

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012438
ARTICLE
Italian Validation of the Healthcare Needs Scale for Youth with Congenital Heart Disease and Its Short-Form Development
1Health Professions Research and Development Unit, IRCCS Policlinico San Donato, San Donato Milanese, Milan, Italy
2Department of Public Health, Experimental and Forensic Medicine, Section of Hygiene, University of Pavia, Pavia, Italy
3Department of Psychology, EngageMinds HUB–Consumer, Food & Health Engagement Research Center, Università Cattolica del Sacro Cuore, Milan, Italy
4Paediatric Cardiology, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples, Italy
5Adult Congenital Heart Disease Unit, Monaldi Hospital, Naples, Italy
6Department of Pediatric and Adult Congenital Heart Disease, IRCCS Policlinico San Donato, San Donato Milanese, Milan, Italy
*Corresponding Author: Rosario Caruso. Email: Rosario.Caruso@grupposandonato.it
Received: 30 June 2020; Accepted: 10 July 2020
Abstract: Aims: This study aimed at providing an Italian short version of the ‘healthcare needs scale for youth with congenital heart disease’ (I-HNS-CHD-s), describing its construct validity and reliability. Methods: A multi-method and multi-phase design were adopted. Phase one referred to the cultural-linguistic validation of the original scale into Italian. Phase two tasted content and face validity of the Italian-translated scale. Phase three included the psychometric validation process of scale, encompassed two different steps: first cross-sectional data collection (sample A) purposed at determining the psychometric characteristics of the I-HNS-CHD-s, using an exploratory factor analysis (EFA). Then, a second round of cross-sectional data collection (sample B) was performed using the version of I-HNS-CHD-s derived from the previous step, and it purposed at confirming the scale factor structure and at assessing its reliability. Results: I-HNS-CHD-s showed evidence of face and content validity, adequate construct and internal consistency and stability. Specifically, I-HNS-CHD-s had 14 items kept by four domains, labelled as follows: Healthcare education, clinical support, emotional support, continuum of care. These domains were predicted by a second-order factor, which was labelled as Healthcare needs. Overall I-HNS-CHD-s encompassed only the items that showed high performance in the psychometric analysis. Accordingly, I-HNS-CHD-s is a shorter form of the original scale (14 items instead of 25). Conclusions: I-HNS-CHD-s is a psychometrically robust measure of the healthcare and psychosocial needs of Italian adolescents and young adults with congenital heart disease.
Keywords: Assessment; congenital heart disease; healthcare needs; instrument; validation
Congenital heart diseases (CHD) are the most prevalent birth defects, representing a major global health problem [1]. With a prevalence of 8 out of 1000 live births [2], the CHD are the leading causes of birth-associated defects [3]. To date, 1.3 million children live with a CHD worldwide [4,5], and approximately 90% of them grown up to adulthood, acknowledging the advancements in treatments [6]. The increasing survival of children with CHD challenges healthcare systems in managing the healthcare transition from pediatric-related services to adult-related ones, which requires to put the attention on the age-specific patients’ healthcare needs [6].
The transition from pediatric-related services to adult-related settings is defined as the transition clinic, which represents a pivotal period for adolescents and young adults with CHD [7,8]. More precisely, transition clinic includes several changes referred to patients and their families [7,9]. Overall, transition clinic aims to enhance the experience of living with CHD [10], the health-related quality of life [11], the perception of social and family support [12], the health literacy [13], the access to care [14], and the patient-provider relationship [15]. However, the assessment of the patients’ psychosocial needs is so far challenging, due to the paucity of valid and reliable self-report measurements that are specific for adolescents and young adults with CHD [16,17]. This undermines the possibility to implement the transition clinic taking into account the general psychosocial needs among youth with CHD, beyond their clinical characteristics.
Furthermore, the psychosocial changes of adolescents with CHD could be complex, acknowledging their increased need to functionally cope with the challenges required by the social demands, given by the increased commitment in social activities, school, and peer-relationships [7,8]. For this reason, the assessment of psychosocial needs of young patients with CHD could allow to implement more supportive and effective care programs [18]. In addition, the assessment of psychosocial needs has to be supported by evidence of validity in the different cultures, as the individual’s perception of psychosocial needs might be influenced by cultural characteristics [19,20].
Overall, there is a lack of available self-report scales allowing the assessment of psychosocial needs of young patients with CHD. Recently, Chen and colleagues developed the ‘healthcare needs scale for youth with CHD’ (HNS-CHD) [21]. The HNS-CHD is the only available scale to assess the healthcare needs of adolescents and young adults with CHD, considering the psychosocial challenges [21]. Thus far, HNS-CHD is available in traditional Chinese and English: It shows evidence of validity and reliability among Taiwanese youth with CHD, and it was translated in English even if there is not yet available a psychometric testing among English-speaking adolescents and young adults [21]. HNS-CHD is not currently available for clinical practice in Italy, as its Italian validation was not yet performed. An accurate validation is required before adopting HNS-CHD in a different context than Taiwan, as the different cultures might reflect different psychometric proprieties (factor structures) [22]. Furthermore, HNS-CHD encompassed 25 items; for this reason, it could result as slightly demanding for responders, as per the need of dedicating time for adequately answering to the questionnaire. For these reasons, this study was aimed at providing the Italian short version of HNS-CHD (I-HNS-CHD-s), describing its translation, adaptation and validation.
This study has a multiphase (three phases) design. Phase one referred to the cultural-linguistic validation of the HNS-CHD into the Italian language, developed by the research team of this study. Phase two tested the content and face validity of the Italian-translated HNS-CHD, involving a panel of experts. Finally, phase three included the psychometric validation process to identify which items required to be modified or deleted, as for resulting ambiguous. More precisely, the psychometric validation phase encompassed two different steps: the first step comprised of the first cross-sectional data collection (sample A) to determine the psychometric characteristics of the I-HNS-CHD-s, using an exploratory factor analysis (EFA). Then, the second step comprised of the second round of cross-sectional data collection (sample B) using the version of I-HNS-CHD-s derived from the previous step to confirm the I-HNS-CHD-s factor structure using a confirmative factor analysis (CFA) and to assess its reliability (i.e., internal consistency).
The HNS-CHD is a self-reported instrument aimed to assess the healthcare and psychosocial needs of young patients with CHD. In the original validation study, the HNS-CHD encompassed 25 items and measured three domains of healthcare needs that adolescents and young adults with CHD may report: health management, health policy, and individual and interpersonal relationship. The young patients with CHD are asked to assign a score based on a 5-point Likert scale (where 1 = not important at all and 5 = very important) for each item of the scale. Higher scores indicated that a need was more perceived as crucial. HNS-CHD was firstly designed and initially validated in the Taiwan setting, and it has been shown to have adequate psychometric characteristics (i.e., stability and reliability) [21].
2.3 Phase One: Cultural and Linguistic Validation
The phase one of this study was aimed to achieve the Italian cultural-linguistic validation of the original instrument (HNS-CHD), as it was unavailable in Italian. Translating the HNS-CHD into Italian use required considerable effort by researchers to maintain the quality of the translation. Consequently, the methodology used throughout this phase strictly followed an adaptation of Brislin’s classic translation model [23], according to some recent Italian cultural-linguistic validations [24]. This phase was performed with a combined translation and bilingual techniques. Translation involved a group of four translators to ensure appropriate back-translation. Specifically, a project manager (RC) was identified by the research team at the beginning of the translation process to control the rigor of the overall translation. Then, two bilingual translators prepared two Italian versions of the HNS-CHD: each Italian version was blindly back-translated into English by two other translators. Finally, the four translators had a meeting to forward translate the two different versions and find consensus on the optimal translation (forward translation).
Subsequently, the final Italian version of HNS-CHD was tested in a group of 10 adult volunteer CHD patients, with good cognitive abilities, assessed using the six-item screener test [25]. This pilot testing was performed to assess the clarity of the items using a four-point Likert scale (1 = not clear, 4 = completely clear). The degree of agreement in the discussion group and among patients involved in pilot testing was assessed using Fleiss’ Kappa index. A value of 0.70 was considered as the cut-off point to indicate adequate consensus [26]. This first phase was conducted in an Italian cardiac research hospital, from August to December 2018.
2.4 Phase Two: Quantitative and Qualitative Content Validity
The Italian-translated HNS-CHD was tested for both quantitative and qualitative content validity, following a standardized methodology. The quantitative content validity followed the methodology developed by Lawshe [27], involving a panel of 16 experts (panelists), who were nurses and physicians specialized in CHD transition care and research methodology. They aimed to rate the pertinence (i.e., essential contents) and the relevance (i.e., appropriateness) of each item with the objective of its measurement. For this reason, two specific index of quantitative content validity were computed to assess the level of agreement among the raters: (a) Content Validity Ratio (CVR) to assess the pertinence through a three-point ordinal scale (1 = not pertinent; 2 = useful but not pertinent; 3 = highly pertinent) and (b) Content Validity Index (CVI) to assess relevance, through a four-point ordinal scale (1 = not relevant; 2 = somewhat relevant; 3 = quite relevant; 4 = highly relevant) [28]. According to CVR’s formula, that is (Ne –N/2)/(N/2), in which the Ne is the number of raters indicating “essential” and N is the total number of raters, each item was considered pertinent when the obtained CVR matched a score >0.60 [29]. The CVI index was calculated using two approaches. Firstly, we computed item level (I-CVIs), followed by scale level (S-CVI) using the average of the I-CVIs scores as described by Polit [28].
Conversely, qualitative content validity (i.e., face validity) was determined based on the same expert panelists’ understanding of the items and their views about the overall concept that they purported to measure [28]. The authors were asked to answer three open-ended questions, and their responses were then transcribed verbatim. The questions sought to explore the clarity of the wording used for each item and to identify areas of ambiguity or possible misinterpretation. All the answers were analyzed using a narrative approach to summarize whatever themes emerged [30]. This phase was performed in an Italian cardiac research hospital in the north of Italy, between January and May 2019.
2.5 Phase Three: Construct Validity (Psychometric Proprieties Assessment)
This phase was performed using a multicenter cross-sectional design with two rounds of convenience samplings. The involved centers were two: one in the north of Italy (Milan) and one in the south of Italy (Naples). The first step (sample A) was carried out to determine the psychometric characteristics of the I-HNS-CHD-s and to delete ambiguous items, while the second step (sample B) was needed to corroborate the most plausible factor structure derived from the analysis on the sample A. The first data collection was made from June to October 2019, and the second collection of data between November 2019 and March 2020 in two cardiac research hospitals.
Adolescents and young adults with CHD were invited to fill the I- HNS-CHD-s (the version derived from content validity for sample A, and the version derived from the analysis on sample A for collecting data on sample B), a sociodemographic form; instead, clinical data were retrieved from medical records. Sociodemographic data were sex, age, marital status, employment, education level of patients. Clinical data were body mass index (BMI), CHD complexity [in accordance with the classification defined by Warnes [31], and New York Heart Association (NHYA) functional classification. Eligible patients have been assessed considering the following inclusion criteria, which were consistent with the ones identified by researchers who developed HNS-CHD: [21] (a) Aged between 15 and 24 years; (b) with a diagnosis of CHD in accordance with 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease [32]. The exclusion criteria were given by the presence of chromosomal abnormalities, such as Down’s syndrome and Marfan’s syndrome, cognitive impairment, assessed using Six Item Screener (SIS: if SIS ≤ 4), and by inadequate knowledge of the Italian language.
Descriptive statistics were performed on the demographic and clinic characteristics of the two samples (i.e., samples A and B) and for the items, including the skewness and kurtosis to ascertain their normality.
For sample A, data were analyzed by exploratory factor analysis (EFA) using maximum likelihood (ML) estimation with a Geomin oblique rotation for maximizing and simplifying factor loadings and their interpretation. EFA is a multivariate technique aimed to explore the underlying structure (factors) of observed variables (items) and the relationships between factors and items [33]. Previous authors suggested that to perform EFA it is recommended a sample size of 10 participants per item [34]. Considering that the items in this stage of scale development were sixteen, the desirable sample comprised of 160 adolescents or young adults with CHD. Before proceeding with the EFA, the Bartlett’s test and the Kaiser-Meyer-Olkin (KMO) index were used to assess the factorability of the correlation matrix. The number of factors to be extracted was based on an analysis of the eigenvalues, the scree test, and the easy of model interpretation [35]. Items whose loading value was >0.30 were kept, and items showing cross-loadings (factor loadings higher or equal to 0.30 in different domains) were removed, as they were considered ambiguous by responders [35]. After the removal of items exhibiting cross-loadings in the most plausible factor structure, a second round of data collection was needed for performing a confirmatory factor analysis (CFA) on sample B to validate the most plausible factor structure model derived from the EFA for Sample A.
According to VanVoorhis and Morgan indications, we needed to enroll 50 CHD patients for each domain (estimated sample = 200 CHD patients) to perform a CFA [36]. The following fit indices were considered to evaluate the CFA model, as well as EFA: omnibus fit index (χ2); χ2/degree of freedom (between 1.5 and 5 is acceptable), comparative fit index (CFI) (values > 0.900 indicated an acceptable fit); root mean square error of approximation (RMSEA) (values < 0.060 indicated an acceptable fit); and the weighted root mean square residual (WRMR) (values 1.0 indicated an acceptable fit). In addition, the possible presence of a single second-order factor was examined, hypothesizing an underlying more general factor of “healthcare and psychosocial needs”, which could explain the intercorrelations between the first-order factors. The χ2 difference tests were performed to evaluate the adequacy of possible competing models, which may explain the observed relationships as well. To compute the χ2 difference tests, we needed to consider both the difference of the χ2 values of the two competing models and the difference of the degrees of freedom. If the χ2 difference is significant, the model with more satisfactory parameters of fit to data is the most suitable solution. In case the χ2 difference is not significant, both models demonstrated to fit the data equally well.
Finally, internal consistency was tested using the McDonald’s ω, as an estimate of the general factor saturation of a test [37]. Omega’s coefficient (ω) was calculated on each domain and the overall scale, both sample A and sample B. A level of ≥0.7 was considered acceptable [38]. All statistics were calculated with α = 5%, using SPSS, version 22 (IBM Corp., Armonk, NY, USA) and Mplus 7.1 (Muthen & Muthen, Los Angeles, CA, USA).
This study was approved by the Research & Ethical Committee of San Raffaele Hospital (Italy) (Protocol n. 9/int/2018 of 8th February 2018), in accordance with the international ethical principles (Good Clinical Practice, GCP) and the Italian legal requirements for non-interventional studies. All participants were informed about the aims and the method of the study, and they were asked to provide their written informed consent. Parents or legal tutors had to sign the written consent of adolescents aged under eighteen. Participants of each phase were also informed about the confidentiality of their.
3.1 Phase One: Cultural and Linguistic Validation
The consensus discussion between four translators required two discussion rounds, which lasted roughly 120 minutes in total, for ensuring the equivalence of the translated concepts [39]. The ratings indicated high agreement between the English and Italian meaning for each item (Fleiss’ K = 0.95 in rating the agreement on the translation). Overall, the items’ wording choice had two principal challenges: The first was referred to the term “illness or disease”, because patients with CHD do not consider their congenital heart defect an actual disease but a “condition”; accordingly, the Italian translation with the term “condition” was preferred. The second challenge was the understanding and translation of “applying for catastrophic illness cards” (item 24), which translation is described in Tab. 1. Then, the pilot test of Italian items of HNS-CHD on the group of 10 adults with CHD assessed the clarity and comprehensibility of each item. The majority of their comments highlight the ‘simplicity’ of items. Participants were mainly male (n = 7; 70%) and the mean age was 34.8 years (standard deviation, SD = 9.1), and their level of agreement computed through the Fleiss’ K index was 0.90.
3.2 Phase Two: Quantitative and Qualitative Content Validity
The involved panelists were mainly females (n = 12; 75%), with a mean age of 37.4 years (SD = 13.14). Eleven of them were nurses and five were physicians. They reported to have a mean of 12.8 years (SD = 9.84) of working experience. All panelists had a postgraduate education. Three rounds of panel discussions were needed to obtain satisfactory CVRs, I-CVIs and S-CVI; during each round of discussion, critical items were identified. As Tab. 1 explains, CVR and I-CVIs’ indices for items 4, 10, 13, 14, 15, 19, 23, 24, and 25 had low values (i.e., <60) [40], suggesting their inadequacy for the Italian context, as their contents were redundant with the meaning of other items. After the removal of redundant items, S-CVI was equal to 0.86, indicating the adequacy of the translated I-HNS-CHD-s (Tab. 1).

3.3 Phase Three: Construct Validity (Psychometric Proprieties Assessment)
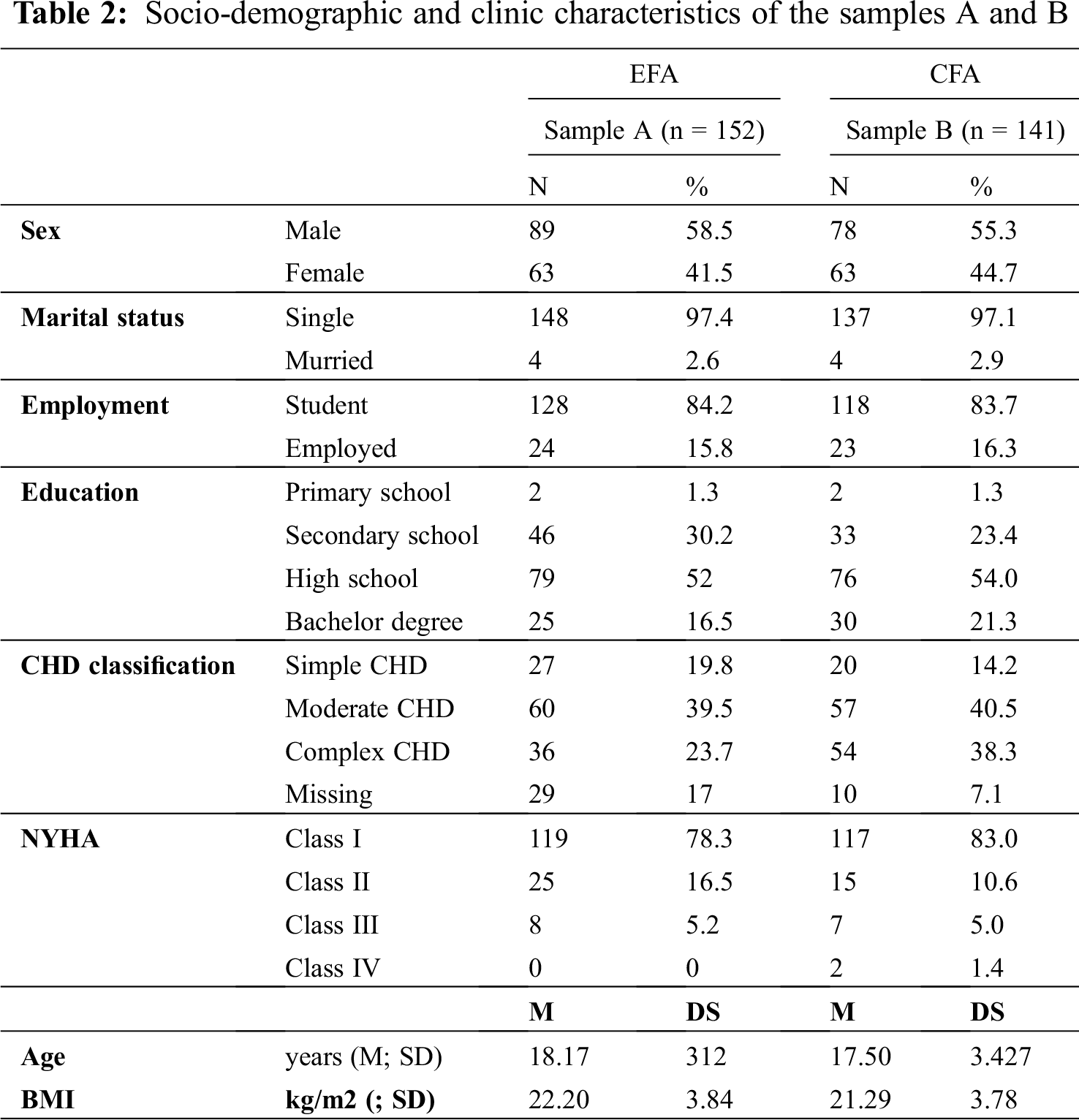
The first data collection (sample A) of the phase three comprised a sample of 152 young patients with CHD. The demographic and clinical characteristics of the sample A are summarized in Tab. 2. The majority of the included patients were males (58.5%), single (97.4%), students (84.2%), and with a mean age equal to 18.17 years (SD = 3.12). According to Warnes classification [31], 22% of the enrolled patients had a simple CHD (n = 27) and 23.7% had a severe complex CHD (n = 36), while 39.5% showed a moderate CHD (n = 60).
The correlation matrix coming from the answers to the I-HNS-CHD-s in sample A was considered suitable for the EFA, due to the Bartlett’s test of sphericity was significant [χ2(120) = 1183.70; p < 0.05], and the KMO was 0.918. The study of the eigenvalues, the scree test and the interpretation of the items kept by their underlying factors suggested that the four-dimension model was adequate to explain data (χ2(62) = 121.381, p < 0.001; χ2/df = 1.95; RMSEA = 0.047; 90% CI [0.009–0.071]; CFI = 0.982; TLI = 0.964; SRMR = 0.029). As shown in Tab. 3, the four-dimension model explained the 77.60% of the total variance; factor loadings and each domain’s variance after rotation are described in Tab. 3. We also tested a concurrent factor model with three-dimension factor structure data, as per the original factor structure of the scale (χ2(41) = 88.68, p < 0.001; χ2/df = 2.16; RMSEA = 0.088; 90% CI [0.069–0.093]; CFI = 0.912; TLI = 0.901; SRMR = 0.059). The χ2 difference test between four-dimension and three-dimension models was significant (Δχ2 = 32.701; Δdf = 21; p-value = 0.049), indicating that only one model was more adequate in explaining data; accordingly, considering the fit indices, the four-dimension model was considered the most plausible solution to determine the psychometric structure of I-HNS-CHD-s.
In sample A, given the interpretation of the relationships between items and latent factors described in Tab. 3, the domains were re-labelled as follows to capture the meaning of the items: (a) Healthcare education; (b) clinical support; (c) emotional support, and (d) continuum of care. However, items 9 (i.e., make an effort to facilitate parent–child interaction, such as communicating worries) and 12 (i.e., improve self-assessment and self-control abilities and engage in a physical activity that enhances health) showed important cross-loadings. Then, items 9 and 12 were considered ambiguous as their wording encompassed meanings ranging from the assessment to the continuum of care. Accordingly, items 9 and 12 were removed before the subsequent data collection for the next validation stage.
The second cross-sectional data collection (sample B) comprised 141 young patients with CHD. Tab. 2 shows the socio-demographic and clinic characteristics of the enrolled patients. The confirmatory model (χ2(73) = 186.801, p < 0.0001; χ2/df = 2.56; RMSEA = 0.105; 90% CI [0.087–0.124]; CFI = 0.956; TLI = 0.921; SRMR = 0.055) showed evidence of the adequacy of the four-factor model, with all items significantly retained on the respective domain (Tab. 3). A second-order CFA was also performed to determine the presence of a single second-order factor for explaining the relationship between first-order factors (χ2(73) = 189.761, p < 0.0001; χ2/df = 2.59; RMSEA = 0.103; 90% CI [0.087–0.125]; CFI = 0.956; TLI = 0.921; SRMR = 0.055). The χ2 difference test between the two models showed no significant difference, thus indicating that both models explained data as well. Given the presence of a second-order factor, a total score of healthcare needs was also computed. Overall, I-HNS-CHD-s showed adequate internal consistency [mean(standardised domain)(SD) = 70.02 (16.41); McDonald’s ω = 0.76], as well as each sub-scale domains: Healthcare education reported the McDonald’s ω = 0.84 [mean(standardised domain)(SD) = 78.33 (17.21)]; clinical support reported the McDonald’s ω = 0.71 [mean(standardised domain)(SD) = 68.20 (21.68)]; emotional support showed the McDonald’s ω = 0.65 [mean(standardised domain)(SD) = 70.80 (20.13)]; continuum of care had the McDonald’s ω = 0.71 [mean(standardised domain)(SD) = 57.80 (23.32)].

This study was designed to validate HNS-CHD into Italian and to provide evidence of the validity and reliability of the adapted, translated and shortened scale (I-HNS-CHD-s). Given that the transition from childhood to adulthood of patients with CHD involves different aspects of life (e.g., morphological, sexual, psychological changes), measuring their healthcare and psychosocial needs is pivotal to address the challenges perceived by patients through specific supportive and educational strategies aimed at enhancing patients’ self-resources [18].
This study demonstrated that I-HNS-CHD-s has adequate validity and reliability. The validation process was aimed at adapting the original scale into the Italian context, maintaining the contents derived from the original items [22]. However, the final number of items included in the last validation step and the dimensionality of I-HNS-CHD-s differ from the original scale [21]. Accordingly, the original scale comprised of 25 items kept by three domains (i.e., health management, health policy, and individual and interpersonal relationships), while the I-HNS-CHD-s encompassed 14 items kept by four domains. The four domains of I-HNS-CHD-s are healthcare education, clinical support, emotional, and continuum of care. Specifically, healthcare education and clinical support kept the items that were retained by the domain of health management in the original study [21]. Likely, emotional support embodied some items that were previously retained by the domain of health policy, specifying a narrower field of policy in the current study, as per the meaning of the items that highlight the patients’ need for being supported by healthcare professionals. The domain of continuum of care is mainly based on the items that were previously kept by the domain of individual and interpersonal relationships; more precisely, it represents a narrower filed of the previously domain of relationships, as it highlights those relationships needed to guarantee a continuity of care [21]. Thus, the Italian version is a shorter version of the original scale. The differences related to the factor structure between I-HNS-CHD-s and HNS-CHD could be explained by two main reasons.
First, according to the study performed by Chen et al., the original HNS-CHD exhibited evidence of initial validation. Its psychometric structure was assessed by a principal component analysis, which is an approach mainly useful for achieving data reduction, by extracting linear composites of observed variables (items) for maximizing their explained total variance [41]. As this approach simplifies the information detected by the items into main components, it helps to achieve an initial validation, but it lacks in determining the nature of a latent variable that linearly influences the items [41]. In other words, the principal component analysis helps to reduce multiple items into fewer components that summarize their variance, while EFA using maximum likelihood determines the nature of and the number of latent variables (theoretical scale domains) that account for observed variation and covariation among items. For this reason, our study further explored the psychometric nature of the scale, as EFA using maximum likelihood allows researchers to identify the domains (latent factors) that predict the patterns of correlation among items, and to determine the psychometric nature of the domains. Furthermore, as this study has a multi-phase design, a second data-collection was used to perform confirmative analysis for corroborating the results of EFA. I-HNS-CHD-s is a shorter version of HNS-CHD where the items that showed lower psychometric performance were deleted to generate the final version of the scale. Second, the context of the investigation could influence the psychometric structure of a self-report scale [42], as cross-national and cross-cultural research is needed to determine whether adolescents and young adults with CHD of different cultures similarly interpret the items.
Overall, higher scores in each domain indicate higher needs. Considering the descriptive statistics derived from the scores in this study, it seems that the need for healthcare education represented a priority for the responders, followed by the need for emotional support and clinical support. The need for interventions to sustain the continuum of care reported the lowest mean score. These results are consistent with previously described evidence about the specific educational interventions aimed at enhancing healthy behaviours among Italian adolescents with CHD, in which the need for healthcare education was considered a priority [7,8], as well as it was also described in other cardiovascular diseases [43]. I-HNS-CHD-s has the potential of assessing and, subsequently, addressing the healthcare and psychosocial needs in a tailored way, as it allows clinicians to detect the needs that are more perceived by adolescents and young adults with CHD.
This study has some limitations. Firstly, caution is required in generalizing the study’s results, as the study was comprised of a sample of Italian patients; for this reason, we have no information about the measurement equivalence of the psychometric structure derived from this research. Future research should test the different levels of the measurement equivalence of I-HNS-CHD-s, such as configural invariance, metric invariance, scalar invariance, and strict invariance. These further tests could clarify whether the adopted psychometric structure of I-HNS-CHD-s could be used in different contexts and cultures. However, we can consider the adopted analytical approach as the most prudent method in assessing the psychometric structure of the scale, even if cross-national research is required to verify whether the items are perceived in the same way by patients when translated in other languages. Secondly, the enrolled samples (samples A and B) were selected using a convenience approach and a cross-sectional data collection; for this reason, we were not able to perform factor analysis with longitudinal data to detect the psychometric structure of the scale over time.
We recommend standardizing each domain score to 0–100, as well as the overall scale score. I-HNS-CHD-s does not include items to be reversed. For this reason, the scoring procedure is described as follows: Healthcare education = [sum (item 1, item 2, items 4–6)–5) × (100/20)]; Clinical support = [sum (item 3, item 8, item 9)–3) × (100/12)]; Emotional support = [sum (item 7, item 10, item 11)–3) × (100/12)]; Continuum of care = [sum (items 12–14)–3) × (100/12)]. Total score I-HNS-CHD-s = [sum (items 1–14)–14) × (100/56)].
I-HNS-CHD-s encompassed 14 items, which were explained by four domains: Healthcare education, clinical support, emotional support, and continuum of care. Overall, I-HNS-CHD-s showed adequate evidence of validity and reliability. I-HNS-CHD-s could be used by clinicians when it needed the assessment of healthcare and psychosocial needs of adolescents with CHD. Each domain represents a cluster of healthcare and psychosocial needs perceived by adolescents with CHD. I-HNS-CHD-s is a short-form of the HNS-CHD and it presents an updated factor structure that allows different scorings. Given that the factor structure derived from this study supports a new approach to score the healthcare and psychosocial needs of adolescents and young adults with CHD, future research could be useful to test at an international level the new factor structure for its measurement equivalence.
CHD = congenital heart disease
HNS-CHD = healthcare needs scale for youth with CHD
I-HNS-CHD-s = shortened and Italian version of the healthcare needs scale for youth with CHD
CVR = content validity ratio
I-CVIs = content validity indices (item-level)
S-CVI = content validity indices (scale-level)
BMI = body mass index
EFA = exploratory factor analysis
CFA = confirmatory factor analysis
KMO = Kaiser-Meyer-Olkin index
CFI = comparative fit index
RMSEA = root mean square error of approximation
WRMR = weighted root mean square residual
SD = standard deviation
Acknowledgement: We wish to thank all the study participants and the data managers for the support in collecting and record the case form reports.
Data Sharing: We intend to make data freely available in the public domain after publication of major findings. Researchers interested in collaborations should contact the corresponding author.
Funding Statement: This research was partially supported by ‘Ricerca Corrente’ funding from Italian Ministry of Health to IRCCS Policlinico San Donato.
Conflicts of Interest: The authors declare no conflict of interest.
1. Flocco, S. F., Lillo, A., Dellaa, F., Goossens, E. (2019). The nursing care handbook Congenital Heart Disease. USA: Springer International Publishing. [Google Scholar]
2. Flocco, S. F., Caruso, R., Barello, S., Nania, T., Simeone, S. et al. (2020). Exploring the lived experiences of pregnancy and early motherhood in Italian women with congenital heart disease: An interpretative phenomenological analysis. British Medical Journal Open, 10(1), 1–9. DOI 10.1136/bmjopen-2019-034588. [Google Scholar] [CrossRef]
3. Pei, L., Kang, Y., Zhao, Y., Yan, H. (2017). Prevalence and risk factors of congenital heart defects among live births: a population-based cross-sectional survey in Shaanxi province, Northwestern China. BMC Pediatrics, 17(1), 1–8. DOI 10.1186/s12887-017-0784-14. [Google Scholar] [CrossRef]
4. van der Bom, T.,Zomer, A. C., Zwinderman, A. H., Meijboom, F. J., Bouma, B. J. et al. (2011). The changing epidemiology of congenital heart disease. Nature Reviews Cardiology, 8(1), 50–60. DOI 10.1038/nrcardio.2010.166. [Google Scholar] [CrossRef]
5. van der Linde, D.,Konings, E. E. M., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of Congenital Heart Disease Worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/J.JACC.2011.08.025. [Google Scholar] [CrossRef]
6. Baumgartner, H., Budts, W., Chessa, M., Deanfield, J., Eicken, A. et al. (2014). Recommendations for organization of care for adults with congenital heart disease and for training in the subspecialty of “Grown-up Congenital Heart Disease” in Europe: A position paper of the Working Group on Grown-up Congenital Heart Disease of the Euro. European Heart Journal, 35(11), 686–690. DOI 10.1093/eurheartj/eht572. [Google Scholar] [CrossRef]
7. Flocco, S. F., Giamberti, A., Micheletti, A., Negura, D. G., Piazza, L. et al. (2018). The effect of the transition care model on health perception among adolescents with congenital heart disease: A quasi-experimental study. Giornale Italiano Di Cardiologia, 19(6), 386–393. DOI 10.1714/2922.29372. [Google Scholar] [CrossRef]
8. Flocco, S. F., Dellafiore, F., Caruso, R., Giamberti, A., Micheletti, A. et al. (2019). Improving health perception through a transition care model for adolescents with congenital heart disease. Journal of Cardiovascular Medicine, 20(4), 253–260. DOI 10.2459/JCM.0000000000000770. [Google Scholar] [CrossRef]
9. Lyon, M. E., Kuehl, K., McCarter, R. (2006). Transition to adulthood in Congenital Heart Disease: missed adolescent milestones. Journal of Adolescent Health, 39(1), 121–124. DOI 10.1016/j.jadohealth.2005.09.008. [Google Scholar] [CrossRef]
10. Mackie, A. S., Rempel, G. R., Islam, S., Rankin, K., Mccurdy, C. et al. (2012). Psychosocial maturity, autonomy, and transition readiness among young adults with congenital heart disease or a heart transplant. Congenit Heart Disease, 11(2), 136–143. DOI 10.1111/chd.12300. [Google Scholar] [CrossRef]
11. Teixeira, F. M., Coelho, R. M., Proença, C., Silva, A. M., Vieira, D. et al. (2011). Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatric Cardiology, 32(8), 1132–1138. DOI 10.1007/s00246-011-0039-0. [Google Scholar] [CrossRef]
12. Sparacino, P. S. A., Tong, E. M., Messias, D. A. K. H., Foote, D., Chesla, C. A. et al. (1997). The dilemmas of parents of adolescents and young adults with congenital heart disease. Heart and Lung: Journal of Acute and Critical Care, 26(3), 187–195. DOI 10.1016/S0147-9563(97)90055-8. [Google Scholar] [CrossRef]
13. Lesch, W., Specht, K., Lux, A., Frey, M., Utens, E. et al. (2008). Disease-specific knowledge and information preferences of young patients with congenital heart disease. Cardiology in the Young, 24(2), 321–330. DOI 10.1017/S1047951113000413. [Google Scholar] [CrossRef]
14. Gurvitz, M. Z., Inkelas, M., Lee, M., Stout, K., Escarce, J. et al. (2007). Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. Journal of the American College of Cardiology, 49(8), 875–882. DOI 10.1016/j.jacc.2006.09.051. [Google Scholar] [CrossRef]
15. Kools, S., Tong, E. M., Hughes, R., Jayne, R., Gilliss, C. L. (2002). Hospital experiences of young adults with congenital heart disease: divergence in expectations and dissonance in care. American Journal of Critical Care, 11(2), 115–127. [Google Scholar]
16. Reid, G. J., Webb, G. D., McCrindle, B. W., Irvine, M. J., Siu, S. C. (2008). Health behaviors among adolescents and young adults with congenital heart disease. Congenital Heart Disease, 3(1), 16–25. DOI 10.1111/j.1747-0803.2007.00161.x. [Google Scholar] [CrossRef]
17. Flocco, S. F., Caruso, R., Dellafiore, F., Orlando, A., Magon, A. et al. (2017). Towards the standardization of transition care models for adolescents with Congenital Heart Disease: A perspective. Journal of Clinical & Experimental Cardiology, 08(01), 1–3. DOI 10.4172/2155-9880.1000495. [Google Scholar] [CrossRef]
18. Chen, C. W., Su, W. J., Chiang, Y. T., Shu, Y. M., Moons, P. (2017). Healthcare needs of adolescents with congenital heart disease transitioning into adulthood: A Delphi survey of patients, parents, and healthcare providers. European Journal of Cardiovascular Nursing, 16(2), 125–135. DOI 10.1177/1474515116643622. [Google Scholar] [CrossRef]
19. Saidi, A., Kovacs, A. H. (2009). Developing a transition program from pediatric- to adult-focused cardiology care: Practical considerations. Congenital Heart Disease, 4(4), 204–215. DOI 10.1111/j.1747-0803.2009.00312.x. [Google Scholar] [CrossRef]
20. Meadows, A. K., Bosco, V., Tong, E., Fernandes, S., Saidi, A. (2009). Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiology Reports, 11(4), 291–297. DOI 10.1007/s11886-009-0042-8. [Google Scholar] [CrossRef]
21. Chen, C. W., Ho, C. L., Su, W. J., Wang, J. K., Chung, H. T. et al. (2017). Initial validation of a healthcare needs scale for youth with congenital heart disease. Journal of Advanced Nursing, 74(1), 223–231. DOI 10.1111/jan.13390. [Google Scholar] [CrossRef]
22. Rattray, J., Jones, M. C. (2007). Essential elements of questionnaire design and development. Journal of Clinical Nursing, 16, 234–243. DOI 10.1111/j.1365-2702.2006.01573.x. [Google Scholar] [CrossRef]
23. Jones, P. S., Lee, J. W., Phillips, L. R., Zhang, X. E., Jaceldo, K. B. (2001). An adaptation of Brislin’s translation model for cross-cultural research. Nursing Research, 50(5), 300–304. [Google Scholar]
24. Caruso, R., Arrigoni, C., Groppelli, K., Magon, A., Pittella, F. et al. (2017). Italian version of Dyspnoea-12: cultural-linguistic validation, quantitative and qualitative content validity study. Acta Bio Medica, 88(6), 1–9. DOI 10.23750/abm.v88i4.6341. [Google Scholar] [CrossRef]
25. Upadhyaya, A. K., Rajagopal, M., Gale, T. M. (2010). The six item cognitive impairment test (6-cit) as a screening test for dementia: comparison with mini-mental state examination. Current Aging Science, 3(2), 138–142. DOI 10.2174/1874609811003020138. [Google Scholar] [CrossRef]
26. Hallgren, K. A. (2012). Computing inter-rater reliability for observational data: an overview and tutorial. Tutorials in Quantitative Methods for Psychology, 8(1), 23–34. [Google Scholar]
27. Lawshe, C. (1975). A quantitative approach to content validity. Personnel Psychology, 28, 563–575. [Google Scholar]
28. Polit, D. F., Beck, C. T. (2006). The content validity index: Are you sure you know what’s being reported? Critique and recommendations. Research in Nursing Health, 29(5), 489–497. DOI 10.1002/nur.20147. [Google Scholar] [CrossRef]
29. Ayre, C., Scally, A. J. (2014). Critical values for Lawshe’s content validity ratio: revisiting the original methods of calculation. Measurement and Evaluation in Counseling and Development, 47(1), 79–86. DOI 10.1177/0748175613513808. [Google Scholar] [CrossRef]
30. Vaismoradi, M., Turunen, H., Bondas, T. (2013). Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nursing & Health Sciences, 15(3), 398–405. DOI 10.1111/nhs.12048. [Google Scholar] [CrossRef]
31. Warnes, C. A., Liberthson, R., Danielson, G. K., Dore, A., Harris, L. et al. (2001). Task force 1: the changing profile of congenital heart disease in adult life. Journal of the American College of Cardiology, 37(5), 1170–1175. [Google Scholar]
32. Stout, K. K., Daniels, C. J., Chair, V., Aboulhosn, J. A., Bozkurt, B. et al. (2018). AHA/ACC guideline for the management of adults with congenital heart disease. Circulation, 139, 698–800. DOI 10.1161/CIR.0000000000000603. [Google Scholar] [CrossRef]
33. Hair, J., Black, W., Babin, B., Anderson, R. (2010). Multivariate data analysis: A global perspective. 7th edition. USA: Pearson Prentice Hall. [Google Scholar]
34. Watkins, M. W. (2018). Exploratory factor analysis: A guide to best practice. Journal of Black Psychology, 44(3), 219–246. DOI 10.1177/0095798418771807. [Google Scholar] [CrossRef]
35. Kim, H., Sefcik, J. S., Bradway, C. (2017). Characteristics of qualitative descriptive studies: A systematic review. Research in Nursing & Health, 40(1), 23–42. DOI 10.1002/nur.21768. [Google Scholar] [CrossRef]
36. Wilson Van Voorhis, C. R., Morgan, B. L. (2007). Understanding power and rules of thumb for determining sample sizes. Tutorials in Quantitative Methods for Psychology. DOI: 10.20982/tqmp.03.2.p043. [Google Scholar]
37. Revelle, W., Zinbarg, R. E. (2009). Coefficients alpha, beta, omega, and the glb: Comments on sijtsma. Psychometrika, 74(1), 145–154. DOI 10.1007/s11336-008-9102-z. [Google Scholar] [CrossRef]
38. Terwee, C. B., Bot, S. D. M., de Boer, M. R.,van der Windt, D. A. W. M.,Knol, D. L. et al. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60(1), 34–42. DOI 10.1016/j.jclinepi.2006.03.012. [Google Scholar] [CrossRef]
39. Jones, P. S., Lee, J. W., Phillips, L. R., Zhang, X. E., Jaceldo, K. B. (2001). An adaptation of Brislin’s translation model for cross-cultural research. Nursing Research, 50(5), 300–304. [Google Scholar]
40. Gilbert, G. E., Prion, S. (2016). Making sense of methods and measurement: Lawshe’s content validity index. Clinical Simulation in Nursing, 12(12), 530–531. [Google Scholar]
41. Alavi, M., Visentin, D. C., Thapa, D. K., Hunt, G. E., Watson, R. et al. (2020). Exploratory factor analysis and principal component analysis in clinical studies: Which one should you use? Journal of Advanced Nursing, 1–4. DOI 10.1111/jan.14377. [Google Scholar] [CrossRef]
42. Putnick, D. L., Bornstein, M. H. (2016). Measurement invariance conventions and reporting: the state of the art and future directions for psychological research. Developmental Review, 41, 71–90. DOI 10.1016/j.dr.2016.06.004.Measurement. [Google Scholar] [CrossRef]
43. Dellafiore, F., Arrigoni, C., Pittella, F., Conte, G., Magon, A. et al. (2018). Paradox of self-care gender differences among Italian patients with chronic heart failure: findings from a real-world cross-sectional study. British Medical Journal Open, 8(9), 1–7. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |