

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.013199
ARTICLE
Percutaneous Occlusion of Right Partial Anomalous Pulmonary Venous Connection with Dual Drainage to the Innominate Vein and the Left Atrium: A Unique Anatomical Finding
1Servicio de Hemodinamia y Cardiología Intervencionista, Hospital Privado Universitario de Córdoba, Córdoba, X5016KEH, Argentina
2Servicio de Cardiología, Hospital Privado Universitario de Córdoba, Córdoba, X5016KEH, Argentina
3Director de Docencia e Investigación, CORDIS, Instituto del Corazón. Resistencia, Chaco, H3508EFR, Argentina
*Corresponding Author: Alejandro R. Peirone. Email: alepeirone@yahoo.com
Received: 29 July 2020; Accepted: 17 August 2020
Abstract: A 43-year-old woman with a past medical history of aortic coarctation surgically repaired at the age of 3 years using an end-to-end anastomosis, presented with 2 years complain of increasing dyspnea and fatigue with exercise associated to frequent palpitations. During extensive work-up, she was found to have a partial anomalous pulmonary venous connection (PAPVC) with “dual drainage” represented by a communication between the right pulmonary veins draining into the left atrium and the innominate vein via an anomalous vein due to a persistence of early connections between the sinus of the right pulmonary veins and the cardinal veins system in the splanchnic plexus and also a persistence of the proximal portion of the left anterior cardinal vein. She was successfully treated through a percutaneous implantation of a vascular plug occluding the vertical portion of the anomalous vein diverting the flow to the left atrium. To the best of our knowledge, this anatomical variant of partial anomalous pulmonary venous connection with dual drainage has not been previously reported.
Keywords: Partial anomalous pulmonary venous connection; dual drainage; percutaneous closure; vascular plug
Partial anomalous pulmonary venous connection (PAPVC) is a rare congenital cardiac anomaly in which some, but not all, of the pulmonary veins connect to the right atrium or to one or more of its venous tributaries such as superior vena cava, inferior vena cava, innominate vein or coronary sinus. The prevalence has been reported to be as high as 0.7% in autopsy series and up to 10% in patients with an atrial septal defect being more frequently described involving veins from the right side [1]. The traditional classification of PAPVC does not categorize “dual drainage” separately [2] and these variants, with combination of supracardiac and cardiac PAPVC, are extremely rare with very few previous case reports encountered [1,3–8]. Moreover, the true incidence of PAPVC cases with dual drainage is difficult to estimate, since patients are often asymptomatic and may escape detection. Surgical correction has been the standard treatment when PAPVC results in right heart volume overload and/or clinical symptoms although in patients with dual drainage, transcatheter therapy may represent an alternative to surgical correction.
A 43-year-old woman was referred to our cardiology clinic for worsening of dyspnea and fatigue with exercise and frequent palpitations during the last few years. She was an amateur cyclist and has had a diagnosis of bicuspid aortic valve with normal function and localized aortic coarctation surgically repaired using an end-to-end anastomosis technique at the age of 3 years. Physical examination showed normal blood pressure in upper and lower limbs (100/60 mm Hg) and a 2/6 systolic ejection murmur on the left upper sternal border as well as wide split second heart sound. The rest of the cardiovascular examination was unremarkable with normal oxygen saturation. The electrocardiogram showed sinus rhythm, right axis deviation and incomplete right bundle branch block. A 2-D and Doppler echocardiogram yield a bicuspid aortic valve with normal function, an aortic arch without residual narrowing and a dilated right atrium and ventricle. In addition, the pulmonary artery trunk was also enlarged. Pulmonary artery pressure was measured within normal limits. A follow-up MRI performed with the aim of portraying the aortic arch, showed no significant abnormality at the site of the arch repair but raise a suspicious of PAPVC from the right upper pulmonary vein to the innominate vein estimating a Qp/Qs of 1.5:1. Both, the right heart chambers and the pulmonary artery trunk were reported as enlarged (Fig. 1).

Figure 1: 3D contrast enhanced MRI shows in a posterior view, the right pulmonary veins entering the left atrium and the anomalous vein with a horizontal portion as well as a vertical portion ascending to the innominate vein. The left pulmonary veins entering the left atrium are also visualized
RPVC: right pulmonary venous collector. VPAV: vertical portion of anomalous vein. HPAV: horizontal portion of anomalous vein. IV: innominate vein. LA: left atrium. SVC: superior vena cava. 1. Superior left pulmonary vein. 2. Inferior left pulmonary vein.
Due to the poorly understood pulmonary vein drainage visualized on MRI, a cardiac catheterization including a rotational angiography with 3D reconstruction was performed. It showed all right‑sided pulmonary veins draining close together in the left atrium and also, a dilated anomalous vein measuring approximately 6,5 mm connecting the upper portion of the confluence to the innominate vein (Figs. 2 and 3).

Figure 2: Angiographic images (a-b-c-d) obtained in an antero-posterior projection showing the venous catheter advanced through the inferior vena cava, right atrium, superior vena cava, innominate vein, anomalous vein into the right pulmonary veins and the left atrium. The left atrium and ventricle are also visualized

Figure 3: Rotational angiography with 3D reconstruction images in an antero-posterior, (a), lateral, (b) and superior, (c) projections, showing the right pulmonary veins and the anomalous vein draining into the innominate vein (in red) as well as the left atrium and ventricle and the ascending aorta (in gray)
A borderline significant left-to-right shunt (Qp/Qs 1.41:1) and a 3-4 mmHg pressure gradient from the left atrium to the innominate vein through this anomalous venous connection were registered. The pulmonary artery pressure was within normal range.
After the vascular occlusion, the patient recovered uneventfully and was discharged the next day. At follow‑up, two months later, the patient remains asymptomatic, receiving clopidogrel daily due to aspirin intolerance and a chest x-ray revealed that the vascular plug remains well seated.
3 Embryologic and Anatomic Considerations
There is a general agreement related to the embryologic origin of the pulmonary veins [9–17]. During the fourth week of development, two lung buds appear at the caudal end of the pulmonary diverticulum originated from the ventral wall of the foregut. These two lung buds are surrounded by a capillary plexus called the splanchnic plexus by which the primitive lungs drain into the systemic circulation. Initially this plexus drain into portions of anterior and posterior cardinal system and the umbilical-vitelline venous systems.
When pulmonary differentiation occurs, part of the splanchnic plexus forms the pulmonary venous plexus, where the pulmonary veins are developing. Simultaneously, an endothelial sprout grows from the dorsal wall of the left atrium toward the pulmonary venous plexus, forming the primordial pulmonary vein, or common pulmonary vein. Eventually, a lumen forms and connects with the pulmonary venous plexus. This structure bifurcates into right and left pulmonary veins, which in turn divide into two further branches.
Once the left atrium and the pulmonary venous plexus are joined, the latter loses its connection with the splanchnic plexus and the cardinal and umbilical-vitelline venous system. The common pulmonary vein is progressively incorporated into the left atrium as well as the branches and the four pulmonary veins end draining separately into the left atrium. The incorporated portions of the pulmonary venous plexus give origin to the smooth portion of the left atrium.
The morphogenetic processes that occurred in this particular and unusual case and its anatomical findings can be summarized as:
(1) Anomaly in the incorporation of the sinus of the right pulmonary veins in the left atrium. This determines the existence of a right pulmonary venous collector partially incorporated into the left atrium.
(2) Persistence of early or primary connections between the sinus of the right pulmonary veins and the cardinal veins system in the splanchnic plexus, which gives rise to the horizontal portion of the anomalous vein connecting the superior right pulmonary vein with the proximal portion of the left anterior cardinal vein.
(3) Anomaly in the process of reabsorption or disappearance of the proximal portion of the left anterior cardinal vein, which gives rise to the vertical portion of the anomalous vein connecting the horizontal portion of this vein with the innominate vein.
(4) The different morphogenetic processes mentioned, determine that the right pulmonary veins have dual drainage in the left atrium and in the innominate vein.
The procedure was performed under conscious sedation with right and left femoral veins access. After confirmation of anatomy using rotational angiography with live 3D reconstruction, the anomalous vein was cannulated using a 6F multipurpose diagnostic catheter. Subsequently, it was exchanged over an extra-stiff exchange wire for a 7‑Fr Shuttle carotid artery access system® (Cook, USA) which was positioned in the anomalous vein, close to the entering of the right pulmonary veins to the left atrium. The anomalous vein was then occluded deploying a 10 mm LepuMemopart vascular plug® (Lepu Medical, China). The occluding device size was selected to be approximately 50% larger than the target vessel. The final position of the device was above the drainage of the right‑sided pulmonary veins without impinging these structures. An angiography performed before device release from the right pulmonary artery using a Pig-tail catheter advanced through the additional femoral vein access, confirmed during levophase, the patency of the right pulmonary veins as well as total occlusion of the anomalous vein (Fig. 4). Redirection of blood flow from the right pulmonary veins to the left atrium was successfully achieved. Pulmonary artery pressure remained unchanged post occlusion. There were no complications.
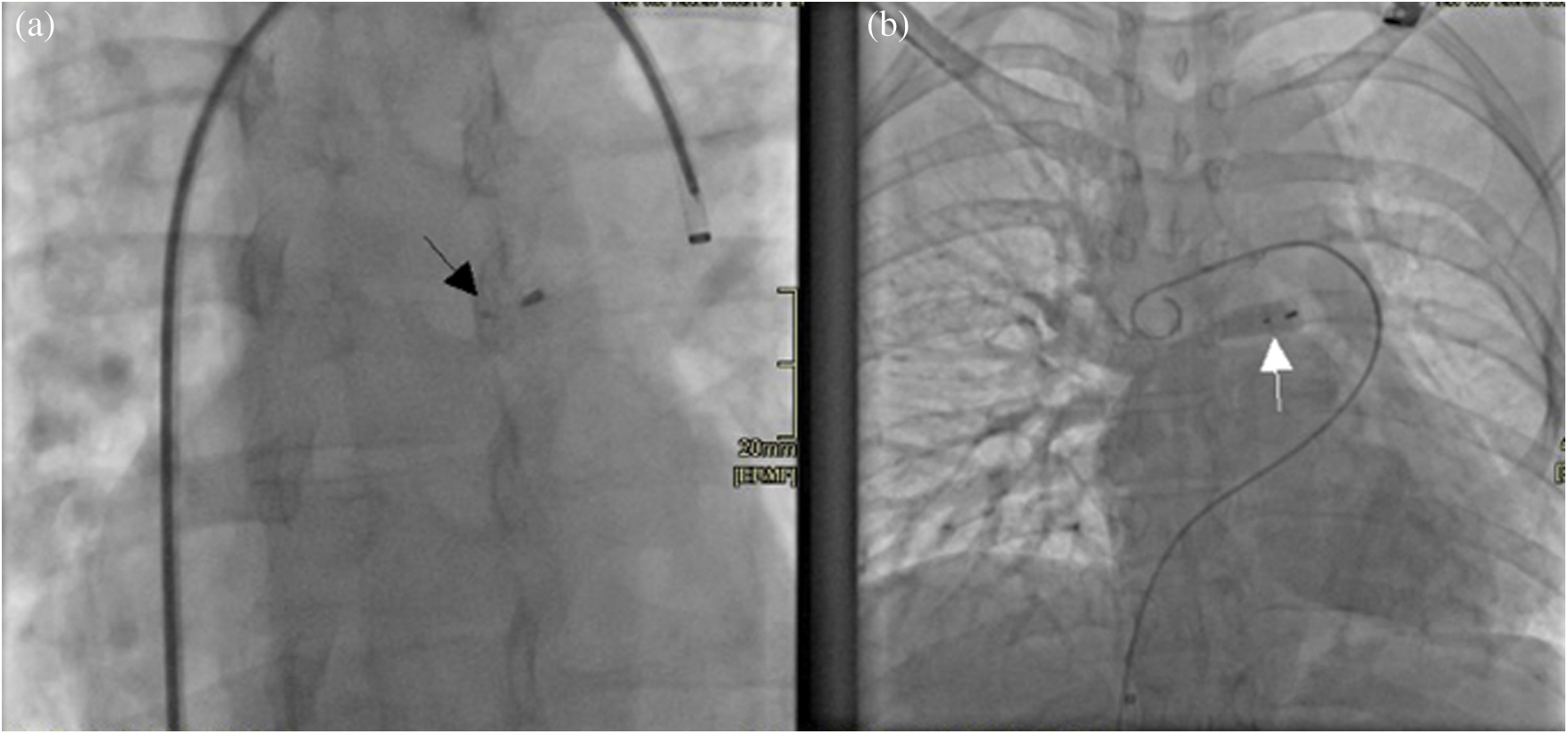
Figure 4: (a) Radioscopy image obtained in an antero-posterior projection showing the vascular plug device in situ (black arrow). (b) A right pulmonary artery angiography shows during levophase, the three right pulmonary veins draining unobstructed into the left atrium after occlusion of the anomalous vein with a device (white arrow)
Approximately 90% of all PAPVC originate from the right lung, 7% originate from the left lung, and 3% of patients are found to have bilateral PAPVC originating from both lungs connecting to the superior vena cava, the inferior vena cava, the right atrium, the innominate vein or the coronary sinus [18]. Although the Toronto group at the Hospital for Sick Children [2] have proposed a classification including five subtypes of PAPVC, it has been difficult to fit the anatomical variant of our patient in the mentioned classification. PAPVC is frequently asymptomatic for many years and the most important variable for the severity of symptoms and development of complications is the magnitude of the left to right shunt. With time, the hemodynamic sequelae of long-standing PAPVC to the right side of the heart includes right atrial and ventricular dilation and pulmonary arterial hypertension leading to occurrence of atrial arrhythmias and right-sided heart failure. Exceptionally, paradoxical embolization may be consider with this type of anatomical finding in the view that any connection between a systemic vein and the left atrium may allow paradoxical embolism.
Two anatomic types of PAPVC with dual drainage have been encountered. The vertical vein type, with dual venous drainage from the upper lobe of the left lung to the innominate vein via a large vertical vein and to the left atrium via another more or less developed left pulmonary vein and the Scimitar vein type, with an anomalous venous connection from the middle and lower pulmonary lobes of the right lung to the inferior vena cava below the level of the diaphragm, associated with an anomalous venous connection of the Scimitar vein to the left atrium and sequestration syndrome [19].
After confirmation of dual drainage or supply, transcatheter occlusion may be considered. The world experience reported by Luciano et al. indicates the after an adequate selection of patients, the interventional procedures with the aim of diverting the flow exclusively to the left atrium have been successful implanting both various coils and self- expandable devices. In their reported experience, no complications occurred, except for device embolization in one patient. Moreover, additional transcatheter procedures were performed including secundum ASD closure, systemic arterial supply occlusion and stent implantation for aortic coarctation. Comparison between occlusion of a vertical vein type or Scimitar vein type of PAPVC showed no difference. Interestingly, patients presenting after 40 year-old tended to have more symptoms [19].
In the uncommon anatomical variant of TAPVC associated to dual drainage or supply, percutaneous closure of the abnormal vertical vein implanting a self-expandable device or coils is possible. This strategy leads to redirect the flow avoiding obstruction to the pulmonary venous circulation to the left atrium.
To our knowledge, there are only two previous reported cases [20,21] of PAPVC in association with coarctation of aorta treated successfully with a percutaneous approach, thereby obviating the need for surgery and its known potential risks. In both previous reports the abnormal veins were left-sided and a left persistent vertical vein was occluded implanting a self-expandable device redirecting the flow to the left atrium. The difference with our case relies on that the PAPVC was diagnosed many years after the initial aortic coarctation surgery; hence, both lesions were treated separately. Moreover, in our patient the abnormality of the pulmonary veins was right-sided, on the contrary, in the other two previous cases reported, those were left-sided.
In summary, so far as we know, this is the first report of a patient suffering from PAPVC involving the right pulmonary veins associated to dual drainage via an anomalous vein with a horizontal portion due to a persistence of early connections between the sinus of the right pulmonary veins and the cardinal veins system in the splanchnic plexus and also a persistence of the proximal portion of the left anterior cardinal vein. The occlusion was planned to be performed proximately at the level of the connection of the anomalous vein to the right pulmonary veins achieving successful diversion of the right pulmonary venous flow to the left atrium. Diligent evaluation and knowledge during clinical diagnostic work-up of the occurrence of PAPVC with dual supply, whether isolated or associated with other cardiac defects that may be amenable to additional interventional procedures, is mandatory to be able to plan a less-invasive transcatheter approach to correct these cardiac defects. Percutaneous closure of the abnormal venous connection is certainly the treatment of choice for PAPVC with dual drainage.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Forbess, L. W., O’Laughlin, M. P., Harrison, J. K. (1998). Partially anomalous pulmonary venous connection: demonstration of dual drainage allowing nonsurgical correction. Catheterization and Cardiovascular Diagnosis, 44(3), 330–335. DOI 10.1002/(SICI)1097-0304(199807)44:3<330::AID-CCD19>3.0.CO;2-O.
2. Alsoufi, B., Cai, S., Van Arsdell, G. S., Williams, W. G., Caldarone, C. A. et al. (2007). Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Annals of Thoracic Surgery, 84(6), 2020–2026. DOI 10.1016/j.athoracsur.2007.05.046.
3. Dähnert, I., Riede, F. T., Kostelka, M. (2007). Partial anomalous pulmonary venous drainage of the left upper pulmonary vein–catheter interventional treatment is sometimes possible. Clinical Research in Cardiology, 96(7), 511–513. DOI 10.1007/s00392-007-0518-8.
4. Gomez, J., Soledispa, C. (2012). Redirection of anomalous venous pulmonary flow to left atrium using a vascular plug II. Journal of Invasive Cardiology, 24(5), E96–E98.
5. Recto, M. R., Sadlo, H., Sobczyk, W. L. (2007). Rare case of persistent left superior vena cava to left upper pulmonary vein: pathway for paradoxical embolization and development of transient ischemic attack and subsequent occlusion with an amplatzer vascular plug. Journal of Invasive Cardiology, 19(10), E313–E316.
6. Wilson, W., Horlick, E., Benson, L. (2015). Successful transcatheter occlusion of an anomalous pulmonary vein with dual drainage to the left atrium. Catheterization and Cardiovascular Interventions, 85(7), 1212–1216. DOI 10.1002/ccd.25734.
7. Kobayashi, D., Forbes, T. J., Delius, R. E., Aggarwal, S. (2012). Amplatzer vascular plug for transcatheter closure of persistent unligated vertical vein after repair of infracardiac total anomalous pulmonary venous connection. Catheterization and Cardiovascular Interventions, 80(2), 192–198. DOI 10.1002/ccd.23497.
8. Craig, J. M., Darling, R. C., Rothney, W. B. (1957). Total pulmonary venous drainage into the right side of the heart; report of 17 autopsied cases not associated with other major cardiovascular anomalies. Laboratory Investigation, 6(1), 44–64.
9. Lucas, R. V., Jr, Anderson, R. C., Amplatz, K., Adams, P., Jr, Edwards, J. E. (1963). Congenital causes of pulmonary venous obstruction. Pediatric Clinics of North America, 10(3), 781–836. DOI 10.1016/S0031-3955(16)31451-1.
10. Sadler, T. W. (1985). Langman’s medical embryology. 5th Edition. Philadelphia: Lippincott Williams & Wilkins Company.
12. Reller, M. D., McDonald, R. W., Gerlis, L. M., Thornburg, K. L. (1991). Cardiac embryology: basic review and clinical correlations. Journal of the American Society of Echocardiography, 4(5), 519–532. DOI 10.1016/S0894-7317(14)80388-X.
13. Valdés-Cruz, L. M., Cayre, R. O. (1999). Echocardiographic diagnosis of congenital heart disease. Philadelphia: Lippincot-Raven Publishers.
14. Blom, N. A., Gittenberger-de Groot, A. C., Jongeneel, T. H., DeRuiter, M. C., Poelmann, R. E. et al. (2001). Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. American Journal of Cardiology, 87(3), 305–309. DOI 10.1016/S0002-9149(00)01363-1.
15. Latson, L. A., Prieto, L. R. (2007). Congenital and acquired pulmonary vein stenosis. Circulation, 115(1), 103–108. DOI 10.1161/CIRCULATIONAHA.106.646166.
16. Murillo, H., Cutalo, M. J., Jones, R. P., Lane, M. J., Fleischmann, D. et al. (2012). Pulmonary circulation imaging: embryology and normal anatomy. Seminars in Ultrasound, CT and MRI, 33(6), 473–484. DOI 10.1053/j.sult.2012.08.001.
17. Poelmann, R. E., Gittenberger-de Groot, A. C., Jongbloed, M. R. M., DeRuiter, M. C. (2016). Congenital heart diseases: the broken heart. Clinical features, human genetics and molecular pathways. Vienna: Springer-Verlag Wien.
18. Jongbloed, M. R. M., Poelmann, R. E., Gittenberger-de Groot, A. C. (2017). The complete reference for scimitar syndrome. anatomy, epidemiology, diagnosis and treatment. London: Academic Press.
19. AboulHosn, J. A., Criley, J. M., Stringer, W. W. (2003). Partial anomalous pulmonary venous return: case report and review of the literature. Catheterization and Cardiovascular Interventions, 58(4), 548–552. DOI 10.1002/ccd.10475.
20. Luciano, D., Laux, D., Boudjemline, Y., Hascoët, S., Lusson, J. R. et al. (2013). Transcatheter therapy in partially abnormal pulmonary venous return with additional drainage to the left atrium. International Journal of Cardiology, 170(2), 221–226. DOI 10.1016/j.ijcard.2013.10.061.
21. Mamas, M. A., Clarke, B., Mahadevan, V. S. (2010). Percutaneous treatment of dual pulmonary venous drainage and coarctation of the aorta in a single patient. Experimental and Clinical Cardiology, 15(1), 11–13.
22. Al Qbandi, M., Thinakar Vel, M. (2018). Transcatheter therapy of partial anomalous pulmonary venous connection with dual drainage and coarctation of the aorta in a single patient. Journal of the Saudi Heart Association, 30(4), 311–315. DOI 10.1016/j.jsha.2018.06.003.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |