) To detect myocardial fibrosis and ventricular dysfunction
(6) To study the aortic arch

4.1 Study Preparations/Requirements

4.2 Performing Study
4.3 Results/Report

Study Specific SOP

The purpose of this SOP is to describe the standards for image acquisition, image analysis and reporting of computed cardiac tomography for assessment of coronary arteries (CTCA) as part of the EPOCH-ASO study.
The SOP is valid for all clinical research functions participating at EPOCH-ASO study and aims to improve the comparability of CTCA exams performed at different investigating sites. The SOP shall set a common standard for image acquisition, image analysis and reporting of results. The CTCA protocol is in compliance with GCP, other SOP and regulatory requirement(s).
The general objectives of the standardized CTCA-protocolas part of the EPOCH-ASO study are:
• To estimate origin and quality of each main coronary arteries (LM, LAD, Cx, RC), cardiac valves, the entire thoracic aorta and pulmonary arteries with enhanced CT. If the thoracic aorta and aortic arch have been comprehensively imaged by means of cardiac/aortic magnetic resonance imaging within the last 3 years prior to CTCA, the inclusion of the thoracic aorta and the aortic arch may be omitted in the CTCA-protocol.
• To detect abnormal origin and course of proximal coronary arteries presenting a risk for potential stenosis and/or occlusion.
• To detect dilatation and all other abnormal geometry features of the thoracic aorta.
• To detect obstruction all along the right ventricular outflow tract, which includes proximal pulmonary arteries?
• To limit X-Ray radiation in this young population.
4.1 Study Preparations/Requirements

4.2 Performing study

Coronary arteries will be analyzed using axial slices and, if necessary, with the aid of post-processing tools such as multiplanar reconstruction, maximum-intensity thin-slab projection and 3-dimensional reconstruction. Analysis will be limited to 6 coronary artery segments: ostia of the left and right coronary artery, left main coronary artery, and the proximal segments of the left anterior descending, left circumflex, and right coronary artery. In both modalities, the following features will be assessed additionally for the purposes of the current study [1]:
(1) Minimum and maximum diameters: at the most narrowed location and the normal distal reference segment; coronary lesions will be graded using visual assessment, and classified as either normal or having >50 or <50% stenosis (3 classes) after being also quantitatively estimated as a percentage of reduction of the coronary artery diameter in % (Fig. 1).
(2) Proximal vessel morphology: categorize proximal vessel morphology as: (i) normal, (ii) ‘oval’ (<50%), and (iii) ‘slit-like’ narrowing (≥50% reduction in minimum diameter in the absence of coronary artery disease). (Fig. 2) [2,3].
(3) Length of narrowing: centerline length of vessel narrowing extending from the most proximal segment to the normal caliber distal reference (Fig. 3) [1].
(4) Acute angle: defined as the presence or absence of acute angle take-off <45° between (a) the plane formed by the ostium centre to a point 5 mm along the vessel centerline, and (b) a plane tangent to the aorta in multiplanar axial reconstruction at the level of the ostium (Fig. 4) [3,4],
(5) Intramural course: defined as (i) presenct, (ii) absent, or (iii) indeterminate (Fig. 4) [1].
(6) Vessel take-off level: categorized as at/above or below the aortic valve commissure (Fig. 4) [1].
(7) Ostia type: defined as (i) separate, (ii) shared, or (iii) branch vessel (Fig. 5) [1].
(8) Coronary pattern will be described using the classification of Yacoub et al. [1,5].
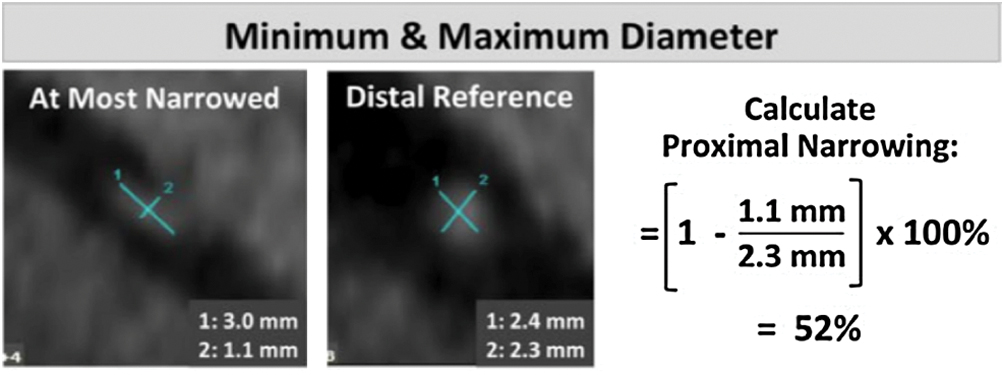
Figure 1: Minimum & Maximum ostial coronary artery diameter
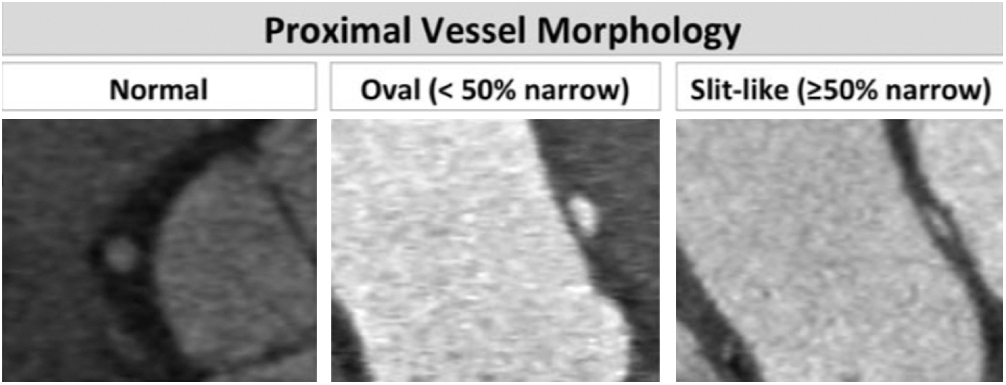
Figure 2: Proximal vessel morphology
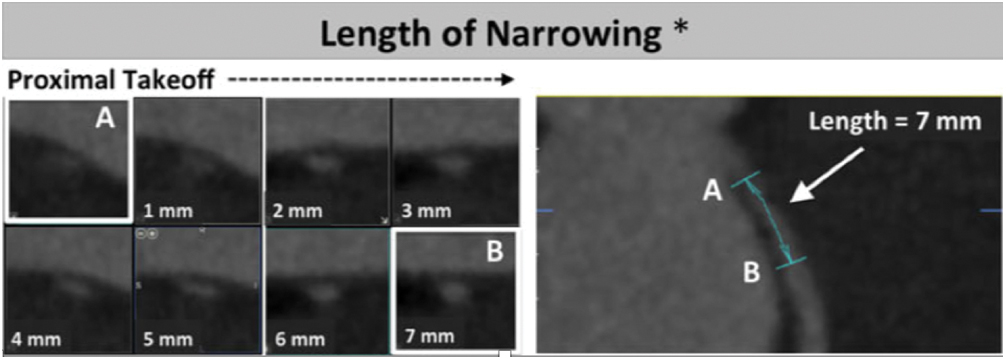
Figure 3: Length of narrowing
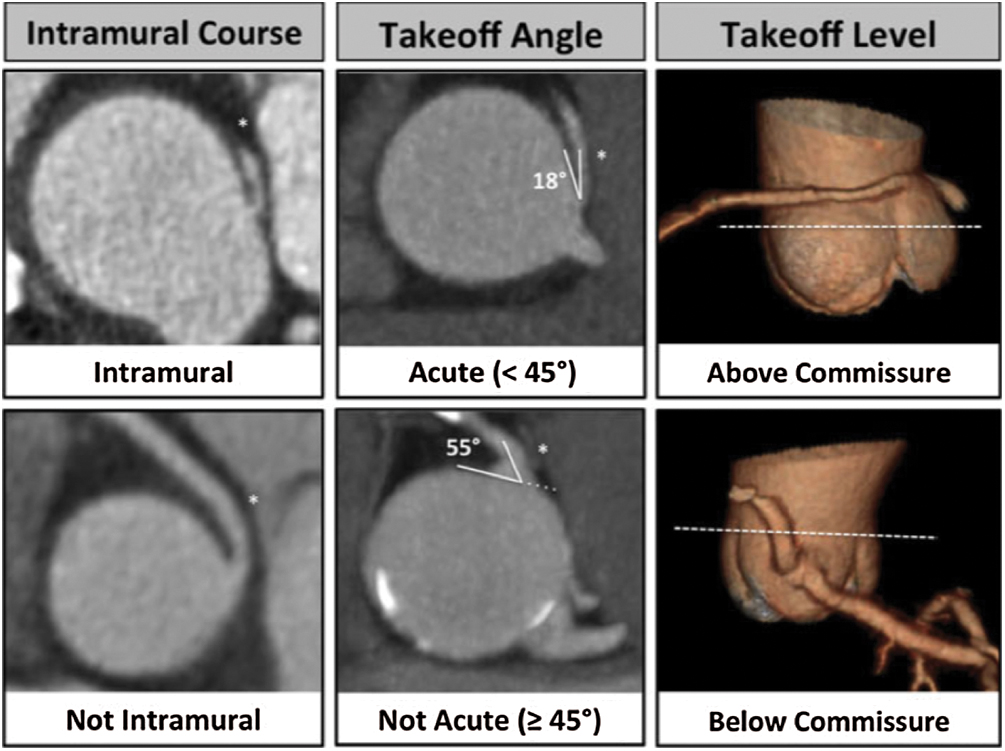
Figure 4: Course, angle and takeoff level of coronary arteries
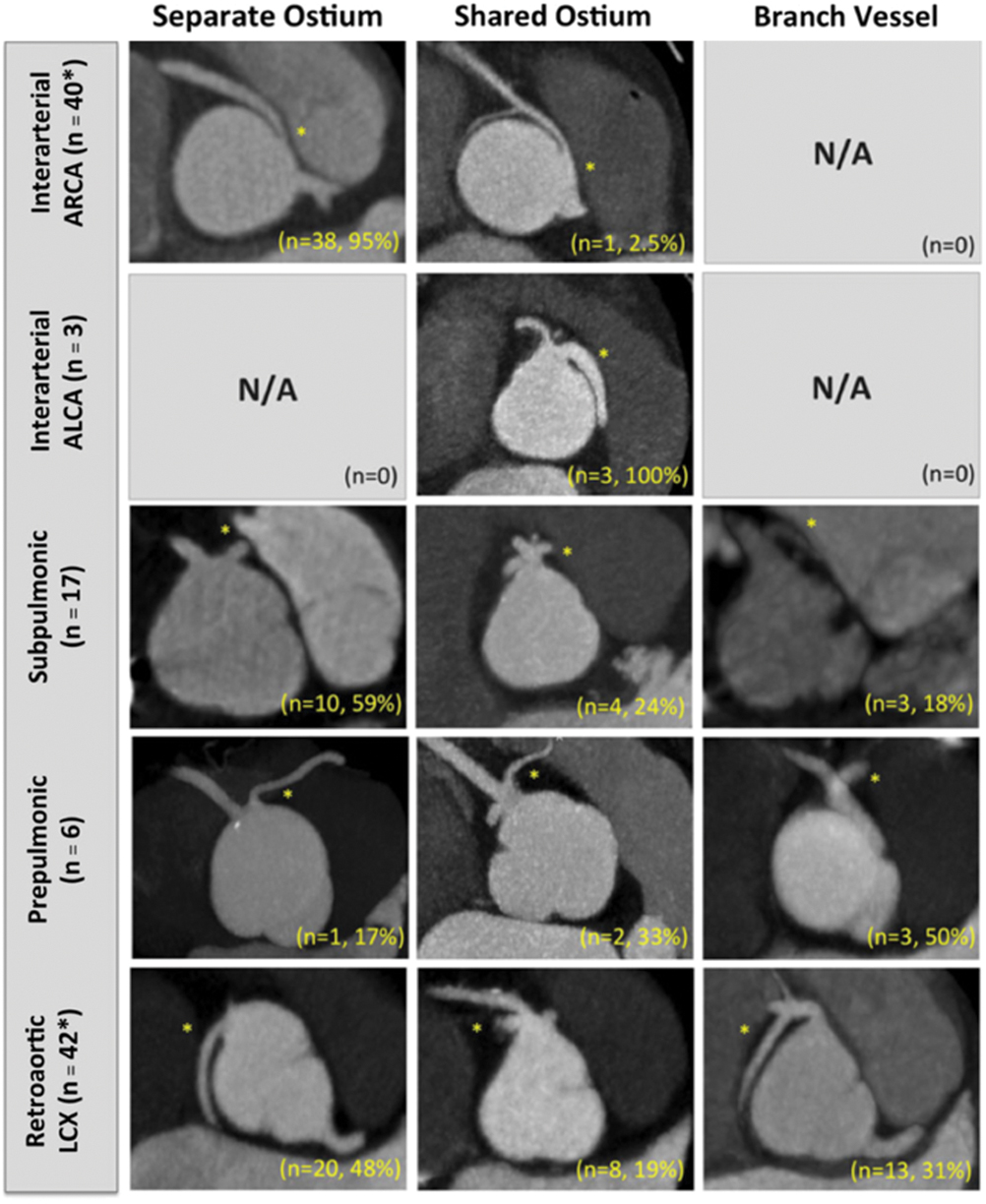
Figure 5: Type and branching of coronary ostias
Right ventricle outflow tract, pulmonary annulus, main pulmonary artery and pulmonary arteries will be also assessed with a measurement of the narrowest diameter at each level in views obtained orthogonal to the centerline of the corresponding vessel. -All diameters will be measured perpendicular to the centreline of the pulmonary artery, during end-diastole
Finally, the maximal diameters at level of aortic annulus, sinus of Valsalva, sino-tubular junction and the ascending and descending aorta (next to the left pulmonary artery) will be measured in orthogonal views of the ascending aorta.
- All diameters will be measured perpendicular to the centreline of the aorta
- All aortic diameters are measured with the inner edge to inner edge convention, during end-diastole
- The dimeter of the sinus of Valsalva will be the maximal diameters among the 3 diameters obtained by measurements sinus to sinus.
- Ascending and descending aorta will be measured in the same plane than the pulmonary artery bifurcation.
4.4 Results/Report
References
1. Cheezum, M. K., Ghoshhajra, B., Bittencourt, M. S., Hulten, E. A., Bhatt, A. et al. (2017). Anomalous origin of the coronary artery arising from the opposite sinus: prevalence and outcomes in patients undergoing coronary CTA. European Heart Journal–Cardiovascular Imaging, 18(2), 224–235. DOI 10.1093/ehjci/jev323.
2. Opolski, M. P., Pregowski, J., Kruk, M., Witkowski, A., Kwiecinska, S. et al. (2013). Prevalence and characteristics of coronary anomalies originating from the opposite sinus of Valsalva in 8,522 patients referred for coronary computed tomography angiography. American Journal of Cardiology, 111(9), 1361–1367. DOI 10.1016/j.amjcard.2013.01.280.
3. Nasis, A., Machado, C., Cameron, J. D., Troupis, J. M., Meredith, I. T. et al. (2015). Anatomic characteristics and outcome of adults with coronary arteries arising from an anomalous location detected with coronary computed tomography angiography. International Journal of Cardiovascular Imaging, 31(1), 181–191. DOI 10.1007/s10554-014-0535-4.
4. Virmani, R., Chun, P. K., Goldstein, R. E., Robinowitz, M., McAllister, H. A. (1984). Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: association with sudden death. Journal of the American College of Cardiology, 3(3), 766–771. DOI 10.1016/S0735-1097(84)80253-3.
5. Yacoub, M. H., Radley-Smith, R. (1978). Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax, 33(4), 418–424. DOI 10.1136/thx.33.4.418.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |