

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011448
ARTICLE
D-Transposition of the Great Arteries after Arterial Switch Operation: Usefulness of 3D-Echocardiography for Left Ventricle Function Evaluation
Pediatric Cardiology & GUCH Unit, Cardiothoracic-Vascular Department, University Hospital S. Orsola-Malpighi, Bologna, 40138, Italy
*Corresponding Author: Ylenia Bartolacelli. Email: ylenia.bartolacelli@gmail.com
Received: 09 May 2020; Accepted: 11 June 2020
Abstract: Objective: The objective of this study was to assess left ventricle (LV) function and remodeling by three-dimensional echocardiography (3DE) in patients who underwent arterial switch procedure (ASO) for transposition of great arteries (TGA) in long-term follow-up. Methods and Results: We studied 54 asymptomatic patients (39 male) who have undergone single-stage ASO for TGA, aged 13.7 ± 4.7 years, with a normal LV ejection fraction (EF), compared to healthy peers. We evaluated LV volume and function in asymptomatic patients with normal ejection fraction by 3DE. All patients had normal EF, measured by modified Simpson’s method (mean 60.9 ± 3.5%) and by 3D method (mean 62.3 ± 3.8%). No statistically significant differences were documented between 2D and 3D measures of age-related LV volumes. Comparison of 3D volumes with reference ones was performed only in pediatric patients (<18 years old). In this subgroup (n = 42) 3D volumes were significantly higher than reference values from the age of 9 years (End-diastolic volume: 9–12 years 79.61 ± 20.29 ml vs. 53.52 ± 13.94 ml, p < 0.001; 13–17 years 107.30 ± 23.28 ml vs. 81.78 ± 26.44 ml, p = 0.0038). Conclusions: Children and young adults late after ASO demonstrate normal ejection fraction, but present subclinical signs of ventricular and myocardial remodeling, such as increased LV dimensions, when using 3D echocardiography. Our findings support the usefulness of 3DE to detect LV remodeling precociously.
Keywords: Echocardiography; congenital heart disease; 3D echocardiography; transposition of great arteries; arterial switch operation
Transposition of the great arteries (TGA) is a conotruncal abnormality in which the aorta arises from the right ventricle (RV) and the pulmonary artery arises from the left ventricle (LV) resulting in discordant ventriculoarterial connections.
Before the 1950’s, TGA was a fatal disease resulting in death in 89% of patients by 1 year of age. After the first successful Arterial switch operation (ASO) by Jatene in 1975 and its modification by Lecompte et al., current perioperative mortality is under 4% and long-term survival is around 97% at 25 years follow-up.
Recent studies have shown that coronary artery abnormalities [1], decreased coronary artery vasoreactivity [2], reduced coronary flow reserve, proximal intimal proliferation [3], and reversible myocardial perfusion defects are present in patients who have undergone ASO [4–6]. Sub-optimal coronary perfusion could lead to chronic ischemia inducing LV damage and remodelling. For these reasons, assessment of ventricular function is an important component of the clinical evaluation after the ASO and echocardiography remains the main diagnostic imaging modality.
LV systolic function in patients who have undergone ASO has largely been found to lie within the normal range when assessed by using standard echocardiographic indices (such as SF, EF) [7,8]. Few data regarding assessment of LV volume and function by 3DE and its reproducibility have been reported.
2DE has important limitations because it makes assumptions of LV shape which can be invalid in pediatric population and, moreover, EF (2DE measured) is load-dependent and 2D-based assessment is dependent on ventricular geometry [9]. In contrast, 3DE doesn’t rely on these assumptions and it is increasingly used in children because of good acoustic windows and the non-invasive nature of the technique [10].
Accurate determination of LV volume and function by 3DE has been reported in adults and children with CHD considering MRI as the gold standard and it is more accurate and reproducible than either M-mode or 2D Simpson’s biplane method and is as feasible as 2DE in children [11].
The capability of 3DE to capture the entire LV volume offers the opportunity to assess global and regional LV function, and so the ventricular dyssynchrony. Recent advances in 3D wall tracking have allowed for assessment of myocardial deformation in three dimensions from a single volume of the LV but this currently remains a research tool, and the role of such analysis in the management of patients with CHD remains to be established.
Our purpose was to assess LV function and remodelling in asymptomatic patients (children and young adults) after one-stage ASO for TGA during long-term follow-up, through 3DE compared with standard echocardiographic measures. We meant to detect subclinical LV dysfunction, expressed as altered 3D LV volumes compared to reference values, and its relation to surgical, clinical and echocardiographic variables in patients who underwent neonatal ASO.
2.1 Study Design and Patients Selection
A prospective cross-sectional cohort study was performed between May 2019 and August 2019. We studied patients who have undergone single-stage ASO for d-TGA, with or without VSD associated, regularly followed at Pediatric Cardiology and Adult Congenital Unit of University of Bologna, Italy.
Inclusion criteria were: age ≥5 years and no symptoms (New YorkHeart Association class I). Exclusion criteria were: presence of LV akinesia or hypokinesia and hypertension and/or diabetes at the time of the study.
Informed consent was obtained from all patients and additional consent by parents if aged <18 years of age.
Patients underwent clinical examination, electrocardiography, standard Doppler echocardiography and 3DE study.
Transthoracic echocardiography was performed using the Philips EPIQ7C machine (Philips, Bothell, Washington, USA) with a 5 MHz transducer. QLAB software version 9.0 (Philips) was used for offline analysis of echocardiographic cine-loops stored. A certified medical analyst (A. B.) with echocardiographic experience performed the echocardiographic exams while offline analysis was performed by a single experienced examinator (G. B.).
LV and left atrial measurements were taken from 2D tracings. LV mass indexed for body surface area (BSA) was calculated using the Devereux-modified American Society of Echocardiography cube equation [12]. LV ejection fraction was computed using a modified Simpson’s biplane method.
3D examination with full-volume data sets obtained from R-wave triggered two-to four-beat acquisitions. Breath holding was done if the child was able; if the child was unable to hold his or her breath, multiple data sets were acquired, and only those with good quality and without respiratory (“stitch”) artifacts were selected. Images were digitally stored for subsequent off-line analysis on QLAB with Advanced Cardiac 3D Quantification software (3DQA). 3Dend-diastolic and end-systolic LV volumes were measured, with ventricular trabeculae and papillary muscles included in the LV cavity, and end systole was defined as the frame before mitral valve opening. Tracing consisted of setting five points (septal, lateral, anterior and inferior mitral annulus and the apex) followed by semi-automated computation with manual correction of the endocardial contour (Fig. 1).
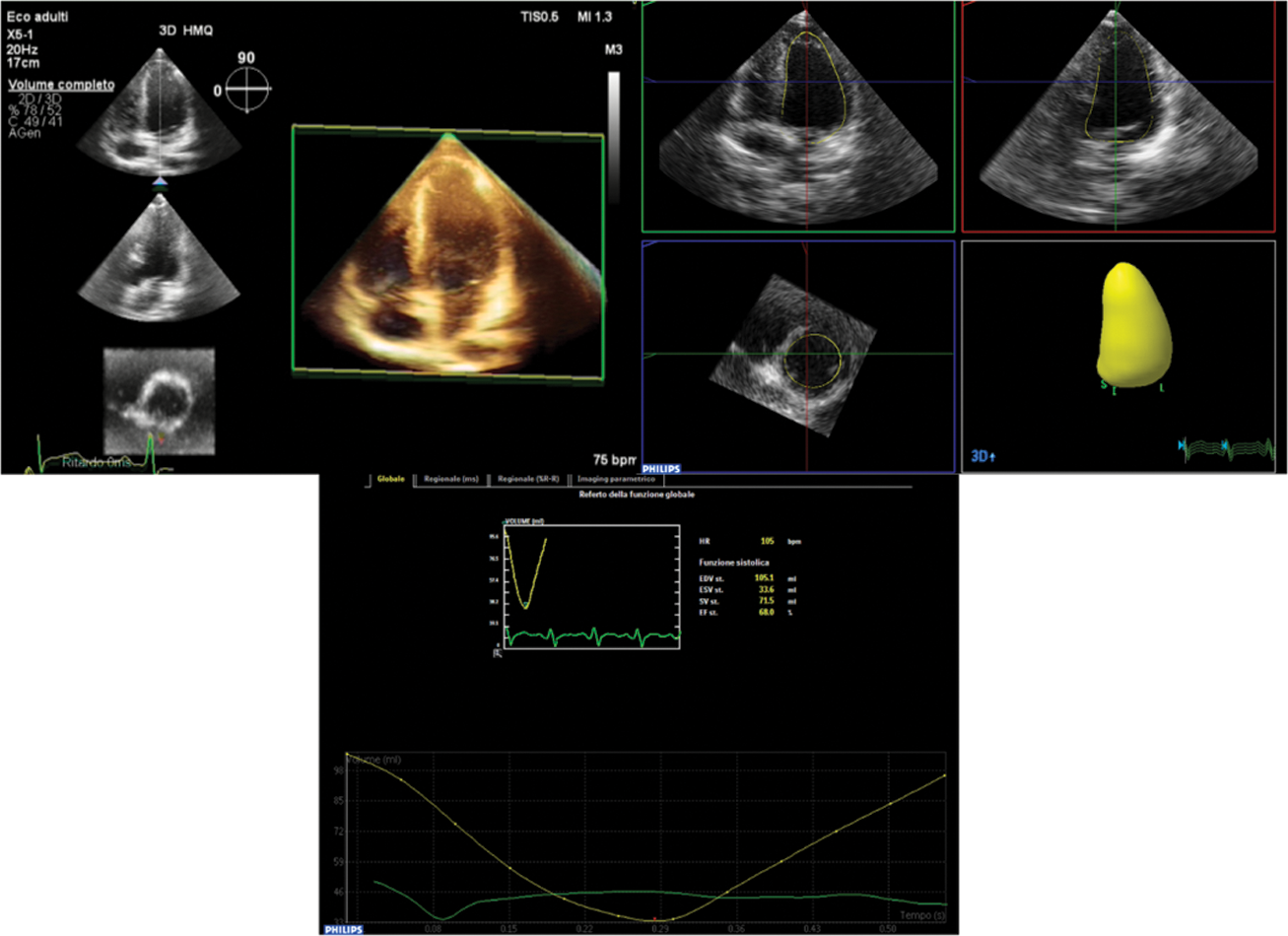
Figure 1: Measurement of Left Ventricle volumes on the basis of semiautomated identification of endocardial surface by 3DE
References values for 3D volumes for patients aged 5–17 years old were obtained from the study of Zhang et al. [13], that reports volumes of healthy children, divided in age group. For patients aged ≥18 years old reference values were obtained from the study of Muraru et al. [14], that reports volumes and indexed volumes of adult healthy subjects, divided by sex and age.
Categorical variables are expressed as percentages, continuous variable are presented as mean ± standard deviation if normally distributed. For comparison of age groups, independent samples t test or ANOVA was used for continuous variables as appropriate and chi-square test for was use for categorical variables.
Univariate and multivariate analyses were performed to assess relationship between 3D data (left ventricle end-diastolic volume and ejection fraction) and demographic, anatomical and surgical factors, based on clinical relevance and potential impact on cardiac function. For all tests, a p-value of <0.05 was accepted as statistically significant.
All analyses were performed using STATA/IC version 15.
A total of 54 consecutive patients participated in this study. Two patients were affected by TGA and coarctation of the aorta and they underwent a complete repair with ASO and coartectomy and end-to-end anastomosis. No residual systemic outflow obstruction was documented.
All 54 patients (39 male, 72%) were asymptomatic and none had undergone further surgery since the initial operation. The median age of participants was 13.7 ± 4.7 years. All were in sinus rhythm without electrocardiographic changes at rest suggesting myocardial scar or ischemia. 5 patients showed complete right bundle branch block. 9 patients were treated with ACE inhibitors or sartans and/or betablockers for aortic dilatation.
At birth 19 (35%) patients had TGA with VSD, 49 (91%) had restrictive PFO and 51 (94%) had PDA. 31 patients (58%) had usual coronary anatomy at diagnosis, 22 (42%) had unusual coronary anatomy at diagnosis according to the Yacoub classification (4 patients type B, 1 type C, 12 type D and 5 type E). One had no anamnestic details about the anatomy at birth, because underwent surgery in another country.
The median age of intervention was 9.5 ± 6.3 days. 10 patients (19%) had a VSD closure with a patch, 4 (8%) by suture. The cardio-pulmonary bypass time aortic cross-clamp time were 139.2 ± 26.6 and 96.9 ± 18.8 min. Few post-procedure complications occurred. One patient underwent sternal closure in a second stage due to hemodynamic instability, 1 had sustained supraventricular arrhythmia and 1 developed pericardial effusion 3 months after the surgery.
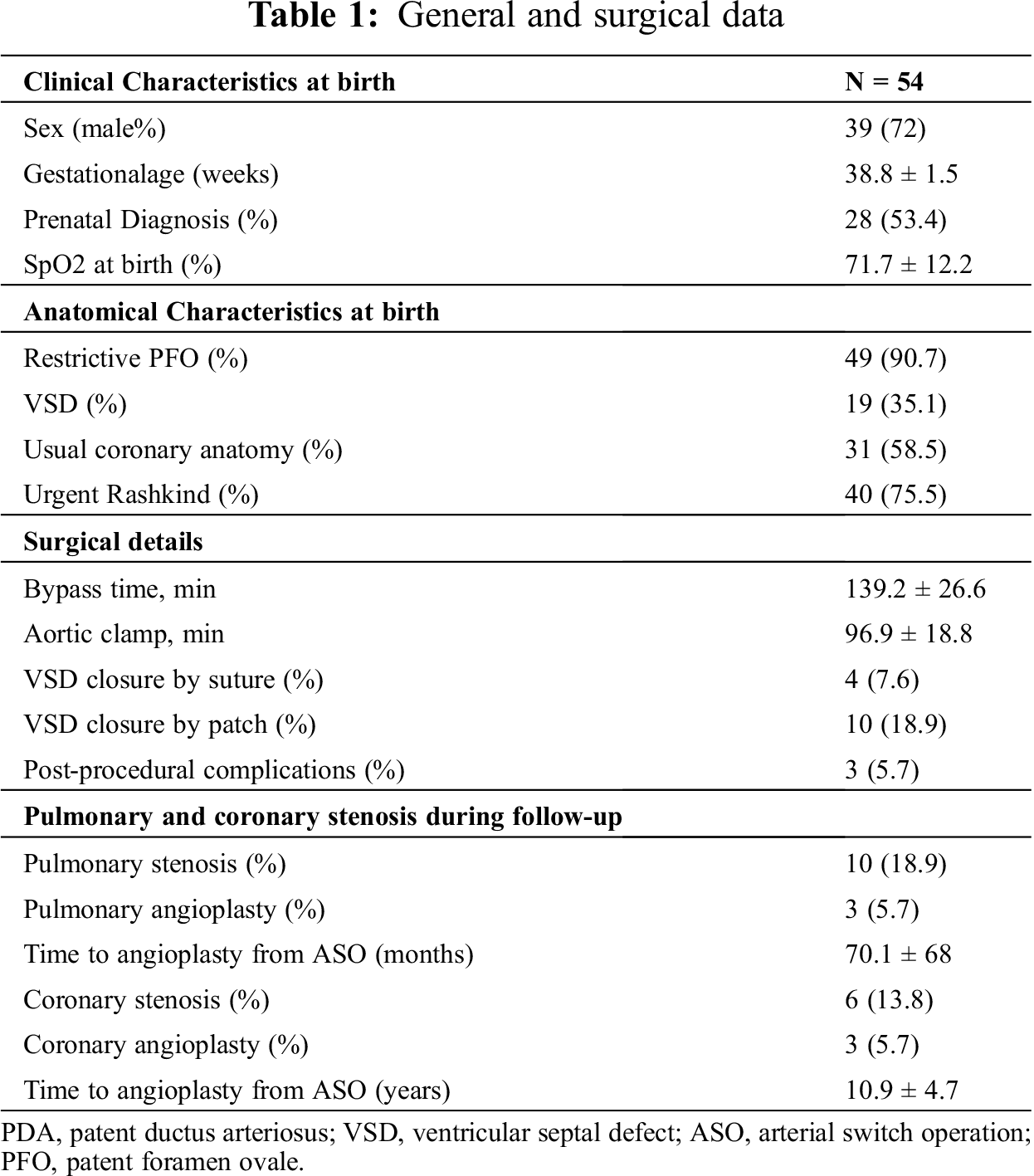
A coronary angiography was performed in all patients between 12 and 24 months after the surgery. At time of first angiographic evaluation, 6 (11%) patients showed coronary occlusion, 3 of these had adequate collateral circulation, the others were re-evaluated when older, because of symptoms, and one underwent left main and anterior descending artery angioplasty (5.7 years after the ASO) and two underwent angioplasty with stenting, one on the anterior descending and right coronaries and one on the right coronary (14.9 and 11.9 years after ASO respectively). 10 patients (18%) developed pulmonary stenosis, of these 2 underwent angioplasty and one angioplasty with stenting (time from ASO 71.06 ± 69 months). Only one patient required a surgical plasty of left pulmonary branch 3 years after. Tab. 1 shows general and surgical data.
3.1 Standard Echocardiographic Evaluation
All patients had normal EF, measured by modified Simpson’s method (mean 60.9 ± 3.5%). LVEDD z-score was within normal range, except for 2 patients, that showed mild LV dilatation (LVEDD z-score +2.57 and +2.64). At the Doppler analysis no one had diastolic dysfunction (E/A mean 2.3 ± 0.8 with E/e’ average mean 8.4 ± 2.2). At Pulsed-wave TDI peak early systolic velocities of medial and lateral basal segments of the LV walls were at the lower normal limit (S’ mit 7.03 ± 1.02 and 8.46 ± 1.48 cm/s respectively). Normal right ventricular longitudinal function was documented in all patients (S’ tric 8.8 ± 1.5 cm/s).
At the Color-doppler analysis, of note, 39 patients had trace-to-mild aortic regurgitation, and 4 (7.4%) had moderate aortic regurgitation. One patient had mild aortic stenosis (1.8%), one patient had moderate aortic stenosis (1.8%), one patient had moderate mitral regurgitation (1.8%) and 19 patients had trace-to-mild mitral regurgitation (35%).
Comparison of main standard echocardiographic data across age groups was performed (Tab. 2).
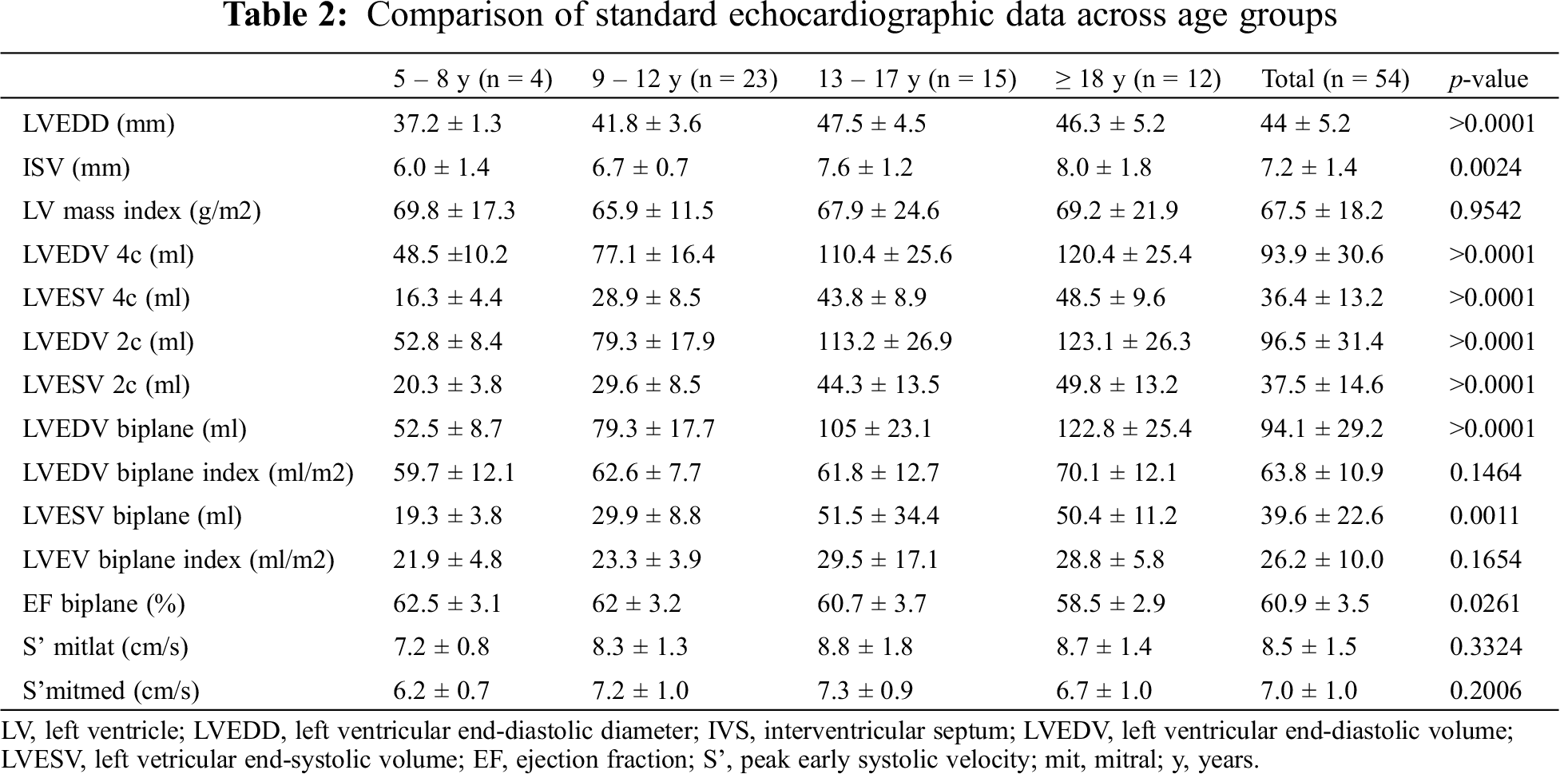
Of note, significant differences were documented in LV dimension parameters, but not in indexed values for BSA (LV mass index 66.76 ± 17.38 g/m2, p = 0.194; LVEDV biplane index 64.87 ± 10.94 ml/m2, p = 0.908).
3.2 Three-Dimensional Echocardiography Study
All patients had normal ejection fraction (mean 60.9 ± 3.5%). Comparison of 3DE data across age groups was performed (Tab. 3).
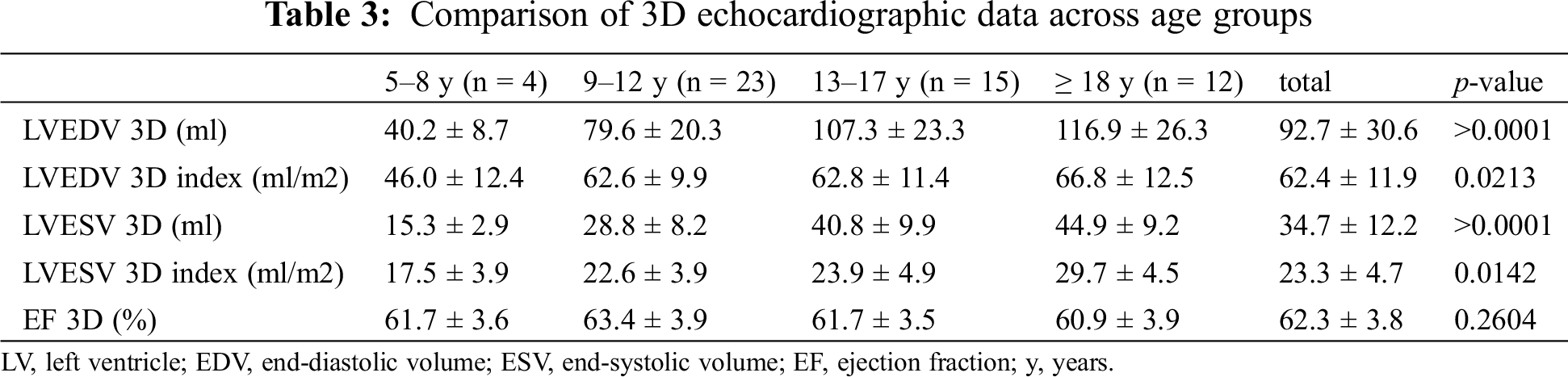
We documented a significant difference in indexed values with an increase of volumes with age, while EF remained in normal range with no significant difference among groups.
No statistically significant differences were documented between 2D and 3D measures of age-related LV volumes (Tab. 4).
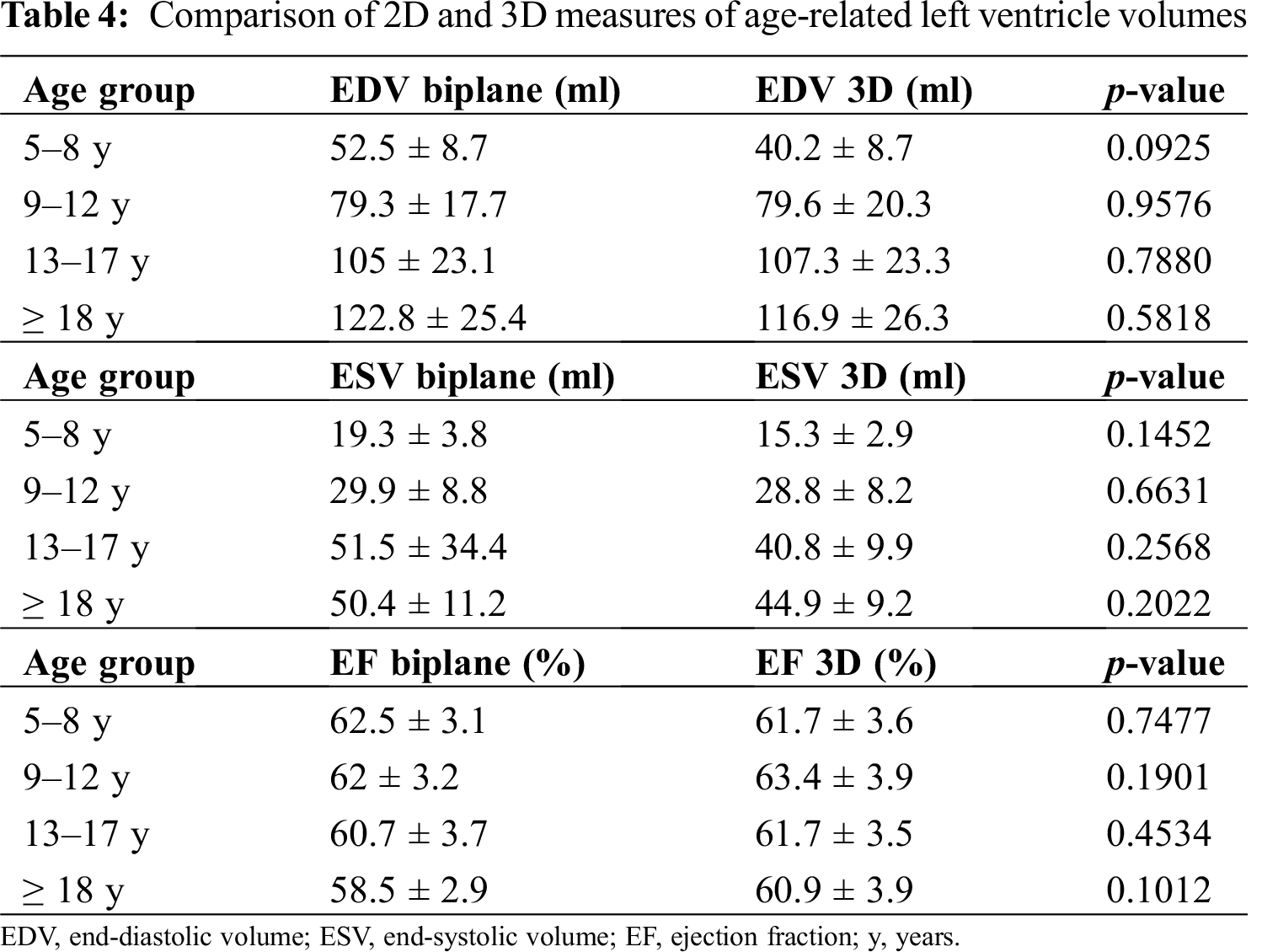
Given the small number of patients >=18 years old (7 males, 5 females, for comparison according to sex and age), we performed a comparison between measured 3D volumes with their reference (according to age) only in pediatric patients (< 18 years old). In this subgroup (n = 42) 3D volumes were significantly higher than reference values from the age of 9 years (Tab. 5 and Figs. 2 and 3).
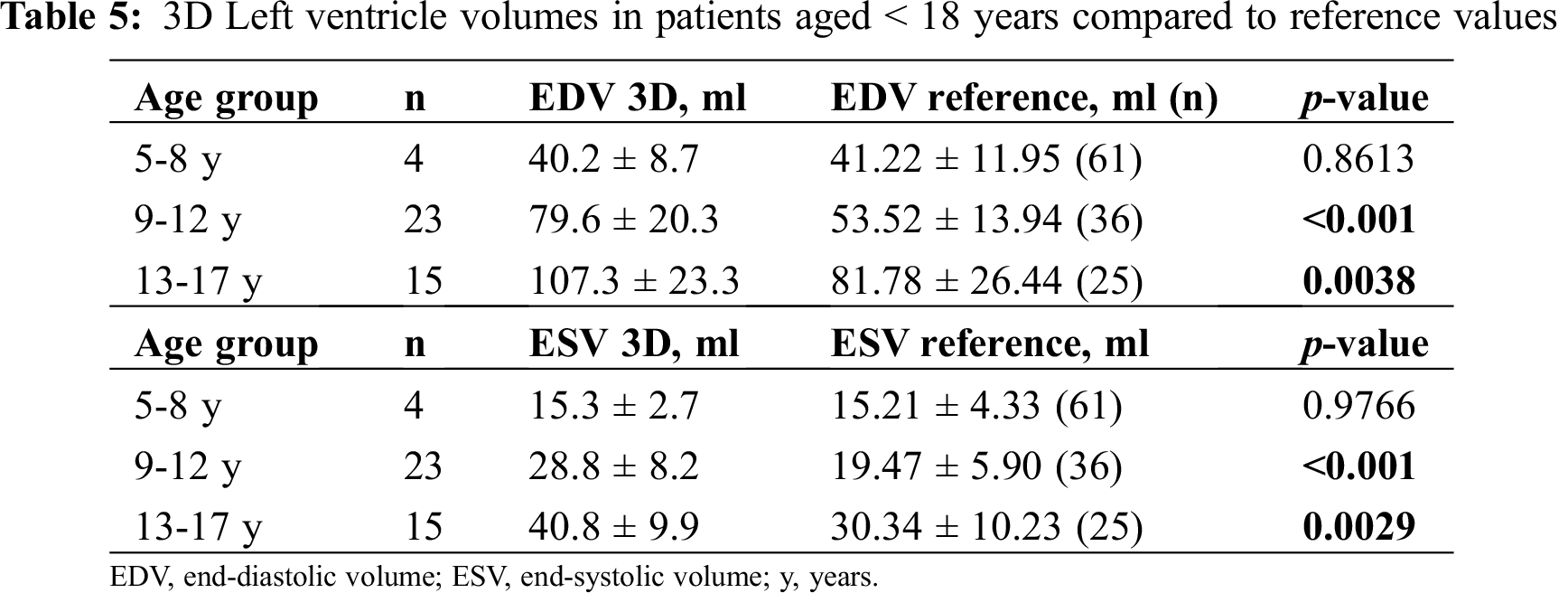
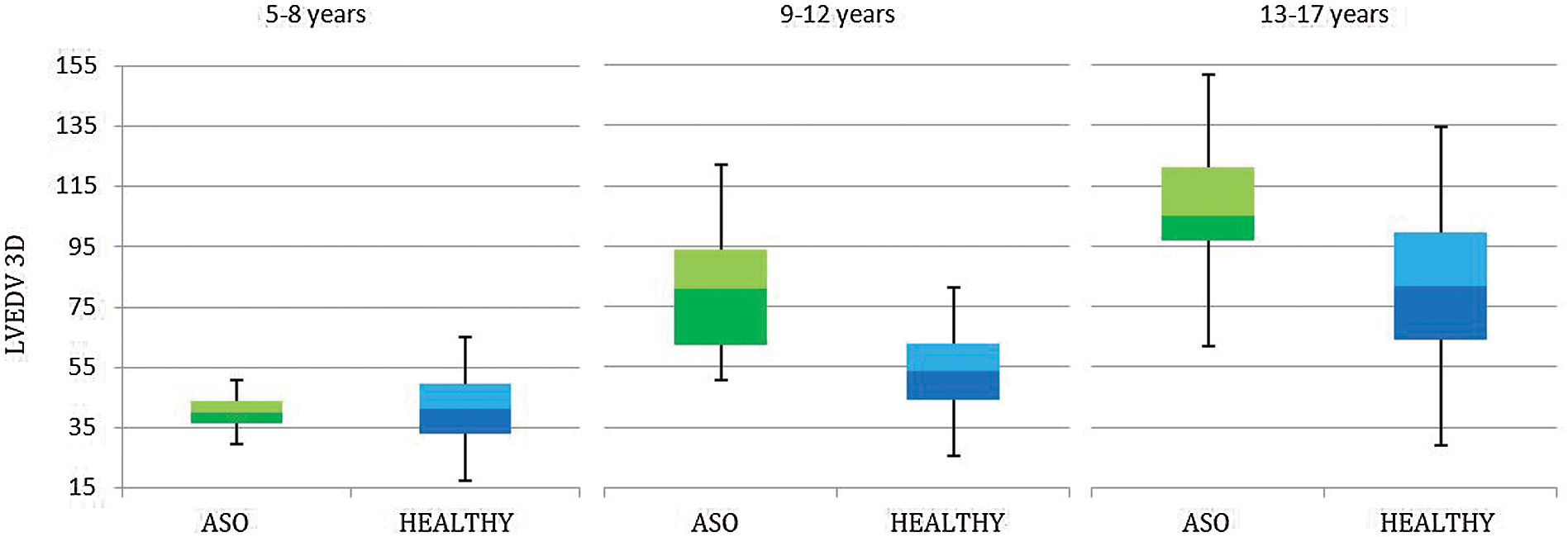
Figure 2: 3D Left ventricle end-diastolic volume (LVEDV) in patients aged <18 years, underwent Arterial Switch Procedure (ASO) and healthy references
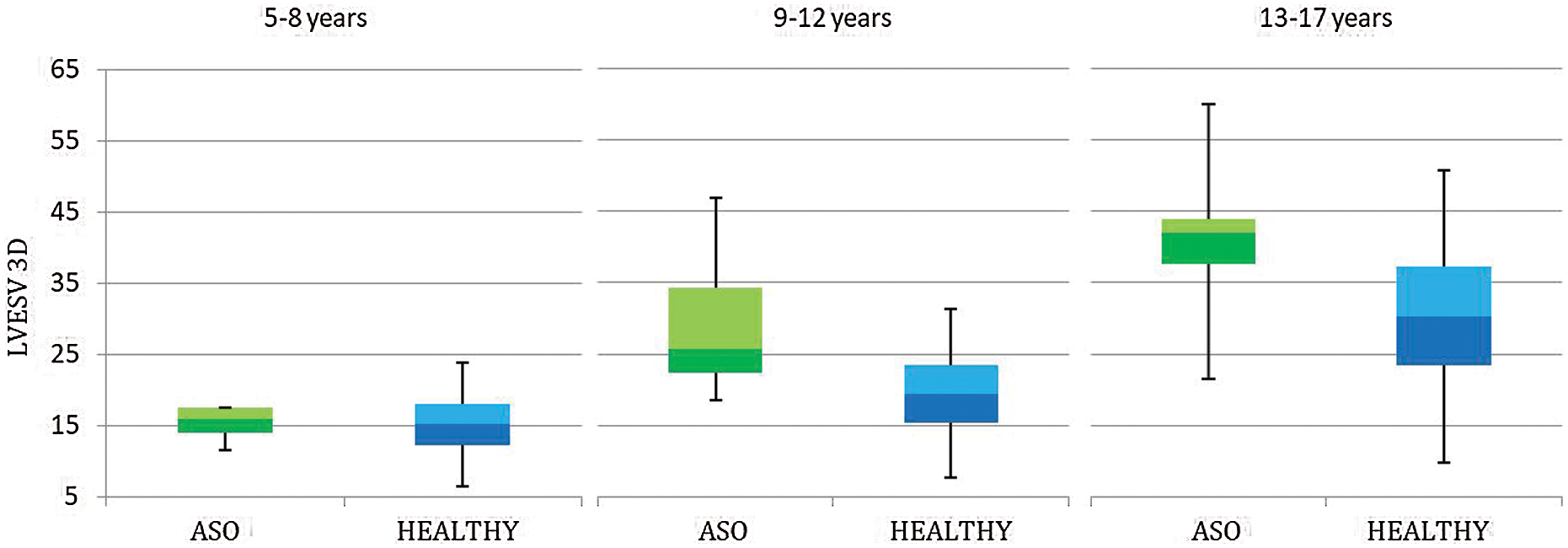
Figure 3: 3D Left ventricle end-systolic volume (LVESV) in patients aged <18 years, underwent Arterial Switch Procedure (ASO) and healthy references
At the univariate analysis LVEDV 3D, but not EF 3D, resulted mildly significantly related to the presence of coronary stenosis and having angioplasty (p = 0.0382 and p = 0.0022 respectively, Tab. 6).
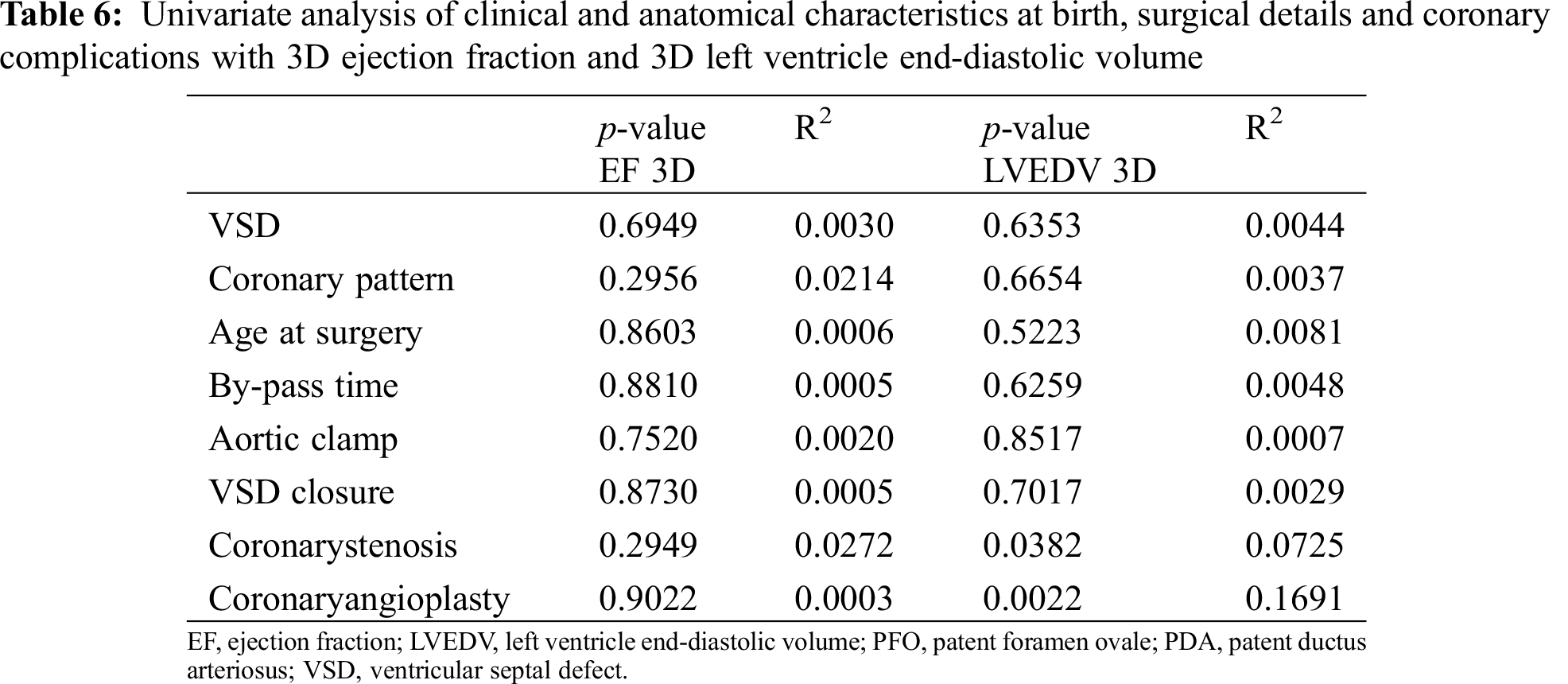
No significant difference was documented at the multivariate analysis.
It is relevant to mention that LVEDV 3D and EF 3D didn’t resulted related to coronary pattern (p = 0.665 and p = 0.295 respectively) nor presence of VSD at birth (p = 0.635 and p = 0.695 respectively), neither surgical time (p = 0.626 and p = 0.881 respectively).
Finally, we found no relation between LVEDV 3D and aortic regurgitation, of any grade (p = 0.251). In particular, only 2 patients in the 3D analysed group had moderate aortic regurgitation, so not justifying the increased LV volumes founded with 3D evaluation.
The presence of coronary stenosis resulted significantly related only to the age at surgery (p = 0.0259), but not the coronary anatomy, by-pass time or prenatal diagnosis and restrictive PFO.
Although long-term outcomes after the ASO for TGA are excellent and the majority of pediatric survivors are asymptomatic and have normal ejection fraction, our study demonstrates that there are significant degrees of myocardial remodelling, expressed as LV dilatation, when compared to healthy reference values.
4.1 LV Dilatation in Arterial Switch Operation Patients
Although LVEDD z-score was normal in our population, except for two patients, we observed an increase of LV volumes at 3DE when compared to healthy ones, from the age of 9 years.
Little information exists about ventricular size and function late after the ASO.
Myocardial ischemia and infarction associated with coronary stenosis related to their transfer to the neoaortic root during the ASO has been a focus of attention. Although several small studies have reported on assessment of the coronary arteries and myocardial viability by echocardiography, computed tomography, nuclear scintigraphy, and CMR, little is known about the prevalence of coronary ischemia sufficient to cause myocardial fibrosis or LV enlargement.
Considering MRI as the gold standard, 3DE has already been reported to be more accurate and reproducible in determining LV volumes and function when compared to 2D in patients with congenital heart disease [11]. However, only few data regarding the assessment of LV volume and function by 3DE and its reproducibility have been reported in patients who underwent ASO. Therefore, we relied mostly on MRI data as main comparison, since this technique has been thoroughly used to investigate patients who underwent ASO. Indeed, our results are in line with a recent study of Grotenhuis et al. [15] performed through CMR on patients who underwent ASO. They found an increase of LV volumes in ASO patients along with longitudinal strain reduction, compared to healthy controls. Moreover, they did not observe myocardial scarring or myocardial perfusion defects, but children and adolescents late after the ASO had mildly elevated T1 values of the LV myocardium, which suggests a subclinical degree of diffuse myocardial fibrosis.
In previous study of Shepard et al. [16], the cohort of patients who underwent CMR late after ASO was noted to have left and right ventricles mildly enlarged compared with published normal controls and to have LGE in ≈20%. However, most had a nonischemic pattern, with the majority having small focal enhancement in the septal-free wall junction. Notably, the presence of LGE, including enhancement of the septal-free wall junctions, was not associated with LV dilatation or dysfunction.
The clinical significance of the observed increased LV volumes, both at end-systole and end-diastole, is not clear, but could be explained by cardiac remodeling in response to a reduced myocardial performance and warrants serial observation for progression.
Moreover, in our study, LVEDV 3D resulted significantly related to the presence of coronary stenosis, but not EF 3D. This is not surprising as late coronary stenosis in ASO patients are usually subclinical, due to the development of an extensive network of collateral coronary circulation. It is known that chronic hypoperfusion induces LV remodeling, expressed as LV dilatation, and that may explain why patients with late coronary stenosis present more frequently with ventricular dysfunction and/or arrhythmias.
We found no significant differences between 2D and 3D measures of LV volume. However, as LVEDD measure is angle-dependent and 2D volume measures are dependent on geometrical assumptions, 3DE seems to be an important tool to study LV remodeling in CHD, that typically show altered geometry, but also in ASO patients that could develop altered loading conditions, such as aortic regurgitation or gothic aortic arch.
In our study the presence of coronary stenosis resulted significantly related only to the age at surgery, but not to the coronary anatomy, by-pass time or prenatal diagnosis. Previous reports showed that the coronary anatomy is not predictive of adverse outcomes after ASO in the current surgical era. Instead, ASO timing seems to have impact on long-term result. Our results suggest that ASO timing may have a clinical influence on long-term outcomes.
Even though long-term clinical outcome is considered excellent in patients after ASO for TGA for the duration of follow-up now available, concerns are rising for long-term preservation of LV function. Clinical evaluation, electrocardiography, and echocardiography have a low sensitivity for detecting coronary insufficiency. Furthermore, ventricular remodeling, as increased LV dimensions, seems to begin early, during pediatric age. All these subclinical signs may help to select patients at higher risk to develop earlier ventricular dysfunction and to provide them with stricter follow-up and/or second level examinations.
Although CMR is the gold-standard for chamber quantification, 3DE has the advantages of being safe, not invasive, more available and not requiring sedation. These factors make it more helpful for clinical practice.
Based on these results LV dimension, assessed by 3DE, should be incorporated in routine evaluations. Long-term longitudinal follow-up studies are needed to confirm if these abnormalities translate into clinically significant dysfunction and negative clinical outcomes.
The present study carries several limitations. First, this study includes a heterogenic patient population of simple and complex TGA patients to best represent long-term outcomes of the entire patient group long after ASO. It also includes patients with aortic regurgitation and/or coronary stenosis. Subgroup analysis showed no significant differences between these groups. Again, we decided to include these patients to provide the best representation of patients after ASO, since those are possible long-term complications. Furthermore, given the cross-sectional nature of our cohort study, any progression of findings or prognostic value in terms of clinical outcome could therefore not be assessed.
Ultimately, as 3DE were assessed by single observer, interobserver variability could not be evaluated.
ASO operative mortality is low and long-term outcomes are generally excellent. Even though this procedure is an anatomical correction, children and young adults late after the ASO demonstrate normal ejection fraction, but present subclinical signs of ventricular remodelling, such as increased LV dimension.
Our findings support the usefulness of 3DE to detect LV remodelling precociously. Further studies are needed to determine whether these abnormalities would have adverse outcomes in these patients.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hauser, M., Bengel, F. M., Kuhn, A., Sauer, U., Zylla, S. et al. (2001). Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and ross operation. Circulation, 103, 1875–1880. [Google Scholar]
2. Gagliardi, M. G., Adorisio, R., Crea, F., Versacci, P., Di Donato, R. et al. (2005). Abnormal vasomotor function of the epicardial coronary arteries in children five to eight years after arterial switch operation, an angiographic and intracoronary doppler flow wire study. Journal of the American College of Cardiology, 46, 1565–1572. [Google Scholar]
3. Pedra, S. R., Pedra, C. A., Abizaid, A. A., Braga, S. L., Staico, R. et al. (2005). Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. Journal of the American College of Cardiology, 45, 2061–2068. [Google Scholar]
4. Pizzi, M. N., Franquet, E., Aguade-Bruix, S., Manso, B., Casaldaliga, J. et al. (2014). Long-term follow-up assessment after the arterial switch operation for correction of dextro-transposition of the great arteries by means of exercise myocardial perfusion-gated spect. Pediatric Cardiology, 35, 197–207. [Google Scholar]
5. Hui, L., Chau, A. K., Leung, M. P., Chiu, C. S., Cheung, Y. F. (2005). Assessment of left ventricular function long term after arterial switch operation for transposition of the great arteries by dobutamine stress echocardiography. Heart, 91, 68–72. [Google Scholar]
6. Vogel, M., Smallhorn, J. F., Trusler, G. A., Freedom, R. M. (1990). Echocardiographic analysis of regional left ventricular wall motion in children after the arterial switch operation for complete transposition of the great arteries. Journal of the American College of Cardiology, 15, 1417–1423. [Google Scholar]
7. Colan, S. D., Boutin, C., Castaneda, A. R., Wernovsky, G. (1995). Status of the left ventricle after arterial switch operation for transposition of the great arteries. Hemodynamic and echocardiographic evaluation. The Journal of Thoracic and Cardiovascular Surgery, 109, 311–321. [Google Scholar]
8. Wernovsky, G., Hougen, T. J., Walsh, E. P., Sholler, G. F., Colan, S. D. et al. (1988). Midterm results after the arterial switch operation for transposition of the great arteries with intact ventricular septum, Clinical, hemodynamic, echocardiographic, and electrophysiologic data. Circulation, 77, 1333–1344. [Google Scholar]
9. Trowitzsch, E., Colan, S. D., Sanders, S. P. (1985). Two-dimensional echocardiographic estimation of right ventricular area changes and ejection fraction in infants with systemic right ventricle (transposition of the great arteries or hypoplastic left heart syndrome). The American Journal of Cardiology, 55, 1153–1157. [Google Scholar]
10. Simpson, J., Lopez, L., Acar, P., Friedberg, M. K., Khoo, N. S. et al. (2017). Three-dimensional echocardiography in congenital heart disease, an expert consensus document from the European association of cardiovascular imaging and the American society of echocardiography. Journal of the American Society of Echocardiography, Official Publication of the American Society of Echocardiography, 30, 1–27. [Google Scholar]
11. Lu, X., Xie, M., Tomberlin, D., Klas, B., Nadvoretskiy, V. et al. (2008). How accurately, reproducibly, and efficiently can we measure left ventricular indices using m-mode, 2-dimensional, and 3-dimensional echocardiography in children? American Heart Journal, 155, 946–953. [Google Scholar]
12. De Simone, G., Daniels, S. R., Devereux, R. B., Meyer, R. A., Roman, M. J. et al. (1992). Left ventricular mass and body size in normotensive children and adults, assessment of allometric relations and impact of overweight. Journal of the American College of Cardiology, 20, 1251–1260. [Google Scholar]
13. Zhang, L., Gao, J., Xie, M., Yin, P., Liu, W. et al. (2013). Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children, feasibility, reproducibility, maturational changes, and normal ranges. Journal of the American Society of Echocardiography, Official Publication of the American Society of Echocardiography, 26, 853–859. [Google Scholar]
14. Muraru, D., Badano, L. P., Pelusom, D., Dal Bianco, L., Casablanca, S. et al. (2013). Comprehensive analysis of left ventricular geometry and function by three-dimensional echocardiography in healthy adults. Journal of the American Society of Echocardiography, Official Publication of the American Society of Echocardiography, 26, 618–628. [Google Scholar]
15. Grotenhuis, H. B., Cifra, B., Mertens, L. L., Riessenkampff, E., Manlhiot, C. et al. (2019). Left ventricular remodelling in long-term survivors after the arterial switch operation for transposition of the great arteries. European Heart Journal Cardiovascular Imaging, 20, 101–107. [Google Scholar]
16. Shepard, C. W., Germanakis, I., White, M. T., Powell, A. J., Co-Vu, J. et al. (2016). Cardiovascular magnetic resonance findings late after the arterial switch operation. Circulation Cardiovasc Imaging, 9, 1–10. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |