

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011576
ARTICLE
Immunomodulatory miRNAs as Potential Biomarkers for the Postoperative Course Following Surgery for the Repair of Congenital Heart Defects in Children
1Pediatric Critical Care Medicine, Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, 5265601, Israel
2Pediatric Cardiology, Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, 5265601, Israel
3Pediatric Cardiac Intensive Care, Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, 5265601, Israel
4Pediatric Cardiac Surgery, Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, 5265601, Israel
*Corresponding Author: Yael Nevo-Caspi. Email: yael.caspi@sheba.health.gov.il
#Contributed equally to this work
Received: 19 May 2020; Accepted: 13 August 2020
Abstract: Objective: To test the hypothesis that circulating miRNAs-146a, -146b, -155, and -21 reflect the inflammatory state of children following heart surgery, and that they may, therefore, correlate with postoperative parameters. We aimed to quantify miRNAs in blood samples from pediatric patients before and 6, 12, and 24 hours after surgery and to evaluate correlations between the miRNA levels and the postoperative course. Setting: PICU. Patients: Forty-two pediatric patients with CHD who underwent cardiac surgery at Safra Children’s Hospital between 2012–2016. Interventions: none. Outcome Measures: The primary outcomes were the postoperative cardiac complications and the secondary outcomes were the length of hospitalization and more than two days of inotropic support. Results: The increase in miRNA-146a and -146b levels correlated with higher troponin, lower lactate, and lower C-reactive protein levels, as well as fewer days on inotropic support, and shorter hospital stay. Conclusions: The increases in the levels of circulating miRNA-146a and miRNA-146b after surgery for the repair of congenital heart defects are potential biomarkers for a better postoperative course in pediatric patients.
Keywords: Biomarker; miRNA; congenital heart disease; pediatric cardiac surgery; immunomodulatory miRNA
There is an ongoing need for expanding our knowledge about the outcomes of congenital heart surgery in children in order to improve treatment as well as to identify the children who are at risk to sustain postoperative complications [1]. MicroRNAs (miRNAs) are a group of small non-coding RNA molecules (~22 nucleotides long) that regulate gene expression at the posttranscriptional level by targeting 3’ untranslated regions of complementary messenger RNAs and consequently inhibit protein synthesis [2]. MiRNAs, which are often tissue-specific, are also found in serum, where they are packaged in exosomes that protect them from ribonucleases and thereby contribute to their stability [3]. Circulating miRNAs have been recently suggested to play a role in cell communication in both immune and non-immune cells, and to possibly have immunoregulatory roles [4]. Those possibilities taken together with the ease by which miRNAs can be detected and quantified have made miRNAs a source of great interest as potential biomarkers for a wide range of diseases [5]. Validated biomarkers are also essential for the evaluation of the postoperative course and identification of patients at high risk for developing complications. The currently available biomarkers have not fully achieved these goals [6].
Advances in the management of children who underwent surgical repair of congenital heart defects have greatly improved the surgical outcome. However, postoperative myocardial and cerebral injuries are still the most common complications after those surgeries and constitute the major causes for morbidity and mortality. Reliable biomarkers of inflammation can improve patient management following those surgeries and help identify the children who are at greater risk for postoperative complications [7]. We had observed that cardiac miRNA-208a can serve as a biomarker for the postoperative course of children undergoing heart surgery [8]. In the current study, we hypothesized that the circulating miRNAs-146a, -146b, -155, and -21 reflect the inflammatory state of those children, and that they may, therefore, correlate with surgical and postoperative clinical parameters following heart surgery for congenital heart defects (CHDs).
2.1 Patient and Laboratory, Surgical, and Postoperative Parameters
Forty-two pediatric patients with CHD who underwent cardiac surgery at Safra Children’s Hospital between the years 2012–2016 and whose legal representative provided informed consent were enrolled. The inclusion criteria were age younger than 18 years and an echocardiographic diagnosis of CHD that required cardiac surgery. We excluded all patients with postoperative infections and those with autoimmune diseases. Clinical data were collected from the patients’ medical files.
The laboratory parameters used in the study were as follows: maximum troponin and maximum C-reactive protein (CRP) levels during the first 24 hours after surgery, lactate levels at 6, 12, and 24 hours after surgery, and maximum lactate levels during the first 24 hours after surgery. The surgical parameters were the lengths of time on cardiopulmonary bypass (CPB) and aortic crossclamp (ACC). The postoperative parameters were invasive and noninvasive ventilation, duration of ventilation, days of hospitalization, need for reintubation, need for extracorporeal membrane oxygenation (ECMO), maximal inotropic support, and days on inotropic support. The maximal inotropic score was calculated as follows: dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100× epinephrine dose (mcg/kg/min).
The primary outcomes were the postoperative cardiac complications, which were defined as the need for cardiorespiratory resuscitation, pericardial and/or pleural effusion requiring treatment or drainage, and postoperative arrhythmia (junctional ectopic, supraventricular, and ventricular tachycardias). The length of hospitalization and more than two days of inotropic support were the secondary outcomes.
2.2 Sample Preparation, RNA Extraction, and cDNA Preparation and Quantification
All samples were processed within 24 hours of surgery. The blood was centrifuged at 4°C for 10 minutes at 1200 g followed by separation of the serum. Centrifugation was then repeated at 4°C for 10 minutes at 10000 g. RNA was extracted from 250 µl serum to which 5.6 × 108 copies of Cel-miRNA39 were added using TRI-Reagent®-LS (Sigma). RNA was resuspended in 45 µl H2O of which 5 µl were taken for cDNA synthesis (TaqMan® MicroRNA-RT-Kit, ABI) using a specific primer. Relative quantification (RQ) was performed on a StepOne™ polymerase chain reaction (PCR). Reactions (10 µl) were run in triplicates: 5 µl PCR-mix (ABI), 0.5 µl of either of the Taqman assays (ABI): cel-miRNA39 (#200), miRNA-146a (#468), miRNA-146b (#1097), miRNA-155 (#2623), miRNA-21 (#397), 1.5 µl of the cDNA and 3 µl H2O. The cycle threshold (Ct) values were calculated with the StepOne software v2.3. ∆Ct values were calculated by subtracting the Ct of the selected miRNA from the Ct of cel-miRNA39 of the same sample. The ∆Ct values were inversely correlated with the amount of template miRNA present in the reaction. The RQ was analyzed by the ∆∆Ct method. ∆∆Ct was calculated by subtracting the ∆Ct of a specific miRNA in the preoperative sample (0 hour) from the ∆Ct of the same miRNA at a specific postoperative time point.
Categorical variables were described as frequency and percentage. Continuous variables were evaluated for normal distribution using histograms and Q-Q plots. Normally distributed continuous variables were described as mean and standard deviation (SD), and non-normally distributed continuous variables as median and interquartile range (IQR). Changes in the amount of miRNA over time were evaluated using the Friedman test and the Wilcoxon test.
Correlations between continuous variables were evaluated using the Spearman correlation coefficient. Variables that exhibited a significant correlation with a specific miRNA at a specific time point were further analyzed as follows: the patients were divided into 2 groups, one below and the other above the median for the RQ of each miRNA at each time point. The variables were compared between the 2 groups by Student’s t test for normally distributed parameters and the Mann–Whitney test for the non-normally distributed parameters. Continuous variables were compared between patients with and without CPB, and with more or less than two days of inotropic support, using the Mann–Whitney test. Univariate and multivariate logistic regressions were used to evaluate the association between the expression of miRNAs and whether the patient received more than two days of inotropic support. The multivariate models were performed twice. The model included age and weight as confounders. The area under the receiver operating characteristic (ROC) curve was used to evaluate the discrimination ability of the potential biomarkers (miRNAs). Odds ratios (OR) with 95% confidence interval (CI) were reported. All statistical tests were 2-tailed, and p ≤ 0.05 was considered statistically significant. All calculations were performed using SPSS (Ver. 23.0).
The protocol of this retrospective study was approved by the Institutional Review Board of the Chaim Sheba Medical Center. Informed consent was obtained from the legal representatives of all subjects.
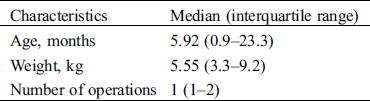
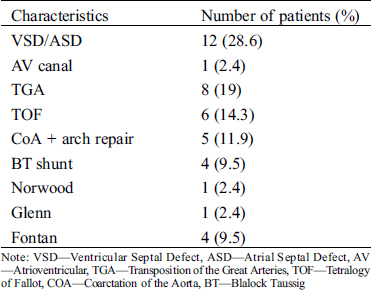
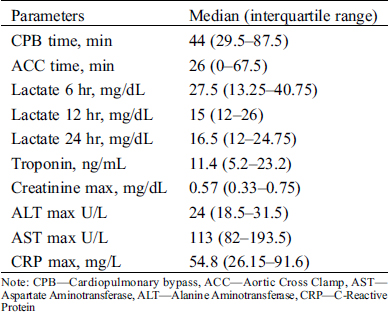
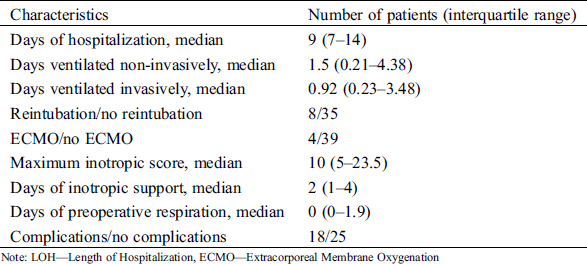
Twenty male (47.6%) and 22 female (52.4%) patients who underwent cardiac surgery due to CHD at Safra Children’s Hospital between the years 2012–2016 were included in this study. Their age at surgery ranged from 3 days to 5.9 years (mean ± standard deviation 1.2 ± 1.48 years). Their weight ranged from 3.3 to 9.2 kg (median 5.55 kg). For 83% of the patients this was their first operation. Nineteen (45.2%) of the patients underwent a non-elective operation. The most frequent malformations were ventricular septal defect (VSD) (n = 12, 28.6%), and transposition of great arteries (TGA) (n = 8, 19%) (Tab. 1). The clinical, laboratory, and postoperative characteristics of these patients are summarized in Tabs. 1, 2, Appendix A and B.
Table 1: Characteristics of the study population
3.1 MiRNA-146a and -146b Expressions Following Surgery for CHD
The miRNA-146a, -146b, -155, and -21 expressions were measured before surgery (0 hours), and at 6, 12, and 24 hours after surgery. The amounts of miRNA-146a and -146b in serum significantly differed over time (p = 0.022 and p = 0.011, respectively). The postoperative amount of miRNA-146a was elevated at 6 hours following surgery and returned to its preoperative level at 24 hours. The expression of miRNA-146b was elevated at 6 hours following surgery and declined at 12 and 24 hours after surgery to levels that were lower than those measured preoperatively. The difference in the amounts of miRNA-155 and -21 was not statistically significant (Fig. 1).
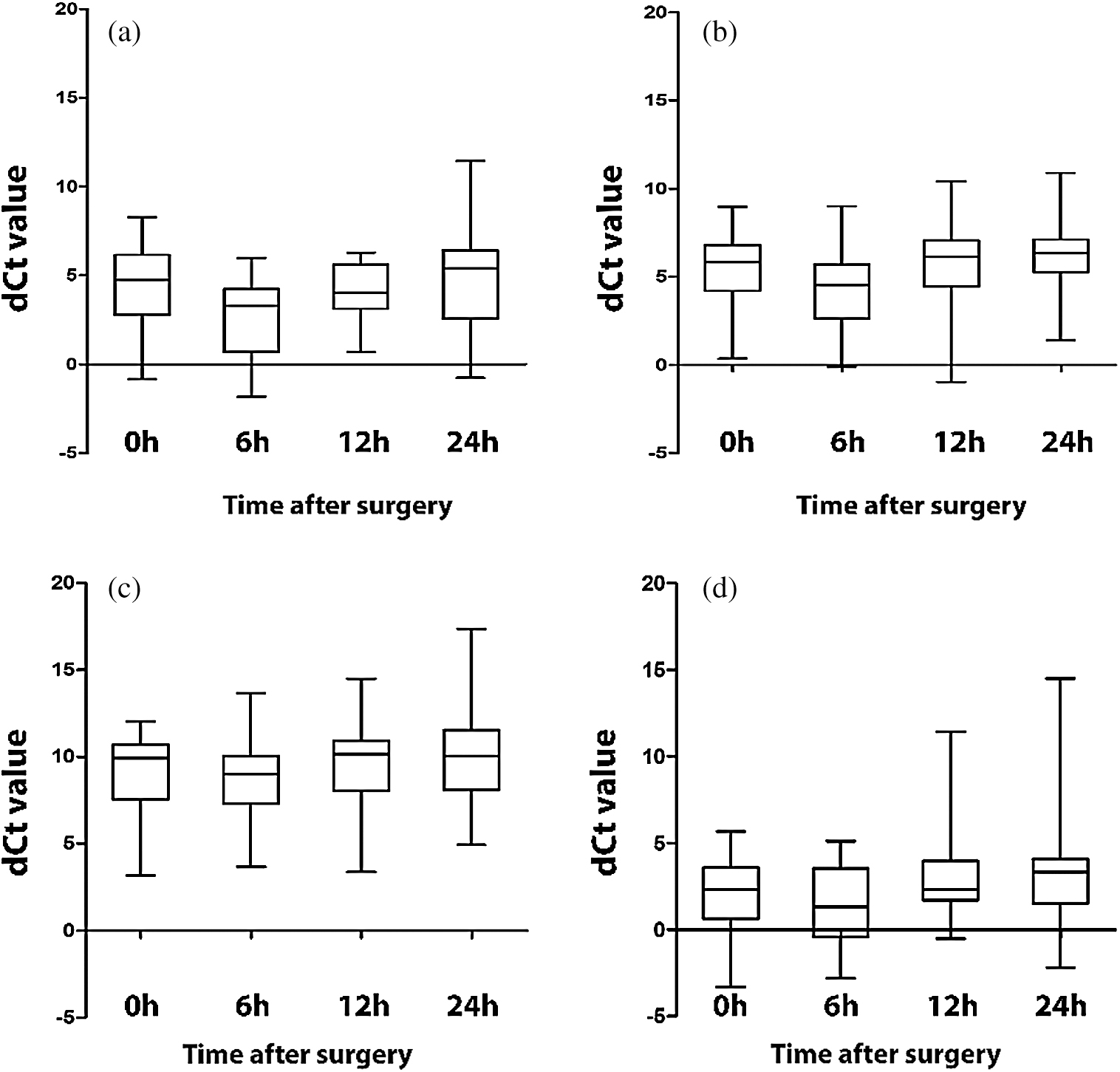
Figure 1: Changes in miRNA expression. a. MiRNA-146a b. MiRNA-146b c. MiRNA-155 d. MiRNA-21. Each box plot shows the 10th, 25th, 50th, 75th, and 90th percentile of all the patients. The amount miRNAs-146a and -146b differed significantly (p < 0.05) over time
3.2 Correlation of miRNA-146a and -146b Expressions with Surgical Parameters
Thirty-four patients were on CPB during surgery. CPB was associated with the increase in the amount of miRNA-146a at 6 hours after surgery (p = 0.01) (Fig. 2). A similar substantial, although not significant result was obtained for miRNA-146b at 6 hours after surgery.
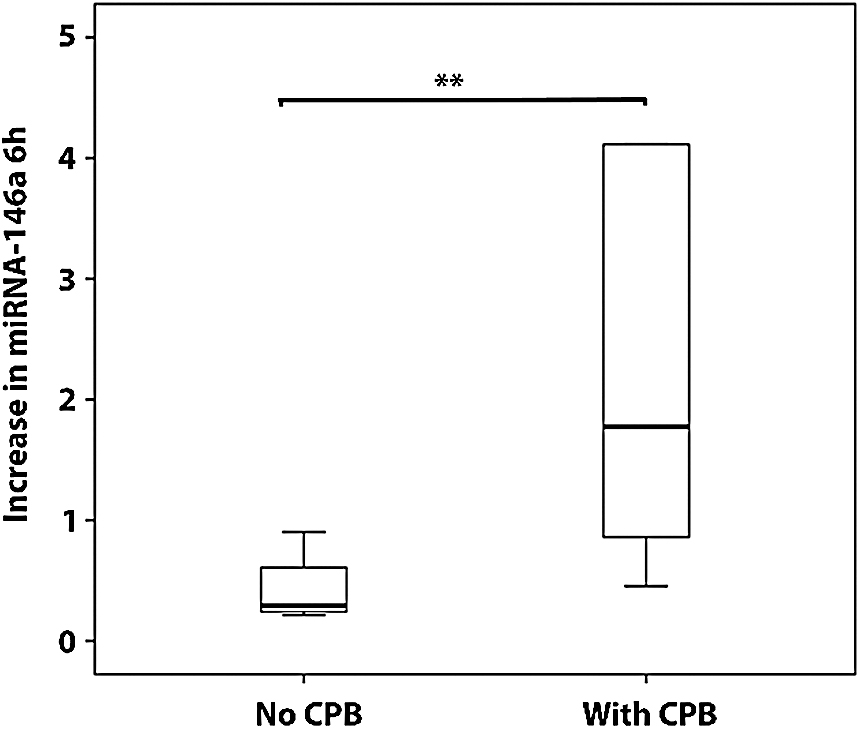
Figure 2: Relative increase in the amount of miRNA-146a 6 hours after surgery. Results are presented as mean ± SD. **p < 0.0
3.3 Correlation of miRNA-146a and -146b Expressions with Laboratory Parameters
There was a significant correlation between a large postoperative increase in miRNA-146a at 6 hours after surgery with higher troponin levels and lower maximal lactate levels (p = 0.034 and p = 0.035 respectively). In addition, there was a larger increase in the levels of miRNA-146a at 24 hours after surgery, which correlated with lower maximal CRP levels (p = 0.005). A large increase in miRNA-146b levels at 6 hours correlated with higher troponin levels (p = 0.01), and that increase at 24 hours correlated with lower maximum CRP levels (p = 0.007).
The correlations that emerged as being significant were studied in greater depth by comparing variables between the group of patients that exhibited a small increase in the expression of a specific miRNA at a specific time point to the values of the same variable in the group of patients that exhibited a large increase in the expression of that miRNA (as described in Methods). These comparisons revealed results similar to those obtained above and reinforced them (Figs. 3a–3e).
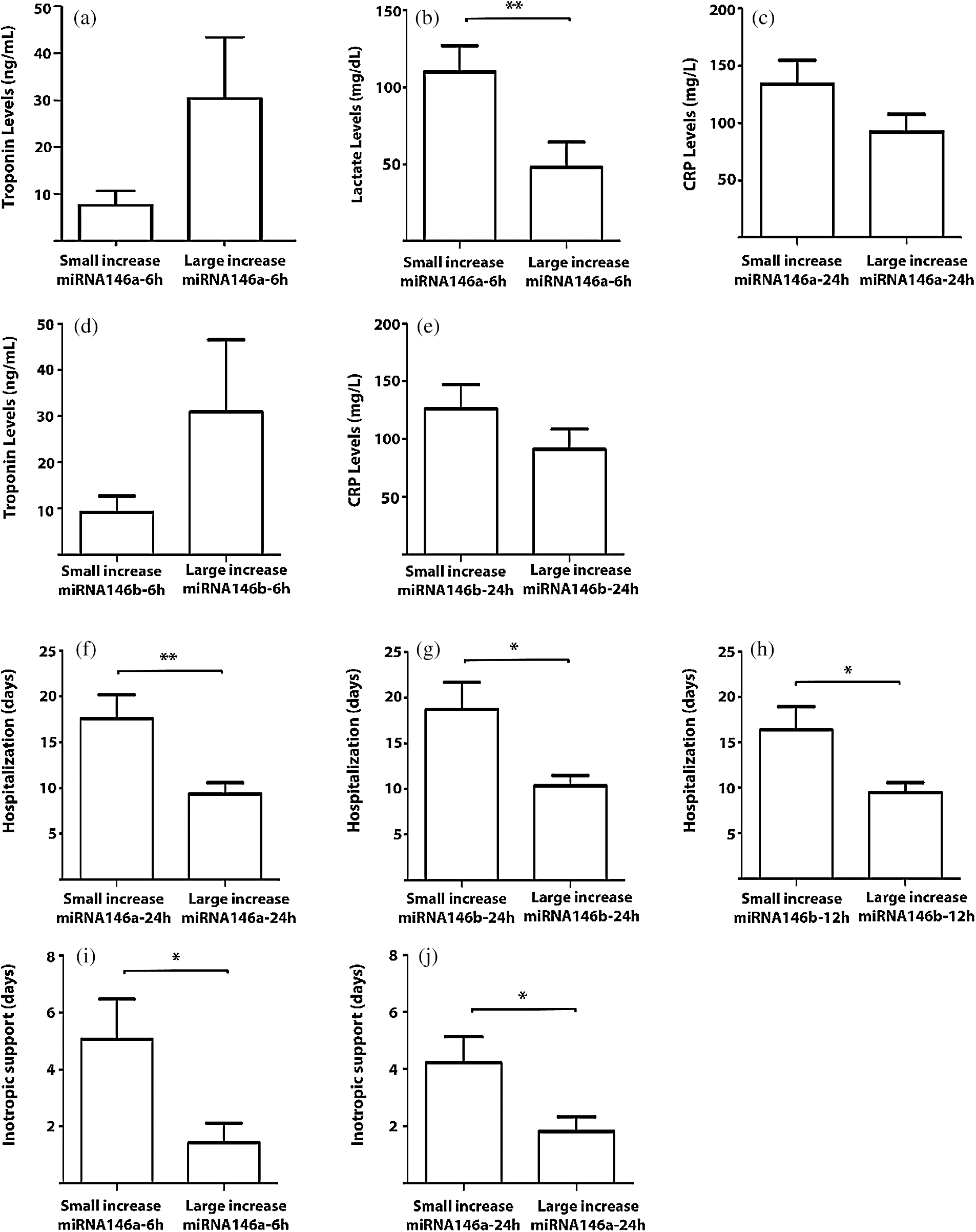
Figure 3: Correlations between the amounts of miRNAs with the postoperative laboratory parameters and the postoperative course. The results are presented as mean ± SEM. * p < 0.05 and ** p < 0.01
3.4 Correlation of miRNA-146a and -146b Expressions with the Postoperative Course
A large postoperative increase in the amounts of miRNA-146a and -146b as measured 24 hours after surgery correlated significantly with shorter lengths of hospitalization (p = 0.021 and p = 0.034, respectively). This correlation for miRNA-146b was also seen as early as 12 hours after surgery (p = 0.029). In addition, the large increases in the amounts of miRNA-146a at 6 and 24 hours following the operation correlated with fewer days on inotropic support (p = 0.017 and p = 0.008, respectively). These results were further confirmed when selected variables were compared between the patients who exhibited a small increase and those who exhibited a large increase in a specific miRNA at a specific time point. The number of days in hospital was greater in patients with a smaller increase in the amounts of miRNA-146a and miRNA-146b at 24 hours (17.6 days vs. 9.3 days and 18.7 days vs. 10.3 days, respectively) and in patients with a smaller increase in the amounts of miRNA-146b at 12 hours (16.3 days vs. 9.4 days), and the number of days on inotropic support was greater for the patients who exhibited a smaller increase in the amount of miRNA-146a at 6 hours (5 days vs. 1.4 days) and at 24 hours (4.23 days vs. 1.82 days) (Figs. 3f–3j). Further analysis showed that lower levels of miRNA-146a at 24 hours were associated with the risk that the patient will receive more than two days of inotropic support (Fig. 4a). Logistic regression analysis showed an association between a smaller increase in miRNA146a levels at 24 h and the need for more than two days of inotropic support for (crude OR 1.60; 95% CI 1.08–2.40; p = 0.02). This association remained significant after adjusting for the child’s age and weight (adjusted OR 1.51; 95% CI 1.00–2.30; p = 0.05). The ability of miRNA-146a at 24 hours to predict the risk of a patient to be in need for more than two days of inotropic support was studied using an ROC curve and the area under the curve (AUC). That analysis reached a level of significance, with an AUC of 71.5% (95% CI 48.7–94.3%; p = 0.04) (Fig. 4b). A RQ value of 0.7 can serve as a cutoff value for the prediction that a patient will receive more than two days of inotropic support. Specifically, RQ < 0.7 predicted that the child will receive more than two days of inotropic support whereas RQ > 0.7 predicted that he/she will not. The sensitivity of this value was 69% and its specificity was 88%. We did not find a correlation between the amounts of the miRNAs that we studied to the appearance of complications.
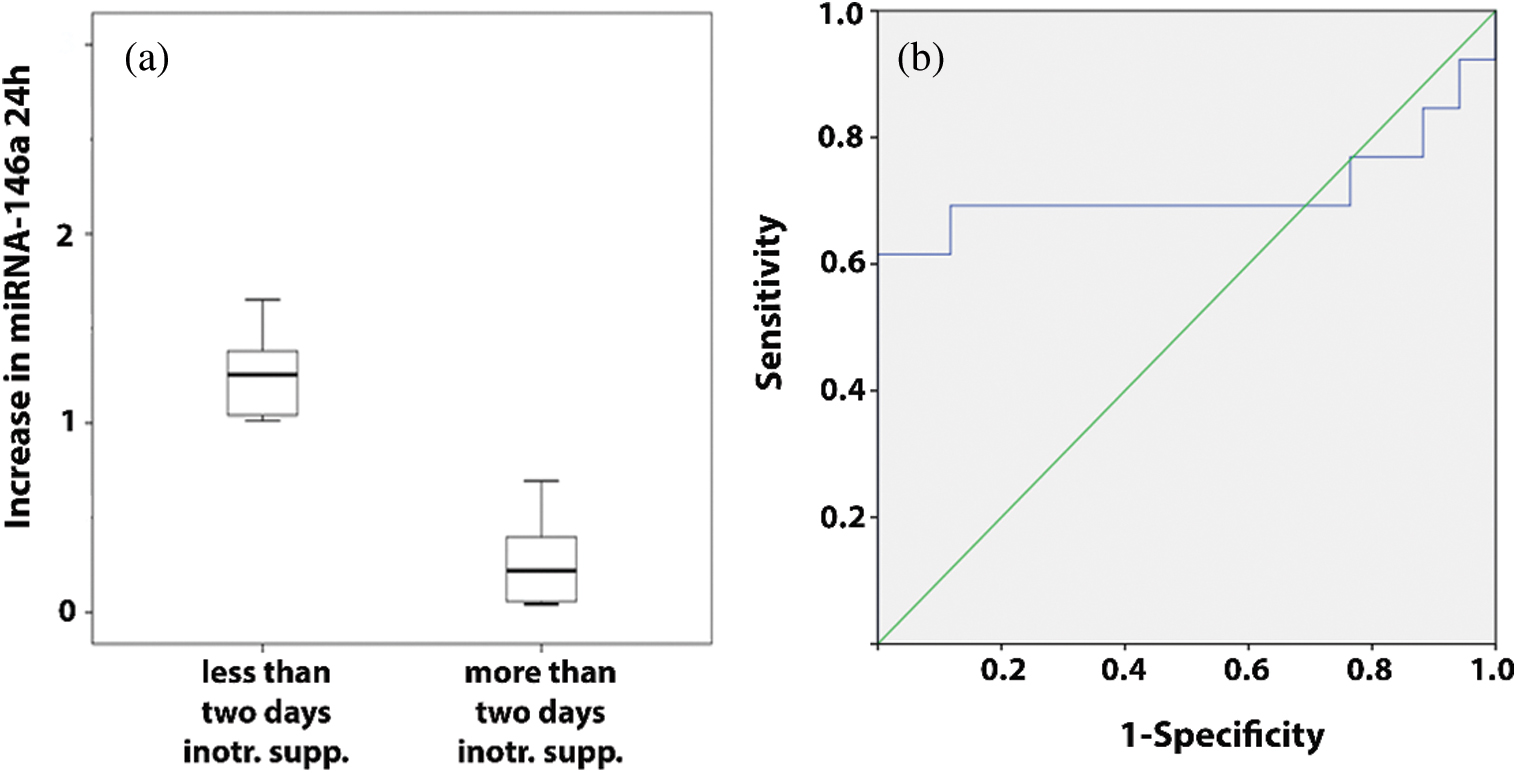
Figure 4: Correlation with the postoperative course. (a) Relative increase in the amount of miRNA-146a 24 hours after surgery in patients that received less or more than two days of inotropic support. (b) ROC curve showing the predictive power of the increase of miRNA-146a levels 24 hours after surgery to predict the risk of receiving more than two days of inotropic support
To the best of our knowledge, only few studies have focused on biomarkers for high-risk patients following heart surgery, and the majority of those studies were done on the adult population [9–11]. This retrospective analysis of the postoperative increase in the amounts of miRNAs-146a and -146b, as measured in serum during the first hours following pediatric heart surgery, revealed that it correlates with the postoperative course of the patient. A larger postoperative increase in the amount of those miRNAs, was in correlation with fewer days during which the child received inotropic support and with shorter hospitalizations. We also demonstrated that the extent of the postoperative increase in the amount of these circulating miRNAs during the hours following surgery correlated with lower CRP levels, as measured during the 24-hour period after the operation. In addition, higher lactate levels correlated to a smaller increase in the amount of miRNA-146a. MiRNAs-146a and -146b can therefore be added to miRNAs-208a, which we had suggested can serve as an early biomarker for children who are at greater risk of experiencing cardiac complications following surgery for CHD and have longer hospitalizations [8]. Early assessment of patients who are at risk for perioperative complications is imperative in order to select the most appropriate therapeutic approach which may lead to improvement in the quality and timing of treatment.
Our results are in line with several studies that showed that miRNAs-146a and -146b are expressed in response to proinflammatory stimuli and have the ability to regulate the inflammatory process (reviewed in [12]). Several groups have studied the importance of CPB in enhancing the proinflammatory response and the compensatory anti-inflammatory response following cardiac surgery [13]. Indeed, we also were able to show a significantly larger increase in miRNA-146a levels in the children that were on CPB during their surgery. A similar result, which was substantial although not statistically significant, was obtained for miRNA-146b. We note that the higher levels of these miRNAs in the children on CPB was obtained despite the fact that such children received, as part of their operation protocol, steroids to reduce their CPB-induced inflammatory response, emphasizing their sensitivity to inflammation.
MiRNA-146a and -146b are known to have an important role in regulating the immune response in a variety of ways. Both miRNAs have been found to inhibit the endothelial inflammatory response [14]. Lu et al. [15] reported that miRNA-146a is essential for the ability of regulatory T cells to restrain pathogenic T helper type 1 cell responses and associated inflammation. In addition, several studies have shown that miRNA-146a and -146b have a protective effect on the myocardium from ischemia/reperfusion injury [16–18]. Although the results of our attempts to study the ability of miRNAs-146a and -146b to predict the child’s risk to sustain postoperative complications did not reach a level of significance, our data suggest that the children who express higher levels of these miRNAs were at less risk to sustain cardiac complications, as reflected by the fewer days they required inotropic support, their shorter hospitalizations after the operation, and their lower levels of lactate, all of which probably reflect their better cardiac function and regulated inflammatory response.
MiRNA-146a and -146b may have other functions in addition to the immunomodulatory roles that have been ascribed to them. For example, Heggermont et al. [19] demonstrated that down-regulation of miR-146a during pressure overload in both mouse models and adult patients resulted in a less severe hypertrophic response and a protective effect that resulted in better cardiac function. Oh et al. [20] demonstrated that up-regulation of miRNA-146a was associated with a reduction in small ubiquitin-like modifier 1 levels in failing hearts, and that inhibition of miRNA-146a expression had beneficial effects on cardiac function. Indeed, there are 2 conflicting views regarding the role of miRNA-146a in the heart: one is a beneficial effect and the other is a deleterious effect, and each of them may be activated depending on the setting. For example, the role of miRNA-146 can differ when inflammation is pathological or physiological. In the setting of pediatric cardiac surgery, we favor the beneficial effect, which suggests that high levels of miRNA-146 are induced in the patients in order to suppress the immune response caused by the pathological activation of inflammation. This activation is probably induced by the surgical procedure and the use of CPB, and the high levels of miRNA-146 might ultimately lead to a better cardiac outcome.
One unique aspect of this study is our ability to shed light on the pathophysiological role of miRNAs-146a and -146b following heart surgery, on the one hand, and the ability of these miRNAs to serve as biomarkers for the postoperative course, on the other. Identifying patients at high risk of complications after surgery for CHDs can help prevent the appearance of such complications and improve therapeutic management for a better outcome. To date, troponin is the most clinically used biomarker for cardiac complications. However, troponin is not specific enough to cardiac damage and does not predict mortality after surgery for CHD, as shown by our group [8] and by others [21,22]. Therefore, a better biomarker is needed to identify patients at high risk. The results of the current study suggest that due to their possible anti-inflammatory role, miRNA-146a and -146b may be used in addition to other previously suggested biomarkers for identifying pediatric patients at high risk for cardiac complications after surgery for CHD. Furthermore, future studies may produce the knowledge needed to provide new therapeutic strategies that will involve these miRNAs for the treatment of inflammatory situations that pose a risk for the patients. This is a pilot study which is intended to serve as the basis of a larger investigation designed to examine immunomodulatory miRNAs as biomarkers for the postoperative course of children following surgery for the repair of CHD.
Several limitations of our study bear mention. Our study group is comprised of patients with diverse CHDs which necessarily affect their medical parameters. The relatively small size of our cohort together with the heterogeneity of their heart defects precluded our results from reaching greater statistical significance.
In conclusion, the results of the current study revealed that a large increase in the levels of miRNA-146a and miRNA-146b at 12 and 24 hours after surgery in pediatric patients undergoing heart surgery for the repair of their CHD is indicative of shorter hospitalizations and fewer days on inotropic support. In addition to influencing the medical decisions regarding the treatment provided to the patients in the first 24 postoperative hours, the use of such a biomarker will reduce expenses significantly. The cost of analyzing miRNAs in the patient’s blood amounts to a few dollars whereas the price of long hospitalizations and complex treatments in the Pediatric Intensive Care Unit can reach thousands of dollars at best. Further studies to assess their applicability on a larger cohort are warranted.
Acknowledgement: We thank Amisragas for their continuous support of the Department of Intensive Care. Esther Eshkol is thanked for editorial assistance.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jenkins, K. J. (2004). Risk adjustment for congenital heart surgery: the RACHS-1 method. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 7(1), 180–184. DOI 10.1053/j.pcsu.2004.02.009.
2. Ambros, V. (2004). The functions of animal microRNAs. Nature, 431(7006), 350–355. DOI 10.1038/nature02871.
3. Kosaka, N., Iguchi, H., Yoshioka, Y., Takeshita, F., Matsuki, Y. et al. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry, 285(23), 17442–17452. DOI 10.1074/jbc.M110.107821.
4. Fernández-Messina, L., Gutiérrez-Vázquez, C., Rivas-García, E., Sánchez-Madrid, F., de la Fuente, H. (2015). Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biology of the Cell, 107(3), 61–77. DOI 10.1111/boc.201400081.
5. Ciesla, M., Skrzypek, K., Kozakowska, M., Loboda, A., Jozkowicz, A. et al. (2011). MicroRNAs as biomarkers of disease onset. Analytical and Bioanalytical Chemistry, 401(7), 2051–2061. DOI 10.1007/s00216-011-5001-8.
6. Januzzi, J. L. (2011). What is the role of biomarker measurement after cardiac surgery?. Minerva Anestesiologica, 77(3), 334–341.
7. Aĝirbaşli, M., Ündar, A. (2013). Monitoring biomarkers after pediatric heart surgery: a new paradigm on the horizon. Artificial Organs, 37(1), 10–15. DOI 10.1111/j.1525-1594.2012.01573.x.
8. Zloto, K., Tirosh-Wagner, T., Bolkier, Y., Bar-Yosef, O., Vardi, A. et al. (2018). MiRNA-208a as a sensitive early biomarker for the postoperative course following congenital heart defect surgery. Pediatric Cardiology, 39(8), 1565–1571. DOI 10.1007/s00246-018-1931-7.
9. Wang, E., Nie, Y., Zhao, Q., Wang, W., Huang, J. et al. (2013). Circulating miRNAs reflect early myocardial injury and recovery after heart transplantation. Journal of Cardiothoracic Surgery, 8, 165. DOI 10.1186/1749-8090-8-165.
10. Zhou, X., Mao, A., Wang, X., Duan, X., Yao, Y. et al. (2013). Urine and serum microRNA-1 as novel biomarkers for myocardial injury in open-heart surgeries with cardiopulmonary bypass. PLoS One, 8(4), e62245. DOI 10.1371/journal.pone.0062245.
11. Yao, Y., Du, J., Cao, X., Wang, Y., Huang, Y. (2014). Plasma levels of microRNA-499 provide an early indication of perioperative myocardial infarction in coronary artery bypass graft patients. PLoS One, 9(8), e104618. DOI 10.1371/journal.pone.0104618.
12. Tahamtan, A., Teymoori-Rad, M., Nakstad, B., Salimi, V. (2018). Anti-inflammatory microRNAs and their potential for inflammatory diseases treatment. Frontiers in Immunology, 9, 1377. DOI 10.3389/fimmu.2018.01377.
13. Bronicki, R. A., Hall, M. (2016). Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatric Critical Care Medicine, 17(8_suppl 1), S272–S278. DOI 10.1097/PCC.0000000000000759.
14. Cheng, H. S., Sivachandran, N., Lau, A., Boudreau, E., Zhao, J. L. et al. (2013). MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Molecular Medicine, 5(7), 1017–1034. DOI 10.1002/emmm.201202318.
15. Lu, L. F., Boldin, P., Chaudhry, A., Lin, L. L., Taganov, K. D. et al. (2010). Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell, 142(6), 914–929. DOI 10.1016/j.cell.2010.08.012.
16. Feng, B., Chen, S., Gordon, A. D., Chakrabarti, S. (2017). miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes. Journal of Molecular and Cellular Cardiology, 105, 70–76. DOI 10.1016/j.yjmcc.2017.03.002.
17. Wang, X., Ha, T., Liu, L., Zou, J., Zhang, X. et al. (2013). Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovascular Research, 97(3), 432–442. DOI 10.1093/cvr/cvs356.
18. Di, Y. F., Li, D. C., Shen, Y. Q., Wang, C. L., Zhang, D. Y. et al. (2017). MiR-146b protects cardiomyocytes injury in myocardial ischemia/reperfusion by targeting Smad4. American Journal of Translational Research, 9(2), 656–663.
19. Heggermont, W. A., Papageorgiou, A. P., Quaegebeur, A., Deckx, S., Cara, P. et al. (2017). Inhibition of microRNA-146a and overexpression of its target dihydrolipoyl succinyltransferase protect against pressure overload-induced cardiac hypertrophy and dysfunction. Circulation, 136(8), 747–761. DOI 10.1161/CIRCULATIONAHA.116.024171.
20. Oh, J. G., Watanabe, S., Lee, A., Gorski, P. A., Lee, P. et al. (2018). miR-146a suppresses SUMO1 expression and induces cardiac dysfunction in maladaptive hypertrophy. Circulation Research, 123(6), 673–685. DOI 10.1161/CIRCRESAHA.118.312751.
21. Tanindi, A., Cemri, M. (2011). Troponin elevation in conditions other than acute coronary syndromes. Vascular Health and Risk Management, 7, 597–603. DOI 10.2147/VHRM.S24509.
22. Momeni, M., Poncelet, A., Rubay, J., Matta, A., Veevaete, L. et al. (2017). Does postoperative cardiac troponin-I have any prognostic value in predicting midterm mortality after congenital cardiac surgery?. Journal of Cardiothoracic and Vascular Anesthesia, 31(1), 122–127. DOI 10.1053/j.jvca.2016.02.018.
Appendix A: Surgical characteristics and laboratory parameters of the patients
Appendix B: Postoperative characteristics
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |