

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011894
ARTICLE
Incidence, Risk Factors, and Outcomes of Hyperferritinemia after Pediatric Cardiac Surgery with Cardiopulmonary Bypass: A Retrospective Study
1Department of Cardiopulmonary Bypass, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100037, China
*Corresponding Author: Jinping Liu. Email: liujinping@fuwai.com; Xin Duan. Email: jackduan2008@163.com
Received: 08 June 2020; Accepted: 03 September 2020
Abstract: Objective: Serum ferritin has been identified as a prognostic marker in patients with a variety of diseases. In the present study we aim to determine the prevalence of risk factors and outcomes for hyperferritinemia in children undergoing cardiac surgery with cardiopulmonary bypass for congenital heart defects. Methods: The serum ferritin levels of 457 children between the ages of twenty-eight days and three years undergoing cardiopulmonary bypass surgery between June 1, 2017 and June 1, 2018 were analyzed. The prevalence of early postoperative hyperferritinemia was investigated; hyperferritinemia was defined as a ferritin level ≥250 ng/ml. Multivariable regression models including candidate risk factors were constructed to determine the independent predictors of serum ferritin levels post-bypass, analyzed as continuous variables (linear regression) and categorized variables (logistic regression). Multivariable logistic regression was applied to assess the relationship between postoperative hyperferritinemia and a composite of in-hospital mortality, acute kidney injury, extracorporeal life support, prolonged postoperative hospital length of stay and prolonged postoperative mechanical ventilation. Results: Of the 457 included patients, frequency of post-cardiopulmonary bypass hyperferritinemia was 59/457 (10.9%). In multivariate logistic analyses, age [odds ratio (OR) 0.776/90 days], maximum cardiopulmonary bypass flow [OR 1.031/(1 ml/kg)], cardiopulmonary bypass duration (OR 1.095/10 mins) and preoperative hemoglobin [OR 1.207/(10 g/L)] were significantly associated with early postoperative day 1 hyperferritinemia. After risk adjustment, hyperferritinemia was independently associated with the composite outcome (OR 6.373; 95%CI 2.863~14.184, p < 0.001), and improved model discrimination, (AUC 0.868; 95%CI 0.821∼0.916) compared with basic clinical prediction alone (AUC 0.840; 95%CI, 0.790∼0.890; ΔAUC = 0.0279, p = 0.0218). Conclusion: In this study, we found early postoperative hyperferritinemia was relatively common in pediatric patients after cardiopulmonary bypass. The occurrence of hyperferritinemia may help identify a population at risk of unfavorable in-hospital outcome.
Keywords: Hyperferritinemia; cardiopulmonary bypass; iron metabolism; inflammation
Ferritin is a multimeric protein with a very high capacity for storing iron. Whilst low serum ferritin levels invariably indicate iron stores, raised serum ferritin level can be due to multiple different aetiologies, including iron overload, inflammation, and cell damage [1–3]. Hyperferritinemia occurrence, regardless of the underlying pathology, was reported as an early indicator of severity and outcome in sepsis [4], hospitalized [5] and lung injury pediatrics [6]. Leaf and colleagues [7] found that increased serum ferritin after CPB is associated with a need for dialysis, or death, in adults. Given the potential importance of hyperferritinemia on outcomes in other patient groups, it is worth gaining an understanding of the frequency of hyperferritinemia in children undergoing cardiac surgery, its risk factors, and outcome associations. Such insights will be of relevance for the identification of new strategies to inhibiting adverse outcomes in this population.
We hypothesized that early postoperative hyperferritinemia is linked to adverse outcomes in children undergoing cardiac surgery. Study goals were to: 1) Describe the frequency of occurrence of hyperferritinemia after pediatric cardiac surgery with CPB; 2) Identify factors associated with hyperferritinemia; and 3) Determine if hyperferritinemia predicts worse in-hospital outcomes.
2.1 Study Setting and Sampling
This study was conducted at Fuwai Hospital (Beijing, China). After institutional research ethics board approval, data were collected from consecutive pediatric CPB cardiac surgery patients (≤3 years) who had undergone surgery from June 1, 2017 to June 1, 2018. Serum ferritin level is from the first day after surgery (POD 1). Neonates were excluded because they have higher normal ferritin values.
General anesthesia technique was at the individual anesthesiologist’s discretion. During induction, inhalational 8% Sevoflurane was used to facilitate peripheral venous cannulation. Appropriate dosages of intravenous agents such as 0.5 μg/kg Sufentanil and 0.2–0.4 mg/kg Cisbenitracurium were combined with facemask oxygen and oral intubation. Both invasive blood pressure monitoring and blood gas analysis were achieved by radial artery catheterization, and the right internal jugular vein was cannulated for central venous pressure. Throughout the procedure, anesthesia maintenance was achieved by the continuous infusion of Dexmedetomidine, 1 μg/kg/h and Cisbenitracurium, 0.5 mg/kg/h; inhalation of 2–3% sevoflurane, and intermittent infusion of 1–3 μg/kg Sufentanil and 0.2 mg/kg Midazolam. Intraoperative monitoring included three-lead electrocardiography, pulse oximetry, rectal and nasopharyngeal temperature, and urinary output.
The cardiopulmonary bypass circuit consisted of a heart-lung machine (LivaNova, Italy), a hollow-fiber membrane oxygenator (Terumo Fx05, Japan for patients less than 10 kg; Medtronic Pixie, USA for patients weight greater than 10 kg ), an arterial filter (Fly Medical Healthcare Co., China), a hemofiltrater (LivaNova, Italy), and an un-coated polyvinyl chloride circuit (Tianjin Plastics Research Institute Co., China). A cellsaver system (Sorin xtra, Italy) was regularly prepared. The priming volume ranged from 220 to 400 ml, determined by the CPB system (crystalloid or colloid). Packed red blood cells were also added to the priming to achieve a hematocrit level of at least 25% if the body weight was less than 10 kg. Before aortic cannulation, 400 IU/kg unfractionated heparin (UFH) was administered (Hemochron Jr. Signature, USA) to obtain an activated clotting time (ACT) longer than 410 seconds. The target temperature for hypothermia was determined by the aortic cross-clamp time and complexity of the surgery. All patients were rewarmed above 36°C rectal temperature before weaning from CPB. A protamine dose of 4 mg/kg was administered to increase heparin activity. During CPB, conventional ultrafiltration and modified ultrafiltration were administered according to patients’ hematocrit level. Standard appropriate operation procedures for pediatric congenital heart disease were selected.
For each patient, the following patient-related variables were recorded: age at surgery, sex, weight, birth weight, preoperative hemoglobin, preoperative serum creatinine, preoperative albumin, preoperative left ventricular ejection fraction (LVEF) and presence of prior cardiac surgeries.
Surgery-related variables were: Risk Adjustment for Congenital Heart Surgery 1 score (RACHS-1 score), CPB duration, use of lower body circulatory arrest, deep hypothermic circulatory arrest (DHCA) and cell salvage, red cell transfusion and albumin administration during CPB, maximum CPB flow rate, minimum blood pressure, maximum vasoactive-inotropic score (VIS) during surgery, maximum pump pressure, total ultrafiltration volume, sodium bicarbonate dosage and need for repeated CPB.
Postoperative variables included serum ferritin, mechanical ventilation duration, postsurgical length of stay, hospital mortality, POD 1 and peak serum creatinine during first 7 days after surgery, and mechanical circulatory support or dialysis requirements.
2.4 Hyperferritinemia and In-Hospital Composite Outcome
Normal pediatric ferritin values range from 35–135 ng/ml [8]. In this study, hyperferritinemia was defined as serum ferritin level ≥250 ng/ml, severe hyperferritinemia as ≥1000 ng/ml.
Adverse in-hospital outcome was defined as a composite of death, acute kidney injury (greater than or equal to a two-fold postoperative increase in creatinine concentration, or need for renal replacement therapy, corresponding to kidney Disease Improving Global Outcomes stage 2 or 3), requirement of extracorporeal life support, prolonged postoperative LOS ≥14 days (90th percentile), and prolonged MV ≥49 hours (90th percentile).
Categorical variables were summarized as frequencies with percentages, continuous variables with normal distribution as ± standard means and continuous variables without normal distribution as medians with interquartile ranges (IQR) 25–75th percentile. To test for statistical significance, we used the Chisquare test or Fisher’s exact test for categorical variables, and the t-test or Wilcoxon ranked-sum test for continuous variables. Significance was defined by a two-sided p value < 0.05.
We performed a Spearman rank correlation test (r) to assess the unadjusted bivariate relationships between interested variables with postoperative serum ferritin. Multivariable linear regression models were built to assess the association of candidate variables with postoperative serum ferritin levels. Covariates that were associated (p < 0.1) with serum ferritin in the bivariate analysis were included in the model. Modeling was by back ward stepwise selection using p < 0.1 as the criterion for variable retention. Multicollinearity was assessed by the variance inflation factor (VIF) of retained variables (VIF > 2.5 indicated presence of multicollinearity). Modeling assumptions were verified. Influential outliers were identified by the Cook’s distance (cutoff > 0.008; 4/n). Multivariable logistic modeling was used to assess the independent relationship of variables with POD1 serum ferritin ≥250 ng/ml, including the covariates that were related (p < 0.1) to the POD1 serum ferritin in the linear regression models and using backward stepwise selection with p < 0.1 as the criterion for covariate retention. Model discrimination and calibration were assessed by the area under the receiver operating characteristic curve (AUC-ROC) and the Hosmer-Lemeshow test, respectively.
To evaluate if POD1 serum ferritin level predicts adverse composite outcome, we built backward stepwise multivariable logistic regression modeling, adjusting for factors that would normally be evaluable in the prediction of outcome of pediatric cardiac surgery [9,10]: age at surgery, duration of CPB, RACHS-1 score. AUC-ROC was determined to be able to assess the ability of basic clinical model and hyperferritinemia model to predict the adverse composite outcome. The base clinical prediction model included age at surgery, sex, duration of CPB, and RACHS-1 score. The hyperferritinemia enriched model added hyperferritinemia to the basic clinical model.
Previous studies have suggested that at least more than 5 events per variable are required for reliable multivariable logistic regression analysis [11]. Based on the number of patients and events, we expected to have adequate power to minimize bias in model estimates for up to 10 covariates in multivariable logistic model taking hyperferritinemia as a dependent variable, and 14 covariates in model taking adverse composite outcome as a dependent variable. All calculations were done using R (version 3.4.5).
The study is based on POD1 serum ferritin measurements of 457 individuals. The number of patients with postoperative hyperferritinemia (serum ferritin level ≥250 ng/mL) and severed hyperferritinemia (serum ferritin level ≥1,000 ng/mL) were 59/457 (10.9%) and 5/457 (1.1%) respectively. The study population characteristics are reported in Tab. 1.
Table 1: Patient-related and surgery-related variables in relation to post-CPB ferritin
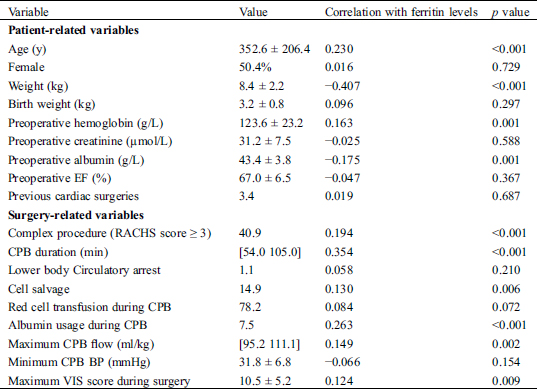
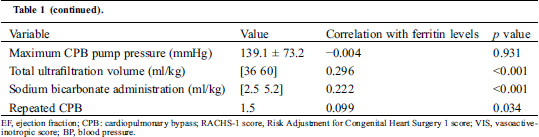
3.2 Risk Factors for Hyperferritinemia
Bivariate relationships between patient-related and surgery-related variables with postoperative ferritin level are shown in Tab. 1. The following risk factors were considered for the postoperative hyperferritinemia prediction model: age, weight, preoperative hemoglobin, preoperative albumin, complex procedure (RACHS score ≥ 3), CPB duration, cell salvage, red cell transfusion during CPB, maximum CPB flow rate, maximum VIS score during surgery, repeated CPB, total ultrafiltration volume, and dose of sodium bicarbonate during CPB. Because of the multicollinearity between age and weight, the latter was not included in the model. Covariates that were independently associated with POD1 ferritin level in the multivariate linear logistic analyses model were age (per day, standard β = −2.846, p = 0.005), baseline hemoglobin concentration (per 1 g/L, standard β = 3.369, p = 0.019 ), CPB duration (per 1 min, standard β = 2.360, p < 0.001), administration of Cell salvage (Yes, standard β = −2.186, p = 0.029 ), total ultrafiltration volume (per 1 ml, standard β = 4.031, p < 0.001) and maximum CPB flow rate (per 1 ml/kg, standard β = 2.769, p = 0.019). Results are shown in Tab. 2. The Model satisfied the assumptions of linear regression and was not affected by influential outliers.
Table 2: Multivariable linear regression analyses for the relationship of patients-related and surgery-related variables with POD1 serum ferritin

Variables included in multivariable logistic regression analysis were age, baseline hemoglobin concentration, CPB duration, maximum CPB flow, Cell salvage and total ultrafiltration volume. Age (odds ratio (OR) 0.776/90 days, p = 0.017), preoperative hemoglobin (OR 1.207/10 g/L, p = 0.013), CPB duration (OR 1.095/10 mins, p = 0.048) and maximum CPB flow rate (OR 1.031/1 ml/kg, p = 0.005) were significantly associated with POD1 Serum ferritin ≥250 ng/ml. The multivariable model for hyperferritinemia was robust, with an AUC-ROC of 0.775 and a Hosmer-Lemeshow goodness-of-fit p value of 0.36. Results are shown in Tab. 3.
Table 3: Multivariable logistic regression analyses for the relationship of patients-related and surgery-related variables with POD1 hyperferritinemia (ferritin ≥250 ng/ml)

3.3 Effect of Hyperferritinemia on Adverse Composite Outcome
29/50 (58%) of patients who developed POD1 hyperferritinemia experienced at least one of the adverse composite outcomes compared to only 13.8% (56/407) without hyperferritinemia (p < 0.0001). Median postoperative LOS and MV were 12.5 days and 27.5 hours, respectively, in hyperferritinemia patients, but only 6.7 days and 7 hours in non-hyperferritinemia patients (p < 0.0001). Results are shown in Tab. 4.
Table 4: Comparison of outcomes between the hyperferritinemia and non-hyperferritinemia population

To identify early predictors of adverse outcome we excluded 16 patients who developed AKI, patients required ECMO or dialysis, or died before POD1. Subsequently, the multivariable analysis for the hyperferritinemia enriched model was performed on 441 patients. The multivariable logistic regression model demonstrated that early postoperative hyperferritinemia (OR, 6.373, 95%CI, 2.863~14.184) predicted an adverse composite outcome, after adjusting for basic clinical predictions (age at surgery, sex, duration of CPB, RACHS-1 score). When serum ferritin was analyzed as continuous variables, it’s also independent risk factor of adverse outcome (OR, 1.003; 95%CI, 1.001~1.005). Results are shown in Tab. 5.
Table 5: Multivariable analyses of POD 1 ferritin for composite outcome prediction (n = 441)

The base clinical model demonstrated good discrimination (AUC, 0.868; 95%CI, 0.821~0.916), with better discrimination in the enriched model containing hyperferritinemia (AUC, 0.840; 95%CI, 0.790~0.890). Results are shown in Tab. 6. The enriched hyperferritinemia model demonstrated improvement on the basic clinical model as measured by the absolute difference in AUC (ΔAUC = 0.0279; p = 0.0218)
Table 6: Area under the receiver operating characteristic curve (AUC) of basic clinical model and enriched model for Composite outcome after cardiac surgery with cardiopulmonary bypass

Elevated serum ferritin as a risk factor with poor outcome has gained a significant amount of attention in recent acute and chronic disease research [12,13]. Very little data are published on the risk factors of elevated serum ferritin, and to our knowledge, this is the first study specifically examining the effect of early postoperative hyperferritinemia as a risk factor of outcome in representative pediatric population who undergo cardiac surgery with CPB.
There are many potential mechanisms by which cardiac surgery with CPB may induce the elevation of ferritin, including exposure of RBCs to non-physiological surfaces resulting in hemolysis [14,15]; shear stress generated by pumps and suction systems; skeletal muscle injury during CPB resulting in the release of iron rich myoglobin into the circulation [16]; ischemia/reperfusion injury to cells and organs [17,18]; and activation of inflammatory response during CPB [4]. This may explain why the common prevalence of hyperferritinemia (10.9%) after CPB in the present study.
In the present study we found that lower age was significantly associated with elevated ferritin, even after adjusting for a large number of demographic factors. Our results are in line with a previous prospective studies, which reported a 25% prevalence of iron overload in infants, 14.3% prevalence in children at 1–5 years, and no children over 5 years of age going into iron overload after CPB [19]. Therefore, we speculate that younger patients are more vulnerable to iron disorder and inflammation causing by CPB. Our findings of a positive correlation between CPB duration (which increases injury by increasing exposure time), hemoglobin concentration (which increases injury by increasing blood viscosity), and maximum CPB flow rate (which increases injury by increasing pump velocity) with postoperative ferritin levels suggest that the raise is at least partially iatrogenic and hence potentially modifiable.
Our finding is not consistent with previous studies that found an association between red cell transfusion and elevated serum ferritin [7,14,20]. These studies, however, were not conducted in the setting of young children undergoing cardiac surgery with CPB where physicians use a small volume RBC priming as a convention intervention (two thirds of the patients in this study were transfused with a single unit of blood), in which transfusion is less likely associated with interoperative hemorrhage and most patients receive blood from one donor. It also should be noted that freshness of red blood cells is given priority to young children undergoing heart surgery in our hospital, which makes our young patients less likely to be exposed to free iron in stored blood [21].
Although not identified in our multivariate regression model for ferritin ≥250 ng/mL, we observed that patients who experienced high POD1 ferritin levels had a higher total ultrafiltration volume and were less likely to receive cell salvage during CPB in multivariate linear model. Red cell injury related to excess ultrafiltration was suggested in a previous study by Okochi et al. [22]. This may be due to the fact that performing diffusion-based solute removal by means of a pressure gradient across the hemoconcentrator membrane is capable of increasing hemolysis of RBCs. The cell-salvage might be reducing the ferritin by removing cytokine load [23] and lipid particles [24], which are associated with postoperative inflammatory response [1,25].
In terms of outcomes associated with hyperferritinemia, we found that those who developed POD1 hyperferritinemia were more likely to remain on mechanical ventilation and stay in the hospital for a longer period of time. POD 1 hyperferritinemia also enabled us to identify patients with a poorer outcome in those undergoing on-pump cardiac surgery. It is an apparent paradox that elevated serum ferritin should be beneficial for minimizing availability of iron to microorganisms and iron chelating mechanism [26], but seems to be an indicator of unfavorable outcomes in variable disease processes [4,27]. The reason for this is likely to be multifactorial. Excess ferritin protein is not of itself toxic in vivo [28], while its level represents an intense inflammatory response and excess released iron [1,3]. Server inflammatory response is reported to be associated with unfavorable postoperative outcome [29,30]. Excess iron participates in Haber-Weiss and Fenton reactions, creating hydroxyl radicals and consequent cell damage and organ injury [31]. Others believe that serum ferritin likely originates from cell leakage and hyperferritinemia and is a direct marker of damaged cells [3,32].
Our study has potential clinical and research implications. Based on our analyses, postoperative measurement of serum ferritin was able to increase the ability to identify a subpopulation of pediatric patients at higher postoperative risk. Understanding the significant and modifiable factors in cardiac surgery associated with hyperferritinemia offers insight into potential risk mitigation approaches. First, a gentler rewarming strategy may be optimal for cardiac surgery, given it is an approach to limit maximum blood flow and reducing red cell injury. Secondly, strategies to limit bypass time may help decrease degree of iron and inflammatory disorder after CPB. Nevertheless, our existing data also suggest that implementing readily available blood management measures, for example administrating cell salvage, moderate hemodilution in severe cyanotic children, and restricting ultrafiltration volume may play beneficial roles for patients undergoing CPB.
There were several limitations to our study. Firstly, due to a lack of standard definition, the present study used a cut-off point of 250 ng/ml for hyperferritinemia according to normal reference of serum ferritin level in pediatrics. Secondly, our data did not consider other sources of iron intake such as formula iron. Finally, because this was a retrospective observational study, the effect of unmeasured confounders cannot be excluded nor can causality be assumed.
This study demonstrates that hyperferritinemia (serum ferritin level ≥250 ng/ml ) is relatively common after cardiac surgery with CPB in pediatrics, and younger age, higher baseline hemoglobin, longer duration of CPB and higher maximum flow are independently associated with elevated ferritin. Development of early postoperative hyperferritinemia provided important prognostic information. Further studies are needed to determine whether attention to certain risk factors can control or prevent this metabolic disturbance and thereby improve prognosis.
Data Sharing: No more data will be shared.
Funding Statement: This study was funded by the National Natural Science Foundation of China (grant number: 81670375; principal investigator: J. L).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kernan, K. F., Carcillo, J. A. (2017). Hyperferritinemia and inflammation. International Immunology, 29(9), 401–409. DOI 10.1093/intimm/dxx031.
2. Cullis, J. O., Fitzsimons, E. J., Griffiths, W. J., Tsochatzis, E., Thomas, D. W. et al. (2018). Investigation and management of a raised serum ferritin. British Journal of Haematology, 181(3), 331–340. DOI 10.1111/bjh.15166.
3. Kell, D. B., Pretorius, E. (2014). Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics: Integrated Biometal Science, 6(4), 748–773. DOI 10.1039/C3MT00347G.
4. Tonial, C. T., Garcia, P. C. R., Schweitzer, L. C., Costa, C. A. D., Bruno, F. et al. (2017). Cardiac dysfunction and ferritin as early markers of severity in pediatric sepsis. Jornal de Pediatria, 93(3), 301–307. DOI 10.1016/j.jped.2016.08.006.
5. Horvat, C. M., Bell, J., Kantawala, S., Au, A. K., Clark, R. et al. (2019). C-Reactive protein and ferritin are associated with organ dysfunction and mortality in hospitalized children. Clinical Pediatrics, 58(7), 752–760. DOI 10.1177/0009922819837352.
6. Papp, A., Bene, Z., Gaspar, I., Nagy, B. J., Kadar, L. et al. (2015). Decreased VEGF level is associated with elevated ferritin concentration in bronchoalveolar lavage fluid of children with interstitial lung diseases. Respiration, 90(6), 443–450. DOI 10.1159/000440888.
7. Leaf, D. E., Rajapurkar, M., Lele, S. S., Mukhopadhyay, B., Rawn, J. D. et al. (2015). Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney International, 87(5), 1046–1054. DOI 10.1038/ki.2014.374.
8. Wiedemann, G., Jonetz-Mentzel, L. (1993). Establishment of reference ranges for ferritin in neonates, infants, children and adolescents. European Journal of Clinical Chemistry and Clinical Biochemistry: Journal of the Forum of European Clinical Chemistry Societies, 31(7), 453–457.
9. Morgan, C. J., Zappitelli, M., Robertson, C. M. T., Alton, G. Y., Sauve, R. S. et al. (2013). Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. Journal of Pediatrics, 162(1), 120–127. DOI 10.1016/j.jpeds.2012.06.054.
10. Brown, K. L., Ridout, D., Pagel, C., Wray, J., Anderson, D. et al. (2019). Incidence and risk factors for important early morbidities associated with pediatric cardiac surgery in a UK population. Journal of Thoracic and Cardiovascular Surgery, 158(4), 1185–1196.e7. DOI 10.1016/j.jtcvs.2019.03.139.
11. Vittinghoff, E., Mcculloch, C. E. (2007). Relaxing the rule of ten events per variable in logistic and Cox regression. American Journal of Epidemiology, 165(6), 710–718. DOI 10.1093/aje/kwk052.
12. Silvestre, O. M., Gonçalves, A., Nadruz, W. J., Claggett, B., Couper, D. et al. (2017). Ferritin levels and risk of heart failure-the Atherosclerosis risk in communities study. European Journal of Heart Failure, 19(3), 340–347. DOI 10.1002/ejhf.701.
13. Yang, G., Hu, R., Zhang, C., Qian, C., Luo, Q. Q. et al. (2016). A combination of serum iron, ferritin and transferrin predicts outcome in patients with intracerebral hemorrhage. Scientific Reports, 6(1), 21970. DOI 10.1038/srep21970.
14. Karkouti, K., Callum, J. L., Acker, J. P., Yip, P., Rao, V. (2017). Red cell transfusion-associated hemolysis in cardiac surgery: an observational cohort study. Anesthesia and Analgesia, 124(6), 1986–1991. DOI 10.1213/ANE.0000000000001807.
15. Vermeulen, W. I., Hanssen, S. J., Buurman, W. A., Jacobs, M. J. (2011). Cardiovascular surgery and organ damage: Time to reconsider the role of hemolysis. Journal of Thoracic and Cardiovascular Surgery, 142(1), 1–11. DOI 10.1016/j.jtcvs.2011.02.012.
16. Benedetto, U., Angeloni, E., Luciani, R., Refice, S., Stefanelli, M. et al. (2010). Acute kidney injury after coronary artery bypass grafting: Does rhabdomyolysis play a role?. Journal of Thoracic and Cardiovascular Surgery, 140(2), 464–470. DOI 10.1016/j.jtcvs.2010.03.028.
17. Skouta, R., Dixon, S. J., Wang, J., Dunn, D. E., Orman, M. et al. (2014). Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. Journal of the American Chemical Society, 136(12), 4551–4556. DOI 10.1021/ja411006a.
18. Christen, S., Finckh, B., Lykkesfeldt, J., Gessler, P., Frese-Schaper, M. et al. (2005). Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radical Biology & Medicine, 38(10), 1323–1332. DOI 10.1016/j.freeradbiomed.2005.01.016.
19. Mumby, S., Chaturvedi, R. R., Brierley, J., Lincoln, C., Petros, A. et al. (2000). Iron overload in paediatrics undergoing cardiopulmonary bypass. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1500(3), 342–348. DOI 10.1016/S0925-4439(00)00003-X.
20. Trevino-Baez, J. D., Briones-Lara, E., Alamillo-Velazquez, J., Martinez-Moreno, M. I. (2017). Multiple red blood cell transfusions and iron overload in very low birthweight infants. Vox Sanguinis, 112(5), 453–458. DOI 10.1111/vox.12528.
21. Shah, A., Brunskill, S. J., Desborough, M. J., Doree, C., Trivella, M. et al. (2018). Transfusion of red blood cells stored for shorter versus longer duration for all conditions. Cochrane Database of Systematic Reviews, 12(12), CD010801.
22. Okochi, S., Cheung, E. W., Barton, S., Zenilman, A., Shakoor, A. et al. (2018). An analysis of risk factors for hemolysis in children on extracorporeal membrane oxygenation. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 19(11), 1059–1066. DOI 10.1097/PCC.0000000000001699.
23. Bauer, A., Hausmann, H., Schaarschmidt, J., Scharpenberg, M., Troitzsch, D. et al. (2018). Shed-blood–separation and cell-saver: An integral Part of MiECC? Shed-blood-separation and its influence on the perioperative inflammatory response during coronary revascularization with minimal invasive extracorporeal circulation systems–a randomized controlled trial. Perfusion, 33(2), 136–147. DOI 10.1177/0267659117728195.
24. Djaiani, G., Fedorko, L., Borger, M. A., Green, R., Carroll, J. et al. (2007). Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation, 116(17), 1888–1895. DOI 10.1161/CIRCULATIONAHA.107.698001.
25. Kell, D. B., Pretorius, E. (2014). Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics, 6(4), 748–773. DOI 10.1039/C3MT00347G.
26. Vercellotti, G. M., Balla, G., Balla, J., Nath, K., Eaton, J. W. et al. (1994). Heme and the vasculature: An oxidative hazard that induces antioxidant defenses in the endothelium. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology, 22(2), 207–213. DOI 10.3109/10731199409117415.
27. Karaboyas, A., Morgenstern, H., Pisoni, R. L., Zee, J., Vanholder, R. et al. (2018). Association between serum ferritin and mortality: Findings from the USA, Japan and European Dialysis Outcomes and Practice Patterns Study. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association–European Renal Association, 33(12), 2234–2244. DOI 10.1093/ndt/gfy190.
28. Wilkinson, J. T., Di, X., Schönig, K., Buss, J. L., Kock, N. D. et al. (2006). Tissue-specific expression of ferritin H regulates cellular iron homoeostasis in vivo. Biochemical Journal, 395(3), 501–507. DOI 10.1042/BJ20060063.
29. Mahle, W. T., Matthews, E., Kanter, K. R., Kogon, B. E., Hamrick, S. E. et al. (2014). Inflammatory response after neonatal cardiac surgery and its relationship to clinical outcomes. Annals of Thoracic Surgery, 97(3), 950–956. DOI 10.1016/j.athoracsur.2013.10.069.
30. Allan, C. K., Newburger, J. W., Mcgrath, E., Elder, J., Psoinos, C. et al. (2010). The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesthesia and Analgesia, 111(5), 1244–1251. DOI 10.1213/ANE.0b013e3181f333aa.
31. Jacob, A. K., Hotchkiss, R. S., Demeester, S. L., Hiramatsu, M., Karl, I. E. et al. (1997). Endothelial cell apoptosis is accelerated by inorganic iron and heat via an oxygen radical dependent mechanism. Surgery, 122(2), 243–253; discussion 254. DOI 10.1016/S0039-6060(97)90015-5.
32. Theil, E. C. (2013). Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorganic Chemistry, 52(21), 12223–12233. DOI 10.1021/ic400484n.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |