

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011523
ARTICLE
Preoperative Risk Assessment and Perioperative Management of Adults with Congenital Heart Disease Undergoing Non-Cardiac Surgery
Adult Congenital Heart Disease Unit, Department of Cardiology, Monaldi Hospital, Naples, 80131, Italy
*Corresponding Author: Giancarlo Scognamiglio. Email: giancascognamiglio@alice.it
Received: 13 May 2020; Accepted: 05 June 2020
Abstract: Adults with congenital heart disease (ACHD) constitute a growing population with complex cardiac physiopathology and frequent extra-cardiac involvement. The recent dramatic improvement of their life expectancy has resulted in an increasing proportion of ACHD patients requiring non-cardiac surgery. While a large body of evidence demonstrated the importance of an accurate risk assessment in patients with acquired heart disease before non-cardiac surgery in order to reduce perioperative morbidity and mortality and detailed algorithms have been released by international societies, no specific guidelines are available for the perioperative management in this population. Nonetheless, understanding the complex anatomy and unusual physiology of both repaired and unrepaired congenital heart disease is paramount to meet the unique needs of these patients and to ensure an adequate perioperative management and prevention of complications. Furthermore, anaesthesiologists and surgeons unfamiliar with congenital heart disease may be not aware of the variety of different disease-related issues, which may arise in ACHD patients during the hemodynamic changes of the perioperative phase, with possible severe adverse effects on cardiac performance. We herein review the limited evidence from the literature and summarize our personal experience in a tertiary ACHD centre in order to propose a first structured approach for preoperative risk assessment and perioperative management to reduce the mortality and morbidity risk in adults with congenital heart disease undergoing non-cardiac surgery.
Keywords: Adult congenital heart disease; non-cardiac surgery; risk stratification; perioperative management
The focus of this review is the management of cardiovascular risk in adults with congenital heart disease (ACHD) undergoing non-cardiac surgery. The necessity of a thorough and practical reappraisal of this issue arises from the demographic change occurred in the last three decades in ACHD population which currently outnumbers the paediatric population with congenital heart disease (CHD), as a result of a dramatic improvement in life expectancy [1]. Consequently, a concomitant steadily rising proportion of ACHD patients with complex physiology requiring non-cardiac surgery has recently been reported [2]. Notably, perioperative mortality represents the third most common cause of death in this population, after sudden cardiac death and progressive heart failure (HF) [3]. ACHD patients constitute a complex and heterogeneous population with high incidence of hospital admissions, arrhythmic events, acute HF episodes and need for cardiac reinterventions. Furthermore, besides the cardiac problem itself they can present multiple comorbidities, either secondary to chronic cardiac-related multi-organ deterioration (i.e., liver failure, impaired lung function, renal insufficiency, alterations of the erythropoietic system) or acquired age-related cardiovascular diseases, such as diabetes, atherosclerosis and hypertension [4,5]. However, to date, only few data on the perioperative management of ACHD patients are available. Surgeons and anaesthesiologists not routinely involved in the care of patients with CHD may not be aware of the unique challenges that may be encountered during non-cardiac surgery in these patients. In the present paper we review the impact of the specific physiopathological features of congenital heart defects on the management of the pre, intra and postoperative phases of a non-cardiac surgical intervention. Furthermore, we propose an approach for comprehensive risk stratification and suggest strategies to minimize the risk in these complex patients based on the limited data available from the literature, current guidelines and our experience in a tertiary ACHD centre.
2.1 Risk Stratification in ACHD
In the recent years, a large body of evidence has demonstrated the importance of an accurate risk assessment in patients with heart disease before non-cardiac surgery in order to reduce perioperative morbidity and mortality. Accordingly, the major Cardiovascular Societies have released specific Guidelines and recommendations [6,7], suggesting different risk indices [8,9] and algorithms based on the underlying cardiac disease and the type of intervention. However, they are not suited for risk evaluation in ACHD patients who may have additional disease-related risk factors and unique hemodynamic conditions. In 1998, one retrospective study in a large cohort demonstrated that patients with complex anatomy, cyanosis, history of congestive HF and patients undergoing operations involving the respiratory or nervous system and urgent procedures are at higher risk [2]. Nevertheless, no further studies have systematically investigated risk factors in this population and risk assessment remains a challenging task, requiring a complete understanding of the previous cardiac surgeries and current cardiac physiology.
2.1.1 Risk Related to the Type of Non-Cardiac Surgery
The 2014 ESC/ESA Guidelines on non-cardiac surgery [6] classify non-cardiac interventions in the general population into three categories according to the 30-day risk of cardiovascular death and myocardial infarction, which are reported in Tab. 1. Perioperative risk is deemed higher in case of urgent procedures. These concepts are generally extended to ACHD patients, despite the fact that those guidelines are mainly focused on traditional cardiovascular risk factors that are not the major concern in this complex population and do not consider specific issues related to CHD physiology. Moreover, it has been demonstrated that this population is at higher risk of mortality and perioperative morbidity even during simple procedures compared to the general population [10]. Accordingly, current guidelines suggest to considering CHD as a risk factor in itself during non-cardiac surgery [6].
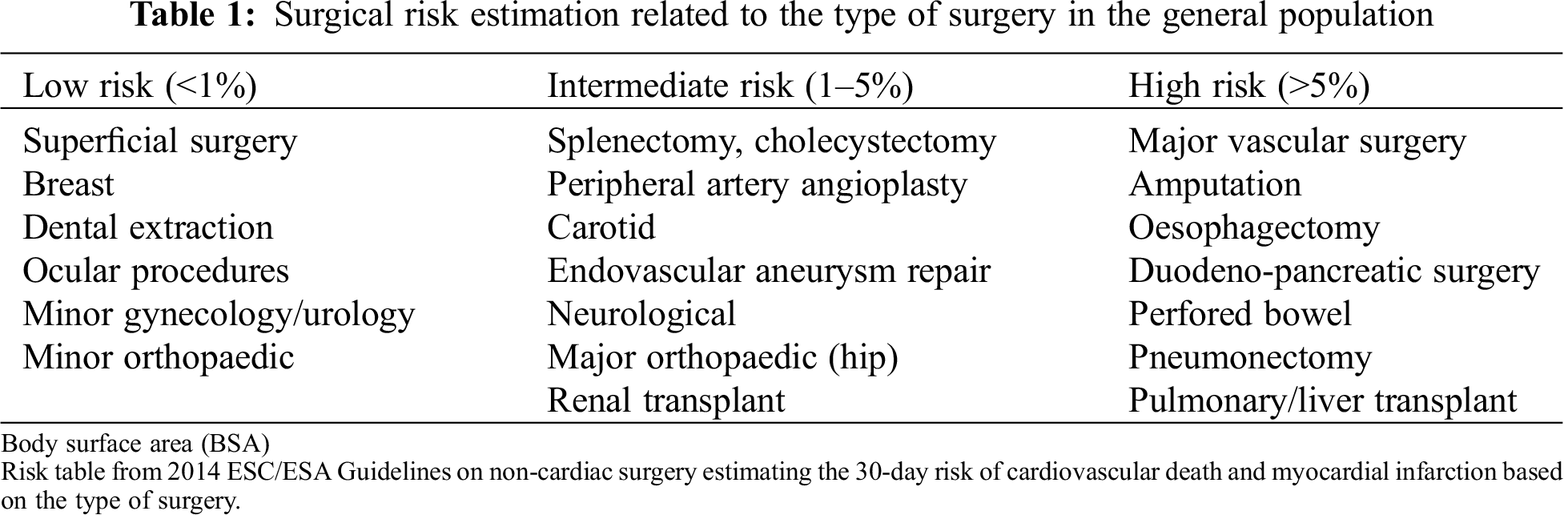
2.1.2 Patient-Related Risk Factors in ACHD Population
Numerous patient-related factors should be taken into account. In particular, knowledge of the baseline anatomy, previous surgeries and current medications is essential and previous medical records should be carefully reviewed. A checklist of issues to consider should include all the following points:
-Disease Complexity Assessment
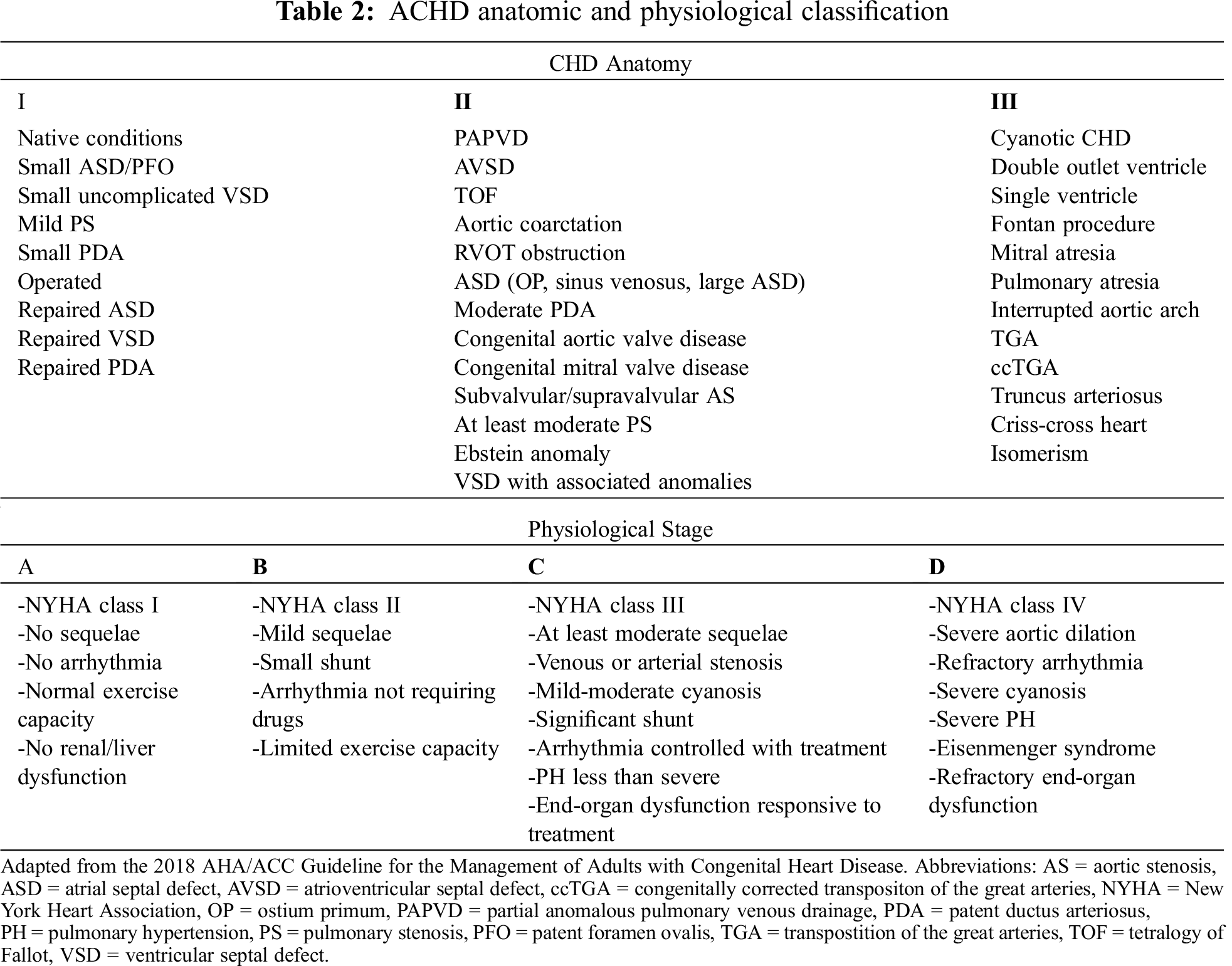
Cardiac disease complexity is the single major determinant of outcome in patients with CHD. The American College of Cardiology Task Force of the 32nd Bethesda Conference developed a classification to categorize disease severity in simple lesions, moderate lesions, and complex lesions [11]. Most recently, the 2018 AHA/ACC guideline for the management of adults with CHD [12] proposed the Adult Congenital Heart Disease Anatomic and Physiological classification (ACHD-AP), which includes the same categorization of the previous classification and encompasses 4 physiopatological stages (A–D), for each class of anatomical severity (I-III), with a total of 12 categories, allowing a more accurate risk stratification [13] (Tab. 2). Invasive procedures in patients with complex anatomy and impaired physiological state are at higher risk [2,14,15]. Therefore ACHD guidelines [12] recommend that, in patients with ACHD-AP class IB-D, II and III, non-cardiac surgical procedures should be performed in a hospital with an ACHD program or in consultation with experts in ACHD (Recommendation class I, level of evidence C).
-Assessment of Ventricular Function and Treatment Optimization
Physical examination allows the identification of signs of decompensated HF such as peripheral oedema, pulmonary rales, hepatomegaly, jugular vein distension. A cardiac murmur should raise the suspicion of valvular disease, ventricular outflow obstruction, and/or the presence of a shunt. Echocardiography can readily provide important information on ventricular function, presence and severity of valvular dysfunction, magnitude and direction of possible native and/or post-operative shunts. Preoperative echocardiographic assessment of ejection fraction for estimation of ventricular function is a powerful predictor of postoperative complications, as well as of ICU length of stay in patients with complex anatomy [14]. In ACHD, natriuretic peptides have demonstrated the same diagnostic and prognostic role showed in acquired heart diseases and, therefore, can represent a further marker of the preoperative hemodynamic status. Current ACHD guidelines recommend optimizing treatment before non-cardiac procedures to reduce the perioperative risk [12]. The management of left ventricular failure with renin-angiotensin-aldosterone system blocking agents, beta-blockers, diuretics and digoxin, is similar to other forms of acquired HF. The same recommendations can be reasonably extended to cases with systemic right ventricle (RV), including congenitally corrected transposition of the great arteries (ccTGA) and Mustard or Senning repair of transposition of the great arteries (TGA). Patients with predominant RV failure and/or pulmonary hypertension (i.e., Eisenmenger syndrome) are at high risk and therefore any invasive procedures, unless urgent, should be performed in presence of durable, relative hemodynamic stability. Diuretics should be cautiously administered to avoid an excessive preload reduction and specific therapy for pulmonary hypertension (PH) should be preferably optimized. In patients with single ventricle and Fontan circulation an optimal volume status should be preserved along with optimization of medical treatment to maintain a normal ventricular filling pressure. Serum albumin levels should be checked in order to exclude a protein losing enteropathy.
-Valvular Residual Lesions and Surgical Sequelae
During follow-up, progressive valvular dysfunction is frequently observed on surgically repaired valves. Typical examples are pulmonary regurgitation late after surgical relief of right ventricular outflow tract obstruction in patients with Tetralogy of Fallot (TOF) or atrio-ventricular (AV) valves disease after repair of atrioventricular septal defect (AVSD). On the other hand, dysfunction of unrepaired valves may be caused by residual/recurrent obstructive lesions (i.e., subaortic stenosis), by unrepaired defects (i.e., aortic regurgitation in patients with small unrepaired perimembranous ventricular septal defect) or may represent a functional sequela of abnormal hemodynamic overload (tricuspid regurgitation in TOF repair, AV valve dysfunction in Fontan circulation and TGA with atrial switch). Residual lesions and surgical sequelae should be systematically searched and recognized in ACHD patients undergoing non-cardiac surgery in order to evaluate the hemodynamic status and optimize medical treatment. In addition, in the presence of significant lesions (i.e., TOF and severe pulmonary regurgitation and right ventricular dysfunction, bicuspid aortic valve with severe stenosis and symptoms) individualized indications on the timing of elective major non-cardiac surgery should be given by the ACHD team, who may defer non-urgent procedures after correction of the cardiac defect.
-Shunting Lesions
Shunting lesions pose unique challenges to the intraoperative management. Blood flow direction across a shunt is mainly driven by pressure difference between systemic and pulmonary vascular resistance (SVR and PVR respectively). Therefore, in absence of PH, the blood flow is directed from the high-pressured left chambers towards the right heart. During induction of anaesthesia, a PVR drop (i.e., due to oxygen administration) or a catecholaminergic-related increase in SVR may increase a left-to-right shunt and contribute to systemic hypoperfusion [16]. Conversely, high doses of induction drugs may result in systemic hypotension, occasionally resulting in flow reversal and right-to-left shunt with subsequent arterial desaturation [17].
-Arrhythmic Burden
Arrhythmic events are frequently encountered in the ACHD population. The main contributing factors are represented by hemodinamically significant sequelae and myocardial scars subsequent to the surgical correction. The most common type is the so-called “incisional tachycardia”, an intra atrial reentrant tachycardia mainly observed in patients with atrio-pulmonary Fontan type and TGA after atrial switch. Since this type of tachycardia is often drug resistant and can cause rapid hemodynamic deterioration, an electrical cardioversion may be required. Supraventricular arrhythmias in patients with Ebstein anomaly may be due to multiple re-entrant atrioventricular accessory pathways, therefore a short PR interval and/or delta waves must always be searched at ECG.
On the other hand, ventricular arrhythmias are more frequent in ACHD patients whose surgical repair required ventriculotomy and patch implantation with hemodinamically significant sequelae and extensive ventricular scarring. The typical example is TOF repair with residual severe pulmonary regurgitation. ECG Holter monitoring may be helpful in assessing the preoperative arrhythmic risk. Accordingly, before non-cardiac procedures in patients with pro-arrhythmic forms of CHD and/or a history of significant tachyarrhythmia, all the cardiac (i.e., ventricular dysfunction, volume overload, significant pressure overload) and extracardiac (electrolyte disorders, thyroid dysfunction, pulmonary diseases, infections) factors that can potentially trigger or exacerbate tachyarrhythmias during or after the intervention must be checked and possibly corrected.
-Pacing Devices
Patients with CHD may need permanent pacemakers for the treatment of severe bradyarrhythmias, which could be secondary to postoperative atrioventricular block (i.e., after correction of perimembranous ventricular septal defect, subaortic stenosis, AVSD), to the cardiac defect itself, (i.e., ccTGA) or part of sick sinus syndrome (TGA after atrial switch). Furthermore, in the last decades the use of implantable cardioverter-defibrillators and cardiac resynchronization devices has substantially increased in the CHD population. The management of patients with intracardiac pacemakers and defibrillators does not differ from the general population. However, it is important to keep in mind that many complex ACHD patients, may require epicardial rather than endocardial pacemaker implantation due to anatomic or technical factors (i.e., Fontan patients with extracardiac conduit), and/or presence of native/residual shunts, so that these devices might be positioned below the diaphragm. The accurate knowledge of the device position is essential in case of abdominal surgery to prevent damages or infections. Moreover, when the use of an electric scalpel is planned, pacemakers must be programmed in V00 mode, to avoid inhibition of the device by electrical impulses. At the end of the procedure, the device can be reprogrammed with the previous modality [18].
-Vascular Disorders
CHD may present as vascular disorders as it is the case of patients with bicuspid aortic valve and aortic coarctation, which are recognized as aortopathies with increased risk of vascular complications such as aortic dissection or rupture of intra-cranial aneurysms. Furthermore, residual obstruction in patients with aortic coarctation may cause increased left ventricular afterload, which should be acknowledged before invasive procedures. Antihypertensive therapy should be optimized in the preoperative phase in patients with aortic coarctation with a view to preventing both perioperative hyper and hypotension. It should be noted that normal blood pressure values assessed in the right arm may cause severe hypotension of the arterial circulation downstream the coarctation site leading to peripheral hypoperfusion and spinal ischemia.
Moreover, CHD may be associated with arteriovenosus malformation, especially in the context of cyanotic CHD, associated with increased bleeding risk. Patients with arterial switch repair of TGA may complicate with coronary obstruction during follow-up. In the latter case, in presence of left ventricle dysfunction or symptoms suggestive of myocardial ischemia, further tests are warranted to assess the coronary patency before invasive procedures [12].
-Pulmonary Sequelae
The pulmonary sequelae of CHD can complicate the perioperative management and can increase the surgical risk. Adults who underwent heart surgery in childhood often have restrictive lung disease, which affects the ventilator management and the timing of extubation [19]. A study by Alonso-Gonzalez et al. [20] demonstrated that the severity of lung function impairment is related to the complexity of underlying CHD, the number of previous thoracotomies, BMI, cardio-thoracic ratio, scoliosis and diaphragm palsy. Accordingly, all these factors should be taken into account during surgical planning. In our experience, patients with complex anatomy and history of multiple cardiac interventions may benefit from a pulmonologist consultation and further preoperative respiratory testing (spirometry, chest x-ray/thoracic CT).
-Renal Function
Chronic kidney disease (CKD) is common among patients with CHD [21], especially those with Eisenmenger physiology and Fontan circulation [22] and is associated with increased mortality [21]. Low cardiac output with impaired renal perfusion, chronic activation of renin-angiotensin-aldosterone system, increased sympathetic activity and raised venous pressure are potential mechanisms for CKD in ACHD. Baseline glomerular filtration rate has a prognostic value and should be assessed before surgery. The renal function vulnerability in ACHD patients warrants judicious use of contrast agents in case imaging tests are requested in the postoperative phase. In addition, reduced end-organ perfusion during anaesthesia and administration of nephrotoxic drugs should be avoided as they may contribute to renal dysfunction possibly leading to acute kidney injury.
-Cyanotic CHD
Cyanosis is common in patients with complex CHD or Eisenmenger syndrome. Providers must consider the multiple organs affected by chronic cyanosis. Cyanotic patients have increased surgical bleeding risk [10] because of multiple collaterals, platelet abnormalities for number and function and alterations in the coagulation cascade [23]. However, the increased risk for bleeding is not protective against thrombosis. In fact, these patients develop secondary erythrocytosis as a compensatory response to chronic hypoxia with resulting increased blood viscosity and decreased flow velocity in the small arterioles and capillaries. This phenomenon is further exacerbated in the setting of iron deficiency and dehydration, conditions frequently occurring after major surgeries. Thus, patients with complex cardiac lesions such as severe PH or Eisenmenger syndrome carry a very high risk for adverse outcomes during non-cardiac surgery and elective surgery is usually best avoided.
-Bleeding and Thrombotic Risk
Patients with CHD have an increased risk of both thrombotic and bleeding complications. Thrombotic events may be related to atrial arrhythmias, residual shunts, prosthetic valves, fibrinolysis factors deficiency and blood hyperviscosity. Intracardiac shunts may also predispose to paradoxical embolism in case of deep venous thrombosis. The risk is related to disease complexity [24], with higher incidence in patients with Fontan circulation (3%–20%) [25,26] or cyanotic CHD (20%–47%) [27,28]. Therefore, administration of anticoagulation or antiplatelet treatment is often required in these patients in primary or secondary prevention. Surgical bleeding and postoperative hemorrhagic complications in CHD are facilitated by arteriovenous malformation, coagulation factors deficiency resulting from liver congestion, platelet dysfunction, thrombocytopenia and chronic anticoagulation, especially when not properly adjusted for impaired renal function or low body weight. Furthermore, in patients with Fontan palliation, the increased risk for bleeding is also attributed to reduced hepatic synthesis of coagulation factors, particularly factor VII [29], due to decreased hepatic function and protein losing enteropathy. Unfortunately, risk factors for both thrombosis and bleeding may be concomitantly present in complex disease such as Eisenmenger syndrome or Fontan circulation, making even more challenging their perioperative management.
Bleeding and thrombotic risk should be carefully evaluated in the preoperative setting reviewing previous medical history and with blood tests including blood cells count, prothrombin time, partial thromboplastin time, activated partial thromboplastin time and renal and liver function. The intrinsic bleeding risk of the type of surgery should also be taken into account: procedures with a low bleeding risk are those in which adequate hemostasis can be achieved (i.e., minor skin procedures) or in which bleeding would have minor impact (i.e., dental extraction, cataract), whereas procedures with a high bleeding risk are those in which bleeding could threaten patients’ life (i.e., neurosurgery).
-Preoperative Management of Oral Anticoagulation in ACHD
The ESC and EHRA guidelines provide a guide for anticoagulation management in patients undergoing invasive procedures. Due to lack of data in ACHD population, it is reasonable to follow the same algorithm used the general population. Anticoagulation should be stopped when the bleeding risk outweighs the thrombotic risk, therefore patients undergoing procedures with low bleeding risk do not require treatment modification. Patients on vitamin K antagonists (VKA) can safely undergo major procedures when INR is ≤ 1.5 [6]. However, patients with high thrombotic risk and patients with metallic prosthetic valves require bridging therapy with unfractionated heparin. In the last years, growing evidence is supporting the safe use of non-vitamin K oral antagonists (NOAC) in the ACHD population [30,31], thus, making the perioperative management easier, without necessity of heparin bridging and of routinely monitoring of coagulative parameters. Patients undergoing high-risk procedures should stop NOAC 48 h before surgery [32] or 72 h in case of reduced kidney function. When an urgent procedure is required, protamine sulphate or frozen plasma for patients on VKA and specific antidotes for NOAC should be considered to reverse anticoagulant effect [6].
-Infective Endocarditis
ACHD patients have an increased risk of infective endocarditis (IE). However, according to current guidelines, antibiotic prophylaxis is indicated only for those at higher risk for perioperative infections [33,34], including patients with complex cyanotic defects, valvular prostheses, intra- and extracardiac conduits, residual shunts and, most recently, patients with previous percutaneous implant of a bioprosthetic pulmonary valve, which show a cumulative incidence of IE ranging from 3.2% to 25.0 [35]. Before the procedure, the patient should stay afebrile with inflammatory markers in the normal range. Special precautions should be taken during venous cannulation and placement of catheters, which should be removed as soon as deemed safe. In presence of postoperative persistent, unexplained fever, IE should be suspected especially in the subgroups above-mentioned and, hence, echocardiography and serial blood cultures should be obtained.
2.2 Preoperative Investigations
ACHD patients, especially those with complex defects, should be referred for additional specialist investigations before undergoing elective non-cardiac surgery, according to the 2014 ESC/ESA Guidelines on non-cardiac surgery [6] (Recommendation class I, level of evidence C). Preoperative exams should aim to evaluate the hemodynamic status and the surgical risk through assessment of cardiac sequelae as well as extracardiac involvement. We here propose an algorithm to guide preoperative investigations based on cardiac disease complexity (Fig. 1). Findings from first level imaging exams provide essential data on ventricular and valvular function, presence of shunts and other residual lesions. Nevertheless, assessment of functional capacity is also important for accurate risk stratification in patients with moderate to complex CHD. Cardiopulmonary exercise (CPET) test is the gold standard functional test providing data on both cardiovascular and respiratory efficiency. CPET parameters have also been related to mortality in the ACHD population [36]. Six-minute walking test may be simple alternative especially useful for patient with inability to cooperate (i.e., Down syndrome). However, the role of testing the exercise capacity in the preoperative setting in this population needs further definition.
Second level imaging tests can be required when a lesion with potential impact on the surgical outcome is suspected and first level imaging is inconclusive or in case of discrepancy between symptoms and findings of the first level imaging. A classical example is the presence of decompensated HF or impaired exercise capacity in a patient with atrial septal defect (ASD). In this clinical scenario, the echo evidence of disproportionate severe dilation of right chambers in presence of a small ASD should raise the suspicion of a partial anomalous pulmonary venous drainage. In this setting, cardiac MRI with measurement of Qp/Qs allows diagnostic clarification and non-invasive functional assessment. Another important indication to second level imaging is to rule out a thrombus in a patient with Fontan circulation. In this context, transthoracic echocardiography may show ambiguous images suggestive of clot inside the circuit. A CT scan with contrast injection in the femoral vein may be required for definite diagnosis in patients with total cavo-pulmonary connection.
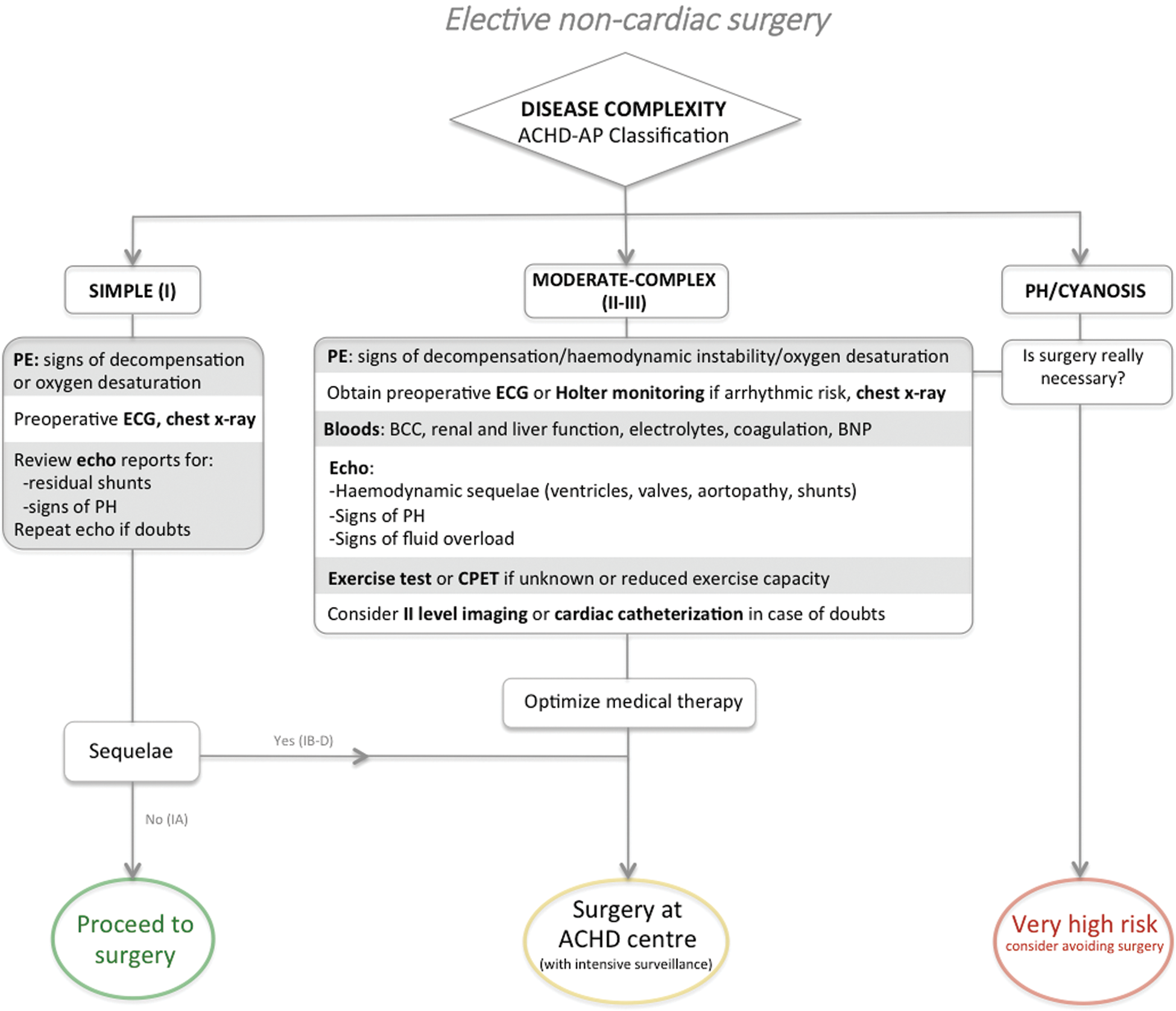
Figure 1: Proposed preoperative algorithm for elective non-cardiac surgery in ACHD patients
ACHD is an independent risk factor for increased perioperative mortality and morbidity, which is consistent with the frailty of these patients. Two main consequences derive from this premise:
1. The importance of a multidisciplinary team including anaesthesiologists familiar with anatomy and physiology of CHD and skilled in managing such patients [37,38]. Multiple reports, in fact, describe the occurrence of perioperative complications due mismanagement related to lack of knowledge of the underlying cardiac physiology.
2. There is consistent evidence supporting lower in-hospital incidence of adverse events in ACHD tertiary centres compared to patients operated in local hospitals [39]. Patients with simple CHD and no surgical sequelae or significant residual lesion will require less aggressive management, while other patients with moderate or complex heart disease will need specific intraoperative management provided by a specialized multidisciplinary team (Fig. 1).
Lesion-related specific issues, which may arise during the peri and postoperative phases of non-cardiac surgery in ACHD patients with some hints on the potential strategies to minimize the risks, are summarized in Fig. 2.
Furthermore, many adults with CHD are familiar with anaesthesia and a surgical environment, since most of them have already undergone previous cardiac surgery. However, a psychological preparation is always important, including a comprehensive and clear explanation of the intervention and the measures that will be taken to minimize the risk of cardiac complications.
All the patients undergoing a major surgical procedure require standard intraoperative non-invasive monitoring including: pulse oximetry, ECG, arterial blood pressure, capnography and temperature. Intraoperative pulse oximetry is of utmost importance in ACHD patients. In presence of cyanotic defects and/or shunting lesions, a drop in oxygen saturation can indicate an increased right-to-left shunt due to excessive systemic vasodilation or a reduced pulmonary perfusion secondary to hemodynamic or ventilatory changes, i.e., increased PVR, hypercapnia or acidosis.
Invasive monitoring tools should be used with caution in these patients, weighting the potential benefit of a more accurate intraoperative surveillance against the risks of complications from invasive procedures, with possible adverse consequences in these patients. Obtaining venous or arterial vascular accesses may be challenging because many of these patients have already undergone previous multiple catheterizations. In patients with shunts or cyanosis, variations of systemic pressure may have substantial hemodynamic consequences and invasive pressure monitoring with intraarterial line is reasonable during major surgery.
Moreover, knowledge of the anatomy and physiology of specific palliative operations is essential: in patients with a classic Blalock-Taussig shunt (end to side anastomosis of the subclavian and pulmonary arteries) or subclavian flap repair of aortic coarctation, systemic blood pressure and SpO2 must be measured on the controlateral side to avoid misdiagnosis of a hypotensive state, which may trigger inappropriate and potentially harmful manoeuvres. In the general population, a central venous catheter (CVC) is usually indicated for central venous pressure (CVP) monitoring and for intravenous drug and fluid administration. In complex ACHD patients, placing a central venous line may be particularly tricky due to special anatomical conditions. In univentricular hearts after Glenn and Fontan palliation, for instance, CVCs, besides the technical challenges of insertion, often provide misleading hemodynamic data with non-univocal interpretation from inexperienced operators. In this context, CVP is usually increased being influenced from pulmonary artery pressure and therefore it should not be used to assess intravascular volume in Fontan circulation. A persistent left superior vena cava is frequently associated with CHD and can pose difficulties during central venous catheterization. This condition should be suspected in presence of coronary sinus dilation on echocardiography.
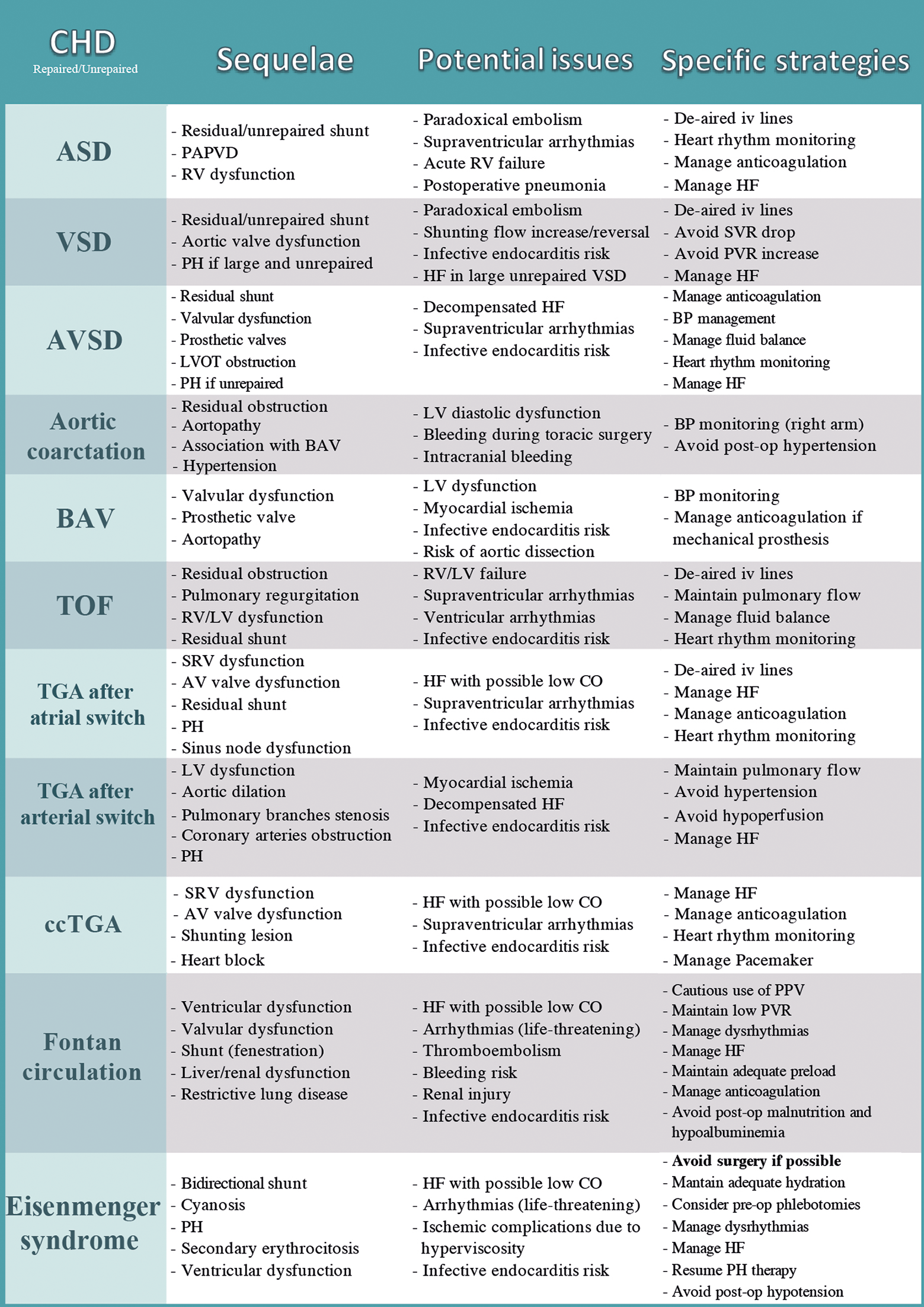
Figure 2: Disease-specific potential issues in the peri and postoperative phases and suggested specific strategies to minimize the risk in ACHD undergoing non-cardiac surgery
Another important consideration pertaining intravascular catheters is that patients with complex CHD frequently have increased thrombotic risk and, in this scenario, a catheter thrombosis can lead to severe complications including pulmonary thrombosis (i.e., Fontan patients) or paradoxical embolism through an intracardiac shunt (i.e., Eisenmenger patients, fenestrated Fontan) with dramatic hemodynamic consequences. Moreover, to avoid air embolism, all the venous lines should be meticulously de-aired and protected with air filters, especially in the context of shunting lesions [40]. An intrapulmonary catheter insertion for invasive monitoring of pulmonary pressure is very rarely required, because of the above-mentioned possible seriously detrimental effects in case of thrombosis. Finally, intraoperative transesophageal echocardiography performed by an ACHD-experienced operator may be helpful for ventricular function and volume status assessment during major surgery in patients with CHD.
3.2 Fluid Balance in the Perioperative Phase
Adequate hydration must be maintained with intravenous fluids administration in complex ACHD patients, whose stroke volume may be preload dependent (e.g., Fontan circulation). At the same time, volume overload should be carefully avoided as it may cause congestion and acute HF. Optimal fluid balance is therefore pivotal before, during and after surgery and warrants a close monitoring.
In some centres, patients with more complex cardiovascular physiology and unstable hemodynamic, are admitted the day before surgery to allow adequate hydration in the setting of a fasting state preceding the intervention. Hydration status is particularly important in cyanotic patients, who have increased blood viscosity and both high bleeding and thrombotic risk. In this setting, if the hematocrit exceeds 65%, preoperative phlebotomies can be considered, followed by adequate replacement with isotonic saline infusion and storage of the removed blood that can be auto-transfused in the postoperative period [41].
3.3 Risk Related to the Anaesthetic Techniques
Management of general anaesthesia in these complex patients should be performed by anaesthesiologists with specific knowledge of ACHD physiology, as this can have a huge impact on the surgical outcome [42]. Recently, sub-specialty training programs in CHD for anaesthesiologists have been developed and are highly recommended by international societies [43]. A major goal of intraoperative management is to ensure an adequate tissue oxygen delivery through prevention of arterial desaturation and hypoperfusion, maintenance of a balanced Qp/Qs and optimization of fluid balance. A comprehensive guide on intraoperative anaesthesiological management of patients with CHD is beyond the purpose of this paper, nevertheless it provides the opportunity to highlight some of the effects of general anaesthesia on the complex cardiac physiology of this population.
3.3.1 Hemodynamic Effects of Anaesthetic Drugs in ACHD Patients
There is paucity of data on the hemodynamic effects of anaesthetic agents in ACHD patients. Premedication with anxiolytics and hypnotics is paramount to reduce sympathetic-related increase in oxygen consumption, however particular attention should be paid to prevent hypoventilation and hypercapnia with subsequent increase in PVR [40], which is deleterious in patients with underlying PH or Fontan circulation. Moreover, it should be noted that the presence of an intracardiac shunt may influence drug distribution of both intravenous and volatile drugs during induction [44], with quicker onset of iv administrated drugs in case of right-to-left shunt. Therefore, dose adjustments may be required to prevent abrupt hemodynamic effects. Most intravenous agents depress myocardial contractility and induce systemic vasodilation, with potential reduction of the tissue oxygen delivery during induction of anaesthesia and frequent necessity of hemodynamic support with vasopressors, especially in those with complex physiology [45]. Patients with Eisenmenger syndrome are particularly susceptible to hemodynamic changes during induction, as they have permanently raised PVR, resulting in impaired efficiency of adaptation. Despite the lack of data, ketamine is frequently used in clinical practice in patients with CHD for its favourable inotropic effect mediated by a central release of catecholamines [46]. Moreover, the results of a recent systematic review suggest that ketamine might be safe in ACHD patients with no significant changes in SVR or PVR [47]. Ketamine administration has also been described in complex patients with cyanosis in combination with other anaesthetic drugs in order to mitigate their effects and support the cardiovascular system [48]. Regional anaesthesia could potentially represent an alternative to general anaesthesia for patients undergoing peripheral procedures; however, spinal or epidural anaesthesia may produce non-negligible decreases in SVR facilitating right-to-left shunting and cyanosis in patients with unrestrictive intracardiac shunts.
3.3.2 Hemodynamic Effects of Invasive Ventilation in Complex CHD
General anaesthesia allows for optimal control of ventilation and may be preferable in patients undergoing high-risk surgery. Ventilation with high airway pressure can compromise venous return, increase PVR, and, thus, exacerbate right-to-left shunting in patients with cyanotic heart disease. Furthermore, higher intrathoracic pressures for prolonged periods will decrease cardiac output in Fontan circulation. Hence, low airway pressure is desirable and anaesthesiologists are encouraged to minimize positive end-expiratory pressure to the lowest level required. On the other hand, in patients with shunting defects, inadequate anaesthesia associated with incomplete suppression of sympathetic activity can raise the SVR with consequent increased overall left-to-right shunt and reduced cardiac output. Single ventricle physiology and Eisenmenger syndrome are probably the most challenging CHD. In both conditions, any factor increasing PVR must be identified and promptly corrected before low cardiac output occurs. In fact, in Eisenmenger patients, an abrupt increase in PVR may precipitate acute right ventricular failure and exacerbate systemic oxygen desaturation, while in patients with Fontan circuit, any increase in PVR causes a reduction of the single ventricle preload and, thus, of the cardiac output. Prevention and treatment of pulmonary hypertensive crisis includes hyperventilation with higher supplemental oxygen levels taking advantages of its potent pulmonary vasodilator effect, correction of acidosis, avoidance of sympathetic nervous system stimulation, maintenance of normothermia and use of inotropic support when indicated. Inhaled nitric oxide should be readily available in the operating theatre to treat low cardiac output in such circumstances [49].
3.4 Risk Related to Specific Surgical Modalities
Some interventions considered at low risk in the general population may have catastrophic consequences in some subsets of ACHD. A typical example is represented by abdominal laparoscopy, a common minimally invasive procedure that nonetheless, which however may undermine the hemodynamic balance in Fontan circulation, in which the pulmonary flow is entirely dependent on adequate preload in the absence of a pumping right ventricle. Gas insufflation, by increasing intra-abdominal pressure, compresses splancnic veins and the inferior vena cava reducing the systemic venous return and potentially precipitating a chronically reduced cardiac output into a severe low-output state. However, use of low intra-abdominal pressures (<10 cmH2O) appears to have minimal impact on oxygenation, ventilation and cardiac output in patients with Fontan circulation [50]. Abdominal gas insufflation may also be responsible of CO2 absorption and diaphragm elevation resulting in hypercapnia, which can raise PVR [14], with adverse effects on Fontan circulation. Additionally, gas emboli from pneumoperitoneum have been reported with severe hemodynamic consequences [51] and possible paradoxical arterial embolization in presence of an intracardiac shunt [52]. Intraoperative TOE is the most useful technique for early recognition of gas embolism [53]. Another rare complication with severe implications is the carbon dioxide pneumothorax due to CO2 displacement into the pleural cavity, most commonly through a congenital defect of the diaphragm [54], with potential devastating hemodynamic effects in ACHD patients.
Furthermore, major surgery involving abdomen, pelvis, spine, cerebral posterior fossa, often requires a specific position (e.g., prone, Trendelenburg), which could significantly impact on the hemodynamic. In patients with Fontan and/or Glenn shunt, a prolonged and steep Trendelenburg position can increase superior vena cava pressure and determine cerebral malperfusion [40]. Prone positioning is often used for spinal surgery and is associated with decreased preload through compression of the inferior vena cava, with deleterious hemodynamic effects on Fontan circulation, especially in case of concomitant massive bleeding [55]. Neck interventions should be cautiously performed in patients with known vascular malformations or with a Glenn anastomosis.
Another issue to consider is the potential gas embolism during neurosurgical interventions performed in the semisitting position. In this position, the venous pressure at wound level may become negative, increasing the risk of gas embolization [56]. The presence of an intracardiac shunt must be excluded before surgery to prevent paradoxical gas embolism [57,58], usually by means of transthoracic bubble test, especially in patients with repaired shunting lesion or intracardiac tunnels (i.e., TGA after atrial switch).
An insufficient postoperative monitoring/care represents one of the major contributors to adverse events [40]. Common postoperative complications such as fever, thromboembolism, bleeding, suboptimal hydration status, muscular deconditioning and infections should be readily addressed as they may have a greater prognostic impact than in the general population. Accordingly, in the first 24 hours after surgery patients with more complex CHD and/or after major surgery should ideally be admitted and managed in a postoperative intensive care unit experienced in the management of ACHD or at least in an intensive care unit that can rely on consultations from an ACHD-specialised staff. Mismanagement from personnel not familiar with CHD physiology may ensue from over- or underestimation of the heart defect and its consequences. Overestimation of CHD can lead intensive care unit medical staff to erroneously focus on a simple or repaired defect, eventually ascribing any postoperative event to the cardiac defect itself [42], with subsequent delay of recognition and treatment of the actual causal mechanism (e.g., failure to recognize the cause of hypoxemia in patients with postoperative pneumonia in the setting of a successfully repaired ASD). Underestimation, on the other hand, by far more frequent, can lead to neglect a possible cardiac source of postoperative complications (e.g., ischaemic stroke in a patient with unrepaired ASD).
The main goals of postoperative care are correction of acidosis, maintenance of normothermia, and normoxia, minimization of intrathoracic pressure, adequate hydration, close surveillance of postoperative arrhythmias and avoidance of excessive sympathetic nervous system stimulation through adequate pain management. In patients with known HF, close monitoring of fluid shifts is paramount, and every effort should be made in agreement with the surgical team to limit parenteral hydration to the minimum required. Deep venous thrombosis prophylaxis should be performed in all cases, by means of early mobilization, mechanical agents or pharmacological therapy, according to the individual risk and the type of surgery. The resumption of anticoagulant and antiplatelet therapy after non-cardiac surgery depends on the balance between thrombotic and bleeding risk. For patients at higher thromboembolic risk, oral anticoagulants can be resumed as soon as deemed safe. In patients with low risk of thrombotic complications or after high bleeding risk interventions, these drugs may safely be recommenced several days later [32].
Some hints may be useful in postoperative management of specific subsets of ACHD. Patients with left-to-right shunting and pulmonary overflow have an increased risk of postoperative pneumonia. In patients with aortic coarctation, adequate and careful postoperative blood pressure monitoring is warranted and sympathetic tone should be minimized to reduce the risk of hypertensive crisis. As previously stated, patients with Eisenmenger syndrome have impaired adaptations to hemodynamic changes. Accordingly, postoperative mobilization should be cautious and gradual to avoid postural hypotension, which could exacerbate a right-to-left shunt, causing arterial hypoxia [59]. Fontan patients are at higher risk of bleeding complications requiring reintervention, have a higher rate of transfusions and can develop low cardiac output syndrome with metabolic acidosis, acute renal failure, arrhythmias and necessity of vasopressor or inotropic support [60]. Careful surveillance for early recognition of complications in the postoperative period is necessary in this subset.
ACHD population is a growing subset of patients with unusual hemodynamic features and frequent comorbidities leading to a higher mortality and morbidity risk in case of non-cardiac surgery compared to the general population. Understanding the complex anatomy and deranged physiology of both repaired and unrepaired congenital heart disease is paramount to meet the unique needs of this population and prevent surgery-related complications. Furthermore, a variety of different disease-related specific challenges may arise in the perioperative phase with possible severe adverse effects on cardiac performance in ACHD patients. Careful preoperative risk stratification, surgical planning and perioperative active surveillance by a multidisciplinary team including ACHD-expert cardiologists, anaesthesiologists and surgeons in ACHD tertiary level centres are essential for risk control through early identification and adequate treatment of potential issues impacting on the final outcome of non-cardiac surgery in this complex population.
Funding Statement: The authors received no specific funding for this study.
Conflict of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Warnes, C. A., Liberthson, R., Danielson, G. K., Dore, A., Harris, L. et al. (2001). Task force 1: the changing profile of congenital heart disease in adult life. Journal of American College of Cardiology, 37(5), 1170–1175. DOI 10.1016/s0735-1097(01)01272-4. [Google Scholar] [CrossRef]
2. Maxwell, B. G., Wong, J. K., Kin, C. (2013). Perioperative outcomes of major noncardiac surgery in adults with congenital heart disease. Anesthesiology, 119(4), 762–769. DOI 10.1097/ALN.0b013e3182a56de3. [Google Scholar] [CrossRef]
3. Rodriguez, F. H. 3rd, Moodie, D. S., Parekh, D. R., Franklin, W. J., Morales, D. L. et al. (2011). Outcomes of hospitalization in adults in the United States with atrial septal defect, ventricular septal defect, and atrioventricular septal defect. American Journal of Cardiology, 108(2), 290–293. DOI 10.1016/j.amjcard.2011.03.036. [Google Scholar] [CrossRef]
4. Billett, J., Cowie, M. R., Gatzoulis, M. A., Vonder Muhll, I. F., Majeed, A. (2008). Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case control analysis. Heart, 94(9), 1194–1199. DOI 10.1136/hrt.2007.122671. [Google Scholar] [CrossRef]
5. Oechslin, E. N., Harrison, D. A., Connelly, M. S., Webb, G. D., Siu, S. C. (2000). Mode of death in adults with congenital heart disease. American Journal of Cardiology, 86(10), 1111–1116. DOI 10.1016/s0002-9149(00)01169-3. [Google Scholar] [CrossRef]
6. Kristensen, S. D., Knuuti, J., Saraste, A., Anker, S., Botker, H. E. et al. (2014). ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). European Heart Journal, 35(35), 2383–2431. DOI 10.1093/eurheartj/ehu282. [Google Scholar] [CrossRef]
7. Fleisher, L. A., Fleischmann, K. E., Auerbach, A. D., Barnason, S. A., Beckman, J. A. et al. (2014). ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/AmericanHeart Association Task Force on Practice Guidelines. Journal of American College of Cardiology, 64(22), e77–e137. DOI 10.1016/j.jacc.2014.07.944. [Google Scholar] [CrossRef]
8. Lee, T. H., Marcantonio, E. R., Mangione, C. M., Thomas, E. J., Polanczyk, C. A. et al. (1999). Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation, 100(101043–1049. DOI 10.1161/01.cir.100.10.1043. [Google Scholar] [CrossRef]
9. Gupta, P. K., Gupta, H., Sundaram, A., Kaushik, M., Fang, X. et al. (2011). Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation, 124(4), 381–387. DOI 10.1161/CIRCULATIONAHA.110.015701. [Google Scholar] [CrossRef]
10. Warner, M. A., Lunn, R. J., O’Leary, P. W., Schroeder, D. R. (1998). Outcomes of noncardiac surgical procedures in children and adults with congenital heart disease. Mayo Clinic Proceedings, 73(8), 728–734. DOI 10.4065/73.8.728. [Google Scholar] [CrossRef]
11. Webb, G. D., Williams, R. G. (2001). Care of the adult with congenital heart disease. Presented at the 32nd Bethesda conference. Journal of American College of Cardiology, 37(5), 1162–1165. [Google Scholar]
12. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019, 2018). AHA/ACC Guideline for the management of adults with congenital heart disease: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines. Journal of American College of Cardiology, 73(12), e81–e192. DOI 10.1016/j.jacc.2018.08.1029. [Google Scholar] [CrossRef]
13. Ombelet, F., Goossens, E., Van de Bruaene, A., Budts, W., Moons, P. (2020). Newly developed adult congenital heart disease anatomic and physiological classification: first predictive validity evaluation. Journal of American Heart Association, 9(5), e014988. DOI 10.1161/JAHA.119.014988. [Google Scholar] [CrossRef]
14. Rabbitts, J. A., Groenewald, C. B., Mauermann, W. J., Barbara, D. W., Burkhart, H. M. et al. (2013). Outcomes of general anesthesia for noncardiac surgery in a series of patients with Fontan palliation. Paediatric Anaesthesia, 23(2), 180–187. DOI 10.1111/pan.1202. [Google Scholar] [CrossRef]
15. Bennett, J. M., Ehrenfeld, J. M., Markham, L., Eagle, S. S. (2014). Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: A review of institutional experience. Journal of Clinical Anesthesia, 26, 286–293. DOI 10.1016/j.jclinane.2013.11.022. [Google Scholar] [CrossRef]
16. Kaye, A. D., Stout, T. B., Padnos, I. W., Schwartz, G., Baluch, A. R. et al. (2012). Left-to-right cardiac shunt: perioperative anesthetic considerations. Middle East Journal of Anaesthesiology, 21(6), 793–806. [Google Scholar]
17. White, M. C., Peyton, J. M. (2012). Anaesthetic management of children with congenital heart disease for non-cardiac surgeryContinuing Education in Anaesthesia Critical Care and Pain, 12(1), 17–22. [Google Scholar]
18. Khairy, P., Van Hare, G. F., Balaji, S., Berul, C. I., Cecchin, F. et al. (2014). PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the pediatric and congenital electrophysiology society (PACES) and the Heart Rhythm Society (HRS). Heart Rhythm, 11(10), e102–e165. DOI 10.1016/j.hrthm.2014.05.009. [Google Scholar] [CrossRef]
19. Ginde, S., Bartz, P. J., Hill, G. D., Danduran, M. J., Biller, J. et al. (2013). Restrictive lung disease is an independent predictor of exercise intolerance in the adult with congenital heart disease. Congenital Heart Disease, 8(3), 246–254. DOI 10.1111/chd.12010. [Google Scholar] [CrossRef]
20. Alonso-Gonzalez, R., Borgia, F., Diller, G. P., Inuzuka, R., Kempny, A. et al. (2013). Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation, 127(8), 882–890. DOI 10.1161/CIRCULATIONAHA.112.126755. [Google Scholar] [CrossRef]
21. Dimopoulos, K., Diller, G. P., Koltsida, E., Pijuan-Domenech, A., Papadopolou, S. A. et al. (2008). Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation, 117(18), 2320–2328. DOI 10.1161/CIRCULATIONAHA.107.734921. [Google Scholar] [CrossRef]
22. Rychik, J., Atz, A. M., Celermajer, D. S., Deal, B. J., Gatzoulis, M. A. et al. (2019). Evaluation and management of the child and adult with Fontan circulation: A scientific statement from the American heart association. Circulation, DOI 10.1161/CIR.0000000000000696. [Google Scholar] [CrossRef]
23. Niwa, K., Perloff, J. K., Kaplan, S., Child, J. S., Miner, P. D. (1999). Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricularheart. Journal of American College of Cardiology, 34(1), 223–232. DOI 10.1016/s0735-1097(99)00153-9. [Google Scholar] [CrossRef]
24. Khairy, P., Aboulhosn, J., Broberg, C. S., Cohen, S., Cook, S. et al. (2016). Anticoagulation therapy in congenital heart disease (TACTIC) investigators and the alliance for adult research in congenital cardiology (AARCC). Thromboprophylaxis for atrial arrhythmias in congenital heart disease: A multicenter study. International Journal of Cardiology, 223, 729–735. DOI 10.1016/j.ijcard.2016.08.223. [Google Scholar] [CrossRef]
25. Firdouse, M., Agarwal, A., Chan, A. K., Mondal, T. (2014). Thrombosis and thromboembolic complications in Fontan patients: A literature review. Clinical and Applied Thrombosis/Hemostasis, 20(5), 484–492. [Google Scholar]
26. Atz, A. M., Zak, V., Mahony, L., Uzark, K., D’agincourt, N. et al. (2017). Longitudinal outcomes of patients with single ventricle after the Fontan procedure. Journal of American College of Cardiology, 69(22), 2735–2744. [Google Scholar]
27. Broberg, C. S., Ujita, M., Prasad, S., Li, W., Rubens, M. et al. (2007). Pulmonary arterial thrombosis in Eisenmenger syndrome is associated with biventricular dysfunction and decreased pulmonary flow velocity. Journal of American College of Cardiology, 50(7), 634–642. DOI 10.1016/j.jacc.2007.04.056. [Google Scholar] [CrossRef]
28. Jensen, A. S., Idorn, L., Thomsen, C., von der Reckes, P., Mortesen, J. et al. (2015). Prevalence of cerebral and pulmonary thrombosis in patients with cyanotic congenital heart disease. Heart, 101(19), 1540–1546. DOI 10.1136/heartjnl-2015-307657. [Google Scholar] [CrossRef]
29. Odegard, K. C., McGowan, F. X. Jr, Zurakowski, D., Dinardo, J. A., Castro, R. A. et al. (2003). Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. Journal of Thoracic and Cardiovascular Surgery, 25(6), 1260–1267. DOI 10.1016/s0022-5223(02)73605-2. [Google Scholar] [CrossRef]
30. Yang, H., Bouma, B. J., Dimopoulos, K., Khairy, P., Ladouceur, M. et al. (2020). Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. International Journal of Cardiology, 299, 123–130. DOI 10.1016/j.ijcard.2019.06.014. [Google Scholar] [CrossRef]
31. Pujol, C., Niesert, A. C., Engelhardt, A., Schoen, P., Kusmenkov, E. et al. (2016). Usefulness of direct oral anticoagulants in adult congenital heart disease. American Journal of Cardiology, 117(3), 450–455. [Google Scholar]
32. Steffel, J., Verhamme, P., Potpara, T. S., Albaladejo, P., Antz, M. et al. (2018). The 2018 European heart rhythm association. Practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. European Heart Journal, 39(16), 1330–1393. DOI 10.1093/eurheartj/ehy136. [Google Scholar] [CrossRef]
33. Habib, G., Lancellotti, P., Antunes, M. J., Bongiorni, M. G., Casalta, J. P. et al. (2015, 2015). ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC). Endorsed by: European association for cardio-thoracic surgery (EACTSthe European association of nuclear medicine (EANM). European Heart Journal, 36(44), 3075–3128. DOI 10.1093/eurheartj/ehv319. [Google Scholar] [CrossRef]
34. Wilson, W., Taubert, K. A., Gewitz, M., Lokhart, P. B., Baddour, L. M. et al. (2007). Prevention of infective endocarditis: guidelines from the American heart association: a guideline from the American heart association Rheumatic fever, endocarditis, and Kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and Anesthesia, and the quality of care and outcomes research interdisciplinary working group. Circulation, 116(15), 1736–1754. DOI 10.1161/CIRCULATIONAHA.106.183095. [Google Scholar] [CrossRef]
35. Abdelghani, M., Nassif, M., Blom, N. A., Van Mourik, M. S., Straver, B. et al. (2018). Infective endocarditis after melody valve implantation in the pulmonary position: A systematic review. Journal of American Heart Association, 7(13), e008163. DOI 10.1161/JAHA.117.008163. [Google Scholar] [CrossRef]
36. Inuzuka, R., Diller, G. P., Borgia, F., Benson, L., Tay, E. L. et al. (2012). Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation, 125(2), 250–259. [Google Scholar]
37. Goeddel, L. A., Jung, Y. H., Patel, P., Upchurch, P., Fernando, R. J. et al. (2020). Analysis of the 2018 American heart association/American college of cardiology guidelines for the management of adults with congenital heart disease: implications for the cardiovascular Anesthesiologist. Journal of Cardiothoracic and Vascular Anesthesia, 34(5), 1348–1365. DOI 10.1053/j.jvca.2019.08.004. [Google Scholar] [CrossRef]
38. Mylotte, D., Pilote, L., Ionescu-Ittu, R., Abrahamowicz, M., Khairy, P. et al. (2014). Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation, 129(18), 1804–1812. DOI 10.1161/CIRCULATIONAHA.113.005817. [Google Scholar] [CrossRef]
39. Maxwell, B. G., Wong, J. K., Lobato, R. L. (2014). Perioperative morbidity and mortality after noncardiac surgery in young adults with congenital or early acquired heart disease: A retrospective cohort analysis of the national surgical quality improvement program database. The American Journal of Surgery, 80(4), 321–326. [Google Scholar]
40. Cannesson, M., Earing, M. G., Collange, V., Kersten, J. R. (2009). Anesthesia for noncardiac surgery in adults with congenital heart disease. Anesthesiology, 111(2), 432–440. DOI 10.1097/ALN.0b013e3181ae51a6. [Google Scholar] [CrossRef]
41. Khairy, P., Poirier, N., Mercier, L. A. (2007). Univentricular heart. Circulation, 115(6), 800–812. DOI 10.1161/CIRCULATIONAHA.105.592378. [Google Scholar] [CrossRef]
42. Maxwell, B. G., Posner, K. L., Wong, J. K., Oakes, D. A., Kelly, N. E. et al. (2015). Factors contributing to adverse perioperative events in adults with congenital heart disease: a structured analysis of cases from the closed claims project. Congenital Heart Disease, 10(1), 21–29. DOI 10.1111/chd.12188. [Google Scholar] [CrossRef]
43. Nasr, V. G., Guzzetta, N. A., Miller-Hance, W. C., Twite, M., Latham, G. J. et al. (2018). Consensus statement by the congenital cardiac anesthesia society: milestones for the pediatric cardiac anesthesia fellowship. Anesthesia & Analgesia, 126(1), 198–207. DOI 10.1213/ANE.0000000000002482. [Google Scholar] [CrossRef]
44. Hasija, S., Chauhan, S., Jain, P., Choudhury, A., Aggarwal, N. et al. (2016). Comparison of speed of inhalational induction in children with and without congenital heart disease. Annals of Cardiac Anaesthesia, 19(3), 468–474. DOI 10.4103/0971-9784.185531. [Google Scholar] [CrossRef]
45. Bennett, J. M., Ehrenfeld, J. M., Markham, L., Eagle, S. S. (2014). Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: A review of institutional experience. Journal of Clinical Anesthesia, 26(4), 286–293. DOI 10.1016/j.jclinane.2013.11.022. [Google Scholar] [CrossRef]
46. Friesen, R. H. (2014). Anesthetic drugs in congenital heart disease. Seminars in Cardiothoracic and Vascular Anesthesia, 18(4), 363–370. DOI 10.1177/1089253214543381. [Google Scholar] [CrossRef]
47. Loomba, R. S., Gray, S. B., Flores, S. (2018). Hemodynamic effects of ketamine in children with congenital heart disease and/or pulmonary hypertension. Congenital Heart Disease, 13(5), 646–654. DOI 10.1111/chd.12662. [Google Scholar] [CrossRef]
48. Goyal, R., Singh, S., Bangi, A., Singh, S. K. (2013). Case series: dexmedetomidine and ketamine for anesthesia in patients with uncorrected congenital cyanotic heart disease presenting for non-cardiac surgery. Journal of Anaesthesiology Clinical Pharmacology, 29(4), 543–546. DOI 10.4103/0970-9185.119142. [Google Scholar] [CrossRef]
49. Bouch, D. C., Allsager, C. M., Moore, N. (2006). Peri-operative trans-oesophageal echocardiography and nitric oxide during general anaesthesia in a patient with Eisenmenger’s syndrome. Anaesthesia, 61(10), 996–1000. DOI 10.1111/j.1365-2044.2006.04758.x. [Google Scholar] [CrossRef]
50. McClain, C. D., McGowan, F. X., Kovatsis, P. G. (2006). Laparoscopic surgery in a patient with Fontan physiology. Anesthesia & Analgesia, 103(4), 856–858. DOI 10.1213/01.ane.0000237294.88298.8e. [Google Scholar] [CrossRef]
51. Uchida, S., Yamamoto, M., Masaoka, Y., Mikouchi, H., Nishizaki, Y. (1999). A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery. Heart and Vessels, 14(4), 197–200. DOI 10.1007/bf02482307. [Google Scholar] [CrossRef]
52. de Jong, K. I. F., de Leeuw, P. W. (2019). Venous carbon dioxide embolism during laparoscopic cholecystectomy a literature review. European Journal of Internal Medicine, 60, 9–12. DOI 10.1016/j.ejim.2018.10.008. [Google Scholar] [CrossRef]
53. Patel, J. H., Szymanski, T. J., Metzler, E. C., Worthington, A. H., Body, S. C. et al. (2012). Rescue transesophageal echocardiography for the diagnosis and management of paradoxical carbon dioxide embolism with hemodynamic compromise during laparoscopic surgery. Journal of Cardiothoracic and Vascular Anesthesia, 27(3), e23–e24. DOI 10.1053/j.jvca.2012.09.020. [Google Scholar] [CrossRef]
54. Wahba, R. W., Tessler, M. J., Kleiman, S. J. (1996). Acute ventilatory complications during laparoscopic upper abdominal surgery. Canadian Journal of Anaesthesia, 43(1), 77–83. [Google Scholar]
55. Vischoff, D., Fortier, L. P., Villeneuve, E., Spilsbury, J., Miller, P. et al. (2001). Anaesthetic management of an adolescent for scoliosis surgery with a Fontan circulation. Paediatric Anaesthesia, 11, 607–610. [Google Scholar]
56. Domaingue, C. M. (2005). Anaesthesia for neurosurgery in the sitting position: A practical approach. Anaesthesia & Intensive Care, 33(3), 323–331. DOI 10.1177/0310057X0503300307. [Google Scholar] [CrossRef]
57. Fathi, A. R., Eshtehardi, P., Meier, B. (2009). Patent foramen ovale and neurosurgery in sitting position: A systematic review. British Journal of Anaesthesia, 102(5), 588–596. DOI 10.1093/bja/aep063. [Google Scholar] [CrossRef]
58. Klein, J., Juratli, T. A., Weise, M., Schackert, G. (2018). A systematic review of the semi-sitting position in neurosurgical patients with patent foramen ovale: how frequent is paradoxical embolism? World Neurosurgery, 115, 196–200. DOI 10.1016/j.wneu.2018.04.114. [Google Scholar] [CrossRef]
59. Ammash, N. M., Connolly, H. M., Abel, M. D., Warnes, C. A. (1999). Noncardiac surgery in Eisenmenger syndrome. Journal of American College of Cardiology, 33(1), 222–227. DOI 10.1016/s0735-1097(98)00554-3. [Google Scholar] [CrossRef]
60. Palumbo, T., Sluysmans, T., Rubay, J. E., Poncelet, A. J., Momeni, M. (2015). Long-term outcome and anaesthetic management for non-cardiac surgery after Fontan palliation: A single-centre retrospective analysis. Cardiology in the Young, 25(6), 1148–1154. DOI 10.1017/S1047951114001814. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |