

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.013057
ARTICLE
Diagnostic Errors in Fetal Echocardiography and the Effect on Neonatal Management: Ten-Year Experience from a Middle-Income Country
Pediatric Department, Hospital Sultanah Aminah, Ministry of Health, Jalan Persiaran Abu Bakar Sultan, Johor Bahru, 80100, Malaysia
*Corresponding Author: Mohd Nizam Mat Bah. Email: nurnizam95@gmail.com
Received: 24 July 2020; Accepted: 12 August 2020
Introduction: Fetal echocardiogram allows early detection of critical congenital heart disease leading to a better outcome. However, data from lower- and middle-income countries is scarce. This study aims to evaluate the diagnostic error of fetal echocardiography and its impact on planned neonatal management. Methods and material: This retrospective observational cohort study includes all high-risk pregnant mothers who had fetal echocardiograms from 2008 to 2017. Fetal and postnatal echocardiograms were compared, while the diagnostic errors were categorized into false positive, false negative, and discrepant diagnoses. The impact of the diagnostic error on planned neonatal management and the long-term outcome was determined by comparing the outcome of expected and actual management. Results: A total of 2622 fetuses were included with the majority in the second half of the study. The mean gestational age of 2622 fetuses was 26.7 ± 3.42 weeks. Of 2622, 191 (7.3%) had congenital heart disease. Of 191, 130 (68%) were major lesions. Confirmatory postnatal echocardiogram was available in 153 fetuses with congenital heart disease and 905 fetuses with a normal heart. Of 1058, 123 were true positives, 30 were false positives, 26 were false negatives, and 879 were true negatives. Hence, the sensitivity, specificity, positive and negative likelihood ratio of fetal echocardiogram for detection of CHD in this study was 82.5% (95% confidence interval [CI]: 75.5% to 88.3%), 96.7% (95% CI: 95.3% to 97.8%), 25.0 (95% CI: 17.5 to 35.8) and 0.18 (95% CI: 0.13 to 0.26) respectively. Most of the false positives and negatives were mild lesions. There were 27% discrepant diagnosis between fetal and postnatal echocardiograms (8.1% partially different, and 18.7% no similarity), leading to 8.9% changes in planned neonatal management and 8.1% severity score. Most of the discrepant diagnoses were complex lesions. Conclusions: Despite relatively new services and limited resources, the diagnostic errors of fetal echocardiography in this study are comparable with centers in high-income countries. However, active participation of all stakeholders, changes in policy, and training are needed to improve fetal echocardiography services further. These are vital before the introduction of the national screening program.
Keywords: Fetal echocardiogram; congenital heart disease; diagnostic error
Congenital heart disease (CHD) is the most common malformation in infants with a worldwide prevalence of 7–10 per 1000 live births [1]. One in four has critical CHD and requires early intervention or surgery within the first year of life [2]. Delayed diagnosis of critical CHD, which occurs in 20–30% of cases [3], causes significant infant morbidity and mortality [4,5]. Hence, early detection is crucial to improve the survival of infants with critical CHD.
Several strategies were introduced to improve the detection of critical CHD. Among them is prenatal screening with a fetal echocardiogram, leading to an increase in the prevalence of prenatally detected critical CHD worldwide [6]. As a non-invasive test, fetal echocardiogram is safe, highly sensitive, and specific in screening CHD among high-risk pregnant mothers [7–9]. Fetal echocardiogram allows prenatal diagnosis of critical CHD, leading to reduced mortality and morbidity [10,11].
Although fetal echocardiogram is well established, the data on the accuracy and reliability of the test tend to be limited [12]. Furthermore, most studies differ in definition, methodology, and patient characteristics leading to different findings [13–16]. The definitive diagnosis is crucial to allow appropriate planning before and after the delivery of the fetus [17–20]. An accurate diagnosis is essential in both complex and critical CHD associated with poor prognoses. However, recent studies from well-established centers in high-income countries (HIC) showed a high rate of discrepant diagnoses between fetal and postnatal cardiac diagnoses leading to a significant change in neonatal management and long-term outcome [12,13].
Due to a lack of resources and expertise, fetal echocardiography services may not be readily available in lower-and middle-income countries (LMIC). As the service is relatively new in our center, we postulate that the discrepant diagnosis is high compared to the centers from HIC. Therefore, this study aims to investigate the diagnostic errors (false positive, false negative, and discrepant diagnosis) of fetal echocardiography and the impact on neonatal management and long-term outcome. This information is essential for future planning of fetal and neonatal services in limited resources countries.
This is a retrospective cohort study that included all consecutive fetuses who had a 2D-echocardiogram from January 2008 to December 2017 in the Pediatric Cardiology Unit, Hospital Sultanah Aminah Johor Bahru, Malaysia. This study was registered with the National Malaysian Research Registry (NMRR-18-1447-42225) and was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia. Medical Research and Ethics Committee waived consent from patients.
Hospital Sultanah Aminah is a tertiary government hospital for the State of Johor, which has a population of 3.5 million and an annual birth rate of 55,000 per year. It also serves as a fetomaternal and cardiac center for the state of Johor. Fetal echocardiography services by the Pediatric Cardiology unit were established in 2007, managed by a single pediatric cardiologist throughout the study period. Due to limited trained obstetricians in fetal echocardiography, a pediatric cardiologist was involved in both screenings and confirmatory CHD among high-risk pregnant mothers. There was no national CHD screening program at 20 weeks of gestation during the study period. The fetal cardiac diagnosis used in this study was based on the confirmatory echocardiography done by the pediatric cardiologist.
Data were retrieved from the Fetal Echocardiography Clinical Information System, a clinical database dedicated to fetal echocardiography. The data collected included gestational age, main indication, fetal cardiac diagnosis, associated non-cardiac anomaly, and fetal outcome. Poor fetal echocardiogram images were excluded from the study. Fetal echocardiography was performed by a pediatric cardiologist using iE33 (Philips Ultrasound, Bothell, WA, USA) in 2008 to 2013 and EPIQ 7 (Philips Ultrasound, Bothell, WA, USA) from 2014 onward, with a 5-1 Mhz transducer. A combination of basic cardiac echocardiography examination, outflow tract view, three and two-vessel views, aortic arch, and ductal view with color and pulse doppler were used to detect CHD [21,22]. All images were digitally archived for review and for comparison with the postnatal echocardiogram.
The indications for fetal echocardiogram were divided into three main groups: family history, maternal and fetal factors [22]. Fetal echocardiogram findings were further divided into two groups: non-CHD and CHD. The non-CHD group includes fetuses with a normal heart or with no significant lesions such as an isolated right arch. A fetus with restricted foramen ovale or ductus arteriosus, causing significant fetal hemodynamic changes (dilated chambers and cardiomegaly), was also classified as a non-CHD. Abnormal findings such as pleural or pericardial effusion, cardiac arrhythmia, changes associated with twin to twin transfusion, or fetal cardiomegaly with normal intracardiac structure also put the fetuses into the non-CHD group.
In this study, both fetal and postnatal CHD were classified according to the Fetal Cardiovascular Severity Scale (FCSS) developed by Davey et al. [23]. Briefly, FCSS grades the severity of CHD into seven, with level one as no significant CHD and level seven as the most severe form of CHD. All lesions with a low risk for single ventricle repair were grouped into level 5, while those at high-risk were grouped into level 6. Additionally, all complex biventricular and univentricular hearts with poor prognosis were grouped into level 7. All lesions with level four and above were considered as major CHD. The presence of a non-cardiac anomaly and reduced cardiac function were also included in the FCSS. This inclusion is important since these factors have a significant impact on the long-term management of CHD in LMIC [24].
2.2 Diagnostic Error Categorization
A false positive, false negative, and discrepant diagnosis were used to categorize diagnostic errors [25]. The Pediatric Cardiology Clinical Information System (PCCIS) was reviewed to obtain the postnatal cardiac diagnosis. PCCIS is a clinical registry for congenital and acquired heart disease for the State of Johor and has been described in detail in our previous publication [26]. Briefly, PCCIS contains all personal, 2d-echocardiography, intervention, and outcome data of children with CHD in the State of Johor. Data was entered at the time of diagnosis, regularly updated, and maintained. The specificity, sensitivity, positive and negative likelihood ratios of fetal echocardiography for detecting CHD were subsequently calculated.
The discrepant diagnosis between fetal and postnatal echocardiograms was based on the differences in anatomical diagnosis, which can be either similar, partially different, or no similarity. A similar diagnosis was defined if the fetal and postnatal anatomical diagnoses were the same. Meanwhile, a partially different diagnosis was made if the fetal and postnatal diagnoses were in a similar group but different in the detailed anatomy. Examples of diagnoses in this category were cases of prenatally diagnosed double outlet right ventricle (DORV) without outflow tract obstruction with a postnatal diagnosis of DORV with outflow tract obstruction. “No similarity” was defined if the fetal and postnatal cardiac diagnosis was different. An example in this category was a case of prenatally diagnosed complete atrioventricular septal defect with a postnatal diagnosis of heterotaxia syndrome. In cases with partial and no similarity, all the images (fetal and postnatal echocardiogram) were reviewed by the same pediatric cardiologist to verify the findings.
2.3 Discrepant Diagnosis and Impact on the Early and Long-Term Neonatal Management
The change in initial neonatal management and the long-term outcome was used to investigate the effects of discrepant diagnosis between fetal and postnatal echocardiograms. A significant change in early neonatal management was defined if a discrepant diagnosis led to a change in planned neonatal medical care. An example includes a fetal diagnosis of Ebstein anomaly (no intravenous prostaglandin required) with a postnatal diagnosis of pulmonary atresia with an intact septum (prostaglandin required and percutaneous balloon valvuloplasty with or without PDA stenting to be done during the early neonatal period).
The impact of a discrepant diagnosis on planned long-term outcome was determined by comparing the outcome of expected and actual management using the FCSS. A negative impact was defined if the severity scale in postnatal cardiac diagnosis was higher than the fetal score and positive impact if the postnatal score was lower than the fetal score.
Statistical data were analyzed using Statistical Package for the Social Sciences version 23 (IBM Corp., Armonk, NY, USA). Group comparisons were made using Pearson chi-square or Fisher’s exact test when any expected cell count was < 5 for categorical data. A p-value < 0.05 represented a statistically significant result.
A total of 2628 pregnant mothers (10 triplets and 69 twin pregnancies) were referred for fetal echocardiogram during the study period. From the 2717 fetuses, 95 (3.5%) were excluded due to poor 2d-echocardiogram images. Of 2622, the majority (92.9%) had a fetal echocardiogram in the second half of the study (Fig. 1), and 191 (7.3%) had CHD.

Figure 1: The trend of referral of fetal echocardiography and congenital heart disease (CHD) findings, 2008–2017
The mean gestational age of 2622 fetuses was 26.7 ± 3.42 weeks. The main indications for fetal echocardiogram were maternal factors followed by family history and fetal factors (Tab. 1).
Table 1: The indications for fetal echocardiography and congenital heart disease detection rate
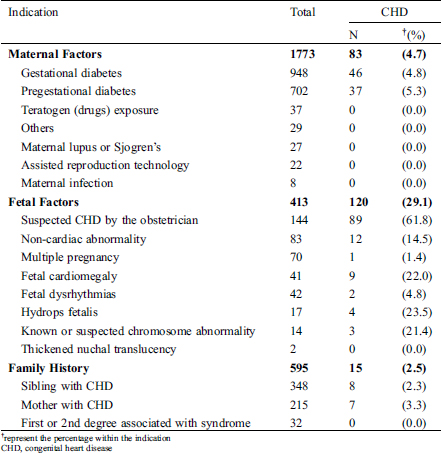
The highest rate of CHD detection was in fetuses who were referred for suspected CHD by the obstetrician (62%), followed by fetuses with features of hydrops fetalis (24%), and fetuses with cardiomegaly (22%). The prevalence of CHD among mothers with pregestational diabetes mellitus was 5.3% and 4.8% for mothers with gestational diabetes mellitus. From the 191 fetal CHD, 55 (28.8%) had complex CHD associated with poor prognosis (Tab. 2). Forty-two percent of fetal CHD were ductal dependent lesions, and 19% were associated with an extracardiac malformation.
Table 2: Fetal Cardiovascular Severity Scale and cardiac diagnosis in 191 fetuses with congenital heart disease detected by fetal echocardiogram
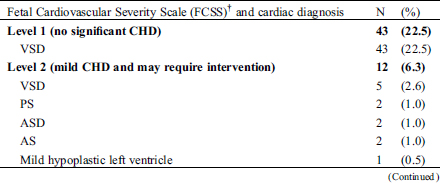
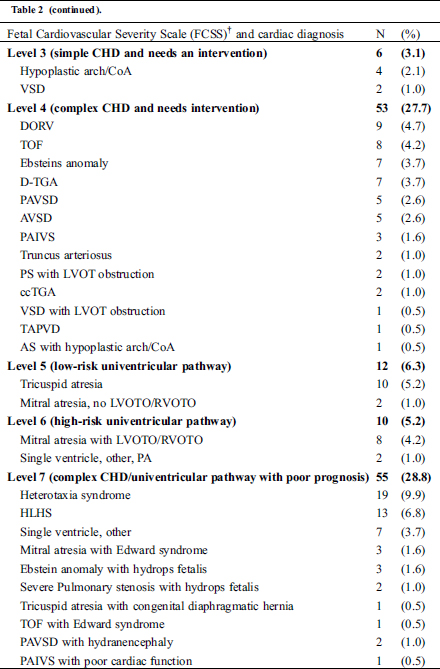

3.1 False Positive and Negative
From the 2622 fetal echocardiograms, 1058 had postnatal echocardiograms available for comparison (Fig. 2). Of 191 fetal CHD, 38 had no confirmatory postnatal echocardiogram due to loss to follow-up and death, either in-utero or after delivery. Of the 153 fetuses with CHD, 30 did not have CHD on postnatal echocardiogram (false positive). Meanwhile, of 905 fetuses with a normal heart on fetal echocardiogram, 26 had CHD (false negative). Hence, the sensitivity, specificity, positive and negative likelihood ratio of fetal echocardiogram for detection of CHD in this study was 82.5% (95% confidence interval [CI]: 75.5% to 88.3%), 96.7% (95% CI: 95.3% to 97.8%), 25.0 (95% CI: 17.5 to 35.8) and 0.18 (95% CI: 0.13 to 0.26) respectively.

Figure 2: The immediate outcome of fetal echocardiography. FE, fetal echocardiogram; CHD, congenital heart disease; HLHS, hypoplastic left heart syndrome; TA, tricuspid atresia, AVSD, atrioventricular septal defect; AVSD, atrioventricular septal defect; VSD, ventricular septal defect; TAPVD, total anomalous venous drainage; TOF, tetralogy of Fallot
There were no critical lesions in the false negative group. Many of the false negatives were small ventricular septal defects, which closed spontaneously during early infancy (Tab. 3).
Table 3: The findings of the false positive (A) and false negative (B) of fetal echocardiogram
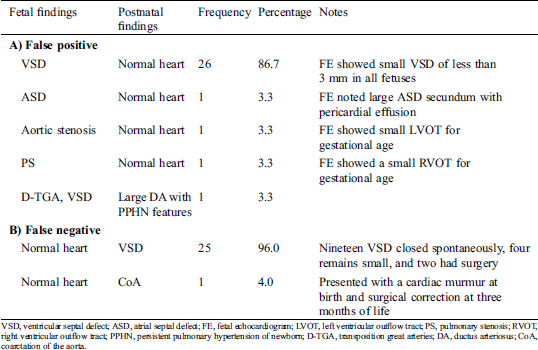
3.2 Discrepant Diagnosis Between Fetal and Postnatal Echocardiograms and Their Impact on the Early Neonatal Management and Long-Term Outcome
There were 26.8% discrepant diagnoses (8.1% partially different, and 18.7% no similarity) between fetal and postnatal echocardiogram (Tab. 4).
Table 4: Level of agreement between fetal and postnatal cardiac diagnosis
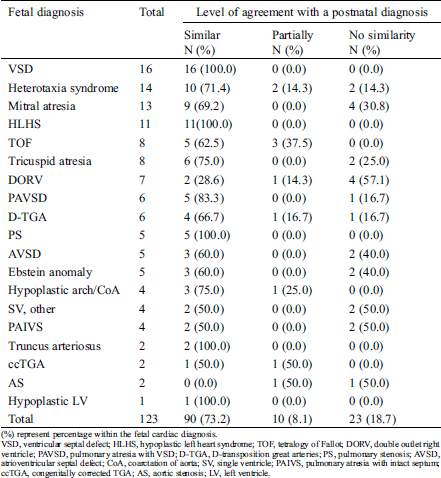
Most of the discrepant diagnoses involved a fetal diagnosis of complex univentricular heart (n = 12, 36.4%). Meanwhile, fetal diagnosis of DORV was associated with the highest rate (71.4%) of discrepant diagnoses.
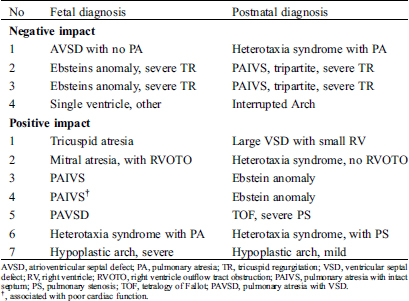
The discrepant diagnosis between fetal and postnatal echocardiogram causes significant changes in neonatal management in 8.9% (Tab. 5). Of 23 cases with no similarity, only nine had significant changes in immediate postnatal management. Meanwhile, of the ten with a partially different diagnosis, only two cases had changes in neonatal management.
Table 5: The fetal and postnatal cardiac diagnosis in infants with significant changes in planned neonatal management
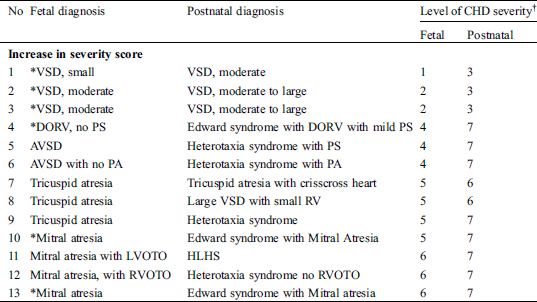
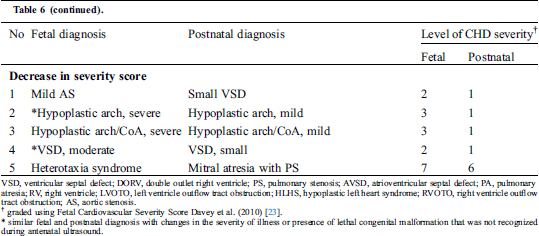
There were 18 (14.6%) significant changes in fetal severity score (Tab. 6). Of the 18, 10 had a discrepant diagnosis between fetal and postnatal echocardiograms, and eight had a similar diagnosis. Overall, discrepant diagnosis led to an 8.1% change in the fetal severity score.
Table 6: The fetal and postnatal cardiac diagnosis with significant changes in Fetal Cardiovascular Severity Score
4.1 Specificity and Sensitivity
In this study, a combination of basic cardiac echocardiography examination, outflow tract view, and three-vessel view were used to detect CHD in high-risk pregnant mothers. The sensitivity, specificity, positive, and negative likelihood ratios of fetal echocardiogram in this study were 82.5%, 96.7%, 25.0, and 0.18, respectively. The result is slightly low compared to that reported in a systematic review and meta-analysis by Zhang et al. [27] in 2015, which showed a pooled sensitivity with basic cardiac echocardiography examination, outflow tract view, and three-vessel view of 83.7%. However, it is encouraging that none of the false negatives and false positives were related to critical CHD. The sensitivity was slightly low due to the inclusion of small ventricular septal defects, which may spontaneously close either during pregnancy or in early infancy. If a small ventricular septal defect which closed spontaneously (26 from fetal and 19 from postnatal echocardiograms) was considered as a normal heart, the sensitivity, specificity, positive and negative likelihood ratio would increase to 94.6%, 99.6%, 220, and 0.05, respectively.
Although the outflow tract and three-vessel views were used in this study, we still missed one case of coarctation of the aorta and over-diagnosed two outflow tract lesions. Nevertheless, this finding is not surprising since most series faced a similar problem [12,16]. A high error of fetal echocardiogram related to outflow tract lesions is due to the dynamics of fetal heart development, which evolves during pregnancy rather than a missed diagnosis per se.
4.2 Discrepant Diagnosis Between Fetal and Postnatal Cardiac Diagnoses
Evaluation of the accuracy and reliability of fetal echocardiography is challenging due to the complexity of some lesions, change of lesion severity over time as well as various definitions applied in earlier studies. In this study, the actual anatomical diagnosis was used to compare fetal and postnatal cardiac diagnosis and to investigate the impact of the discrepant diagnosis on planned neonatal management and long-term outcome. This study showed a 73% similarity between fetal and postnatal cardiac diagnoses, which is almost similar compared to Bensemlali et al. at 71% [13]. However, using the Wald and Aristotle score, Clur et al. [16] noted a high similarity rate of 81% to 86%. Meanwhile, Velzen et al. [12] recorded that 82% of their fetal cardiac diagnoses were correct. Nonetheless, this suggests that approximately one in five fetuses may have partial- or completely different cardiac diagnoses following delivery.
Despite a comparable rate of similarity with previous studies, unfortunately, the rate of completely different diagnosis is high (20%) compared to Bensemlali et al. [13] (2.9% of liveborn) and Velzen et al. [12] (8.1%). The possible explanation could be due to error in the interpretation of complex single ventricle anatomy, or a part of the disease continuum. Furthermore, differentiation between DORV with pulmonary stenosis and tetralogy of Fallot with a small pulmonary artery in utero is challenging. Meanwhile, some lesions may have similar features, e.g., pulmonary atresia with an intact septum may have abnormal tricuspid valve mimicking Ebstein anomaly, and Ebstein anomaly may be associated with functional pulmonary atresia. Nevertheless, this illustrates the importance of follow-up echocardiogram during antenatal and postnatal periods as well as proper parental counseling.
4.3 Impact on the Planned Neonatal Management and Severity Score
In this study, fetal CHD was graded according to the level of severity using the modified FCSS. This CHD severity classification allowed planning of place of delivery, early neonatal, and long-term management. Fortunately, despite having a significant discrepant diagnosis, less than 10% of the cases caused a considerable impact on planned neonatal management, which is comparable to Bensemlali et al. [13]. However, there was a 10% negative impact on CHD severity following delivery. The percentage is rather high compared to that reported by Bensemlali et al. (4.9%) [13] and attributed to undiagnosed lethal congenital malformations. Hence, all fetuses with suspected CHD should have a detailed scan to exclude non-cardiac malformations or syndromes, which may significantly alter the overall outcome and vice-versa.
4.4 Indication for Fetal Echocardiogram
Most of the indications of fetal echocardiogram in our study were due to pre-existing or gestational diabetes mellitus, which is a well-known risk factor for developing a conotruncal defect in their offspring [28,29]. However, data for gestational diabetes mellitus tend to be limited. Our study shows that the risk of CHD in infants of mothers with gestational diabetes mellitus to be 4.8%, which is almost similar to infants of pre-existing diabetes mellitus mothers of 5.3%. This rate is also similar to a recent publication by Hunter et al. [30]. Hence, a mother with gestational diabetes mellitus is also at risk of having a baby with CHD and should be included in the current guidelines for fetal echocardiography.
Although to our knowledge, this is the first large scale study over a ten-year duration in Malaysia, our study was limited by lack of post-mortem in fetuses who died in utero or immediately after delivery due to legislation and cultural issues. Secondly, a higher rate of lost to follow up was observed in cases with normal fetal heart findings which may be contributed by the change in our protocol from 2015 onward, i.e., a postnatal echocardiogram was no longer offered for all normal fetal echocardiogram findings which could have led to an underestimation of our findings.
Another limitation in this study is the risk of bias in the interpretation of the fetal and postnatal echocardiograms by the same pediatric cardiologist. Unfortunately, this is unavoidable as there is only a single pediatric cardiologist serving the entire state of Johor during the study period who can confirm the diagnosis. To make matters worse, there were limited obstetricians trained in fetal echocardiography. This highlights that fetal echocardiography services in LMIC are still at the infancy stage. Consequently, only 3% of CHD in Johor were detected antenatally [26]. Therefore, training of obstetricians and pediatric cardiologists in fetal echocardiography is vital before the introduction of the National CHD Screening program.
Despite insufficient resources and expertise, the sensitivity, specificity, positive and negative likelihood ratio of fetal echocardiogram in detecting CHD, and the discrepant diagnosis between fetal and postnatal diagnosis in this study are comparable with centers in a high-income country. As fetal echocardiography is not perfect, counseling of parents with prenatally diagnosed CHD should be done carefully.
Active involvement of all stakeholders, significant changes in policy, and training are needed to improve fetal echocardiography services in LMIC.
Acknowledgement: We would like to thank the Director-General of Health Malaysia for his permission to publish this article.
Data Sharing: Data is available upon reasonable request.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Van Der Linde, D., Konings, E. E. M., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/j.jacc.2011.08.025.
2. Mahle, W. T., Newburger, J. W., Matherne, G. P., Smith, F. C., Hoke, T. R. et al. (2009). Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics, 124(2), 823–836. DOI 10.1542/peds.2009-1397.
3. Liberman, R. F., Getz, K. D., Lin, A. E., Higgins, C. A., Sekhavat, S. et al. (2014). Delayed diagnosis of critical congenital heart defects: trends and associated factors. Pediatrics, 134(2), e373–e381. DOI 10.1542/peds.2013-3949.
4. Eckersley, L., Sadler, L., Parry, E., Finucane, K., Gentles, T. L. (2016). Timing of diagnosis affects mortality in critical congenital heart disease. Archieve Disease of Childhood, 101(6), 516–520. DOI 10.1136/archdischild-2014-307691.
5. Fixler, D. E., Xu, P., Nembhard, W. N., Ethen, M. K., Canfield, M. A. (2014). Age at referral and mortality from critical congenital heart disease. Pediatrics, 134(1), e98–e105. DOI 10.1542/peds.2013-2895.
6. Bakker, M. K., Bergman, J. E. H., Krikov, S., Amar, E., Cocchi, G. et al. (2019). Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. British Medical Journal Open, 9(7), e028139. DOI 10.1136/bmjopen-2018-028139.
7. Li, Y., Hua, Y., Fang, J., Wang, C., Qiao, L. et al. (2013). Performance of different scan protocols of fetal echocardiography in the diagnosis of fetal congenital heart disease: a systematic review and meta-analysis. Public Library of Science, 8(6), e65484. DOI 10.1371/journal.pone.0065484.
8. Oggè, G., Gaglioti, P., Maccanti, S., Faggiano, F., Todros, T. et al. (2006). Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound in Obstetrics and Gynecology, 28(6), 779–784. DOI 10.1002/uog.3830.
9. Carvalho, J. S., Mavrides, E., Shinebourne, E. A. E., Cambell, S., Thilaganathan, B. et al. (2002). Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart, 88(4), 387–391. DOI 10.1136/heart.88.4.387.
10. Levey, A., Glickstein, J. S., Kleinman, C. S., Levasseur, S. M., Chen, J. et al. (2010). The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatric Cardiology, 31(5), 587–597. DOI 10.1007/s00246-010-9648-2.
11. Holland, B. J., Myers, J. A., Woods, C. R. (2015). Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound in Obstetrics and Gynecology, 45(6), 631–638. DOI 10.1002/uog.14882.
12. Van Velzen, C. L., Clur, S. A., Rijlaarsdam, M. E. B., Pajkrt, E., Bax, C. J. et al. (2016). Prenatal diagnosis of congenital heart defects: accuracy and discrepancies in a multicenter cohort. Ultrasound in Obstetrics and Gynecology, 47(5), 616–622. DOI 10.1002/uog.15742.
13. Bensemlali, M., Stirnemann, J., Le Bidois, J., Bidois, M., Raimondi, F. et al. (2016). Discordances between prenatal and postnatal diagnoses of congenital heart diseases and impact on care strategies. Journal of the American College of Cardiology, 68(9), 921–930. DOI 10.1016/j.jacc.2016.05.087.
14. Bakiler, A. R., Ozer, E. A., Kanik, A., Kanit, H., Aktas, F. N. (2007). Accuracy of prenatal diagnosis of congenital heart disease with fetal echocardiography. Fetal Diagnosis and Therapy, 22(4), 241–244. DOI 10.1159/000100782.
15. Berkley, E. M., Beth Goens, M., Karr, S., Rappaport, V. (2009). Utility of fetal echocardiography in postnatal management of infants with prenatally diagnosed congenital heart disease. Prenatal Diagnosis, 29(7), 654–658. DOI 10.1002/pd.2260.
16. Clur, S. A., Van Brussel, P. M., Ottenkamp, J., Bilardo, C. M. (2012). Prenatal diagnosis of cardiac defects: accuracy and benefit. Prenatal Diagnosis, 32(5), 450–455. DOI 10.1002/pd.3837.
17. Fuchs, I. B., Müller, H., Abdul-Khaliq, H., Harder, T., Dudenhausen, J. W. et al. (2007). Immediate and long-term outcomes in children with prenatal diagnosis of selected isolated congenital heart defects. Ultrasound in Obstetrics and Gynecology, 29(1), 38–43. DOI 10.1002/uog.3900.
18. Randall, P., Brealey, S., Hahn, S., Khan, K. S., Parsons, J. M. (2005). Accuracy of fetal echocardiography in the routine detection of congenital heart disease among unselected and low risk populations: a systematic review. BJOG: An International Journal of Obstetrics and Gynaecology, 112(1), 24–30. DOI 10.1111/j.1471-0528.2004.00295.x.
19. Peake, L. K., Draper, E. S., Budd, J. L., Field, D. (2015). Outcomes when congenital heart disease is diagnosed antenatally versus postnatally in the UK: a retrospective population-based study. BMC Pediatrics, 15, 58. DOI 10.1186/s12887-015-0370-3.
20. Landis, B. J., Levey, A., Levasseur, S. M., Glickstein, J. S., Kleinman, C. S. et al. (2013). Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatric Cardiology, 34(3), 597–605. DOI 10.1007/s00246-012-0504-4.
21. Carvalho, J., Allan, L., Chaoui, R., Copel, J., DeVore, G. et al. (2013). ISUOG practice guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound in Obstetrics and Gynecology, 41(3), 348–359. DOI 10.1002/uog.12403.
22. Donofrio, M. T., Moon-Grady, A. J., Hornberger, L. K., Copel, J. A., Sklansky, M. S. et al. (2014). Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation, 129(21), 2183–2242. DOI 10.1161/01.cir.0000437597.44550.5d.
23. Davey, B. T., Donofrio, M. T., Moon-Grady, A. J., Fifer, C. G., Cuneo, B. F. et al. (2014). Development and validation of a fetal cardiovascular disease severity scale. Pediatric Cardiology, 35(7), 1174–1180. DOI 10.1007/s00246-014-0911-9.
24. Mat Bah, M. N., Sapian, M. H., Jamil, M. T., Alias, A., Zahari, N. (2018). Survival and associated risk factors for mortality among infants with critical congenital heart disease in a developing country. Pediatric Cardiology, 39(7), 1389–1396. DOI 10.1007/s00246-018-1908-6.
25. Benavidez, O. J., Gauvreau, K., Jenkins, K. J., Geva, T. (2008). Diagnostic errors in pediatric echocardiography: development of taxonomy and identification of risk factors. Circulation, 117(23), 2995–3001. DOI 10.1161/CIRCULATIONAHA.107.758532.
26. Mat Bah, M. N., Sapian, M. H., Jamil, M. T., Abdullah, N., Alias, E. Y. et al. (2018). The birth prevalence, severity, and temporal trends of congenital heart disease in the middle-income country: a population-based study. Congenital Heart Disease, 13(6), 1012–1027. DOI 10.1111/chd.12672.
27. Zhang, Y. F., Zeng, X. L., Zhao, E. F., Lu, H. W. (2015). Diagnostic value of fetal echocardiography for congenital heart disease. Medicine, 94(42), e1759. DOI 10.1097/MD.0000000000001759.
28. Øyen, N., Diaz, L. J., Leirgul, E., Boyd, H. A., Priest, J. et al. (2016). Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation, 133(23), 2243–2253. DOI 10.1161/CIRCULATIONAHA.115.017465.
29. Wren, C., Birrell, G., Hawthorne, G. (2003). Cardiovascular malformations in infants of diabetic mothers. Heart, 89(10), 1217–1220. DOI 10.1136/heart.89.10.1217.
30. Hunter, L. E., Sharland, G. K. (2015). Maternal gestational diabetes and fetal congenital heart disease: an observational study. Journal of Pregnancy and Child Health, 2, 132. DOI 10.4172/2376-127X.1000132.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |