

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012197
ARTICLE
Minimally Invasive Congenital Cardiac Surgery: A Large Volume European Experience
1Labatt Family Heart Centre, Department of Cardiovascular Surgery, The Hospital for Sick Children, University of Toronto, Toronto, M5G 1X8, Canada
2Pediatric and Congenital Cardiac Surgery Unit, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padua, Padua, 35128, Italy
3Department of Cardiac Surgery, Boston Children’s Hospital, Department of Surgery, Harvard Medical School, Boston, 02115, USA
4Department of Surgery, Columbia University Medical Center, New York, NY, 10032, USA
*Corresponding Author: Vladimiro L. Vida. Email: vladimiro.vida@unipd.it
Received: 19 June 2020; Accepted: 09 July 2020
Abstract: Background: In an effort to reduce postoperative trauma and achieve more cosmetic results, minimally invasive approaches to correct congenital heart anomalies have been recently proposed and increasingly adopted. Here we describe our experience for the past 23 years. Methods: Patients who underwent a surgical procedure between February 1996 and March 2019 with a minimally invasive approach for the correction of congenital heart disease in our center were included in this study. A statistical analysis was carried out to compare the results of the different minimally invasive techniques. A meta-analysis was conducted to compare our results in patients undergoing atrial septal defect repair with those from other groups. Results: There were 1002 patients included. A midline lower mini-sternotomy was performed in 45% of patients (n = 455), a right anterior mini-thoracotomy in 36% (n = 356) and a right lateral mini-thoracotomy in 19% (n = 191). The procedures were atrial septal defect repair (n = 575, 57%), ventricular septal defect repair (n = 218, 22%), and correction of atrioventricular defect (n = 82, 8%) or partial anomalous pulmonary venous return (n = 70, 7%). Post-cardiotomy syndrome was the most frequent complication (n = 40, 4%). No difference was observed between the approaches in terms of complications and peri-operative outcomes, and when these were compared with the results of other centers. Conclusions: Patients undergoing surgical repair of congenital heart disease through a minimally invasive approach have excellent outcomes, regardless of the approach used.
Keywords: Congenital heart disease; minimally invasive surgery; surgical outcomes
In 1897, decades before conventional cardiac surgery had even reached its infancy, a median sternotomy was proposed as the best surgical approach to the heart [1]. After the advent and eventual proliferation of open-heart surgery in the mid-1950s, median sternotomy became the most common approach. Through a median sternotomy, the heart is easily accessed and visualized. Median sternotomy has thus been the standard approach for surgical treatment of all congenital and acquired heart diseases for many years [2,3]. More recently minimally invasive cardiac surgery (MICS) has emerged with aims to minimize physical trauma, particularly in the pediatric population, and enhance postoperative recovery [4]. Progress in MICS has been made possible through the parallel development of surgical instrumentation and perfusion strategies that are specifically tailored to MICS [5,6].
To our knowledge, there are minimal reports of large volume, long term experiences with MICS for congenital heart disease (CHD) in North America. We have treated CHD, both simple and complex, through MICS strategies for over 20 years and in more than 1000 patients. From the beginning of our experience, there has been a constant evolution with a progressive improvement and introduction of new access strategies including lower midline mini-sternotomies (MS), right anterior mini-thoracotomies (RAMT) and right lateral mini-thoracotomies (RLMT) [6–13].
The purpose of this study is to describe our vast experience with MICS and provide a broad overview of the multiple techniques used in our center for MICS in the CHD population.
A review of medical records was approved by the hospital committee on clinical investigation and publication was approved by the institutional ethics committee chair (protocol number 4482/AO/18). All patients in our center who underwent a surgical procedure for CHD between February 1996 and March 2019 via MICS were included.
2.2 Minimally Invasive Surgery Protocol
Patients requiring a surgical procedure for CHD repair are regularly evaluated for MICS in our institution. A thorough discussion is held with patients and families on the various surgical approaches available and a specific informed consent process is followed if a minimally invasive strategy is chosen.
The surgical approach is chosen based on numerous demographic characteristics including age, sex and patient preferences. When feasible, we also employ a “sex-differentiated approach” in which we attempt to honor the preferences of the different genders based on cosmetic considerations [8].
Patients are all managed under the same postoperative protocol. This includes early extubation (<6 hours after surgery) and early discharge from the intensive care unit (ICU <24 hours) when appropriate. Postoperative pain is controlled by intravenous nonsteroidal anti-inflammatory medications (ketorolac 0.2 mg/kg) every 6 hours. Upon discharge, all patients are then followed up at 1 month, 1 year, and 3 years after surgery, at which point an assessment of cosmesis is included in the clinical evaluation. In patients who underwent mini-thoracotomies, the postoperative evaluations include a specific assessment of the presence of scoliosis, any limitations of shoulder mobility, and any sensory deficits in mammary development.
Cardiopulmonary bypass (CPB) via direct aortic cannulation and bicaval cannulation under mild hypothermia (rectal temperature 34°C) is the standard, and mainly used in small infants. Vacuum-assisted venous drainage (between –30 and –50 mmHg maximum pressure) is routinely utilized to reduce the caliber of the venous cannula that is necessary. In some cases, the inferior vena cava cannula is inserted into the chest through a separate 5 mm skin incision below the primary incision. This site is then used for chest drainage at the end of the operation.
In patients with a body weight above 15 kg a remote cardiopulmonary bypass strategy with peripheral cannulation is used. In such cases, arterial and venous femoral vessels are surgically isolated through a 1.5 to 2 cm incision in the inguinal fold. The right common femoral artery is cannulated directly while the femoral vein is accessed under 2D-echo guidance with the Seldinger technique to position the tip of the venous cannula at the inferior vena cavo-atrial junction. The venous return of the upper part of the body is generally drained by cannulating the superior vena cava. The internal jugular vein is the preferred access site for venous drainage from the upper body in case of partial anomalous pulmonary venous return (PAPVR) [12]. Near-infrared spectroscopy with a cerebral oximeter (INVOS 5100, Medtronic, Dublin, Ireland) is used to continuously monitor oxygen saturation in the lower extremities during CPB, when peripheral arterial cannulation is used.
Intracardiac repair in patients with atrial septal defect (ASD) is achieved in simple CHD by using induced ventricular fibrillation (IVF) with an epicardial lead. In more complex procedures aortic cross-clamping (CC) with cardioplegic arrest if preferred.
All patients weighing more than 5 kg are monitored with transesophageal echocardiography, particularly when attempting to wean from CPB, to visualize any retained air in the left side of the heart. For patients weighing less than 5 kg, intraoperative epicardial echocardiography is performed.
MS, RAMT, and RLMT are used variably depending on the anatomic lesions and can be employed for the surgical correction of simple CHDs such as ASD, ventricular septal defects (VSD), or PAPVR. Generally, MS or RAMT, are used for more complex CHD lesions such as partial atrioventricular septal defects (pAVSD) or procedures on the left ventricular outflow tract.
2.3.1 Midline Lower Mini-Sternotomy (MS)
A straight 3–4 cm skin incision is made in the midline, starting about 2 cm below the transverse nipple line. The sternum is then divided longitudinally in its lower third and retracted to expose the aorta [8,9]. In patients over 20 kg an additional transverse incision is made in the lower third of the sternum, creating a T-shape to overcome the stiffness of the mature sternum and allow for adequate retraction.
2.3.2 Right Anterior Mini-Thoracotomy (RAMT)
A semilunar 4 to 5 cm semilunar incision is created on the sub-mammary sulcus entering the chest of the fourth intercostal space. In the prepubertal age, this incision is kept very low under the right nipple (usually above the 5th intercostal space) to avoid interference with the development of the future breast tissue [9,11]. Subcutaneous fat and mammary tissue can usually be spared by gently dissecting such tissue away from the point of entry. The incision in the intercostal space is usually carried approximately 1 cm beyond the length of the skin incision at each end.
2.3.3 Right Lateral Mini-Thoracotomy (RLMT) or Subaxillary Approach
A transverse semi-lunar incision of 3–4 cm in length is created in the right thorax in the subaxillary position. The incision is initiated in the midaxillary line, then extended towards the anterior axillary line, following the fourth or fifth intercostal space according to the anatomic lesion being addressed. The incision in the intercostal space is usually carried approximately 1 cm beyond the length of skin incision on each end [7,13].
Continuous variables are expressed as median ± interquartile range (IQR), Categorical variables are presented as absolute and relative frequencies (%). All continuous variables were normally distributed as tested by Shapiro-Wilk test and graphically represented by histograms and Q-Q plots. Variables were analyzed with regard to the surgical access (MS, RAMT and RLMT). Between-group comparisons were performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s correction for continuous variables. Fisher’s exact test or Pearson’s chi-square were used for nominal variables. Multivariate linear or logistic regression was used to assess the correlation between possible risk factors and the continuous or binomial dependent variable, respectively. The level of significance was set at p-value ≤ 0.05. Statistical analyses were performed with STATA 15.0 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Odds ratio (ORs) and 95% confidence intervals (CI) were calculated by 2 × 2 tables for each categorical outcome; OR > 1 denoted an outcome more frequently present in the available literature. Hedge’s g statistic with its 95% CI were calculated for length of stay; Hedge’s g > 0 corresponded to larger values in the available literature. Between-study heterogeneity was assessed through Cochran Q statistic and by estimating I2. We used Random-effects (DerSimonian-Laird) models to calculate pooled effect estimates.
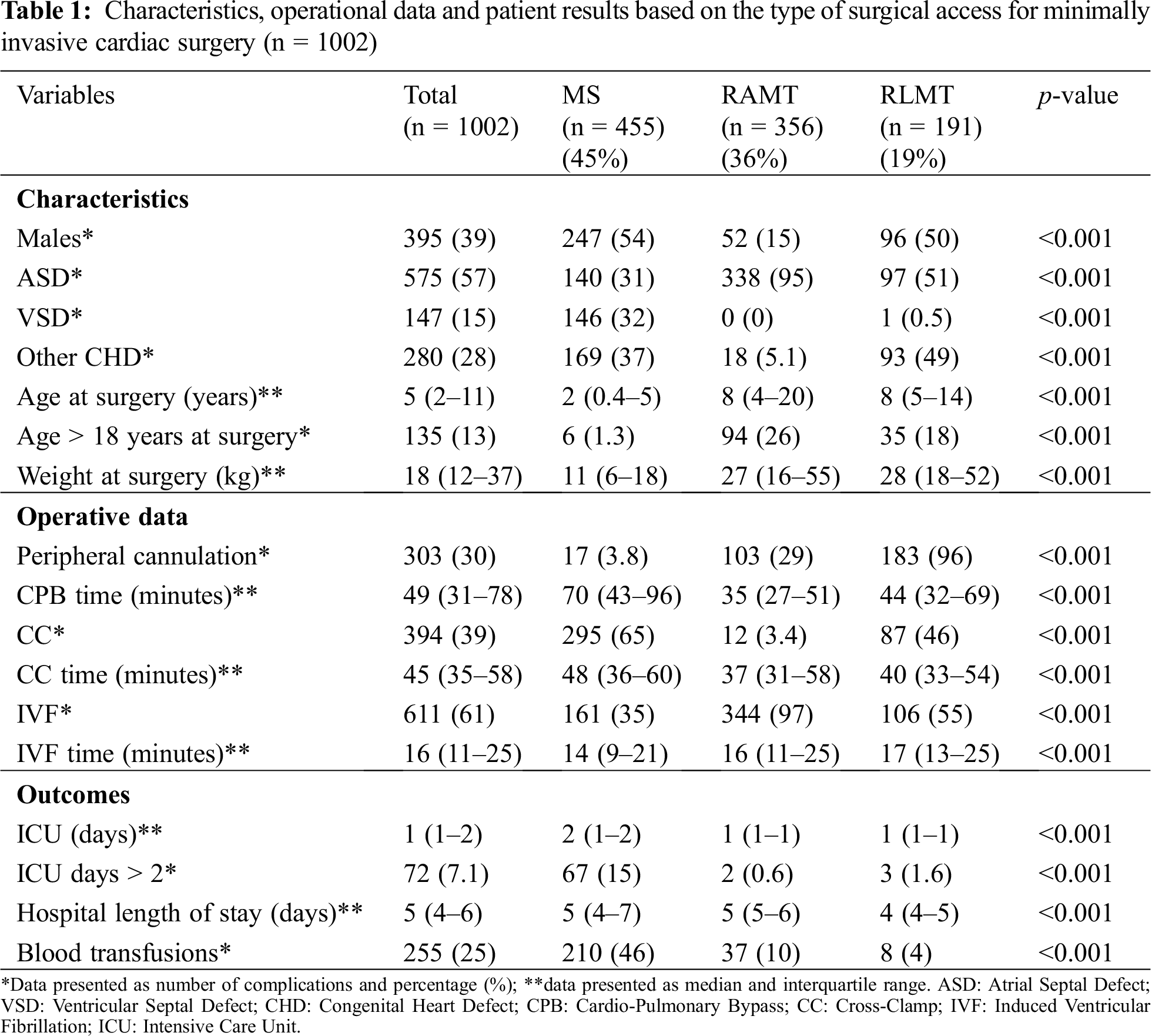
A total of 1,002 patients were included. Of these, 455 (45%) underwent MS, 356 (36%) underwent RAMT and 191 (19%) underwent RLMT (Fig. 1). No case required conversion to full median sternotomy (FMS). Genders were equally distributed in the MS and RLMT group (54% and 50%, respectively), while females were predominant in the RAMT group (85%) (p < 0.001). Median age at surgery was 2 years (IQR 0.4–5) in the MS group, and 8 years both for RAMT (IQR 4–20) and RLMT (IQR 5–14) (p < 0.001). Weight at surgery was 11 kg in the MS group (IQR 6–18), 27 kg in the RAMT group (IQR 16–55) and 28 kg in the RLMT group (IQR 18–52) (p < 0.001) (Tab. 1).
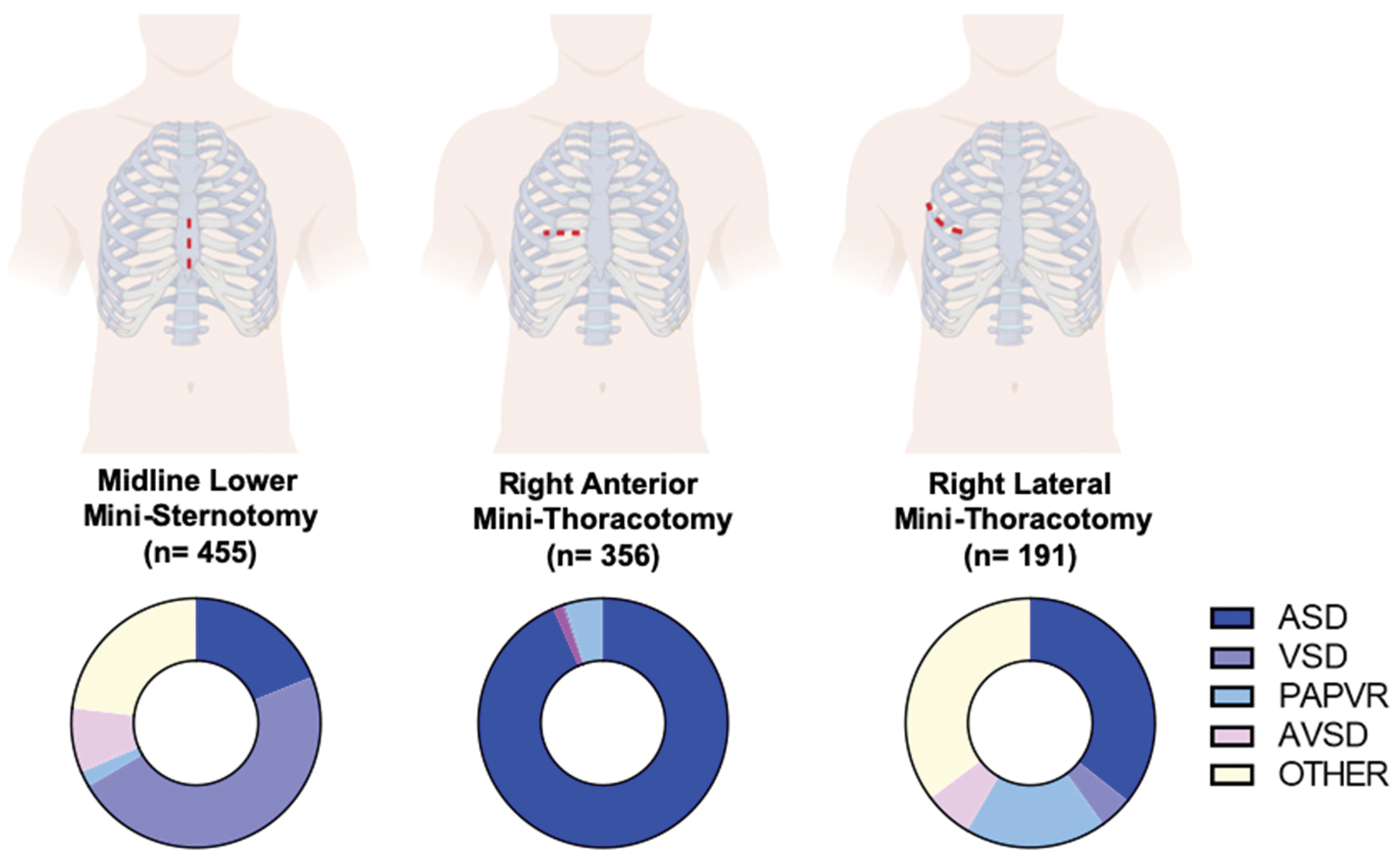
Figure 1: Access for minimally invasive cardiac surgery and diagnoses (Graphical representation of the three different type of access for minimally invasive cardiac surgery used. On the bottom, pie charts illustrate the distribution of diagnoses according to surgical access. ASD: Atrial Septal Defect; VSD: Ventricular Septal Defect; PAPAVR: Partial Anomalous Pulmonary Venous Return; pAVSD: Partial Atrioventricular Septal Defect)
The percentage of minimally invasive procedures in our center (based on the total number of major surgeries) was 16.1% during the first decade (1996–2007) and 22.3% during the second part of the study (2008–2019). Between 1996–1997, 55% of ASD closures were performed through MICS. Since 1998 all ASD patients have been treated with MICS.
MS was the MICS incision of choice until 2009 and was particularly dominant during the period 2005–2009 (Fig. 2). RAMT was equally distributed throughout the study period. RLMT has become increasingly more prevalent approach since 2015. There was a significant correlation between the type of surgical access and the year in which the surgery was performed (p < 0.001).
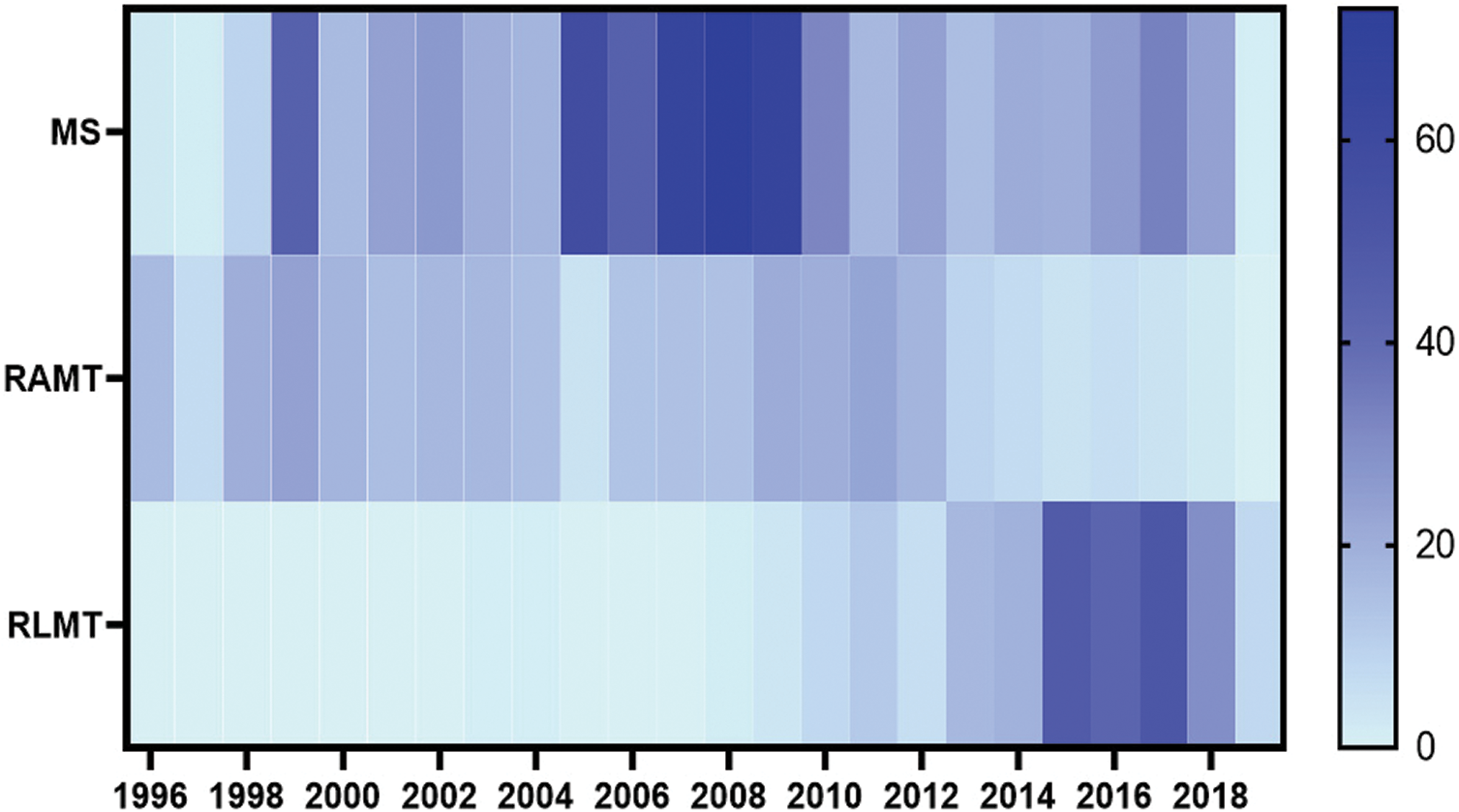
Figure 2: Timeline of surgical access for minimally invasive cardiac surgery (Heatmap illustrating the distribution of the MS, RAMT and RLMT for minimally invasive cardiac surgery throughout the study period (1996–2019). MS: Mini-sternotomy; RAMT: Right anterior mini thoracotomy; RLMT: Right lateral mini-thoracotomy)
Atrial septal defect was the most common diagnosis in the RAMT and RLMT groups (338/356, 95% and 97/191, 51%, respectively) (p < 0.001). VSD was most common (48%) in the MS group. As for complex CHD, pAVSD was prevalent in less than 10% of the population regardless of the surgical access, whereas PAPVR was present in 50/191 (26%) of the RLMT cases (Tab. 1). Other diagnoses were less commonly approached via MICS (Fig. 1S, Appendix A).
3.4 Peri-Operative Characteristics
Central cannulation was commonly used in MS and RAMT (438/455, 96%; 253/356, 71%, respectively), while peripheral cannulation was utilized in the majority of the RLMT group (183/191, 96%) (p < 0.001). Median CC time ranged between 37 min to 48 min in the three groups, and time of IVF ranged between 14 min to 17 min (p < 0.001). Blood transfusions were required in 210/455 (46%), 37/356 (10%) and 8/191 (4%) patients in the MS, RAMT and RLMT groups, respectively (p < 0.001). Median ICU stay was 1 day for RAMT (IQR 1–1) and RLMT (IQR 1–1), and 2 days for MS (IQR 1–2) (p < 0.001). Median length of stay was 5 days for the RAMT (IQR 5–6) and MS (IQR 4–7) and was 4 days for RLMT (IQR 4–5) (p < 0.001) (Tab. 1).
3.5 Mortality and Postoperative Complications
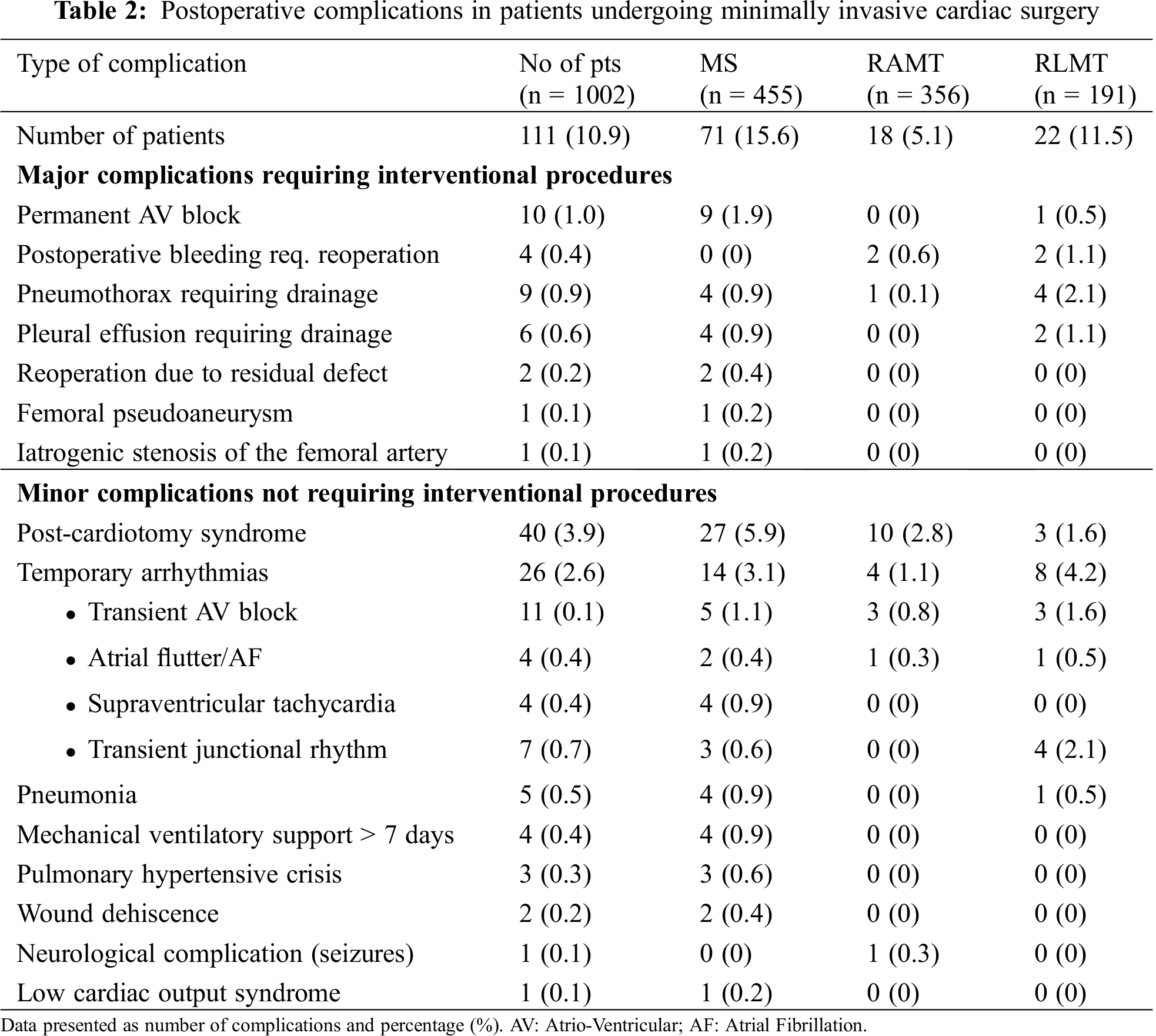
No peri-operative or in-hospital deaths occurred. Overall, 111 patients (11%) had at least one postoperative complication, with post-cardiotomy syndrome being the most common (40/1,002, 3.9%). Temporary arrhythmia was the second most common complication (2.6%) and was most common in the RLMT group (4.7%). Other minor complications included pneumonia, pneumothorax, postoperative bleeding requiring re-operation, mechanical ventilatory support for more than 7 days, neurological complications, wound dehiscence and low cardiac output syndrome, each having an incidence lower than 1% (Tab. 2).
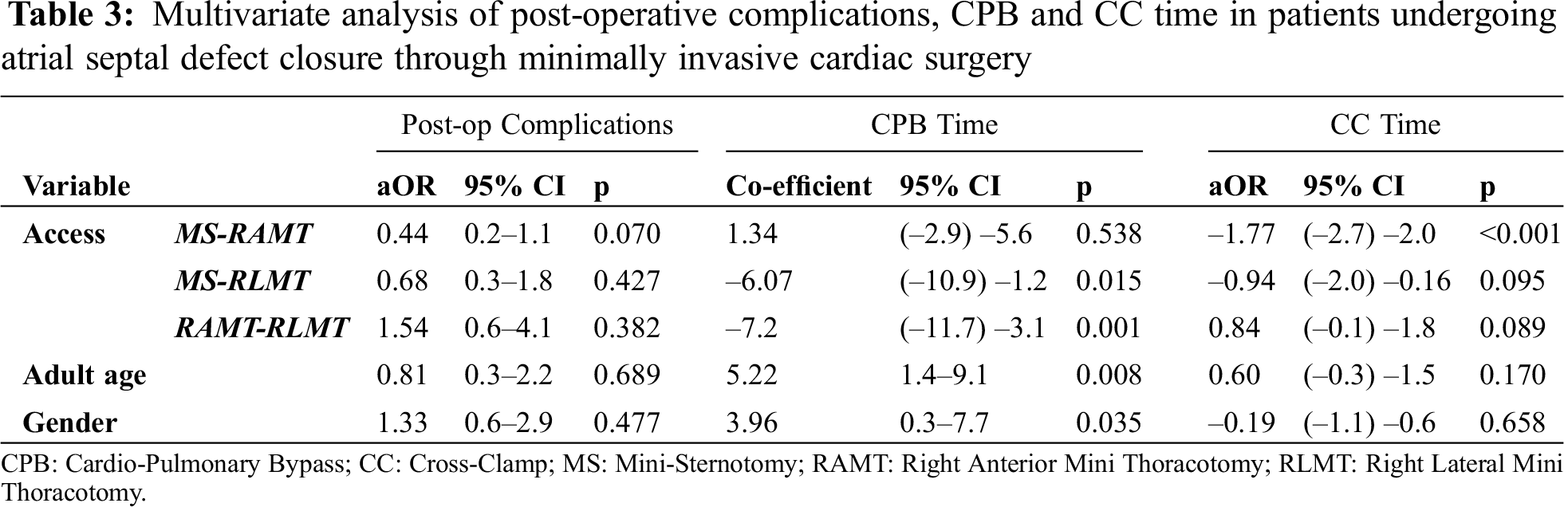
3.6 Multivariate Analysis of ASD Cases
In order to control for any confounding effect of the different diagnoses and confirm our results, a multivariate logistic regression analysis of only patients who underwent ASD closure was performed (Tab. 3). No difference in the percentage of postoperative complications was noted between MS, RAMT and RLMT when adjusted for age and gender. Similarly, no difference was found in the CC time. CPB time was shorter in RLMT than in the MS and RAMT (p = 0.015 and p = 0.001, respectively).
3.7 Meta-Analysis of ASD Cases
Meta-analysis of the existing literature demonstrated that our cohort of ASD patients had a shorter CPB time (Hedge’s g: 1.96; 95% CI: 1.10–2.81, p < 0.01), IVF time (Hedge’s g: 2.31; 95% CI: 0.25–4.38, p = 0.03) and ICU stay (Hedge’s g: 1.32; 95% CI: 0.00–2.64, p = 0.05) (Fig. 3). No differences were noted in CC time, post-operative complications and pericardial effusions (Fig. 2S, Appendix A).
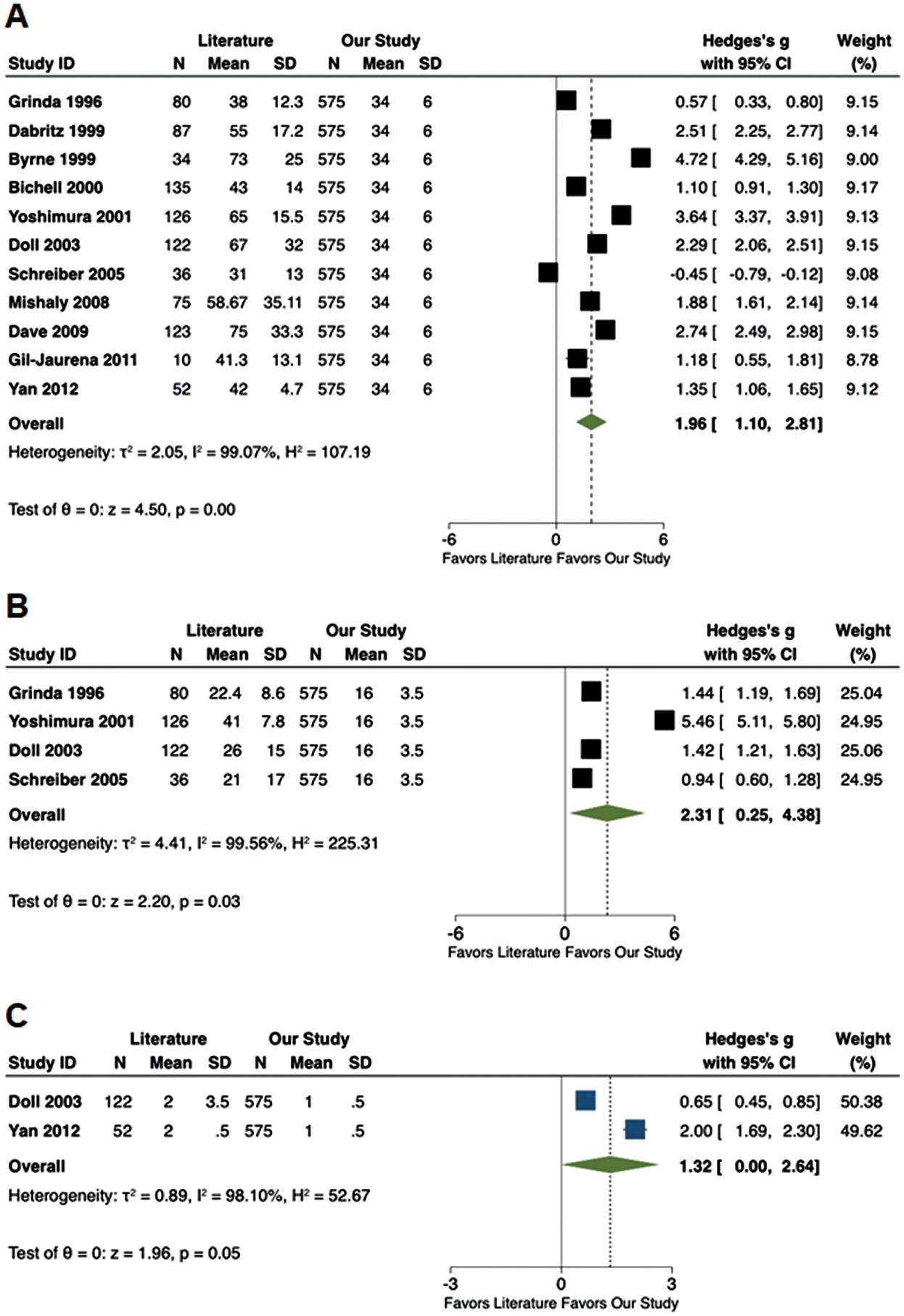
Figure 3: Forest plots comparing available literature vs. the results of the current study. (A) Cardiopulmonary bypass time (min); (B) Induced ventricular fibrillation time (min); (C) Intensive care unit stay (d)
MICS is currently emerging in the field as a means to reduce the psychological and physical trauma of surgery, aspects that are particularly important in the pediatric population [4,22,23].
While some groups dispute the long-term benefits and safety of MICS in the CHD population [24], we and others have demonstrated excellent aesthetic results without sacrificing clinical outcomes [4,5,9,25].
The first challenge in the implementation of MICS was reducing the size of the cannula during CPB to optimize the visual field. To augment drainage with smaller sized cannulas, vacuum-assisted venous drainage is routinely used in our institution [6,12,26]. Similarly, peripheral access for CPB is a standard practice for MICS cases. At the onset of our experience with MICS, peripheral CPB was used only in patients with a body weight >30 kg, largely in part due to the limitations of access via the femoral vessels. Our current protocol includes the routine use of femoral arterial cannulation in patients with body weight ≥15 kg and the use of femoral venous cannulation in patients with body weight ≥7 kg [12].
In our first MICS cases, patient gender was the primary determinant in the surgical approach. In fact, a MS was favored in males and was rarely used in patients >35 kg. This approach was used mainly in the middle period of our 20-year experience and has produced optimal results and an extremely low incidence of postoperative complications [6]. A relatively high percentage of patients required blood transfusions in the MS group, but this was probably related to the younger age of this group.
In contrast, RAMT is preferred in females to maximize the aesthetic result without sacrificing the clinical outcome [8]. We now use RAMT in children and adults with CHD, mainly for ASD and PAPVR repair in females, as an alternative to MS [11].
Starting in 2013, we included RLMT to our MICS armamentarium, demonstrating excellent clinical results comparable to a classic sternotomy and the other MICS approaches [7]. Indeed, no difference was noted in CBP, IFV, aortic CC time and length of hospital stay between MICS and FMS, suggesting the feasibility and efficacy of MICS (Tab. 1S, Appendix A). Moreover, a meta-analysis of the current literature on MICS for ASD closure showed shorter CPB time and ICU length of stay in our center, and comparable CC time, rates of postoperative complications and pericardial effusions.
Previously, we showed that the vast majority of patients (92–98%) treated with MICS were satisfied with the cosmetic result of the operation. The satisfaction rate was higher with RAMT or RLMT than in MS. None of our patients showed skeletal problems such as scoliosis or chest deformities [8,13].
Early in our experience, the application of MICS was restricted to operations requiring intracardiac access through the right atrium. Therefore, our initial experience is comprised of simple CHD lesions such as ASD, PAPVR or VSD. More recently, these techniques have also been applied to pAVSD, tetralogy of Fallot, subaortic stenosis or other CHD [27,28].
MICS would not be possible without a coordinated multidisciplinary approach and buy-in from all of the members of the operative and postoperative team [4]. We believe that morbidity and mortality will continue to be the most important outcomes and should continue to be the greatest factor in decision making for the management of patients with CHD. However, as outcomes have continued to improve for all CHD patients, the potential for emerging technologies to reduce postoperative trauma, and improve secondary considerations such as aesthetic outcomes, will play an increasingly important role in cardiac surgery strategies.
This study has several limitations. First, it is a retrospective examination of a heterogeneous cohort of patients with multiple diagnoses who underwent different procedures at different ages and at different times in our institutional experience. The population of patients treated with RLMT is proportionately smaller and only short-term results were analyzed. This limits the ability to draw definitive conclusions about long-term efficacy and safety. The population of patients treated with FMS was derived from a historical cohort from previous studies and this may have determined an era effect. However, previously published data from our experience on this topic reveal no significant complications such as arrhythmias at midterm follow-up [29].
The post-operative results of MICS are excellent and comparable between the different techniques used for the surgical correction of CHD. Confirmatory prospective randomized clinical trials and studies with longer follow-up are needed to compare the various approaches.
Data sharing: Data are available on reasonable request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The author Vladimiro L. Vida have conflicts of interest with the manuscripts submitted to CONGENITAL HEART DISEASE since he is the Editor of the journal.
1. Julian, O. C., Lopez-Belio, M., Dye, W. S., Javid, H., Grove, W. J. (1957). The median sternal incision in intracardiac surgery with extracorporeal circulation: a general evaluation of its use in heart surgery. Surgery, 42(4), 753–761. DOI 10.5555/uri:pii:0039606057903422. [Google Scholar] [CrossRef]
2. Reser, D., Caliskan, E., Tolboom, H., Guidotti, A. (2015). Median sternotomy. Multimedia Manual of Cardiothoracic Surgery, 2015, mmv017. DOI 10.1093/mmcts/mmv017. [Google Scholar] [CrossRef]
3. Hagl, C., Stock, U., Haverich, A., Steinhoff, G. (2001). Evaluation of different minimally invasive techniques in pediatric cardiac surgery: Is a full sternotomy always a necessity? Chest, 119(2), 622–627. DOI 10.1378/chest.119.2.622. [Google Scholar] [CrossRef]
4. Bacha, E., Kalfa, D. (2014). Minimally invasive paediatric cardiac surgery. Nature Reviews Cardiology, 11(1), 24–34. DOI 10.1038/nrcardio.2013.168. [Google Scholar] [CrossRef]
5. del Nido, P. J., (2007). Minimal incision congenital cardiac surgery. Seminars in Thoracic and Cardiovascular Surgery, 19(4), 319–324. DOI 10.1053/j.semtcvs.2007.12.004. [Google Scholar] [CrossRef]
6. Vida, V. L., Stellin, G. (2018). Fundamentals of congenital minimally invasive cardiac surgery. London, UK: Elsevier.DOI 10.1016/c2016-0-01630-3. [Google Scholar]
7. Vida, V. L., Padalino, M. A., Bhattarai, A., Stellin, G. (2011). Right posterior-lateral minithoracotomy access for treating congenital heart disease. Annals of Thoracic Surgery, 92(6), 2278–2280. DOI 10.1016/j.athoracsur.2011.06.069. [Google Scholar] [CrossRef]
8. Vida, V. L., Padalino, M. A., Boccuzzo, G., Veshti, A. A., Speggiorin, S. et al. (2009). Minimally invasive operation for congenital heart disease: a sex-differentiated approach. Journal of Thoracic and Cardiovascular Surgery, 138(4), 933–936. DOI 10.1016/j.jtcvs.2009.03.015. [Google Scholar] [CrossRef]
9. Vida, V. L., Padalino, M. A., Motta, R., Stellin, G. (2011). Minimally invasive surgical options in pediatric heart surgery. Expert Review of Cardiovascular Therapy, 9(6), 763–769. DOI 10.1586/erc.11.69. [Google Scholar] [CrossRef]
10. Vida, V. L., Tessari, C., Cristante, A., Nori, R., Pittarello, D. et al. (2016). The role of regional oxygen saturation using near-infrared spectroscopy and blood lactate levels as early predictors of outcome after pediatric cardiac surgery. Canadian Journal of Cardiology, 32(8), 970–977. DOI 10.1016/j.cjca.2015.09.024. [Google Scholar] [CrossRef]
11. Vida, V. L., Tessari, C., Fabozzo, A., Padalino, M. A., Barzon, E. et al. (2013). The evolution of the right anterolateral thoracotomy technique for correction of atrial septal defects: cosmetic and functional results in prepubescent patients. Annals of Thoracic Surgery, 95(1), 242–247. DOI 10.1016/j.athoracsur.2012.08.026. [Google Scholar] [CrossRef]
12. Vida, V. L., Tessari, C., Putzu, A., Tiberio, I., Guariento, A. et al. (2016). The peripheral cannulation technique in minimally invasive congenital cardiac surgery. International Journal of Artificial Organs, 39(6), 300–303. DOI 10.5301/ijao.5000505. [Google Scholar] [CrossRef]
13. Vida, V. L., Zanotto, L., Zanotto, L., Tessari, C., Padalino, M. A. et al. (2019). Minimally invasive surgery for atrial septal defects: a 20-year experience at a single centre. Interactive CardioVascular and Thoracic Surgery, 28(6), 961–967. DOI 10.1093/icvts/ivz017. [Google Scholar] [CrossRef]
14. Gil-Jaurena, J. M., Zabala, J. I., Conejo, L., Cuenca, V., Picazo, B. et al. (2011). Minimally invasive pediatric cardiac surgery. Atrial septal defect closure through axillary and submammary approaches. Revista Española de Cardiología (English Edition), 64(3), 208–212. DOI 10.1016/j.rec.2010.08.009. [Google Scholar] [CrossRef]
15. Grinda, J. M., Folliguet, T. A., Dervanian, P., Macé, L., Legault, B. et al. (1996). Right anterolateral thoracotomy for repair of atrial septal defect. Annals of Thoracic Surgery, 62(1), 175–178. DOI 10.1016/0003-4975(96)00182-8. [Google Scholar] [CrossRef]
16. Bichell, D. P., Geva, T., Bacha, E. A., Mayer, J. E., Jonas, R. A. et al. (2000). Minimal access approach for the repair of atrial septal defect: the initial 135 patients. Annals of Thoracic Surgery, 70(1), 115–118. DOI 10.1016/S0003-4975(00)01251-0. [Google Scholar] [CrossRef]
17. Schreiber, C., Bleiziffer, S., Kostolny, M., Hörer, J., Eicken, A. et al. (2005). Minimally invasive midaxillary muscle sparing thoracotomy for atrial septal defect closure in prepubescent patients. Annals of Thoracic Surgery, 80(2), 673–676. DOI 10.1016/j.athoracsur.2005.03.020. [Google Scholar] [CrossRef]
18. Doll, N., Walther, T., Falk, V., Binner, C., Bucerius, J. et al. (2003). Secundum ASD closure using a right lateral minithoracotomy: five-year experience in 122 patients. Annals of Thoracic Surgery, 75(5), 1527–1530. DOI 10.1016/S0003-4975(02)04889-0. [Google Scholar] [CrossRef]
19. Yoshimura, N., Yamaguchi, M., Oshima, Y., Oka, S., Ootaki, Y. et al. (2001). Repair of atrial septal defect through a right posterolateral thoracotomy: a cosmetic approach for female patients. Annals of Thoracic Surgery, 72(6), 2103–2105. DOI 10.1016/S0003-4975(01)03086-7. [Google Scholar] [CrossRef]
20. Byrne, J. G., Adams, D. H., Mitchell, M. E., Cohn, L. H. (1999). Minimally invasive direct access for repair of atrial septal defect in adults. American Journal of Cardiology, 84(8), 919–922. DOI 10.1016/S0002-9149(99)00466-X. [Google Scholar] [CrossRef]
21. Däbritz, S., Sachweh, J., Walter, M., Messmer, B. J. (1999). Closure of atrial septal defects via limited right anterolateral thoracotomy as a minimal invasive approach in female patients. European Journal of Cardio-Thoracic Surgery, 15(1), 18–23. DOI 10.1016/S1010-7940(98)00267-X. [Google Scholar] [CrossRef]
22. Burke, R. P., Hannan, R. L. (2001). Reducing the trauma of congenital heart surgery. The Surgical Clinics of North America, 80(5), 1593–1605. DOI 10.1016/S0039-6109(05)70247-4. [Google Scholar] [CrossRef]
23. Bleiziffer, S., Schreiber, C., Burgkart, R., Regenfelder, F., Kostolny, M. et al. (2004). The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. Journal of Thoracic and Cardiovascular Surgery, 127(5), 1474–1480. DOI 10.1016/j.jtcvs.2003.11.033. [Google Scholar] [CrossRef]
24. Mavroudis, C., Backer, C. L., Stewart, R. D., Heraty, P. (2005). The case against minimally invasive cardiac surgery. Pediatric Cardiac Surgery Annual, 8(1), 193–197. DOI 10.1053/j.pcsu.2005.01.017. [Google Scholar] [CrossRef]
25. Del Nido, P. J. (2020). Minimally invasive cardiac surgical procedures in children. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery, 15(2), 95–98. DOI 10.1177/1556984520914283. [Google Scholar] [CrossRef]
26. Zanella, F., Ceccato, L., Vida, V. L. (2018). Cardiopulmonary bypass strategies: vacuum assisted venous drainage. Fundamentals of congenital minimally invasive cardiac surgery. London, UK: Elsevier.DOI 10.1016/b978-0-12-811355-4.00003-4. [Google Scholar]
27. Gil-jaurena, J. M., Pita-fernández, A., González-lópez, M. T., Pérez-caballero, R. (2018). Other less commonly used minimally invasive surgical approaches. Fundamentals of congenital minimally invasive cardiac surgery. London, UK: Elsevier.DOI 10.1016/b978-0-12-811355-4.00021-6. [Google Scholar]
28. Lee, T., Weiss, A. J., Williams, E. E., Kiblawi, F., Dong, J. et al. (2018). The right axillary incision: a potential new standard of care for selected congenital heart surgery. Seminars in Thoracic and Cardiovascular Surgery, 30(3), 310–316. DOI 10.1053/j.semtcvs.2018.02.011. [Google Scholar] [CrossRef]
29. Castaldi, B., Vida, V. L., Argiolas, A., Maschietto, N., Cerutti, A. et al. (2015). Late electrical and mechanical remodeling after atrial septal defect closure in children: surgical versus percutaneous approach. Annals of Thoracic Surgery, 100(1), 181–186. DOI 10.1016/j.athoracsur.2015.03.017. [Google Scholar] [CrossRef]
Appendix A
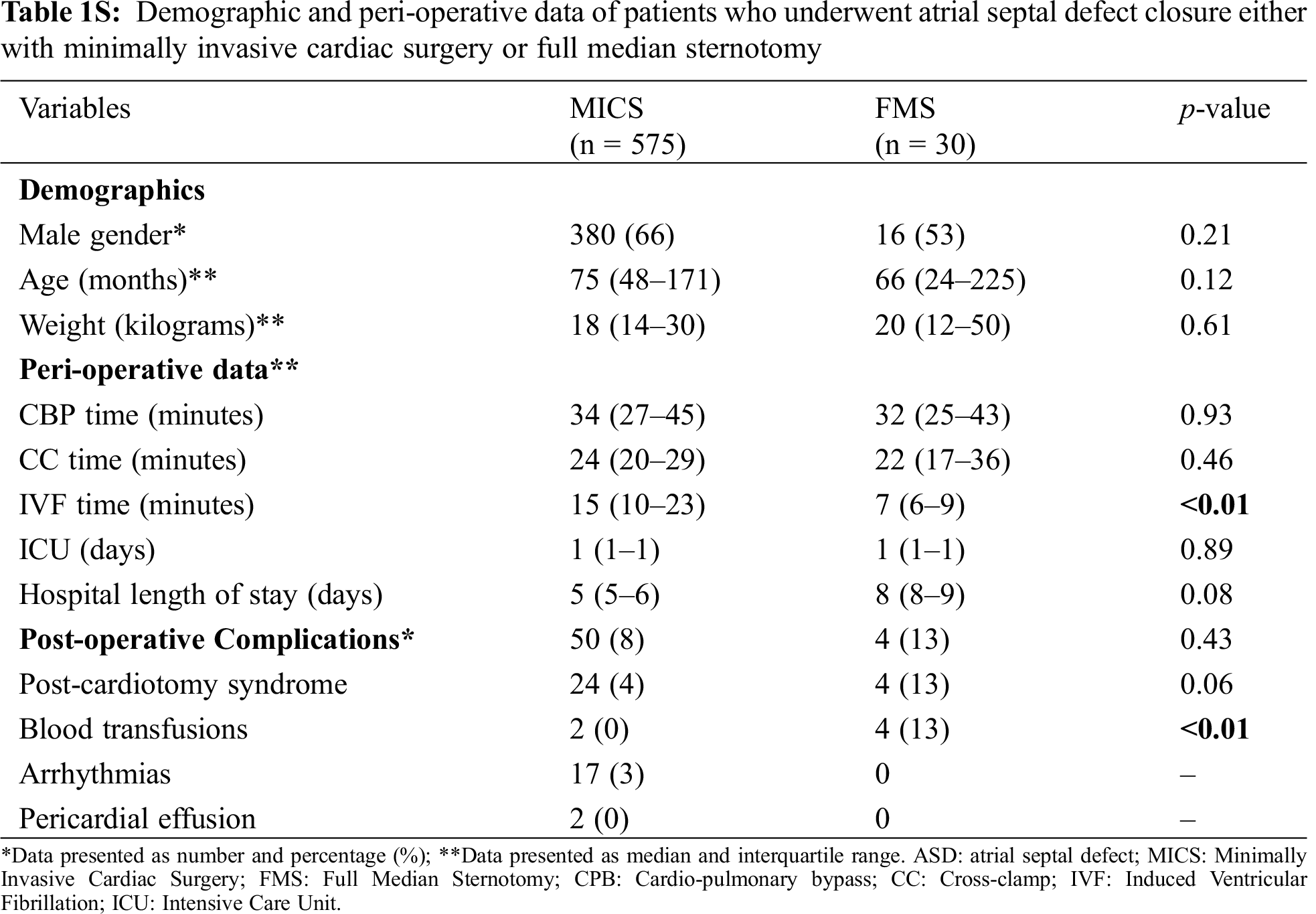
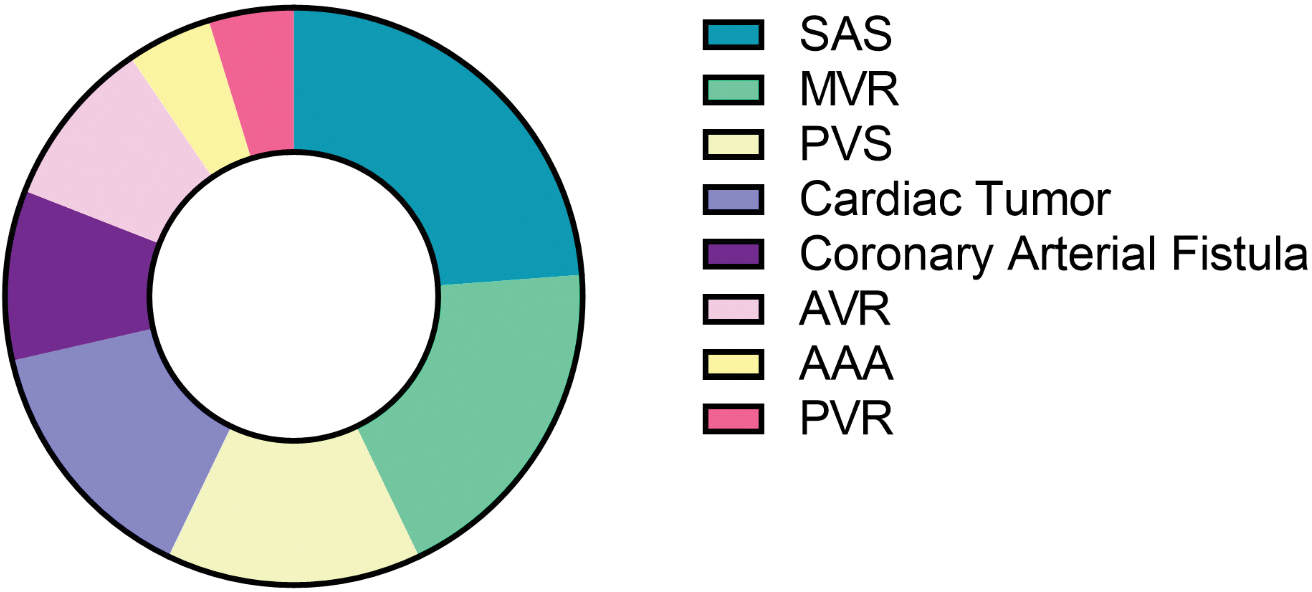
Figure 1S: Other diagnoses of the patients that underwent minimally invasive cardiac surgery. (Pie chart illustrating other diagnoses of the patients that underwent minimally invasive cardiac surgery apart from atrial septal defect, ventricular septal defect, partial anomalous pulmonary vein return and atrioventricular septal defects. SAS: Subaortic stenosis; MVR: Mitral valve regurgitation; PVS: Pulmonary valve stenosis; AVR: Aortic valve regurgitation; AAA: Ascending aortic aneurysm; PVR: Pulmonary valve regurgitation (n = 280))
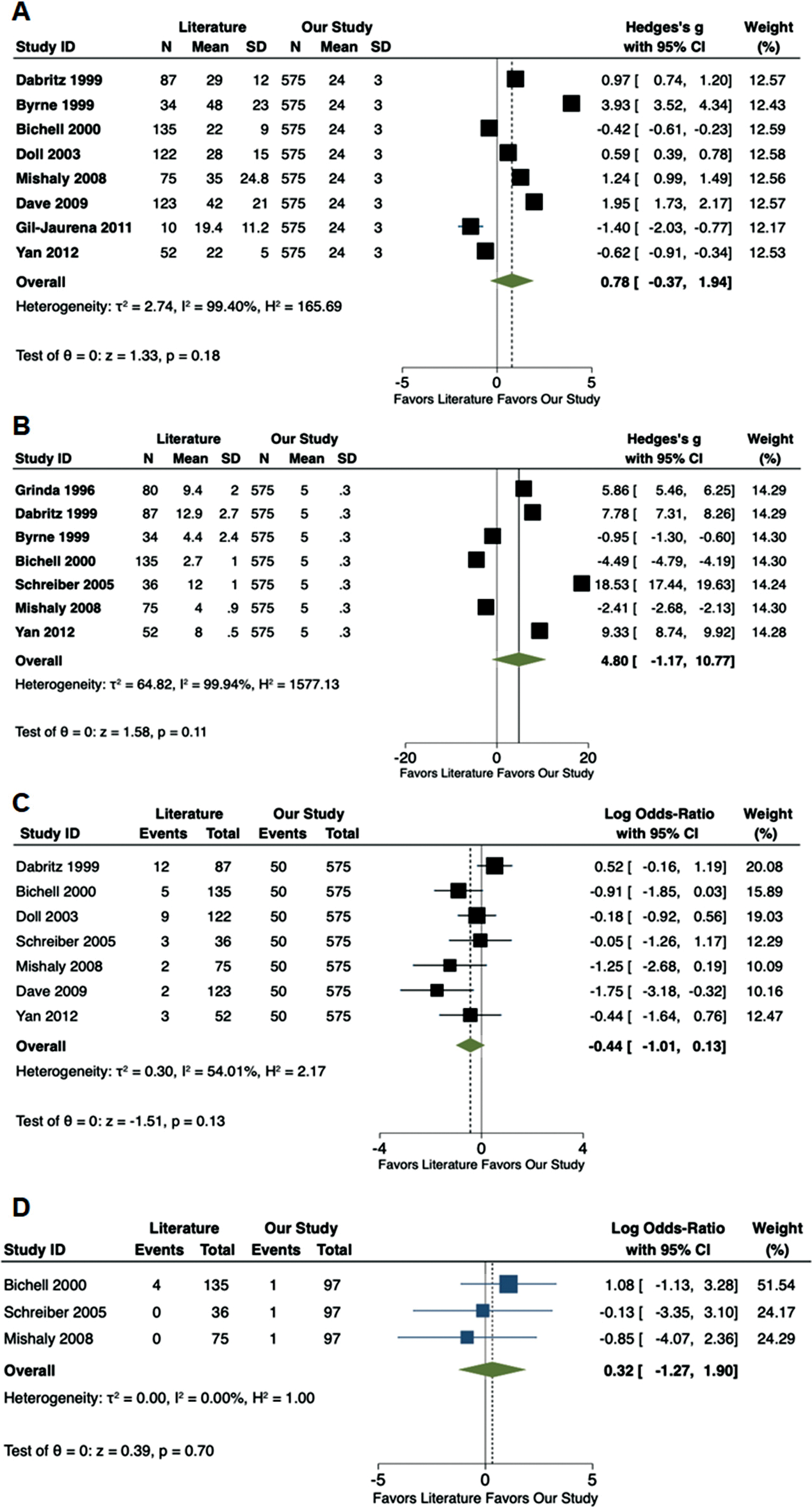
Figure 2S: Forest plots comparing available literature vs. the results of the current study. (A) Aortic cross-clamp time (min); (B) Length of hospital stay (d); (C) Post-operative complications and (D) Post-operative pericardial effusions
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |