

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011977
ARTICLE
Atrial Septal Defect in Children: The Incidence and Risk Factors for Diagnosis
1Department of Clinical Sciences, Unit of Pediatrics, Umeå University, Umeå, Sweden
2Department of Public Health and Clinical Medicine, Unit of Research, Education and Development Östersund Hospital, Umeå University, Umeå, Sweden
3Department of Pediatric Cardiology, Skåne University Hospital, Lund, Sweden
4Department of Clinical Sciences, Lund University, Lund, Sweden
5Department of Women’s and Children’s Health, Karolinska Institute, Stockholm, Sweden
*Corresponding Author: Gustaf Tanghöj. Email: gustaf.tanghoj@regionjh.se
Received: 09 June 2020; Accepted: 14 August 2020
Abstract: Objective: Secundum atrial septal defect (ASD II) is a common congenital heart defect, and interatrial communications among preterm children is even more common. The objective of this study was to calculate the incidence of ASD II in children, with assessment to gestational age at birth. Further, to assess maternal, prenatal and postnatal risk factors associated with ASD II among children of different gestational age at birth. Design: This national registry based retrospective incidence study was supplemented with a national case-control study, using the Swedish Register of Congenial Heart Disease, Swedish Medical Birth Register and Statistics Sweden. All children, 0–18 years of age, born in Sweden and diagnosed with an ASD II between 2010 and 2015 were included in the study and compared with children without diagnosis of ASD II. Results: The yearly overall incidence of ASD II was 150 per 100 000 live births. However, this incidence ranged from 449 per 100 000 live births to 1737 per 100 000 live births, with higher incidence among preterm children. ASD II was associated with a presence of persistent ductus arteriosus; OR = 8.11 (Cl 95% 2.80–16.69), female gender; OR = 1.39 (Cl 95% 1.18–1.63) and being small for gestational age; OR = 1.86 (Cl 95% 1.29–2.68). Born preterm was also associated with ASD II; born at 32–36 gestational children; OR = 3.21 (Cl 95% 2.46–4.19), and born <32 gestational weeks; OR = 4.02 (Cl 95% 2.80–7.12). Conclusion: Preterm children have a higher incidence of ASD II than previously found, increasing with lower gestational age at birth. Preterm birth is an independent risk factor for ASD II diagnosis with three to four times, suggesting that this group of children may need new structured follow up program with careful assessment of indication when need of treatment and closure.
Keywords: Preterm infants; heart septal defects; atrial; incidence; epidemiology
Atrial septal defects is the third most common congenital heart defect (CHD), with an estimated incidence of 56 in 100 000 live births [1]. Most atrial septal defects occur sporadic, with some genetic exceptions [2,3]. Secundum atrial septal defects (ASD II) situated within the fossa ovalis accounts for 75% of all atrial septal defects (1). Most ASD II are asymptomatic during infancy and spontaneous closure is common [4]. Larger ASD II cause a significant left to right shunt with subsequent volume overload of the right atrium and ventricle and, less commonly, myocardial cell injury with development of myocardial fibrosis and altered pump function [5–7]. Early closure of ASD II is indicated in the presence of symptoms such as heart failure, reduced growth, recurrent infections or right side atrial or ventricular enlargement. Asymptomatic ASD II with hemodynamic significant shunt are preferably closed electively when the child has reached the age of three to four years [8,9].
Worldwide 12.9 million children are born prior to 37 completed gestational weeks and in Sweden 6% are born preterm [10,11]. CHD is present in 12,5 per 1000 preterm births and interatrial shunts (ASD II and patent foramen ovale [PFO]) are seen in 40% of children with very low birth weight. This is almost five times as common compared to term children [12,13]. The preterm child´s heart show both morphological and functional alterations compared to the heart of children born term and these alterations are withstanding throughout infancy [14–18].
Preterm children with an ASD II are at risk for pulmonary hypertension (PH), severe bronchopulmonary dysplasia (BPD) and increased mortality [19–22]. This has been in part resulted in a shift towards earlier ASD II closure in symptomatic preterm children, with beneficial clinical results and without increase of adverse events [23–26]. Preterm children appear to be more common among children in need of ASD II closure, indicating a prevalence between 17 and 20% [27–29].
The aim of this study was to calculate the incidence of ASD II in Sweden, both for all children and for children born at different gestational weeks, and to assess maternal, perinatal and paediatric factors associated with the diagnosis of ASD II in preterm and term children born in Sweden.
A national registry based retrospective incidence study as well as and national case-control study using the Swedish Registry of Congenial Heart Disease (SWEDCON) [30] and Swedish Medical Birth Register (MBR) and Statistics Sweden (SCB) was conducted [31].
Children diagnosed with an ASD II (European paediatric cardiac code (EPCC) 05.04.02) and born in Sweden between 2010 and 2015 were retrieved from the SWEDCON register for the incidence study. These patients were compared to children age 0–18 years retrieved from the MBR register, excluding those with a diagnosis of an ASD II by checking against the SWEDCON register. The retrieval was performed by the National Board of Health and Welfare and anonymized with regard to personal identification before referral to the research group.
Cases in the case-control study were defined as children with ASD II (EPCC 05.04.02) diagnosis retrieved from the Swedcon register, while controls were defined as children without diagnosis of ASD II. Controls were matched to cases by birth hospital and month and year of birth. Demographic data, potential maternal and neonatal risk factors and gestational age were retrieved from MBR. Type and date of diagnosis for cases were retrieved from SWEDCON.
Preterm children and term children were stratified according to the World Health Organization’s definition; term (>37 weeks), late premature (32 to <37 weeks), very premature (28 to <32 weeks) and extreme premature (<28 weeks) [32]. The corrected (adjusted) age of preterm born children were calculated by subtracting the number of weeks of the child´s birth prior to 40 weeks, and was registered as the chronological age. These calculations were made to adjust maturational age of diagnosis for preterm children. Maternal weight status was classified according to the World Health Organization’s definition as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), pre-obesity (BMI 25.0–29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) and was measured at the first antenatal clinic visit [33].
SWEDCON is a national based quality register for collecting data on patients with congenital heart diseases. The register was founded in 2009 and consists of four parts: foetal cardiology, paediatric cardiology, GUCH (Grown-Up Congenital Heart disease) and congenital heart surgery. The parts share a common base with information on each patient, including personal data, diagnosis, operations and catheter interventions. The SWEDCON register makes it possible to follow patients with CHD throughout their entire lives and collect and combine data from different parts of the register [34]. Only children who have passed an echocardiac assessment due to clinical signs are registered in SWEDCON.
This applies to all types of CHD and Sweden, like other countries, has not a mandatory extrauterine screening program for CHD. The included data from the paediatric section of SWEDCON are diagnosis (EPPC codes) and date of diagnosis, retrieved January 22nd 2019. The register has been validated and shown to be in accordance with medical records [35].
The MBR register was established in 1973 and includes data on more than 99% of all births in Sweden and is held by the National Board of Health and Welfare [36]. The first registration of each individual occurs at the first visit to the antenatal clinic and is completed when the mother and the new-born infant are discharged from the hospital. Information on maternal demographics, reproductive history and smoking habits, as well as on pregnancy, delivery and neonatal data are included for all births [37].
All data are presented as mean (std.), median (IQR) or percentage (%) depending on the type and distribution of the data. Student’s t-test (unpaired two-sided) was used for parametrically distributed variables, Man-Whitney U test for non-parametric distributed variables, Kruska Wallis test for multiple categorical groups with non-paramedic distributed variables and Person’s χ2 for categorical data and Fisher’s exact test when cell values below five was present. Overall a p < 0.05 was considered to be significant.
Incidence proportion was assessed by number of individuals with diagnosis in each gestational age-group calculated as per one hundred thousand live births per year using Swedish National Statistics Database for livebirths between 2010 and 2015 (SCB) [38].
Conditional logistic regression was performed to evaluate the association between premature birth and ASD II diagnosis adjusting for predefined confounding risk factors. Very preterm children and extremely preterm children were merged to one group and defined as children born at less than 32 gestational weeks. Confounding factors were defined according to clinical importance and time causality. Maternal confounding factors (maternal smoking at first visit, diabetes mellitus and maternal weight-classes) and neonatal confounding factors (gestational subclasses, small for gestational age, persistent ductus arteriosus and congenital heart defect) were included in the adjusted logistic regression model. Down’s syndrome (DS) was not included as a confounding factor for ASD II, due to the low number of children with DS among the controls. Persistent pulmonary hypertension (PPHN) and bronchopulmonary dysplasia (BPD) were not included into the regression model, as ASD II is already present before PPHN as well as BPD is developed. Maximum-likelihood estimates of the odds ratio (OR) and 95% confidence interval (CI) were obtained. The IBM SPSS Statistics, Version 25 Software (IBM Corporation, New York, USA) was used.
This study was conducted according to the ethical standards of the 1964 Helsinki Declaration with amendments and approved by the ethics committee of human research at Umeå University (D-nr 2017/86-31)
This study included 978 children with ASD II and 8866 controls. Children with a diagnosis of ASD II had a lower birth weight (3124.6 g ± 885.3 std) compared to children without ASD II (birthweight 3511.7 g ± 593.6 std, p < 0.001). Children with an ASD II diagnosis were born at an earlier gestational age (38.1 weeks ± 3.6 std.) compared to children without diagnoses (gestational age 39.7 weeks ± 2.0 std, p < 0.001) (Tab.1).
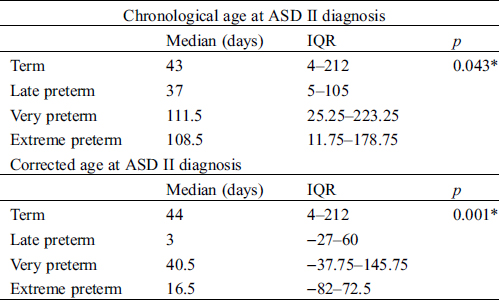
Table 1: Age at diagnosis of ASD II
The annual birth rate during 2010–2015 had a range between 110004 and 115352. Between the years 2010 and 2015 the yearly overall incidence of ASD II was 150 per 100 000 live births (Fig. 1). The incidence of ASD II decreased with increasing gestational age. In late preterm children the incidence of ASD II was 449 per 100 000 live births, in very preterm children 1215 per 100 000 live births and in extremely preterm children 1737 per 100 000 livebirths (Fig. 1).
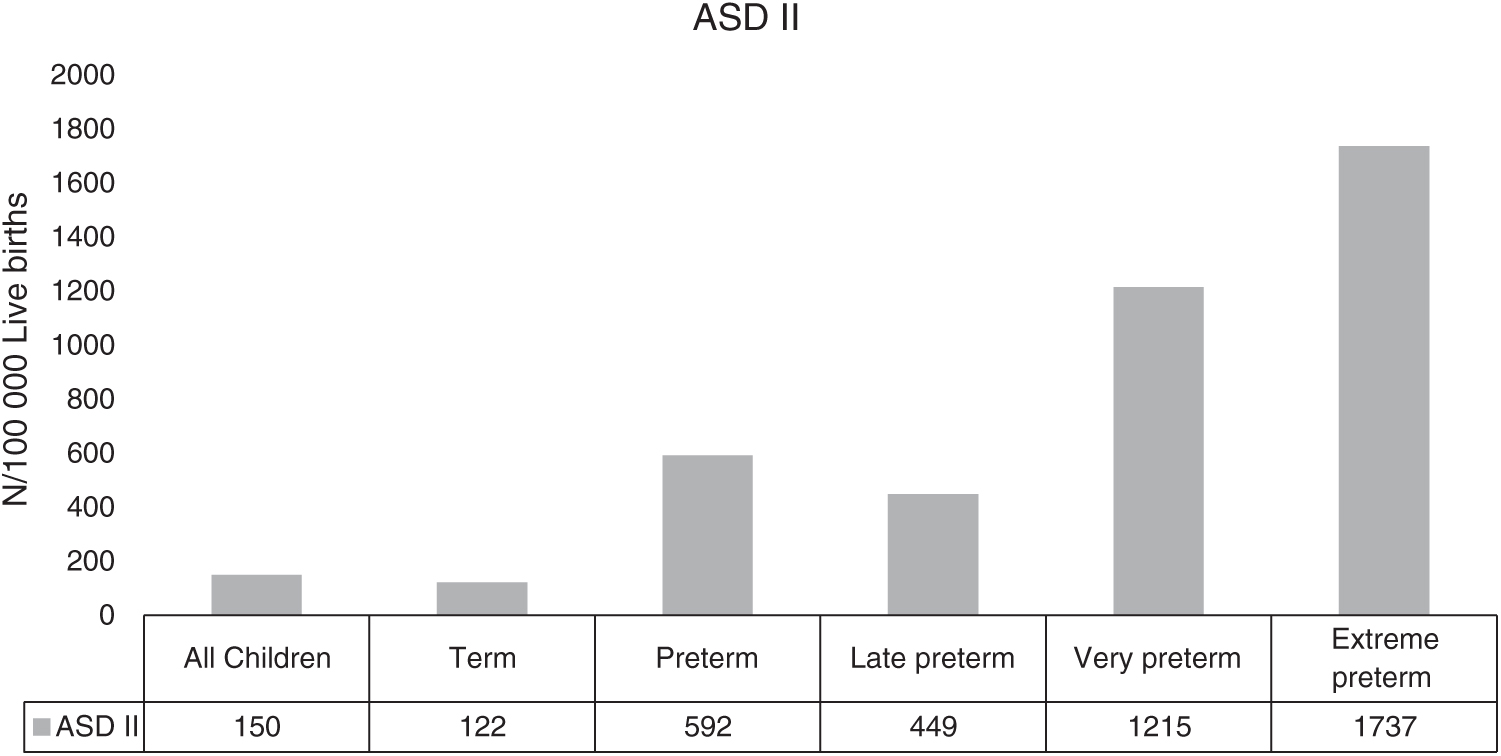
Figure 1: Incidence of ASD II in relation to gestational age ASD II = ASD secundum
The median chronological age of an ASD II diagnosis for preterm children was 55.0 days (interquartile range (IQR) 6.0–136 days) and for term children 43 days (IQR 4.0–212.0 days), p = 0.927. Further subgroup analyses indicated that very- and extreme-preterm children were older at time of ASD II diagnosis than term children (Tab. 1). However, using corrected gestational age, all subgroups of preterm children were younger at time of ASD II diagnosis compared to term born children (Tab. 1).
3.3 Risk Factors for ASD II Diagnosis
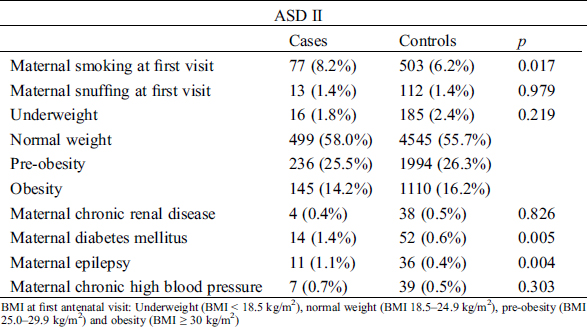
Maternal smoking at first visit to maternal care and diagnosis of maternal diabetes mellitus and epilepsy were more common among mothers to children with ASD II compared to children without ASD II (Tab. 2).
Table 2: Demographic information on maternal factors
Children with ASD II were more often born preterm, small for gestational age or with a very low birth weight. They also more commonly had associated diseases such as non-specific neonatal infections, BPD, infant respiratory distress syndrome, persistent pulmonary hypertension, persistent ductus arteriosus (PDA), CHD and DS, compared to children born without ASD II (Tab. 3). Girls were more common among children with ASD II compared to children without ASD II (Tab. 3). The female to male ratio for ASD II was 1.3:1.
Table 3: Demographic information on factors related to the child
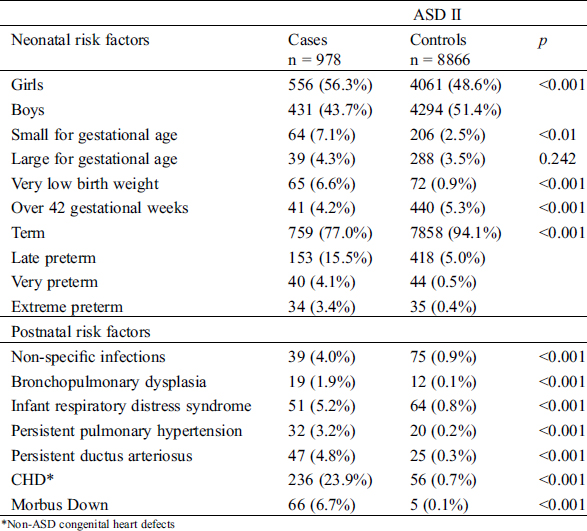
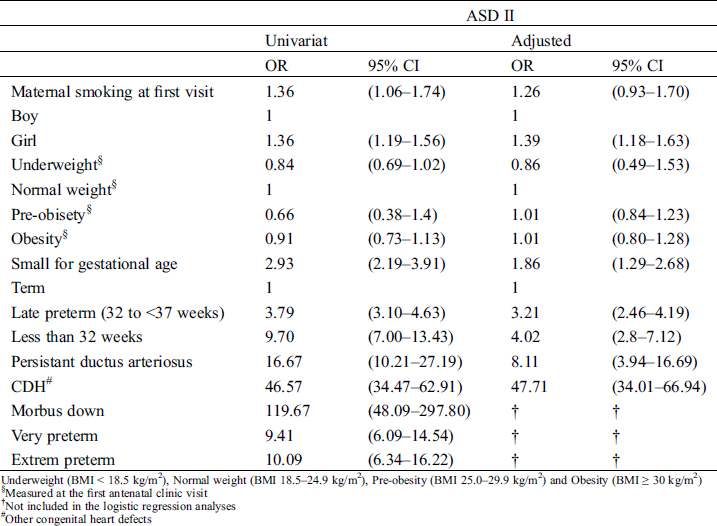
Maternal risk factors were not associated with ASD II after adjusting for potential confounding factors (Tab. 4). The risk of ASD II was independently associated with a presence of other CHD; OR = 47.71 (Cl 95% 34.01–66.94), PDA; OR = 8.11 (Cl 95% 2.80–16.69), female gender; OR = 1.39 (Cl 95% 1.18–1.63) and small for gestational age; OR = 1.86 OR 1.39 (Cl 95% 1.29–2.68). Late preterm children had a threefold independent association; OR = 3.21 (Cl 95% 2.46–4.19), and children born less than 32 gestational weeks had a fourfold association, OR = 4.02 (Cl 95% 2.80–7.12) to ASD II diagnosis compared to term born children (Tab. 4).
Table 4: Risk factors for diagnosis of ASD II
4.1 Incidence of ASD II among Children after Term and Preterm Live Births
The overall incidence of ASD II in children born in Sweden in this national population-based register study was 150 per 100 000 live births. This incidence is almost three times higher than previously reported [1,39]. As the ASD II diagnosis is highly influenced by the timing of and access to echocardiographic examinations and along with an improved detection of ASD II the last decades, the higher incidence in our study is in line with the suggested trend in other studies [39,40]. ASD II is usually asymptomatic with insignificant or no murmur. This may have contributed to a previous underestimation of the true incidence in children and probably not all children with ASD II have been detected in our study population [41].
Preterm children in our study have, depending on degree of prematurity, a three to fourteen times higher incidence of ASD II diagnosis, compared to term children (Fig. 1). Estimates of incidence of all types CHD among children with very low birth weight or preterm birth is increased, even when ASD II are excluded [28]. The prevalence of atrial septal communication is over of 40% in preterm children [12]. The combination of CHD, a risk factor for both preterm birth and ASD II, and an early presence of atrial septal communication, may contribute to the high incidence of ASD II among preterm in our study [28,42]. A presence of a CHD could be part of the causal mechanism leading to preterm delivery. However, this pathway is less likely among children born with ASD II without additional CHD as, primarily an interatrial communication is a basis for intrauterine life and secondly the defects becomes hemodynamically significant only after birth. Thus, it is most unlikely ASD II is the origin to induced preterm birth [43–45].
Persistent ductus arteriosus (PDA) and CDH are common in preterm children [46,47]. PDA usually creates a left to right shunt increasing pulmonary blood flow which subsequently stretches the left atrial wall [12]. It can be speculated that a stretched PFO may develop to an ASD II or possibly wrongly diagnosed as an ASD II. One can speculated that chronic pulmonary disease, common among premature born children and more likely to require ventilating support, combined with residual postnatal pulmonary hypertension could contribute to poor right ventricular compliance and elevated right atrial pressure. This might keep the flap of the foramen ovale open [48]. Reduced lung compliance in premature infants could lead to greater phasic respiratory changes in intrathoracic, left atrial, and right atrial pressures [49]. This may theoretically impose misclassification of a stretched PFO as an ASD II.
Preterm children are more likely to be exposed to several echocardiographic exams compared to term born children and we expected preterm children to be diagnosed at an earlier age compared to term children. The calculated corrected age at diagnosis for preterm born children indicated that diagnosis of ASD II was set at a younger age, although the chronological age, days between birth and diagnosis, was longer (Tab. 1). This implies that the diagnosis of a true ASD II is set after the first intensive period of care at the neonatal ward. The younger corrected age at diagnosis rules out the risk of misclassification of an ASD II to a PFO, serendipitously found during the neonatal period, to influence the increased incidence rates of ASD II among preterm children. However, one can speculate that an atrial septal communication was identified at the neonatal care unit as part of an overall cardiac evaluation related to preterm morbidity, and then followed with several echocardiographic evaluations before the ASD II diagnosis was set. This careful follow-up may have influenced the incidence rates. On the other hand, we, along with one other previous study, have reported increased number of preterm born children among those with an early ASD closure [50,51]. Thus, the increased incidence rate among premature born children in our study may be true.
4.2 Maternal Factors and Association to ASD II
Maternal smoking, maternal diabetes, maternal epilepsy and overweight are known risk factors for CHD in general as well as for ASD II [27,52–54]. These factors are also known to increase the risk for preterm birth [55]. Amongst children with ASD II maternal smoking, diabetes mellitus and epilepsy were more common compared to controls in our study, but maternal overweight was not, which is in contrast to previous studies [52]. The dose response pattern previously described with higher prevalence of ASD among offspring to mothers with BMI > 40 have probably multifactorial origin with complex interplays between genetic and environmental factors (such as smoking, alcohol usage and socioeconomic status), and is not evaluated in our study [52,56]. Overall only 13 (1.3%) of all mothers in our study had a BMI > 40, and thus too few too incorporate as a single risk factor in the conditional regression model. In our study there was no difference between maternal overweight (pre-obesity and obesity) for cases and controls. The overall overweight at first antenatal visit in our study was in line with the overall presence of overweight among women in Sweden [57].
Maternal epilepsy was three times as common among children with ASD II compared children without ASD II, which also has been reported by others [54]. Maternal epilepsy, and antiepileptic drug treatment, has previously been linked to congenital malformations as well as premature birth [54,58,59]. The MBR register lacks information on antiepileptic drugs. Antiepileptic drugs may have a synergetic effect to the overall risk for ASD II in our study, and may further enforce the advocated caution of antiepileptic drugs during pregnancy. However, very few cases (1.1%) and controls (0.4%) were exposed to maternal epilepsy in our study, which destabilized the multivariate regression model and the adjusted risk calculation of epilepsy could not be done (Tab. 2).
Several maternal factors are well known risk factors for preterm birth. However, none of maternal risk factors were independently associated with diagnosis of ASD II in our study. Thus, we believe that premature birth, rather than maternal factors, is associated with ASD II.
4.3 Paediatric Risk Factors and Association to ASD II
Almost all risk factors related to the child were more common among children with ASD II compared to controls. An ASD II is probably already present prior to birth as the septation of the atrial cavities and formation of foramen ovale is completed around the 12th gestational week [45]. ASD II might be a risk factor for neonatal conditions such as PPHN and BPD, and not the other way around. Therefore, neither PPHN nor BPD was included in the regression analysis. We speculate that the presence of ASD II can be linked to neonatal morbidity in presence of PPNH and BPD. Down Syndrome (DS), a known risk factor for ASD II as well as for preterm birth, was more common among children with ASD II (6.7%) than controls (0.1%) in our study, but could not be included in the multivariate regression model due to the few exposed controls [60]. Among children with both ASD II and DS, in our study population, a total of 16 (25%) had another CHD.
Low birth weight, premature birth and chromosomal abnormalities are reported risk factors for ASD II by others [61,62]. This study adds that being born prior to 32 gestational weeks (very or extremely premature) further increases the association, but we can only speculate about the mechanism behind this. We believe that the withstanding morphological and functional alterations of the heart of a preterm born child, may contribute to the risk [14–18]. Being small for gestational week was associated with ASD II in our study; OR = 1.86 (95% CI 1.29–2.68) and thus it can be speculated that intra-uterine growth retardation may influence the heart’s growth and development and thereby increase the risk of ASD II.
4.4 Burden of an ASD II among Preterm Children
Previous studies report an increased association between preterm birth and pulmonary hypertension as well as BPD, and that the combination of ASD II, BPD and pulmonary hypertension increases the risk of neonatal death [19,20,22]. An early identification of ASD II with intervention may be beneficial in symptomatic preterm children [24,50,63]. However, the physiological normalization of pulmonary vascular resistance and improved pulmonary blood flow during the postnatal period may be interoperated as the interventional improvement.
Spontaneous closure of ASD II is known to occur within the first year, while others may grow in size [4]. Choosing the optimal timing of ASD closure can be discussed [64]. The increased association between ASD II diagnosis and incidence among preterm children in our study indicates that a structured follow-up program in preterm born children is needed, assessing a development of cardiac and pulmonary morbidity.
The SWEDCON register relies solely on voluntarily based registration of data by physicians of divergent experience. Previous studies on incidence have used population-based registry classification according to the International classification of disease (ICD), where ASD II and PFO share the same classification (Q21.1) [39]. Data in the SWEDCON register is using the European paediatric cardiac code which separates ASD II diagnosis from a PFO [34]. We believe that by using the EPCC classification, we reduced the risk of misclassification, selection bias and missing of data in our study [30,35]. A good concordance between medical records and SWEDCON data further reduces this risk [35]. A limitation to this study and the use of SWEDCON is that the ASD II diagnosis is registered after an echo cardiac evaluation due to clinical signs. Thus, small asymptomatic ASD II might not be diagnosed among healthy full term born children with no need of cardiac assessment and with an overestimation of the incidence-difference between preterm and term born children. On the other hand, the prospectively collected data in this large cohort enabled us to assess multiple risk factors, and the national coverage of all registers provided linkage between the registers, which strengthens the study [35].
We chose not to assess the diagnosis of PFO in this study and this may be regarded as a limitation. However, due to the uncertainties concerning the validity in the method of PFO diagnosis, commonly found in healthy children and often considered as a normal variation and left without a diagnosis in the register, the exclusion of PFO reduces the risk of not accurately assessing the incidences of PFO and its potential risk factors.
Preterm children have a higher incidence of ASD II than previously found, increasing with lower gestational age, compared to term children. Preterm birth is an independent risk factor for ASD II diagnosis with three to four times the risk, suggesting that this group of children may need new structured follow-up programs with careful assessment of the indications and need for treatment and closure. Further national studies to assess the risk of ASD II intervention in preterm children are needed.
Authors Contribution: Concept and design, data collection, drafting of the manuscript and statistical analyses, approval of the article: Tanghoj. G Concept and design, critical revision of the article, approval of the article: Naumburg. E and Lindam. A Critical revision, approval of the article: Liuba. P and Sjöberg. G.
Funding Statement: This study was funded by the Unit of Research, Education and Development, Östersund Hospital, Region Jämtland Härjedalen.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report in this study.
1. Hoffman, J. I., Kaplan, S., Liberthson, R. R. (2004). Prevalence of congenital heart disease. American Heart Journal, 147(3), 425–439. DOI 10.1016/j.ahj.2003.05.003.
2. Caputo, S., Capozzi, G., Russo, M. G., Esposito, T., Martina, L. et al. (2005). Familial recurrence of congenital heart disease in patients with ostium secundum atrial septal defect. European Heart Journal, 26(20), 2179–2184. DOI 10.1093/eurheartj/ehi378.
3. Maitra, M., Schluterman, M. K., Nichols, H. A., Richardson, J. A., Lo, C. W. et al. (2009). Interaction of Gata4 and Gata6 with Tbx5 is critical for normal cardiac development. Developmental Biology, 326(2), 368–377. DOI 10.1016/j.ydbio.2008.11.004.
4. Hanslik, A., Pospisil, U., Salzer-Muhar, U., Greber-Platzer, S., Male, C. (2006). Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. PEDIATRICS, 118(4), 1560–1565. DOI 10.1542/peds.2005-3037.
5. Hoffman, J. I., Rudolph, A. M., Heymann, M. A. (1981). Pulmonary vascular disease with congenital heart lesions: pathologic features and causes. Circulation, 64(5), 873–877. DOI 10.1161/01.CIR.64.5.873.
6. Sugimoto, M., Kuwata, S., Kurishima, C., Kim, J. H., Iwamoto, Y. et al. (2015). Cardiac biomarkers in children with congenital heart disease. World Journal of Pediatrics, 11(4), 309–315. DOI 10.1007/s12519-015-0039-x.
7. Campbell, M. (1970). Natural history of atrial septal defect. Heart, 32(6), 820–826. DOI 10.1136/hrt.32.6.820.
8. Feltes, T. F., Bacha, E., Beekman, R. H., Cheatman, J. P., Feinstein, J. A. et al. (2011). Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation, 123(22), 2607–2652. DOI 10.1161/CIR.0b013e31821b1f10.
9. Baumgartner, H., Bonhoeffer, P., De Groot, N. M., de Haan, F., Deanfield, J. E. et al. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010): the task force on the management of grown-up congenital heart disease of the european society of cardiology (ESC). European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249.
10. Beck, S., Wojdyla, D., Say, L., Betran, A. P., Merialdi, M. et al. (2010). The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization, 88(1), 31–38. DOI 10.2471/BLT.08.062554.
11. Morken, N. H., Kallen, K., Hagberg, H., Jacobsson, B. (2005). Preterm birth in Sweden 1973-2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstetricia et Gynecologica Scandinavica, 84(6), 558–565. DOI 10.1111/j.0001-6349.2005.00765.x.
12. Lee, C., Lim, G., Kim, W. S., Han, H. S. (2014). Clinical characteristics and outcome of incidental atrial septal openings in very low birth weight infants. Neonatology, 105(2), 85–90. DOI 10.1159/000356164.
13. Ghiglia, S., Fesslova, V. (2008). Patency of foramen ovale in fullterm and preterm neonates. A follow-up study. Medical and Surgical Pediatrics, 30(4), 192–196.
14. Broadhouse, K. M., Finnemore, A. E., Price, A. N., Durgheil, G., Cox, D. J. et al. (2014). Cardiovascular magnetic resonance of cardiac function and myocardial mass in preterm infants: a preliminary study of the impact of patent ductus arteriosus. Journal of Cardiovascular Magnetic Resonance, 16(1), b2325. DOI 10.1186/s12968-014-0054-4.
15. Schubert, U., Muller, M., Abdul-Khaliq, H., Norman, M. (2016). Preterm birth is associated with altered myocardial function in infancy. Journal of the American Society Echocardiography, 29(7), 670–678. DOI 10.1016/j.echo.2016.03.011.
16. Levy, P. T., Dionedam, B., Holland, M. R., Sekarski, T. J., Lee, C. K. et al. (2015). Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. Journal of the American Society Echocardiography, 28(5), 559–569. DOI 10.1016/j.echo.2015.01.024.
17. Lewandowski, A. J., Bradlow, W. M., Augustinem, D., Davis, E. F., Francis, J. et al. (2013). Right ventricular systolic dysfunction in young adults born preterm. Circulation, 128(7), 713–720. DOI 10.1161/CIRCULATIONAHA.113.002583.
18. Lewandowski, A. J., Augustine, D., Lamata, P., Davis, E. F., Lazdam, M. et al. (2013). Preterm heart in adult life. Circulation, 127(2), 197–206. DOI 10.1161/CIRCULATIONAHA.112.126920.
19. Vyas-Read, S., Guglani, L., Shankar, P., Travers, C., Kanaan, U. (2018). Atrial septal defects accelerate pulmonary hypertension diagnoses in premature infants. Frontiers in Pediatrics, 6, e682. DOI 10.3389/fped.2018.00342.
20. Kumar, K. R., Clark, D. A., Kim, E. M., Perry, J. D., Wright, K. et al. (2018). Association of atrial septal defects and bronchopulmonary dysplasia in premature infants. Journal of Pediatrics, 202, 56–62.e2. DOI 10.1016/j.jpeds.2018.07.024.
21. Vyas-Read, S., Kanaan, U., Shankar, P., Stremming, J., Travers, C. et al. (2017). Early characteristics of infants with pulmonary hypertension in a referral neonatal intensive care unit. BMC Pediatrics, 17(1), 839. DOI 10.1186/s12887-017-0910-0.
22. Choi, E. K., Jung, Y. H., Kim, H. S., Shin, S. H., Choi, C. W. et al. (2015). The impact of atrial left-to-right shunt on pulmonary hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Pediatrics & Neonatology, 56(5), 317–323. DOI 10.1016/j.pedneo.2014.12.006.
23. Bishnoi, R. N., Everett, A. D., Ringel, R. E., Owada, C. Y., Holzer, R. J. et al. (2014). Device closure of secundum atrial septal defects in infants weighing less than 8 kg. Pediatric Cardiology, 35(7), 1124–1131. DOI 10.1007/s00246-014-0905-7.
24. Wood, A. M., Holzer, R. J., Texter, K. M., Texter, K. M., Hill, S. L. et al. (2011). Transcatheter elimination of left-to-right shunts in infants with bronchopulmonary dysplasia is feasible and safe. Congenital Heart Disease, 6(4), 330–337. DOI 10.1111/j.1747-0803.2011.00540.x.
25. Zaqout, M., De Baets, F., Schelstraete, P., Suys, B., Panzer, J. et al. (2010). Pulmonary function in children after surgical and percutaneous closure of atrial septal defect. Pediatric Cardiology, 31(8), 1171–1175. DOI 10.1007/s00246-010-9778-6.
26. Tanghoj, G., Liuba, P., Sjoberg, G., Naumburg, E. (2019). Risk factors for adverse events within one year after atrial septal closure in children: a retrospective follow-up study. Cardiology in the Young, 30, 1–10.
27. Petrossian, R. A., Kuehl, K. S., Loffredo, C. A. (2015). Relationship of birth weight with congenital cardiovascular malformations in a population-based study. Cardiology in the Young, 25(6), 1086–1092. DOI 10.1017/S1047951114001644.
28. Laas, E., Lelong, N., Thieulin, A. C., Houyel, L., Bonnet, D. et al. (2012). Preterm birth and congenital heart defects: a population-based study. PEDIATRICS, 130(4), e829–e837. DOI 10.1542/peds.2011-3279.
29. Tanghoj, G., Odermarsky, M., Naumburg, E., Liuba, P. (2017). Early complications after percutaneous closure of atrial septal defect in infants with procedural weight less than 15 kg. Pediatric Cardiology, 38(2), 255–263.
30. Thilen, U. (2019). National quality registry for congenital heart disease. http://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforcongenitalheartdiseaseswedcon.2279.html.
31. Socialstyrelsen, The Swedish Medical Birth Register. http://www.socialstyrelsen.se/register/halsodataregister/medicinskafodelseregistret/inenglish.
32. WHO-Preterm Bith (2018). https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
33. WHO-Body mass index-BMI (2019). World Health Organization-Europe. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
34. SWEDCON, Thilen, U. (2018) \xC5rstapport SWEDCON 2018. http://www.ucr.uu.se/swedcon/.
35. Bodell, A., Björkhem, G., Naumburg, E. (2017). National quality register of congenital heart diseases – can we trust the data?. Journal of Congenital Cardiology, 1(11), 749. DOI 10.1186/s40949-017-0013-7.
36. Gottvall, K. (2010). Pregnancies, deliveries and newborn infants the swedish medical birth register 1973-2009 assisted reproduction, treatment 1991-2008, Socialstyrelsen. https://www.socialstyrelsen.se/globalassets/sharepointdokument/artikelkatalog/statistik/2011-3-19.pdf.
37. Cnattingius, S., Ericson, A., Gunnarskog, J., Kallen, B. (2016). A quality study of a medical birth registry. Scandinavian Journal of Social Medicine, 18(2), 143–148. DOI 10.1177/140349489001800209.
38. SCB Statistical Databas of Sweden (2015). Population livbirth 2010-2015. http://www.socialstyrelsen.se/statistik/statistikdatabas/graviditeter-forlossningarochnyfodda.
39. Botto, L. D., Correa, A., Erickson, J. D. (2001). Racial and temporal variations in the prevalence of heart defects. PEDIATRICS, 107(3), e32–e32. DOI 10.1542/peds.107.3.e32.
40. Wilson, P. D., Correa-Villasenor, A., Loffredo, C. A., Ferencz, C. (1993). Temporal trends in prevalence of cardiovascular malformations in Maryland and the district of Columbia, 1981–1988. Epidemiology, 4(3), 259–265. DOI 10.1097/00001648-199305000-00010.
41. Seldon, W. A., Rubinstein, C., Fraser, A. A. (1962). The incidence of atrial septal defect in adults. Heart, 24(5), 557–560. DOI 10.1136/hrt.24.5.557.
42. Shuler, C. O., Tripathi, A., Black, G. B., Park, Y. M., Jerrell, J. M. (2013). Prevalence of treatment, risk factors, and management of atrial septal defects in a pediatric Medicaid cohort. Pediatric Cardiology, 34(7), 1723–1728. DOI 10.1007/s00246-013-0705-5.
43. Saito, T., Ohta, K., Nakayama, Y., Hashida, Y., Maeda, A. et al. (2012). Natural history of medium-sized atrial septal defect in pediatric cases. Journal of Cardiology, 60(3), 248–251. DOI 10.1016/j.jjcc.2012.05.005.
44. Fesslova, V., Nava, S., Villa, L. (1999). Evolution and long term outcome in cases with fetal diagnosis of congenital heart disease: Italian multicentre study. Fetal cardiology study group of the Italian society of pediatric cardiology. Heart, 82(5), 594–599.
45. Jensen, B., Wang, T., Moorman, A. F. M. (2018). Evolution and Development of the Atrial Septum. Anatatomical Record, 302(1), 32–48. DOI 10.1002/ar.23914.
46. Tanner, K., Sabrine, N., Wren, C. (2005). Cardiovascular malformations among preterm infants. PEDIATRICS, 116(6), e833–e838. DOI 10.1542/peds.2005-0397.
47. Hamrick, S. E., Hansmann, G. (2010). Patent ductus arteriosus of the preterm infant. PEDIATRICS, 125(5), 1020–1030. DOI 10.1542/peds.2009-3506.
48. Gouyon, J. B., Iacobelli, S., Ferdynus, C., Bonsante, F. (2012). Neonatal problems of late and moderate preterm infants. Seminars in Fetal and Neonatal Medicin, 17(3), 146–152. DOI 10.1016/j.siny.2012.01.015.
49. Davis, R. P., Mychaliska, G. B. (2013). Neonatal pulmonary physiology. Seminars in Pediatric Surgery, 22(4), 179–184. DOI 10.1053/j.sempedsurg.2013.10.005.
50. Wyss, Y., Quandt, D., Weber, R., Stainsy, B., Weber, B. et al. (2016). Interventional closure of secundum type atrial septal defects in infants less than 10 kilograms: indications and procedural outcome. Journal of Interventional Cardiology, 29(6), 646–653. DOI 10.1111/joic.12328.
51. Tanghoj, G., Liuba, P., Sjoberg, G., Naumburg, E. (2020). Predictors of the need for an atrial septal defect closure at very young age. Frontiers in Cardiovascular Medicine, 6, 2607. DOI 10.3389/fcvm.2019.00185.
52. Persson, M., Razaz, N., Edstedt Bonamy, A. K., Villamor, E., Cnattingius, S. (2019). Maternal overweight and obesity and risk of congenital heart defects. Journal of the American College of Cardiology, 73(1), 44–53. DOI 10.1016/j.jacc.2018.10.050.
53. Persson, M., Norman, M., Hanson, U. (2009). Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care, 32(11), 2005–2009. DOI 10.2337/dc09-0656.
54. Lagana, A. S., Triolo, O., D’Amico, V., Cartelle, S. M., Sofo, V. et al. (2016). Management of women with epilepsy: from preconception to post-partum. Archives of Gynecology and Obstetrics, 293(3), 493–503. DOI 10.1007/s00404-015-3968-7.
55. Goldenberg, R. L., Culhane, J. F., Iams, J. D., Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet, 371(9606), 75–84. DOI 10.1016/S0140-6736(08)60074-4.
56. Correa, A., Marcinkevage, J. (2013). Prepregnancy obesity and the risk of birth defects: an update. Nutrition Reviews, 71((Suppl 1), ), S68–S77. DOI 10.1111/nure.12058.
57. Folkhälsomyndigheten (2020). (in Swedish) Övervikt och fetma. Public Health Agency of Sweden. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/halsa/overvikt-och–fetma/.
58. Tomson, T., Battino, D., Bonizzoni, E., Craig, J., Lindjour, D. et al. (2018). Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurology, 17(6), 530–538. DOI 10.1016/S1474-4422(18)30107-8.
59. Soontornpun, A., Choovanichvong, T., Tongsong, T. (2018). Pregnancy outcomes among women with epilepsy: a retrospective cohort study. Epilepsy & Behavior, 82, 52–56. DOI 10.1016/j.yebeh.2018.03.001.
60. Stoll, C., Dott, B., Alembik, Y., Roth, M. P. (2015). Associated congenital anomalies among cases with Down syndrome. European Journal of Medical Genetics, 58(12), 674–680. DOI 10.1016/j.ejmg.2015.11.003.
61. Kramer, H. H., Trampisch, H. J., Rammos, S., Giese, A. (1990). Birth weight of children with congenital heart disease. European Journal of Pediatrics, 149(11), 752–757. DOI 10.1007/BF01957272.
62. Wogu, A. F., Loffredo, C. A., Bebu, I., Luta, G. (2014). Mediation analysis of gestational age, congenital heart defects, and infant birth-weight. BMC Research Notes, 7(1), 926. DOI 10.1186/1756-0500-7-926.
63. Khemani, E., McElhinney, D. B., Rhein, L., Andrade, O., Lacro, R. V. et al. (2007). Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. PEDIATRICS, 120(6), 1260–1269. DOI 10.1542/peds.2007-0971.
64. Riggs, T., Sharp, S. E., Batton, D., Hussey, M. E., Weinhouse, E. (2000). Spontaneous closure of atrial septal defects in premature vs. full-term neonates. Pediatric Cardiology, 21(2), 129–134. DOI 10.1007/s002469910020.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |