

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012097
ARTICLE
Prevalence and Risk Factors Associated with Renal Dysfunction in Patients with Single Ventricle Congenital Heart Disease after Fontan Palliation
1Division of Pediatric Cardiology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, 60611, USA
2Northwestern University Feinberg School of Medicine, Chicago, IL, 60611, USA
3Division of Pediatric Cardiology, Stanford University School of Medicine, Palo Alto, CA, 94304, USA
4Division of Pediatric Cardiology, Nationwide Children’s Hospital, Columbus, OH, 43205, USA
5Institute of Public Health, Charité–Universitaetsmedizin Berlin, Berlin, 10177, Germany
6Division of Kidney Diseases, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, 60611, USA
*Corresponding Author: Sheetal R. Patel. Email: spatel@luriechildrens.org
Received: 17 June 2020; Accepted: 30 July 2020
Abstract: Objectives: The Fontan operation has increased survival in patients with single-ventricle congenital heart defects. However, Fontan survivors are at risk of other organ dysfunctions, such as renal dysfunction (RD). The objectives of this study are to assess the prevalence of and potential risk factors for RD among Fontan survivors. Design, setting, and patients: We performed a two-center, cross-sectional study that included Fontan survivors evaluated in outpatient-clinics for routine follow up between 01/08-12/16. Outcome measures: The primary outcome was the presence of RD defined by estimated glomerular filtration rate (eGFR) of <90 ml/min/1.73 m2 derived using the serum creatinine-based Full Age Spectrum equation. Chi-square and t-tests were used to compare groups with and without RD. A multivariable logistic regression model was derived to identify risk factors associated with the presence of RD using stepwise variable selection methods. Additionally, using eGFR as a continuous variable, a linear regression model was derived to evaluate risk factors that negatively correlate with eGFR. Results: We included 402 Fontan survivors; 61% male; median age 13.7 (2.3–49.9) years; median time since initial Fontan 9.8 (0.1–36.9) years. RD was present in 27.4% (110/402) of patients. Risk factors for RD included single ventricle with right ventricular morphology [odds ratio 2.04; 95% CI (1.26,3.3)], ascites [2.99 (1.04,8.59)] and sildenafil therapy [2.22 (1.05,4.67)]. Risk factors that negatively correlate with eGFR included history of Stage 1 Norwood palliation (−7.6 ml/min/1.73 m2; p = 0.003); “failing Fontan physiology” defined by ascites, protein-losing enteropathy and/or plastic bronchitis (−8.9 ml/min/1.73 m2; p = 0.01) and moderate or greater ventricular dysfunction (−16.7 ml/min/1.73 m2; p = 0.02). Conclusions: One-fourth of Fontan survivors demonstrate RD within ten years after Fontan. Risk factors for RD included right ventricular morphology of the single ventricle, history of Stage 1 Norwood palliation, “failing Fontan physiology,” or ventricular dysfunction. Therefore, comprehensive screening for RD in Fontan survivors is needed, particularly in those identified at a higher risk for RD.
Keywords: Fontan; renal dysfunction; single ventricle; congenital heart disease; risk factors
Single ventricle congenital heart disease (CHD) is a group of complex congenital cardiac defects where only one of the ventricles is adequately developed. Staged surgical procedures culminating in Fontan palliation have allowed survival in many single ventricle patients [1]; however, Fontan survivors are at risk of secondary organ dysfunction, including renal dysfunction (RD) later in life [2–7]. Although Fontan related renal dysfunction is reported to be tolerated well without significant difference in outcomes in one study of young adults with Fontan palliation that were followed for one decade [7], the presence of RD is associated with worse long-term outcomes in Fontan patients. Multiple studies demonstrate the association of RD with adverse events such as unplanned hospital admissions [8], early post-operative mortality after heart transplantation [9,10], and higher rates of overall mortality [8,11,12]. A systematic review of 28 studies found an association between RD and late mortality after Fontan. While 85/1000 deaths (12%) were attributed to RD, a distinction between RD as part of a multiorgan system failure or intrinsic RD was not made [11]. Despite the association of RD with higher mortality and morbidity, the studies reporting the prevalence of RD in Fontan survivors are limited, with discrepant prevalence rates ranging from 10% to 55% [2–5,13]. The prevalence of RD reported by these smaller or single-center cohort studies has not been confirmed in larger multi-center Fontan cohorts. Most of these previous studies evaluated the presence of RD by using different eGFR equations for children and adults. However, as these patients transition to adult care, change in the eGFR equation for children vs. adults is shown to result in a relatively large increase in their calculated eGFR [14]. Recently, the Full Age Spectrum equation (FAS) has been developed to derive an estimated glomerular filtration rate (eGFR) using serum creatinine (Scr) and adjusting for age and height in children, adolescents, young adults, and older adults [15]. This FAS equation has been validated externally [16,17], and is particularly useful in patients transitioning from pediatric to adult care. It prevents artificial eGFR deviation from the change of an eGFR equation for children to one for adults. Until now, the FAS equation has not been utilized to evaluate renal function in the Fontan population. There is a poor understanding of the risk factors associated with RD in Fontan survivors. Risk factors for RD in adults with any form of CHD include older age, cyanosis, and certain medications [6]. However, the risk factors for RD may differ between single ventricle Fontan patients and two-ventricle CHD patients, due to the unique hemodynamic arrangement in the Fontan circulation that includes the absence of a pumping sub-pulmonary chamber, higher central venous pressure (CVP) [18] and reduced renal perfusion pressure [19]. Two prior studies have reported an association of increased CVP [5] and a longer time since Fontan palliation [3] with RD in Fontan survivors. Apart from these two small cohort studies that included 67 and 152 patients, respectively, the risk factors for RD in Fontan survivors remain unclear. While it is clear that RD in Fontan survivors impacts outcomes, data regarding its prevalence and associated risk factors remain very limited. A more complete understanding and assessment of RD prevalence and associated risk factors may allow for the development of interventions to improve long-term outcomes in single-ventricle patients requiring the Fontan palliation. This study aimed to evaluate the prevalence of and potential risk factors for RD in Fontan survivors. Specifically, we sought to evaluate the association between demographic, cardiac morphologic, surgical, clinical, and hemodynamic factors with RD in Fontan survivors.
We performed a cross-sectional analysis of a two-center cohort approved by the institutional review boards at both the Ann & Robert H. Lurie Children’s Hospital of Chicago and the Lucile Packard Children’s Hospital Stanford with a waiver of informed consent (IRB 2016-342)
We included all single ventricle CHD patients who had undergone Fontan palliation, attending outpatient pediatric cardiology or adult CHD clinic for their routine outpatient follow up, and who had concurrent Scr assessment at one of the two participating tertiary care cardiac centers between January 2008 and December 2016. We excluded patients with known congenital structural renal anomalies. Data pertaining to renal function for patients who had undergone Fontan completion but had a subsequent heart transplant were collected from their last routine outpatient clinic visit before receiving their heart transplant.
Data were collected for each patient during their most recent routine outpatient clinic visit that included a concurrent Scr assessment. Data sources consisted of electronic medical records, surgical, echocardiography and catheterization databases, including clinic notes, laboratory test results, and echocardiogram reports performed within six months of the clinic visit. Hemodynamic data were obtained from the cardiac catheterization reports prior to their Fontan surgery (Pre-Fontan cath) and after their Fontan surgery (Post-Fontan cath), if available.
Independent variables included demographic characteristics, cardiac morphologic diagnoses, surgical factors, anthropometric measurements, laboratory data, clinical symptoms, medications (diuretics, sildenafil, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), echocardiographic findings, and catheterization-based hemodynamics as listed in Tabs. 1–3. Surgical factors included prior Stage 1 Norwood operation, initial Fontan type (extracardiac, lateral tunnel, atrio-pulmonary or other), fenestration at the time of initial Fontan surgery, a history of Fontan conversion surgery, as well as the current Fontan type at the time of the study (extracardiac, lateral tunnel, atrio-pulmonary or other). Clinical symptoms of ascites, protein-losing enteropathy, and plastic bronchitis were recorded if they were indicated either as symptoms or included in the problem list in their electronic medical records. “Failing Fontan physiology” was defined as having ascites, protein-losing enteropathy, and/or plastic bronchitis (any one of the three). Study data were collected and managed using REDCap electronic data capture tools [20] hosted at Northwestern University Feinberg School of Medicine.
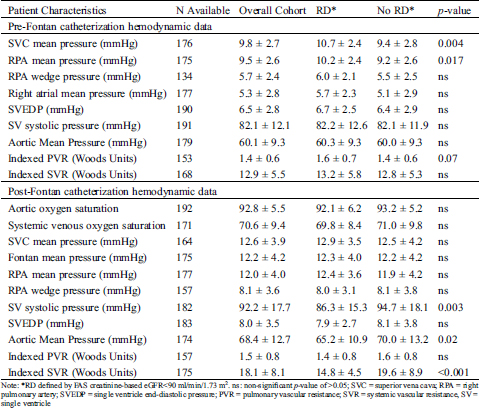
Table 1: Comparison of demographic, cardiac morphologic and surgical factors between Fontan survivors with and without renal dysfunction (RD)
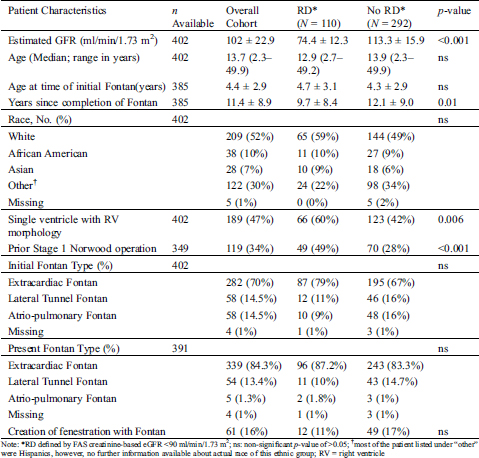
Table 2: Comparison of anthropometric, laboratory, clinical and echocardiographic factors between Fontan survivors with and without renal dysfunction (RD)
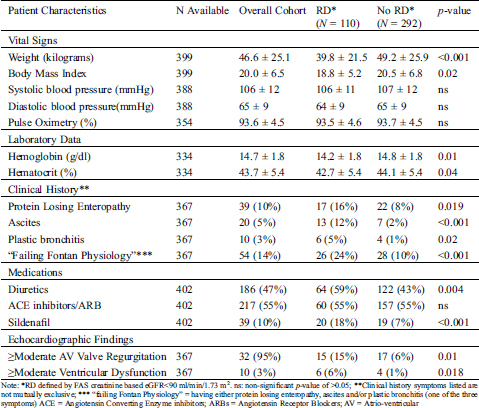
Table 3: Comparison of catheter-based hemodynamic factors between Fontan survivors with and without renal dysfunction (RD)
The primary outcome was the prevalence of RD in Fontan survivors. We defined RD as a reduced eGFR of less than 90 mL/min/1.73 m2. We calculated eGFR for each patient using the Scr-based FAS equation, which adjusts for age and height [15]. The Scr level was measured by Roche/Hitachi Cobas-C enzymatic assay at Lurie Children’s Hospital and by isotope dilution mass spectrometry method using a Siemens RxL Dimension instrument at Lucile Packard Children’s Hospital. Renal function was categorized according to the eGFR cut-off values suggested by the National Kidney Foundation [21]: normal if eGFR ≥90 mL/min/1.73 m2 or having RD if eGFR <90 mL/min/1.73 m2. The RD group was further sub-classified according to severity: mild RD for eGFR between 60 and 89 mL/min/1.73 m2, moderate RD for eGFR between 30 and 59 mL/min/1.73 m2, and severe RD for eGFR <30 mL/min/1.73 m2.
Variables with a discrete distribution were summarized as counts and percentages, while those with a continuous distribution were presented as mean and standard deviation, or median and range. The study cohort was divided based on eGFR into RD or no-RD groups. Univariate analyses were performed for an initial comparison of candidate factors between these two groups using the Chi-square test for categorical variables and the two-sample t-test with unequal variances and Satterthwaite’s approximation for degrees of freedom for continuous variables. Additionally, to evaluate the relationship between eGFR and catheter-based hemodynamic variables, Pearson correlation coefficients were calculated. Statistically significant risk factors identified by these univariate analyses were then used to create two separate multivariable models using all candidate risk factors using a stepwise selection method. A multivariable logistic regression model was created to evaluate risk factors associated with RD. Additionally, a multivariable linear regression model was created to evaluate risk factors that negatively correlate with eGFR using eGFR as a continuous variable. Statistical significance was established at a two-sided 5% alpha level, and there were no adjustments for multiple testing. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC).
A total of 402 Fontan survivors were included, with baseline demographic characteristics shown in (Tab. 1).
The median age of Fontan survivors was 13.7 (2.3–49.9) years, 68% were younger than 18 years, and 61% were male. Nearly half (47%) of the cohort had a single ventricle with right ventricular morphology of their single ventricle (single RV), 70% had an extracardiac-type of Fontan palliation as their initial Fontan, and 84% were non-fenestrated. Out of the 58 patients with initial atrio-pulmonary or similar type of Fontan palliation, 53 had undergone a Fontan conversion surgery to an extracardiac non-fenestrated Fontan. At the time of data collection, 84% had an extracardiac Fontan, 13% had a lateral tunnel Fontan, and only five patients (1.3%) had an atrio-pulmonary Fontan.
3.2 Prevalence of RD in Fontan Survivors
RD was noted in 27.4% (110/402) of our cohort. Most patients with RD had mild RD (94/110; 85.5%), and a small percentage had moderate RD (13/110; 11.8%) or severe RD (3/110; 2.7%).
3.3 Risk Factors for RD in Fontan Survivors
Comparison of demographic, cardiac morphologic, and surgical characteristics between groups with and without RD is shown in Tab. 1. Age at the time of initial Fontan operation and age at the time of the study did not differ significantly by RD status. Median duration since the completion of the initial Fontan was about two years less for RD patients. Fontan survivors with RD were more likely to have a single RV. Of the 349 patients with the availability of a prior surgical description of the initial surgical palliation, 119 had Stage 1 Norwood operation. Patients with RD were more likely to have had a prior Stage 1 Norwood palliation as compared to those without RD. Initial Fontan type, Fontan type at the time of the study, and Fontan fenestration at the time of surgery did not differ between groups. (Tab. 2) describes group comparison for anthropometric, laboratory, clinical, and echocardiographic risk factors between patients with and without RD.
Although groups did not differ significantly in age, patients with RD were shorter, weighed less, and had lower body mass index as compared to those without RD. Blood pressure and pulse oximetry recorded during the clinic visit were not different in patients with or without RD. Although the hemoglobin and hematocrit were slightly lower in the RD group, the difference in the mean values (14.2 g/dl vs. 14.8 g/dl) was not deemed clinically significant. Patients in the RD group were 2.5-times more likely to have symptoms of “failing Fontan physiology” and twice more likely to be on sildenafil therapy as compared to those without RD. Although indications for sildenafil therapy were not consistently recorded in the medical records, sildenafil therapy was commonly used for the patients exhibiting symptoms of “failing Fontan physiology” at both participating institutions. Diuretic therapy was associated with RD, although the use of ACE inhibitors or Angiotensin II Receptor blockers was not. Patients with RD were more likely to have echocardiographic findings of moderate or greater regurgitation of their systemic atrioventricular valve and moderate or greater systolic dysfunction of their single ventricle as compared to those without RD.
A comparison of catheter-based hemodynamic variables between the two groups is shown in Tab. 3.
Pre-Fontan catheterization hemodynamic data were available in approximately half of the patients (191/402). The group with RD had higher pre-Fontan mean pressures in the superior vena cava (SVC) and right pulmonary artery, as well as a trend towards higher pulmonary vascular resistance (PVR) as compared to those without RD. Univariate linear regression analysis further evaluating the relationship between these pre-Fontan catheter-based hemodynamic factors and eGFR is shown in Fig. 1. No violations of the linearity assumption were identified in this analysis. The eGFR is negatively correlated with the mean SVC pressures (correlation r = −0.29; p-value <0.001); mean right atrial pressures (r = −0.19; p-value 0.009); and mean right pulmonary artery pressures (r = −0.24; p-value = 0.001) documented on the pre-Fontan cath.
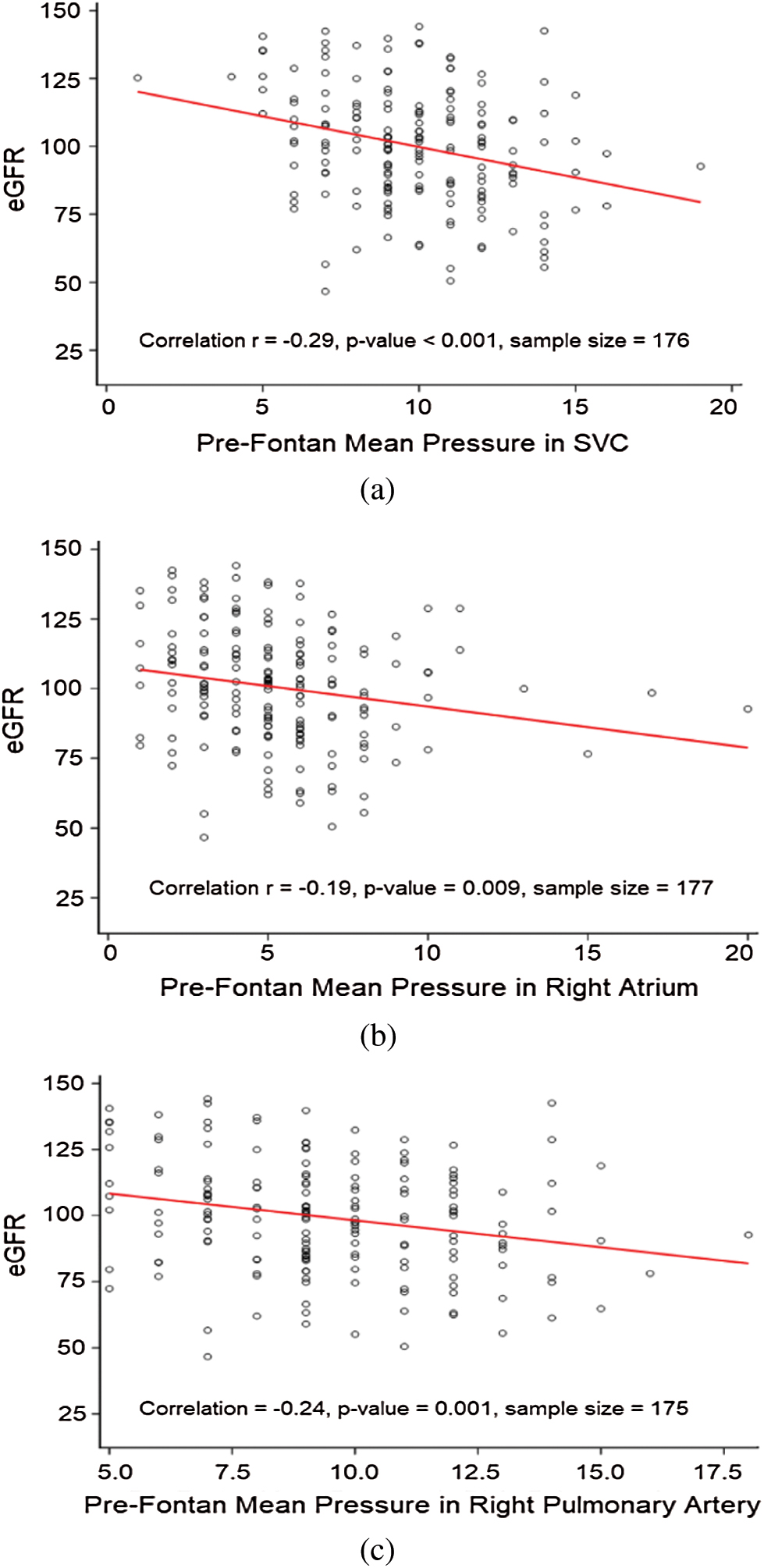
Figure 1: Linear regression analysis evaluating the relationship of eGFR with pre-Fontan catheter-based hemodynamic characteristics
Post-Fontan cardiac catheterization data after Fontan palliation was typically acquired for hemodynamic evaluation in patients with clinical concerns or for hemodynamic assessment before planned surgical or catheter-based procedures at the two participating institutions Post-Fontan catheterization data were available in approximately half of the patients (192/402). Fontan survivors with RD had lower systemic ventricular systolic pressure, lower aortic mean pressure, and lower systemic vascular resistance as compared to those without RD documented on the post-Fontan cath. Univariate linear regression analysis further evaluating the relationship between these post-Fontan catheter-based hemodynamic factors and eGFR is shown in Fig. 2. The eGFR is positively correlated with the systemic ventricular systolic pressure (r = 0.20; p-value = 0.009) and aortic mean pressure (r = 0.15; p-value = 0.038) after the Fontan operation.
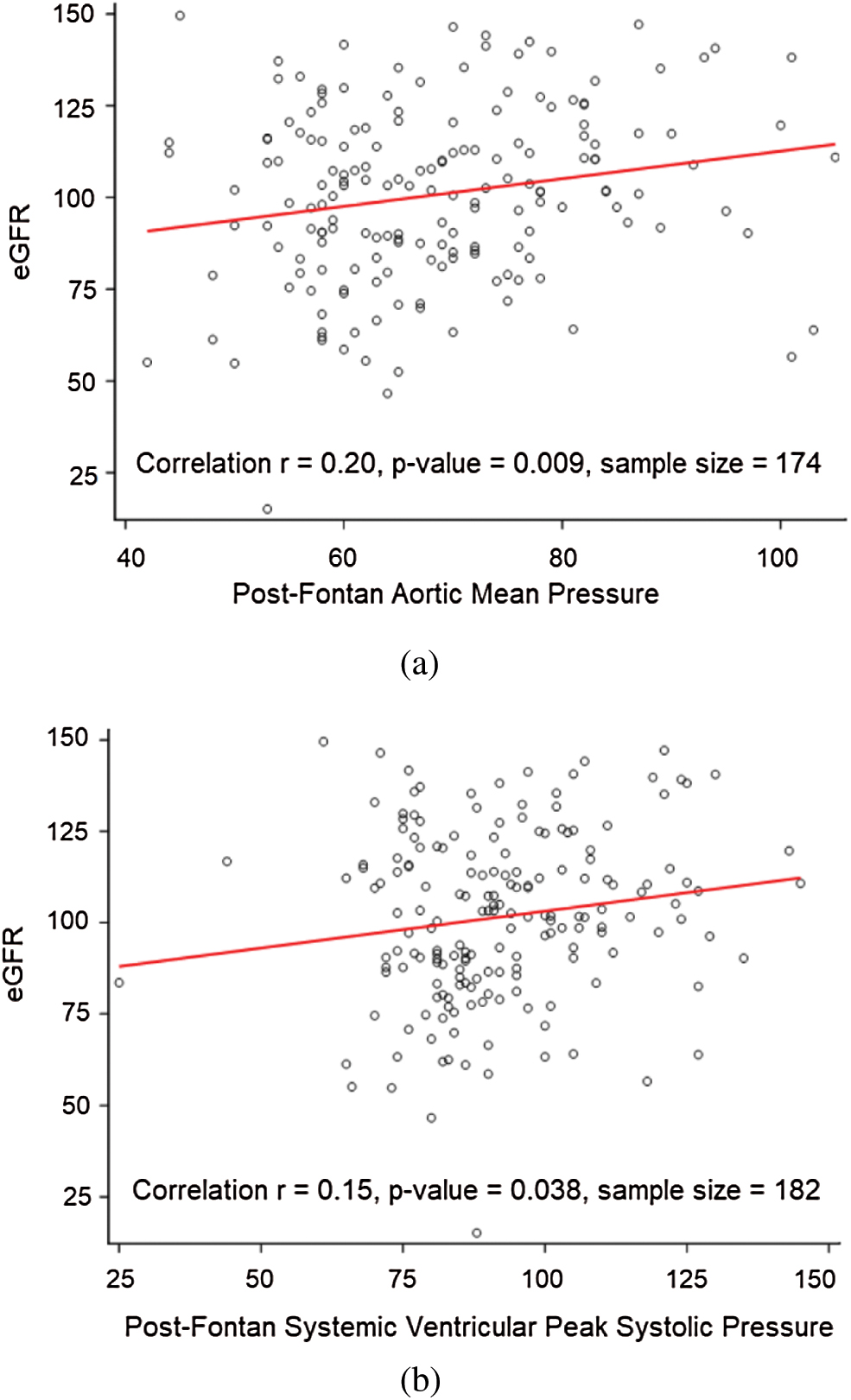
Figure 2: Linear regression analysis evaluating the relationship of eGFR with post-Fontan catheter-based hemodynamic characteristics
Independent risk factors derived by multivariable logistic regression analyses associated with RD are listed in Tab. 4.
Table 4: Summary of the multivariable logistic regression model for the presence of RD
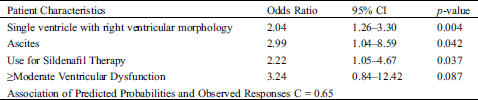
Having a single RV was associated with a more than 2-fold increase in odds of having RD. The presence of ascites was associated with an almost 3-fold increase in odds of having RD. Patients on sildenafil therapy had more than a 2-fold increase in the odds of experiencing RD. Although it did not reach statistical significance, there was a trend for higher risk for RD in patients with moderate or greater ventricular systolic dysfunction.
Independent risk factors that negatively correlate with eGFR are listed in Tab. 5.
Table 5: Summary of multivariable linear regression analysis to identify risk factors that negatively correlate with eGFR (ml/min/1.73 m2)
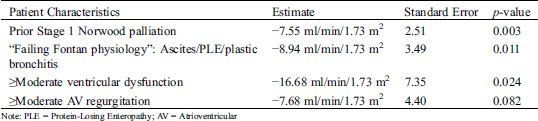
A history of prior Stage 1 Norwood palliation was associated with an average reduction in eGFR by 7 ml/min/1.73 m2 while having symptoms of “failing Fontan physiology” was associated with an almost 9 ml/min/1.73 m2 reduction in eGFR. The most considerable eGFR reduction was predicted in patients with moderate or greater ventricular dysfunction, with eGFR being 16.7 ml/min/1.73 m2 lower than those with no or only mild ventricular dysfunction. There was a trend for lower eGFR in patients with moderate or greater regurgitation of the atrioventricular valve, which did not reach statistical significance.
We present the largest study to date that evaluates the prevalence and risk factors for RD after Fontan palliation showing that with a median follow-up duration of 10 years, at least one out of four Fontan patients develop RD. We have identified several independent factors associated with the presence of RD in Fontan survivors, including the right ventricular morphology of the single ventricle, clinical symptoms of “failing Fontan physiology,” and being on sildenafil therapy. As identified by our study, independent factors that negatively correlate with eGFR included prior Stage 1 Norwood palliation, symptoms of “failing Fontan physiology,” such as either protein-losing enteropathy, ascites, and/or plastic bronchitis; and the presence of moderate or greater ventricular dysfunction.
Our study showed that RD is common in Fontan survivors. In our study, 27% of Fontan survivors had RD defined by reduced Scr-based eGFR at the time of the study. Previous single-center or smaller cohort studies have reported a wide range of RD prevalence ranging from 10% up to 55% based on either estimated or measured GFR, as summarized in Tab. 6.
Table 6: Comparison of our study findings with previous studies reporting the prevalence of renal dysfunction (RD) in Fontan survivors
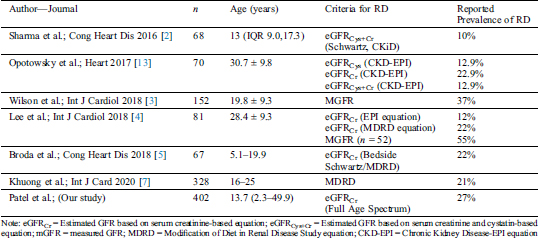
The current study evaluated a larger cohort than seen in previous studies, included children, adolescents, and adult Fontan survivors, and used a Scr-based FAS equation for eGFR. Our study provides additional evidence that RD is common in Fontan survivors, and the prevalence of RD in Fontan survivors is significantly higher as compared to the general population [22]. Our cohort had a higher prevalence of RD in children and adolescents Fontan survivors (29%) as compared to the adult Fontan survivors (23%). We attribute this finding to the fact that this specific subgroup’s survival to adulthood may indicate some selection bias. Previous studies have shown higher mortality in Fontan survivors with RD [6]. As a result, Fontan patients who survived to adulthood without transplantation and qualified to be in our study are likely to have a lower prevalence of RD.
Our study identified several cardiac morphologic, clinical, echocardiographic, and hemodynamic factors associated with RD and is the first to demonstrate that having a single RV is independently associated with the presence of RD in Fontan survivors. Previous studies have reported that having a single RV is a risk factor for Fontan failure defined as death, transplantation, New York Heart Association III/IV symptoms, protein-losing enteropathy, plastic bronchitis, and/or ascites [23–25]. We hypothesize that the association of a single RV with RD is multifactorial. Single RV has a morphologic disadvantage compared to the single LV. For instance, the tripartite shape of the ventricular cavity and trabeculated myocardial architecture predisposes the single RV to have reduced tolerance to the afterload of systemic vascular resistance over time and resulting in reduced cardiac output [26]. Also, patients with single RV need Norwood operation in the neonatal period, which is known to have a more complicated post-operative course and a higher risk for acute kidney injury (AKI), possibly contributing to later development of RD. In addition to the association of single RV with RD, our study showed that a surgical history of Stage 1 Norwood operation is also independently associated with a lower eGFR. Previously, the presence of a single RV or a Stage 1 Norwood operation has been shown to be associated with the development of acute post-operative kidney injury [27] with single RV patients being more likely to need renal replacement therapy following their Fontan completion as compared to those with a single left ventricular morphology [24,27]. Our study shows that having a single RV and history of Stage 1 Norwood operation are associated with reduced renal function years after Fontan completion in the form of RD and lower eGFR. Cardiovascular providers caring for patients with single RV or history of Stage 1 Norwood palliation should monitor for later development of RD or lower eGFR as their patient age into adolescence and adulthood.
Clinical risk factors associated with RD in Fontan survivors include symptoms of ascites, any signs or symptoms of “failing Fontan physiology” or need for sildenafil therapy. One of the possible etiologies for “failing Fontan physiology” is having elevated pulmonary vascular resistance (PVR) and resulting in elevated central venous pressure (CVP) [28–30]. An elevation in CVP causes a diminished effective renal perfusion pressure, as well as reduced lymphatic drainage into the central venous circulation. Sildenafil is commonly used for the management of “failing Fontan physiology” to lower their PVR, reduce CVP, and improve organ perfusion pressure and lymphatic drainage [31–34]. Sub-group analysis to compare the prevalence of RD in patients with “failing Fontan physiology” who are on sildenafil versus those who are not on sildenafil was not performed due to an inadequate sub-group sample size, as well as, variation in cardiac anatomy, surgical strategies, and differences in late complications in our cohort of Fontan survivors. However, future studies should attempt to study the effects of sildenafil in patients with “failing Fontan physiology” relative to the development of RD.
Echocardiographic risk factors for RD in our cohort of Fontan survivors included moderate or greater systolic dysfunction of the single ventricle. As described above, having a single RV is also an independent risk factor for RD. These two factors are possibly interrelated, as previous studies have shown that the single RV has reduced myocardial contractility as compared to the single LV. Therefore, Fontan patients with a single RV are at a higher risk of developing systolic ventricular dysfunction [26]. Although it did not reach statistical significance, there was a trend for lower eGFR in patients with moderate or greater regurgitation of the atrioventricular valve. Previous studies have reported that atrioventricular valve regurgitation in single ventricle CHD is associated with higher mortality [35–37] and higher morbidity such as protein-losing enteropathy, plastic bronchitis, serious thromboembolic event or tachyarrhythmia following the Fontan completion [38]. In addition to these prior studies indicating an association of atrioventricular valve regurgitation with other Fontan comorbidities, our study suggests a possible association of atrioventricular regurgitation with RD in Fontan survivors.
Catheter-based hemodynamic risk factors for RD in Fontan survivors include factors that lead to a higher CVP and a lower aortic pressure. In our cohort, Fontan survivors with RD had a higher SVC mean pressure, a higher pulmonary arterial mean pressure and a trend towards a higher PVR noted during their pre-Fontan catheterization and a lower peak systolic pressure of the single ventricle and a lower aortic mean pressure noted at post-Fontan catheterization as compared to those without RD. By definition, Fontan survivors have an elevated CVP from the moment the Fontan operation is completed. Further elevation in CVP due to the risk factors, as described above, may result in renal vein hypertension and decreased renal perfusion pressure. Prior studies have reported that higher CVP is associated with renal dysfunction in adults with biventricular CHD [39], advanced decompensated heart failure [40], and a broad spectrum of other cardiovascular diseases such as acquired valvular stenosis or regurgitation, coronary artery diseases, heart transplantation or pulmonary hypertension [41]. Prior studies have also reported that having a lower aortic mean pressure correlated with lower creatinine clearance in adults with biventricular congenital heart diseases [39]. Our study shows that similar to the adults with biventricular CHD and acquired heart conditions, higher CVP, and lower aortic pressure are also associated with RD in single ventricle CHD patients with Fontan palliation. Although our study did not directly assess the potential pathophysiology for the development of the RD, we speculate that elevated CVP and lower aortic pressure may result in increased renal venous pressure and reduced renal arterial pressure, respectively, resulting in reduced renal perfusion pressure and predisposing the Fontan survivors to develop RD. Our study did not find statistically significant associations between post-Fontan higher SVC mean pressure or higher RPA wedge pressure and renal dysfunction. Only 192 out of 402 Fontan patients had a post-Fontan cardiac catheterization as post-Fontan cardiac catheterization was typically performed for clinical concerns or for hemodynamic assessment before planned surgical or catheter-based procedures. Given that the post-Fontan cardiac catheterization data likely represents a Fontan population with suboptimal anatomy and/or hemodynamic derangement and is a smaller subset of our entire cohort, statistically significant associations between higher SVC mean pressure or higher RPA wedge pressure and renal dysfunction may be less likely to be found. There are some limitations of our study, which cumulatively would attribute to an underestimation of the true prevalence of RD in this population. We only included patients seen in outpatient clinics who were otherwise considered to be in a stable clinical status, and therefore may have chosen a biased healthier subset of the Fontan population. Older patients included in our study were more likely to have had an initial atrio-pulmonary Fontan and referred for Fontan conversion surgery. As one of the referral criteria for Fontan conversion surgery is having an acceptable renal function to withstand major cardiac surgery, there is also selection bias in this age group. Our cohort had more patients with extracardiac non-fenestrated Fontan as compared to other types due to the primary surgical technique preference at both participating centers. A multi-center study to include different Fontan surgical variations would overcome this limitation in the future. Information related to the post-operative acute kidney injury after prior palliative surgeries was not available for our analyses. Only a subset of Fontan patients had a post-Fontan catheterization performed for clinical indications as described above, and therefore, the post-Fontan cardiac catheterization data may not be representative of the entire Fontan cohort. We recognize that low muscle mass in Fontan survivors may impact the accuracy of any creatinine-based eGFR equation and that RD prevalence assessment would be superior with cystatin C based eGFR equations, the inclusion of information on micro-albuminuria or a measured GFR. However, measurement of cystatin C, micro-albuminuria, or measured GFR is not presently standard clinical practice, and therefore, those results were not available for retrospective analyses of our study. Lastly, we were only able to calculate one eGFR due to the nature of our retrospective cross-sectional study and recognize that a second eGFR calculation in longitudinal study design may have been preferred to identify patients with chronic kidney disease.
At least one out of four Fontan survivors developed RD within ten years in our two-center cohort, which included children, adolescents, and adult Fontan survivors. Our study identified several risk factors for RD, including single RV, history of a Stage 1 Norwood operation, ascites, the sildenafil therapy, any signs or symptoms of “failing Fontan physiology,” and factors that lead to elevated CVP or reduced aortic pressure.
Despite the high prevalence and known risk factors for RD in Fontan survivors, there are no guidelines for screening and ongoing evaluation of renal function in this high-risk population. Understanding the etiologies and risk factors for RD in single ventricle CHD patients will help derive strategies for primary and secondary prevention of renal complications such as chronic kidney disease and improve overall outcomes and long-term prognosis.
Author contributions: Sheetal R. Patel: Concept/Design, Data collection, Data interpretation, Drafting original draft of the article, Critical revision of the article. David M. Kwiatkowski: Data collection, Data interpretation, review, and edit of the article. Adin-Cristian Andrei: Formal statistical analysis; Methodology; Validation; Visualization; Writing-review & editing. Ankita Devareddy: Data collection. Hangzhi Shi: Formal statistical analysis, review of the article. Catherine D. Krawczeski: Concept and design, data interpretation, critical revision of the article. Natalie Ebert: Concept and design, data interpretation, critical revision of the article. Barbara J. Deal: Concept and design, data interpretation, critical revision of the article. Craig B. Langman: Concept and design, data interpretation, critical revision of the article. Bradley S. Marino: Concept and design, data interpretation, critical revision of the article.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Fontan, F., Baudet, E. (1971). Surgical repair of tricuspid atresia. Thorax, 26(3), 240–248. DOI 10.1136/thx.26.3.240.
2. Sharma, S., Ruebner, R. L., Furth, S. L., Dodds, K. M., Rychik, J. et al. (2016). Assessment of kidney function in survivors following Fontan palliation. Congenital Heart Disease, 11(6), 630–636. DOI 10.1111/chd.12358.
3. Wilson, T. G., d’Udekem, Y., Winlaw, D. S., Cordina, R. L., Celermajer, D. S. et al. (2018). Hepatic and renal end-organ damage in the Fontan circulation: a report from the Australian and New Zealand Fontan Registry. International Journal of Cardiology, 273, 100–107. DOI 10.1016/j.ijcard.2018.07.118.
4. Lee, D., Levin, A., Kiess, M., Sexsmith, G., Chakrabarti, S. et al. (2018). Chronic kidney damage in the adult Fontan population. International Journal of Cardiology, 257, 62–66. DOI 10.1016/j.ijcard.2017.11.118.
5. Broda, C. R., Sriraman, H., Wadhwa, D., Wang, Y., Tunuguntla, H. et al. (2018). Renal dysfunction is associated with higher central venous pressures in patients with Fontan circulation. Congenital Heart Disease, 13(4), 602–607. DOI 10.1111/chd.12617.
6. Dimopoulos, K., Diller, G. P., Koltsida, E., Pijuan-Domenech, A., Papadopoulou, S. A. et al. (2008). Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation, 117(18), 2320–2328. DOI 10.1161/CIRCULATIONAHA.107.734921.
7. Khuong, J. N., Wilson, T. G., Grigg, L. E., Bullock, A., Celermajer, D. et al. (2020). Fontan-associated nephropathy: predictors and outcomes. International Journal of Cardiology, 306, 73–77. DOI 10.1016/j.ijcard.2020.01.014.
8. Ohuchi, H., Yasuda, K., Miyazaki, A., Iwasa, T., Sakaguchi, H. et al. (2014). Comparison of prognostic variables in children and adults with Fontan circulation. International Journal of Cardiology, 173(2), 277–283. DOI 10.1016/j.ijcard.2014.03.001.
9. Davies, R. R., Sorabella, R. A., Yang, J., Mosca, R. S., Chen, J. M. et al. (2012). Outcomes after transplantation for “failed” Fontan: a single-institution experience. Journal of Thoracic & Cardiovascular Surgery, 143(5), 1183–1192 e1184. DOI 10.1016/j.jtcvs.2011.12.039.
10. Backer, C. L., Russell, H. M., Pahl, E., Monge, M. C., Gambetta, K. et al. (2013). Heart transplantation for the failing Fontan. Annals of Thoracic Surgery, 96(4), 1413–1419. DOI 10.1016/j.athoracsur.2013.05.087.
11. Pundi, K. N., Johnson, J. N., Dearani, J. A., Pundi, K. N., Li, Z. et al. (2015). 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. Journal of the American College of Cardiology, 66(15), 1700–1710. DOI 10.1016/j.jacc.2015.07.065.
12. Alsaied, T., Bokma, J. P., Engel, M. E., Kuijpers, J. M., Hanke, S. P. et al. (2017). Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart, 103(2), 104–110. DOI 10.1136/heartjnl-2016-310108.
13. Opotowsky, A. R., Baraona, F. R., Mc Causland, F. R., Loukas, B., Landzberg, E. et al. (2017). Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart, 103(6), 434–442. DOI 10.1136/heartjnl-2016-309729.
14. Pottel, H., Bjork, J., Bokenkamp, A., Berg, U., Asling-Monemi, K. et al. (2019). Estimating glomerular filtration rate at the transition from pediatric to adult care. Kidney International, 95(5), 1234–1243. DOI 10.1016/j.kint.2018.12.020.
15. Pottel, H., Hoste, L., Dubourg, L., Ebert, N., Schaeffner, E. et al. (2016). An estimated glomerular filtration rate equation for the full age spectrum. Nephrology Dialysis Transplantation, 31(5), 798–806. DOI 10.1093/ndt/gfv454.
16. Bjork, J., Nyman, U., Berg, U., Delanaye, P., Dubourg, L. et al. (2019). Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatric Nephrology, 34(6), 1087–1098. DOI 10.1007/s00467-018-4185-y.
17. Pottel, H., Dubourg, L., Goffin, K., Delanaye, P. (2018). Alternatives for the bedside Schwartz equation to estimate glomerular filtration rate in children. Advances in Chronic Kidney Disease, 25(1), 57–66. DOI 10.1053/j.ackd.2017.10.002.
18. Hsia, T. Y., Khambadkone, S., Deanfield, J. E., Taylor, J. F., Migliavacca, F. et al. (2001). Subdiaphragmatic venous hemodynamics in the Fontan circulation. Journal of Thorac and Cardiovascular Surgery, 121(3), 436–447. DOI 10.1067/mtc.2001.112527.
19. Patterson, T., Hehir, D. A., Buelow, M., Simpson, P. M., Mitchell, M. E. et al. (2017). Hemodynamic profile of acute kidney injury following the Fontan procedure: impact of renal perfusion pressure. World Journal for Pediatric and Congenital Heart Surgery, 8(3), 367–375. DOI 10.1177/2150135117701376.
20. Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N. et al. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. DOI 10.1016/j.jbi.2008.08.010.
21. Levey, A. S., Coresh, J., Balk, E., Kausz, A. T., Levin, A. et al. (2003). National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of Internal Medicine, 139(2), 137–147. DOI 10.7326/0003-4819-139-2-200307150-00013.
22. Levy, A. R., Perkins, R. M., Johnston, K. M., Sullivan, S. D., Sood, V. C. et al. (2014). An epidemiologic model to project the impact of changes in glomerular filtration rate on quality of life and survival among persons with chronic kidney disease. International Journal of Nephrology and Renovascular Disease, 7, 271–280. DOI 10.2147/IJNRD.S58074.
23. Schumacher, K. R., Stringer, K. A., Donohue, J. E., Yu, S., Shaver, A. et al. (2015). Fontan-associated protein-losing enteropathy and plastic bronchitis. Journal of Pediatrics, 166(4), 970–977. DOI 10.1016/j.jpeds.2014.12.068.
24. Nordmeyer, S., Rohder, M., Nordmeyer, J., Miera, O., Peters, B. et al. (2017). Systemic right ventricular morphology in the early postoperative course after extracardiac Fontan operation: is there still a need for special care?. European Journal of Cardiothoracic Surgery, 51(3), 483–489. DOI 10.1093/ejcts/ezw374.
25. d’Udekem, Y., Iyengar, A. J., Galati, J. C., Forsdick, V., Weintraub, R. G. et al. (2014). Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation, 130(11_Suppl_1), S32–S38. DOI 10.1161/CIRCULATIONAHA.113.007764.
26. Kato, A., Riesenkampff, E., Yim, D., Yoo, S. J., Seed, M. et al. (2017). Pediatric Fontan patients are at risk for myocardial fibrotic remodeling and dysfunction. International Journal of Cardiology, 240, 172–177. DOI 10.1016/j.ijcard.2017.04.073.
27. Wong, J. H., Selewski, D. T., Yu, S., Leopold, A. E., Roberts, K. H. et al. (2016). Severe acute kidney injury following Stage 1 Norwood palliation: effect on outcomes and risk of severe acute kidney injury at subsequent surgical stages. Pediatric Critical Care Medicine, 17(7), 615–623. DOI 10.1097/PCC.0000000000000734.
28. Yu, J. J., Yun, T. J., Yun, S. C., Leopold, K. E., Roberts, K. H. et al. (2013). Low pulmonary vascular compliance predisposes post-Fontan patients to protein-losing enteropathy. International Journal of Cardiology, 165(3), 454–457. DOI 10.1016/j.ijcard.2011.08.848.
29. McMahon, C. J., Nihill, M. R., Reber, A. (2001). The bronchial cast syndrome after the fontan procedure: further evidence of its etiology. Cardiology in the Young, 11(3), 345–351. DOI 10.1017/S1047951101000385.
30. Ohuchi, H., Yasuda, K., Miyazaki, A., Kitano, M., Sakaguchi, H. et al. (2013). Haemodynamic characteristics before and after the onset of protein losing enteropathy in patients after the Fontan operation. European Journal of Cardio-Thoracic Surgery, 43(3), e49–e57. DOI 10.1093/ejcts/ezs714.
31. Reinhardt, Z., Uzun, O., Bhole, V., Ofoe, V., Wilson, D. et al. (2010). Sildenafil in the management of the failing Fontan circulation. Cardiology in the Young, 20(5), 522–525. DOI 10.1017/S1047951110000648.
32. Morchi, G. S., Ivy, D. D., Duster, M. C., Claussen, L., Chan, K. C. et al. (2009). Sildenafil increases systemic saturation and reduces pulmonary artery pressure in patients with failing Fontan physiology. Congenital Heart Disease, 4(2), 107–111. DOI 10.1111/j.1747-0803.2008.00237.x.
33. Rychik, J. (1998). Management of protein-losing enteropathy after the Fontan procedure. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 1(7), 15–22. DOI 10.1038/ncpcardio1220.
34. Rychik, J., Goldberg, D., Rand, E., Semeao, E., Russo, P. et al. (2013). End-organ consequences of the Fontan operation: liver fibrosis, protein-losing enteropathy and plastic bronchitis. Cardiology in the Young, 23(6), 831–840. DOI 10.1017/S1047951113001650.
35. Naito, Y., Hiramatsu, T., Kurosawa, H., Agematsu, K., Sasoh, M. et al. (2013). Long-term results of modified Fontan operation for single-ventricle patients associated with atrioventricular valve regurgitation. Annals of Thoracic Surgery, 96(1), 211–218. DOI 10.1016/j.athoracsur.2013.02.029.
36. Podzolkov, V. P., Chiaureli, M. R., Yurlov, I. A., Zelenikin, M. M., Kovalev, D. V. et al. (2015). Results of Fontan operation in patients with atrioventricular valve regurgitation. European Journal of Cardio-Thoracic Surgery, 48(2), 308–315. DOI 10.1093/ejcts/ezu489.
37. Imai, Y., Takanashi, Y., Hoshino, S., Terada, M., Aoki, M. et al. (1997). Modified Fontan procedure in ninety-nine cases of atrioventricular valve regurgitation. Journal of Thoracic and Cardiovascular Surgery, 113(2), 262–269. DOI 10.1016/S0022-5223(97)70322-2.
38. Allen, K. Y., Downing, T. E., Glatz, A. C., Rogers, L. S., Ravishankar, C. et al. (2017). Effect of Fontan-associated morbidities on survival with intact Fontan circulation. American Journal of Cardiology, 119(11), 1866–1871. DOI 10.1016/j.amjcard.2017.03.004.
39. Damman, K., Voors, A. A., Hillege, H. L., Navis, G., Lechat, P. et al. (2010). Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. European Journal of Heart Failure, 9(9), 974–982. DOI 10.1093/eurjhf/hfq118.
40. Mullens, W., Abrahams, Z., Francis, G. S., Sokos, G., Taylor, D. O. et al. (2009). Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. Journal of the American College of Cardiology, 53(7), 589–596. DOI 10.1016/j.jacc.2008.05.068.
41. Damman, K., van Deursen, V. M.,Navis, G., Voors, A. A., van Veldhuisen, D. J. et al. (2009). Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. Journal of the American College of Cardiology, 53(7), 582–588. DOI 10.1016/j.jacc.2008.08.080.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |